SUMMARY
The effects of an oral fish oil treatment regimen on sensorimotor, blood-brain barrier, and biochemical outcomes of traumatic brain injury (TBI) were investigated in a juvenile rat model. Seventeen-day old Long-Evans rats were given a 15 mL/kg fish oil (2.01 g/kg EPA, 1.34 g/kg DHA) or soybean oil dose via oral gavage 30 minutes prior to being subjected to a controlled cortical impact injury or sham surgery, followed by daily doses for seven days. Fish oil treatment resulted in less severe hindlimb deficits after TBI as assessed with the beam walk test, decreased cerebral IgG infiltration, and decreased TBI-induced expression of the Mmp9h gene one day after injury. These results indicate that fish oil improved functional outcome after TBI resulting, at least in part from decreased disruption of the blood-brain barrier through a mechanism that includes attenuation of TBI-induced expression of Mmp9.
Keywords: traumatic brain injury, juvenile, rat, fish oil, blood-brain barrier, matrix metalloproteinase 9
INTRODUCTION
Traumatic brain injury (TBI) is a major cause of death and acquired disability in young children [1]. Despite having a high degree of neuroplasticity, young children tend to have poorer outcomes after TBI than adults [2]. Children can also exhibit differential responses to pharmacological interventions including altered bioavailability, metabolism, and drug response [3, 4]. It is thus critical that both the effect of TBI and potential therapeutics be investigated in an age-appropriate model [5].
The n-3 long-chain polyunsaturated fatty acids (LC-PUFA) eicosapentaenoic acid (EPA, 20:6n-3) and docosapentaenoic acid (DHA, 22:6n-3), the main constituents in fish oil, are biologically active with many neuroprotective properties. When consumed in the diet or via supplementation, these n-3 fatty acids are incorporated into the phospholipids that form cell membranes where they can alter the physicochemical and membrane-signaling properties of the cell [6, 7]. DHA, EPA and their metabolites also have anti-excitotoxic [8], antioxidant [9], anti-apoptotic [10, 11], and anti-inflammatory properties [12]. During development, N-3 fatty acids, particularly DHA, accumulate in the brain during late gestation and early post-neonatal life in humans and rats, a time at which children have a high risk for sustaining traumatic brain injuries [13–15]. Additionally, EPA and DHA can be metabolized into several families of molecules including NPD1, docosanoids, resolvins, etc., which have been shown be neuroprotective through their anti-inflammatory and inflammation-resolving activities [16–18]. LC-PUFA can also directly or indirectly modulate gene expression through activation or suppression of cell signaling pathways and transcription factors (e.g., PI3K/Akt, NF-κB, PPAR and RXR) [19–21].
DHA and EPA, the primary active constituents in fish oil, readily cross the blood-brain barrier [22]. Studies of various neural injury models including TBI and spinal cord injuries in adult animals indicate that fish oil, or DHA or EPA alone produce beneficial effects [23–25]. Furthermore, in a case report, high dose fish oil supplementation (19.2 g/day) was associated with substantial clinical improvement in a young patient with severe, potentially lethal, head trauma [26]. However, the effects of LC-PUFA or fish oil treatment have not been investigated in juvenile brain injury. Accordingly, this study investigated the use of oral fish oil treatment in a juvenile rat model on sensorimotor and biochemical outcomes of TBI, including blood-brain barrier disruption and levels of Ccl2, Gfap, and Mmp9 mRNAs, representative key mediators involved in immune cell recruitment, astrocytosis, and blood-brain barrier disruption after TBI. We will show that fish oil resulted in improved functional outcome, at least in part, by limiting disruption of the blood-brain barrier through a mechanism that includes attenuation of TBI-induced expression of Mmp9.
MATERIALS AND METHODS
All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Animals, husbandry, and treatment
Long-Evans rats were housed in a temperature- and humidity-controlled facility with a 14–10 hour light-dark cycle (on at 06:00 h) with ad libitum access to water and chow (Teklad Global diet 2016, Harlan Laboratories, Inc., Indianapolis, IN). The chow contained 4% fat by weight and was formulated with soybean oil resulting in a fatty acid composition of 0.5% 16:0, 0.1% 18:0, 0.7% 18:1n-9, 2% 18:2n-6, 0.1% 18:3n-3. Breeding stock (females 75–85 days; male proven breeders; Harlan Laboratories, Inc. Indianapolis, IN) were obtained a minimum of 5 days prior to the beginning of the experiment and were handled regularly. Litters were culled to eight pups with preference for males on postnatal day (PND) one. Male pups (n = 4–12/group, depending on endpoint, and each from a different litter) received either a controlled cortical impact (CCI) injury or sham surgery on PND 17 and were returned to the dam until weaning. Pups were weaned on PND 20 and housed in groups of two to four, TBI and sham-injured together, for the remainder of the study.
Rats were treated with either 15 mL/kg of fish oil (2.01 g/kg EPA, 1.34 g/kg DHA; Nature Made 1200 mg fish oil capsules, Mission Hills, CA) or unhydrogenated soybean oil via oral gavage 30 minutes prior to the initial TBI or Sham surgery, and then daily for seven days. On days behavioral testing occurred (see below), oil was administed after testing to avoid any confounding effect the gavage procedure might have on behavior. On the day of euthanasia, rats were decapitated without anesthesia approximately six hours after oil administration. Soybean oil was used as the comparator in this study to control for the caloric content of the fish oil, and, because it is the oil used in the formulation of the Teklad Global #2016, it does not introduce any fatty acids not already consumed by rats in both treatment groups. The fatty acid composition of the oils is shown in Table 1.
Table 1.
Fish and soybean oil fatty acid composition.
| Percent of Total Fatty Acids (wt%) | ||
|---|---|---|
| Fatty Acid | Fish Oil | Soybean Oil |
| 14:0 | 7.73 | ND |
| 16:0 | 15.95 | 11.12 |
| 16:1 | 13.63 | 0.10 |
| 18:0 | 5.18 | 5.05 |
| 18:1n-9c | 10.26 | 20.99 |
| 18:2n-6c | 3.63 | 50.10 |
| 18:3n-6 | 0.45 | ND |
| 18:3n-3 | 0.77 | 9.74 |
| 20:1n-9 | 1.04 | 0.13 |
| 20:2n-6 | 0.37 | 0.32 |
| 20:3n-6 | 0.23 | ND |
| 20:4n-6 | 1.70 | 0.02 |
| 22:1 | 0.22 | 0.04 |
| 20:5n-3 | 11.57 | ND |
| 22:2n-6 | 0.13 | 0.27 |
| 22:5n-6 | 0.36 | ND |
| 22:6n-3 | 16.39 | ND |
ND: not detected
Rats used for assessment of sensorimotor function were tested 1, 4, and 7 days after surgery and then euthanized on day 7 by transcardial perfusion under pentobarbital anesthesia, followed by removal of the brain for IgG immunohistochemistry. Rats used for determination of mRNA levels were euthanized on day 1 (28 hrs after surgery) or day 4 after surgery by decapitation. Brains from these rats were rapidly removed and then dissected on ice. The frontal cortex, a cortical region remote from the contusion site, which was not needed for mRNA analyses and has a fatty acid composition representative of whole brain [27], was frozen on dry ice for later fatty acid analysis. The injured motor cortex was preserved in RNAlater (Life Technologies/Ambion, Gaithersburg, MD) for mRNA analysis.
Controlled cortical Impact
CCI was performed as previously described [28, 29]. Briefly, rats were stabilized in a Cunningham stereotaxic frame (Stoelting, Wood Dale, IN) after being anesthetized with isoflurane (induction, 3.0%; maintenance, 2.0%). A 4×4 mm craniotomy was performed lateral (right side) to the mid-sagittal suture, centered at: AP = 0, ML = 2.5 from bregma. The impactor device, previously described in detail [30] was equipped with a 3.0 mm-diameter tip. The impactor tip was centered within the craniotomy and lowered until the tip just contacted the dura over motor (M1, M2) and sensory (S1FL, S1HL) cortical areas [31, 32]. The parameters of the impact were as follows: 3.0 mm depth, 1.5 m/sec strike velocity, 300 msec contact time. The scalp incision was then closed with a 6–0 silk suture and the animal was allowed to recover from anesthesia.
Sham procedures involving the use of a trephine or drill to produce craniotomy have been shown to cause brain injury distinct from that caused by the impact [33, 34]. Though cortical damage caused by TBI significantly outweighs damage produced by the craniotomy [35], the sham surgery consisted of an incision in the scalp with no craniotomy or impact from the CCI device to avoid potential experimental confounds that might be caused by any additional intervention.
All rats received 0.05 mg/kg of buprenorphine approximately thirty minutes after surgery and again 24 hours after surgery, after day 1 behavioral testing was completed. The administration of buprenorphine analgesia after CCI surgery was required by the Institutional Animal Care and Use Committee. Buprenorphine is a μ-opioid receptor partial agonist and is thus analgesic, but not anti-inflammatory [36]. Opioid analgesics are clinically contraindicated for use in TBI because they can increase intracranial pressure thus exacerbating the effects of injury; however, the craniotomy required for the CCI likely minimizes this effect. Furthermore, the administration of buprenorphine to all treatments groups ensures that any effects it may produce are controlled for.
Beam walk test
Rats were tested for their ability to traverse a 75 cm-long wooden dowel (15 mm diameter) elevated 30 cm and ending in a dark goal box as previously described in detail [29]. Video-taped sessions were scored for ipsilateral and contralateral foot slips, time required to reach the goal box, and total number of steps taken by the right (uninjured) hind foot. Data are reported as the percent of contralateral foot slips relative to the total number of steps needed to traverse the beam.
Brain Total Phospholipid Fatty Acid Composition
Brain total phospholipid fatty acid composition was analyzed as previously described [27]. Briefly, phospholipids were extracted from frontal cortex and isolated by thin layer chromatography. The phospholipids were then transmethylated with boron trifluoride methanol (Sigma, St. Louis, MO) to produce fatty acid methyl esters. Individual fatty acid methyl esters were analyzed using a Varian 3400 gas chromatograph with an SP-2330 capillary column (30 m, Supelco, Inc., Belfonte, PA), using helium as the carrier gas. Peaks were identified by comparing to authentic standards (PUFA 1 and 2, and Supelco 37, Supelco, Inc. and 22:5n-6, Nu-Chek Prep, Elysian, MN) and corrected for response factors. Individual fatty acids were expressed as weight percent of total fatty acids on the basis of peak area.
IgG Immunohistochemistry
Frozen coronal sections (30 μm) through the site of impact (+2.0 mm to −2.0 mm bregma), or the corresponding area in shams, collecting every tenth section, and mounted on Fisherbrand Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were stained for IgG using a Vectastain Rat IgG ABC Kit (Vector Labs, Burlingame, CA) according to the manufacturer’s protocol. IgG immunoreactivity was visualized using ABC (streptavidin-HRP) and diaminobenzidine tetrahydrochloride (Vector Labs, Burlinggame, CA). ImageJ [37] was used to determine the ipsilateral cortical IgG staining density and total area of IgG staining (both hemispheres) from macro-level digitized grayscale images of each section. The total area of IgG staining was calculated from IgG staining in the entirety of both hemispheres. A baseline grayscale value threshold was set at 75 based on the background level of nonspecific staining in sham sections. Any areas darker than the threshold were considered positive for IgG staining. The volume of IgG staining was calculated using the following equation: IgG area * section thickness * distance between sections = Subvolume. Total IgG Volume = Σ Subvolume (section1 + section2 + …sectionn).
Quantitative Real-Time PCR
Quantitative real-time PCR was performed as previously described in detail [28]. Total RNA was isolated using a Trizol (Life Technologies/Ambion) phenol-chloroform extraction according to the manufacturer’s protocol and precipitated overnight with 75% isopropyl alcohol. The quality of isolated RNA quality was determined using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) with adequate quality being an OD 260/280 greater than 1.8. mRNA quality was further verified using a Agilent Bioanalyzer 2011 (Agilent Technologies, Inc., Santa Clara, CA). First strand cDNAs were synthesized using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) per the manufacturer’s protocol using a PTC-100 Peltier Thermal Cycler (MJ Research, Waltham, MA). Exon spanning, gene-specific primers (Supplemental Table 1) were prepared using the NCBI’s Primer-BLAST [38] and purchased from Integrated DNA Technologies, Inc. (Coralville, IA). Primer specificity was determined by the presence of a single peak in the melt curve. Relative gene expression was calculated using the 2−ΔΔCt method [39]. Data are expressed as fold change in gene expression compared to the reference gene beta-2-microglobulin (B2m), which was experimentally determined to be the most stably expressing gene in our experimental model and brain region of interest [40].
Statistical Analysis
All data are expressed at the mean ± SEM. Data were analyzed for effects of injury (TBI or Sham-injured) and oil (Soybean oil or Fish oil) by ANOVA with factors of TBI, oil, and day after injury (1–7 days after injury) (Systat, v.12). Time after injury was analyzed as repeated measure for the sensorimotor function studies. Outliers identified by Systat were discarded from subsequent analyses. Post-hoc comparisons were made using 1-way ANOVA and Fisher’s Least Significant Difference test, or Student’s-t test. Differences were considered significant if P < 0.05.
RESULTS
Effects on Growth, Development, and Brain Phospholipid Fatty Acid Composition
Overall growth and development of the rats was not affected by TBI or oil treatment (Figure 1).
Figure 1. Effects of injury and fish oil treatment on body weight.
Data are the mean ± SEM (n = 11–12 per group). Rate of weight gain did was not altered by TBI or oil treatments as assessed by repeated-measures ANOVA.
Total phospholipid fatty acid composition was assessed in the frontal cortex of each brain four days after injury (Table 2). In Sham-injured rats, administration of fish oil reduced the percentage of the monounsaturated fatty acid (MUFA) 24:1 and Other MUFA by 16% (P < 0.05) and 57% (P < 0.05), respectively, compared to soybean oil. Fish oil administration also significantly reduced the Other n-6 and 17% (P < 0.01) compared to soybean oil-treated shams.
Table 2.
Effects of TBI and fish or soybean oil treatment on total phospholipid fatty acid composition of frontal cortex four days after initiation of treatment with fish oil or soybean oil.
| Percent of Total Fatty Acids (wt%) | ||||
|---|---|---|---|---|
| Soybean Oil | Fish Oil | |||
| Fatty Acid | Sham | TBI | Sham | TBI |
| 16:0 | 24.14 ± 0.85 | 27.27 ± 1.07 | 26.90 ± 1.66 | 25.72 ± 0.73 |
| 18:0 | 23.07 ± 0.67 | 23.16 ± 0.76 | 17.98 ± 3.99 | 24.48 ± 0.76 |
| Other SFA | 1.42 ± 0.16 | 2.60 ± 0.34 | 2.32 ± 0.53 | 2.02 ± 0.31 |
| 18:1n9 | 9.17 ± 1.67 | 10.17 ± 1.27 | 11.46 ± 0.77 | 10.13 ± 0.51 |
| 24:1 | 4.74 ± 0.25 | 4.04 ± 0.26 | 3.99 ± 0.22b | 3.43 ± 0.20b |
| Other MUFA | 2.27 ± 0.12 | 1.44 ± 0.26a | 0.97 ± 0.17b | 1.58 ± 0.35 |
| 20:4n6 | 14.60 ± 0. 41 | 13.57 ± 0.56 | 15.26 ± 0.79 | 13.39 ± 0.44 |
| 22:5n6 | 1.97 ± 0.16 | 1.66 ± 0.10 | 1.64 ± 0.10 | 1.40± 0.10b |
| Other n-6 | 2.28 ± 0.07 | 2.28 ± 0.06 | 1.75 ± 0.17b | 1.75 ± 0.21bc |
| 22:6n3 | 17.09 ± 1.72 | 13.98 ± 0.70 | 18.49 ± 1.20 | 15.86 ± 0.83 |
| Other n-3 | 0.08 ± 0.04 | 0.05 ± 0.03 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| ΣSFA | 48.64 ± 1.61 | 53.02 ± 1.59 | 47.20 ± 2.11 | 52.21 ± 0.66 |
| ΣMUFA | 16.18 ± 1.47 | 15.65 ± 1.50 | 16.42 ± 0.99 | 15.14 ± 0.42 |
| Σn-6 | 18.85 ± 0.56 | 17.51 ± 0.59 | 18.64 ± 0.92 | 16.54 ± 0.47 |
| Σn-3 | 17.17 ± 1.74 | 14.05 ± 0.70 | 18.55 ± 1.20 | 15.92 ± 0.83 |
| ΣPUFA | 36.02 ± 2.17 | 31.54 ± 1.21 | 37.20 ± 1.95 | 32.46 ± 1.05 |
Other saturated fatty acids (SFA): 13:0, 14:0, 15:0, 17:0, 18:0, 20:0, 21:0, 22:0, 23:0, 24:0; Other monounsaturated fatty acids (MUFA): 15:1, 16:1, 20:1n-9; Other n-6: 18:2n-6c, 18:3n-6, 20:2n-6, 20:3n-6, 22:2n-6; Other n-3: 18:3n-3, 20:5n-3. Data are presented as the mean ± SEM (n = 6-7 per group selected at random from the total sample size).
P < 0.05 vs. Sham, Same Oil;
P < 0.05 vs. Sham, Different Oil;
P < 0.05 vs. TBI, Different Oil by ANOVA and Fisher’s LSD test.
Two-way ANOVA indicated that TBI produced a significant main effect of decreased Total PUFA (P < 0.05), Total n-3 (P < 0.05) and Total n-6 (P < 0.05), resulting from significant decreases in 22:6n-3 (P < 0.05), 20:4n-6 (P < 0.05) and 22:5n-6 (P < 0.05), and an increase in Total SFA (P<0.01); however, these effects were not quite statistically significant in post hoc comparisons (P = 0.05 – 0.09).
TBI resulted in a significant main effect of decreased Total PUFA (P < 0.05), Total n-3 (P < 0.05) and Total n-6 (P < 0.05), resulting from significant decreases in 22:6n-3 (P < 0.05), 20:4n-6 (P < 0.05) and 22:5n-6 (P < 0.05), and an increase in Total SFA (P < 0.01); however, these effects were not quite statistically significant in post hoc comparisons (P = 0.05 −0.09).
Effects on Sensorimotor Function
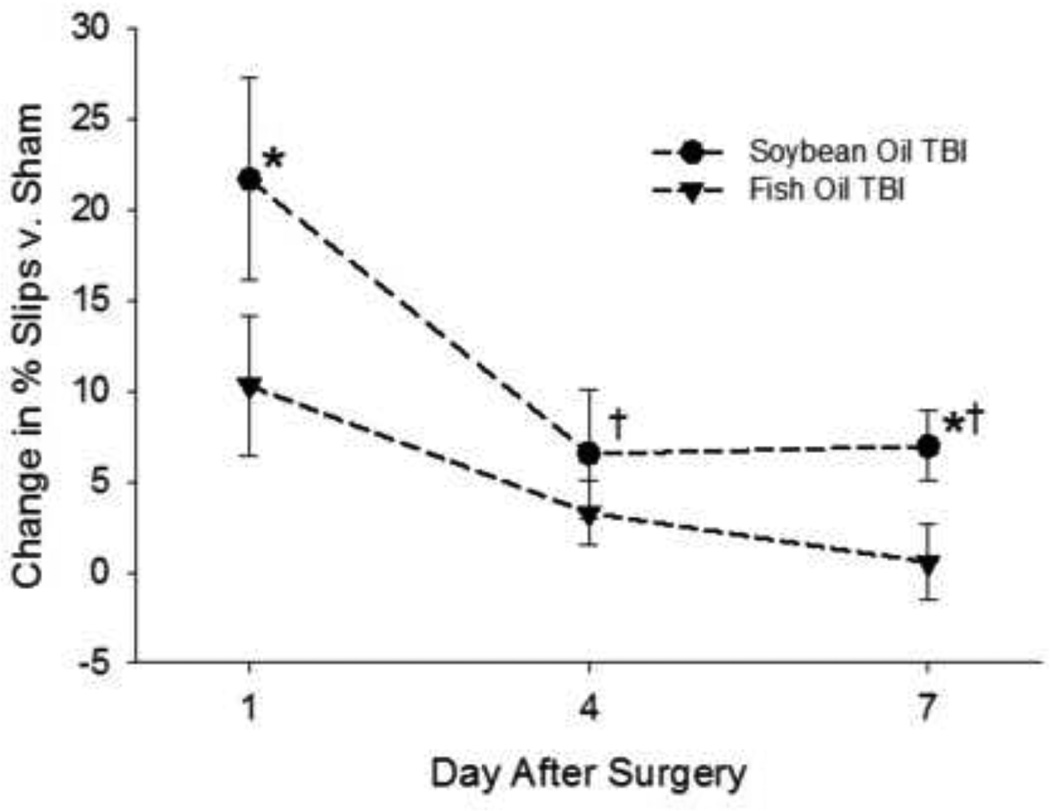
Sensorimotor function was altered in all rats that sustained a TBI as indicated by an overall significant increase in the percentage of unilateral beam walk slips (Figure 2), indicating deficits in hindlimb function [41]. Repeated-measures ANOVA indicated that rats treated with either oil exhibited significant improvement from day one to day seven; however, post -hoc analysis indicated that rats treated with fish oil had less functional deficit on days one and seven after injury than those treated with soybean oil (P < 0.05).
Figure 2. Effects of fish oil treatment of TBI-induced hindlimb deficits.
assessed using the beam walk test. Data are the mean ± SEM (n = 11–12 per group). *P < 0.05 vs. same day, different oil; †P < 0.05 vs. same oil, day one by repeated-measures ANOVA and Fisher’s LSD test.
Effects on IgG staining
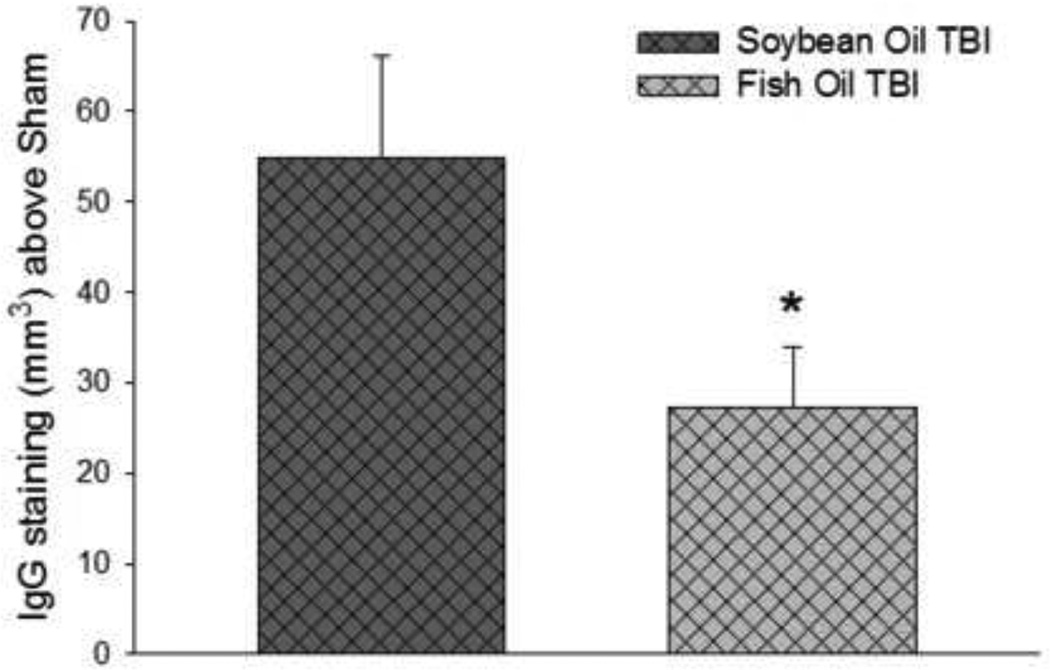
Rats with TBI exhibited extensive IgG staining seven days after TBI. There was no significant difference in the density of IgG staining between the injured rats in both oil treatment groups (not shown). There was, however, a significant interaction with the oil treatments such that IgG infiltrated a smaller volume of brain area in rats treated with fish oil than with soybean oil (P < 0.05) (Figure 3). The volume of IgG stained tissue was not different in sham-injured rats treated with either oil (not shown).
Figure 3. Effects of fish oil treatment on TBI-induced cerebral IgG Infiltration.
Data are the mean ± SEM (n = 10–11 per group). *P < 0.05 by Student’s-t test.
Effects on TBI-induced mRNA levels
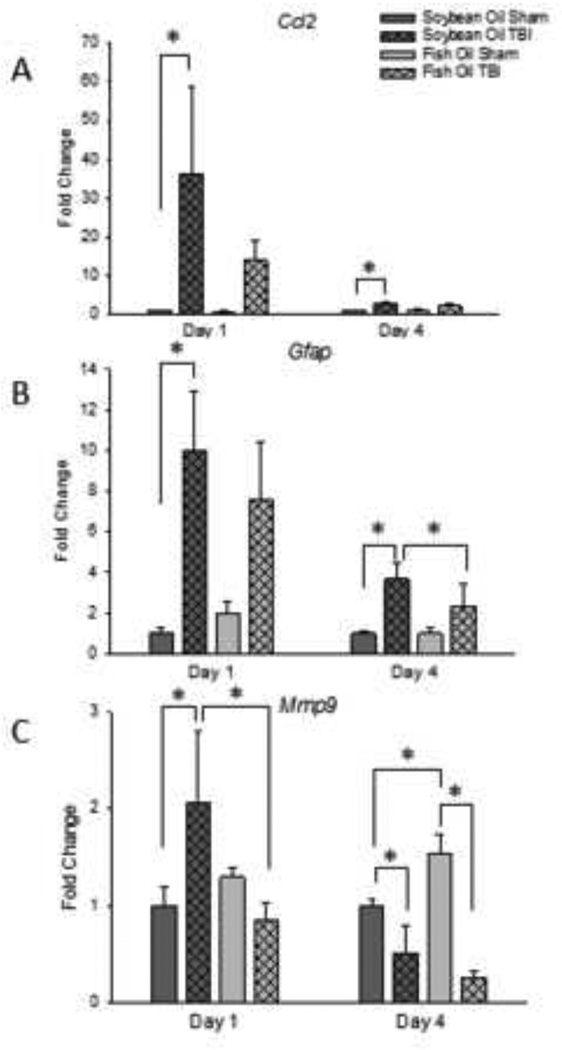
Chemokine CC ligand-2 (Ccl2) mRNA level was increased on day 1 after TBI approximately 35-fold (P < 0.05 v. Sham) in rats treated with soybean oil. A non-significant increase in Ccl2 mRNA level of approximately 15-fold was also observed in rats with TBI treated with fish oil (P = 0.091 v. Sham) (Figure 4A). In rats treated with fish oil, the TBI-induced increase in Ccl2 mRNA level returned to near-baseline levels on day 4 after TBI, but were still significantly increased in the TBI soybean oil group compared to soybean oil-treated sham controls (P < 0.01).
Figure 4. Effects of fish oil on TBI-induced changes in Ccl2 (A), Gfap (B), and Mmp9 (C) mRNA levels.
Data are the mean ± SEM (n = 4–7 per group). *P < 0.05 by ANOVA and Fisher’s LSD test.
Glial fibrillary acidic protein (Gfap) mRNA level was also increased by TBI by 10-fold on day 1 after TBI in the soybean oil-treated group (P < 0.05 v. Sham). An 8-fold increase was also observed in the fish oil treated group, but this was not quite significant and was not different from the increase observed in soybean oil-treated rats (P = 0.053 v. Sham) (Figure 4B). Gfap mRNA level decreased to 3–4-fold on day 4 after TBI (P < 0.05 v. Sham) and was not different between soybean oil- and fish oil-treated rats.
Matrix metalloproteinase-9 (Mmp9) mRNA level was increased on day 1 after TBI approximately 2-fold in rats treated with soybean oil (P < 0.05) (Figure 4C). In contrast, mRNA level for Mmp9 was not increased on day 1 after TBI in rats treated with fish oil and was significantly different from those treated with soybean oil (P < 0.05). On day 4 after surgery, Mmp9 mRNA level in rats with TBI was lower than in Sham-injured rats in both soybean oil- and fish oil-treated rats (P < 0.001). In addition, there was an effect of fish oil in the Sham-injured rats such that levels of Mmp9 mRNA were 50% higher in Sham-injured rats treated with fish oil than in those treated with soybean oil (P < 0.01)
DISCUSSION AND CONCLUSIONS
LC-PUFA or fish oil treatment has been beneficial in treating adult neural injuries in several models, including humans [24–26, 42–44]. This study investigated the effects of oral fish oil treatment in 17-day old rats, which are comparable to human toddlers with respect to motor development [45, 46], on the functional and molecular outcomes of TBI. The treatment paradigm used, with oil treatment beginning 30 min prior to injury, was designed to ensure increased levels of n-3 LC-PUFA during all early phases of the events triggered by TBI to best test the hypothesis that fish oil mitigates the effects of TBI, rather than model a clinical treatment scenario.
Injury to the primary motor cortex of 17 day-old rats produced deficits in hindlimb function as assessed using the beam walk test (Figure 2), a sensorimotor test previously validated for use with this CCI model of juvenile TBI (Russell et al. 2011). Consistent with previous studies [47, 48], TBI also increased expression of the Ccl2 and Gfap genes (Figure 4), mediators involved in immune cell recruitment and astrocytosis, respectively, and Mmp9, a mediator of blood-brain barrier disruption [28, 49]. Also in agreement with previous studies [50, 51], TBI caused significant infiltration of IgG, a serum protein, into the brain parenchyma (Figure 3), indicating disruption of the blood-brain barrier [52]. Together, these data indicate that an injury specific to the sensorimotor cortex with persisting functional deficits was achieved.
In addition to the effects of TBI on sensorimotor function and gene expression, TBI significantly altered the fatty acid composition of the frontal cortex. Four days after injury, TBI resulted in a main effect of decreased percentage of LC-PUFA in brain membranes, which was accompanied by an increase in saturated fatty acids (Table 2). Because this study used semi-quantitative methods which assess the fatty acid composition of brain phospholipids, rather that the absolute amounts of each fatty acid, these changes could be due to substitution of saturated fatty acids for LC-PUFA or changes in the absolute amounts of specific fatty acids. However, other studies indicate that TBI causes a rapid, sustained increase of free fatty acids [53], including DHA (Chris Butt, personal communication). Therefore, it is likely that the cleavage of n-6 and n-3 fatty acids from the membrane, as a result of TBI, is responsible for the observed decrease in the total percentage of membrane LC-PUFAs
In sham-injured rats, administration of either fish oil or soybean oil did not cause any alterations in growth (Figure 1), behavior (Figure 2), IgG infiltration in to the CNS (Figure 3), or expression of the Ccl2 or Gfap, genes (Figure 4). This indicates that soybean oil and fish oil administration was well-tolerated. Interestingly, fish oil increased Mmp9 mRNA level in sham-injured rats after 4 days of administration (Figure 4), the physiological importance of which must be determined in future studies.
In rats with a CCI injury, fish oil administration decreased the magnitude and persistence of TBI-induced motor function deficits, reduced the extent of IgG infiltration into the brain parenchyma (Figure 3), and produced some reduction in TBI-induced Gfap gene expression (Figure 4B) when compared to rats treated with soybean oil. Furthermore, fish oil treatment prevented the early TBI-induced increase in Mmp9 mRNA level (Figure 4C), a key mediator in the breakdown of the blood-brain barrier, and attenuated the TBI-induced decrease in brain DHA (Table 2) that was observed in soybean oil-treated rats. Beneficial effects of dietary supplementation with n-3 LC-PUFA have been reported in studies using adult models of TBI on end points such as TBI-induced apoptosis and deficits in the Morris water maze. [23, 42, 44, 54–57]. In other models of neural injury such as ischemia-reperfusion, injections of DHA decreased blood-brain barrier permeability [24], as well as improving injury-induced deficits in neurological score and infarct volume [58, 59]. Furthermore, DHA and EPA have been shown to decrease protein levels and activity of MMP-9 [60], suggesting that the changes in Mmp9 mRNA observed in this study would be consistent with decreased MMP-9 activity, and thus decreased disruption of the blood-brain barrier. Thus, the present findings generally agree with the literature and support the use of fish oil as a treatment for TBI in both juveniles and adults.
The present findings also suggest that n-3 LC-PUFA in fish oil enhance outcomes after TBI through a mechanism that includes limiting blood-brain barrier damage after TBI and/or expediting its repair. The blood-brain barrier is a dynamic, complex structure made up of vascular endothelial cells surrounded by support cells and astrocytic foot processes [61]. A growing body of evidence implicates MMP-9 in the breakdown of the blood-brain barrier after neural injury in both juvenile and adult animal models [62–66]. MMP-9 disrupts the blood-brain barrier by degrading collagen IV and laminin in the basal lamina [67], as well as MMP-dependent cleavage of blood-brain barrier tight junction proteins leading to significant disruption of cell-cell contact [68].
Mmp9 is regulated at the level of gene transcription, primarily through an NF-κB site in the promoter region [69]. LC-PUFA are known to regulate NF-κB signaling through binding to and activating PPAR receptors thereby antagonizing the NF-κB signaling pathway [70] or through directly inhibiting activation of NF-κB independently of PPAR [21, 71]. From this we can hypothesize that fish oil may limit gene expression of Mmp9 in activated astrocytes, microglia, and perhaps other cells, through inhibition of NF-κB signaling early after injury; however, this must be determined in future studies.
In conclusion, the present data show that treatment with fish oil improved TBI outcomes in juvenile rats, resulting, at least in part, from decreased disruption of the blood-brain barrier through a mechanism that included attenuation TBI-induced expression of Mmp9.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Andrew J. Ralya, Ph.D. and Heather Spalding for technical and editorial assistance.
Supported by NIH R03 MH059939 (BL), P30 HD02528 (BL, NEJB), P20 RR016475 (BL), T32 ES007079 (KLR), T32 HD007523 (KLR), the University of Kansas Medical Center Biomedical Research Training Program (KLR), and the American Society for Pharmacology and Experimental Therapeutics (ASPET) Zannoni Summer Undergraduate Research Fellow (SURF) Program (PRAG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
K. L. Russell, Email: kristinlrussell@gmail.
N. E. J. Berman, Email: nberman@kumc.edu.
P. R. A. Gregg, Email: pragregg@gmail.com.
B. Levant, Email: blevant@kumc.edu.
REFERENCES
- 1.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the united states: Emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Giza CC, Prins ML. Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Dev Neurosci. 2006;28:364–379. doi: 10.1159/000094163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy S, McIntyre J, Choonara I, Stephenson T. Drug trials in children: Problems and the way forward. Br J Clin Pharmacol. 2000;49:93–97. doi: 10.1046/j.1365-2125.2000.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 5.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 6.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 7.Shaikh SR. Biophysical and biochemical mechanisms by which dietary n-polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem. 2012;23:101–105. doi: 10.1016/j.jnutbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogyes E, Nyakas C, Kiliaan A, Farkas T, Penke B, Luiten PG. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999–1012. doi: 10.1016/s0306-4522(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 9.Hossain MS, Hashimoto M, Masumura S. Influence of docosahexaenoic acid on cerebral lipid peroxide level in aged rats with and without hypercholesterolemia. Neurosci Lett. 1998;244:157–160. doi: 10.1016/s0304-3940(98)00147-5. [DOI] [PubMed] [Google Scholar]
- 10.Sinha RA, Khare P, Rai A, Maurya SK, Pathak A, Mohan V, Nagar GK, Mudiam MK, Godbole MM, Bandyopadhyay S. Anti-apoptotic role of omega-3-fatty acids in developing brain: Perinatal hypothyroid rat cerebellum as apoptotic model. Int J Dev Neurosci. 2009;27:377–383. doi: 10.1016/j.ijdevneu.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Florent S, Malaplate-Armand C, Youssef I, Kriem B, Koziel V, Escanye MC, Fifre A, Sponne I, Leininger-Muller B, Olivier JL, Pillot T, Oster T. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem. 2006;96:385–395. doi: 10.1111/j.1471-4159.2005.03541.x. [DOI] [PubMed] [Google Scholar]
- 12.Bazan NG, Marcheselli VL, Cole-Edwards K. Brain response to injury and neurodegeneration: Endogenous neuroprotective signaling. Ann N Y Acad Sci. 2005;1053:137–147. doi: 10.1196/annals.1344.011. [DOI] [PubMed] [Google Scholar]
- 13.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: Implications for fatty acid requirements. Early Hum Dev. 1980;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 14.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 15.Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31:859–865. doi: 10.1007/BF02522981. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazan NG. Neuroprotectin d1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and alzheimer's disease. J Lipid Res. 2009;50(Suppl):S400–S405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SA, Vanden Heuvel JP. Role of nuclear receptors in the regulation of gene expression by dietary fatty acids (review) . J Nutr Biochem. 2003;14:554–567. doi: 10.1016/s0955-2863(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 20.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: A positive modulator of akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draper E, Reynolds CM, Canavan M, Mills KH, Loscher CE, Roche HM. Omega-fatty acids attenuate dendritic cell function via nf-kappab independent of ppargamma. J Nutr Biochem. 2011;22:784–790. doi: 10.1016/j.jnutbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Edmond J. Essential polyunsaturated fatty acids and the barrier to the brain: The components of a model for transport. J Mol Neurosci. 2001;16:181–193. doi: 10.1385/JMN:16:2-3:181. discussion 215–121. [DOI] [PubMed] [Google Scholar]
- 23.Mills JD, Hadley K, Bailes JE. Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery. 2011;68:474–481. doi: 10.1227/NEU.0b013e3181ff692b. discussion 481. [DOI] [PubMed] [Google Scholar]
- 24.Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL, Raung SL, Wu CW, Chiang AN, Chen CJ. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–725. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 25.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26:4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis M, Ghassemi P, Hibbeln J. Therapeutic use of omega-3 fatty acids in severe head trauma. Am J Emerg Med. 2013;31:273, e275–e278. doi: 10.1016/j.ajem.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levant B, Ozias MK, Jones KA, Carlson SE. Differential effects of modulation of docosahexaenoic acid content during development in specific regions of rat brain. Lipids. 2006;41:407–414. doi: 10.1007/s11745-006-5114-6. [DOI] [PubMed] [Google Scholar]
- 28.Russell KL, Berman NE, Levant B. Low brain dha content worsens sensorimotor outcomes after tbi and decreases tbi-induced timp1 expression in juvenile rats. Prostaglandins Leukot Essent Fatty Acids. 2013;89:97–105. doi: 10.1016/j.plefa.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell KL, Kutchko KM, Fowler SC, Berman NE, Levant B. Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: Assessment of sex differences. J Neurosci Methods. 2011;199:214–222. doi: 10.1016/j.jneumeth.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: Characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. Berkeley and Los Angeles: University of California Press, Ltd.; 1970. [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press, Inc.; 1986. [Google Scholar]
- 33.Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O'Neill JT, Grunberg NE, Dalgard CL, Frank JA, Watson WD. Craniotomy: True sham for traumatic brain injury, or a sham of a sham? J Neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokely ME, Orr EL. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- 35.Wu JC, Chen KY, Yo YW, Huang SW, Shih HM, Chiu WT, Chiang YH, Shiau CY. Different sham procedures for rats in traumatic brain injury experiments induce corresponding increases in levels of trauma markers. J Surg Res. 2013;179:138–144. doi: 10.1016/j.jss.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher MA, Basbaum AI, Way WL. Opioid analgesics & antagonists. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic & clinical pharmacology. New York: McGraw Hill Lange; 2012. pp. 543–565. [Google Scholar]
- 37.Rasband WS. Imagej. 1997–2012 Available from: http://imagej.nih.gov/ij/
- 38.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. Primer-blast: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Harris JL, Reeves TM, Phillips LL. Injury modality, survival interval, and sample region are critical determinants of qrt-pcr reference gene selection during long-term recovery from brain trauma. J Neurotrauma. 2009;26:1669–1681. doi: 10.1089/neu.2009.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: An evaluation of selected measures. J Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- 42.Mills JD, Bailes JE, Sedney CL, Hutchins H, Sears B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model. J Neurosurg. 2011;114:77–84. doi: 10.3171/2010.5.JNS08914. [DOI] [PubMed] [Google Scholar]
- 43.Javierre C, Vidal J, Segura R, Lizarraga MA, Medina J, Ventura JL. The effect of supplementation with n-3 fatty acids on the physical performance in subjects with spinal cord injury. J Physiol Biochem. 2006;62:271–279. doi: 10.1007/BF03165756. [DOI] [PubMed] [Google Scholar]
- 44.Shin SS, Dixon CE. Oral fish oil restores striatal dopamine release after traumatic brain injury. Neurosci Lett. 2011;496:168–171. doi: 10.1016/j.neulet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- 46.Westerga J, Gramsbergen A. The development of locomotion in the rat. Brain Res Dev Brain Res. 1990;57:163–174. doi: 10.1016/0165-3806(90)90042-w. [DOI] [PubMed] [Google Scholar]
- 47.Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of ccl2 (mcp-1) in traumatic brain injury (tbi): Evidence from severe tbi patients and ccl2−/− mice. J Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- 49.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 50.Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: A behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 51.Aihara N, Tanno H, Hall JJ, Pitts LH, Noble LJ. Immunocytochemical localization of immunoglobulins in the rat brain: Relationship to the blood-brain barrier. J Comp Neurol. 1994;342:481–496. doi: 10.1002/cne.903420402. [DOI] [PubMed] [Google Scholar]
- 52.Seitz RJ, Heininger K, Schwendemann G, Toyka KV, Wechsler W. The mouse blood-brain barrier and blood-nerve barrier for igg: A tracer study by use of the avidin-biotin system. Acta Neuropathol. 1985;68:15–21. doi: 10.1007/BF00688950. [DOI] [PubMed] [Google Scholar]
- 53.Homayoun P, Parkins NE, Soblosky J, Carey ME, Rodriguez de Turco EB, Bazan NG. Cortical impact injury in rats promotes a rapid and sustained increase in polyunsaturated free fatty acids and diacylglycerols. Neurochem Res. 2000;25:269–276. doi: 10.1023/a:1007583806138. [DOI] [PubMed] [Google Scholar]
- 54.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize bdnf levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 55.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- 56.Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of dha dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28:2113–2122. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailes JE, Mills JD. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27:1617–1624. doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- 58.Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eady TN, Belayev L, Khoutorova L, Atkins KD, Zhang C, Bazan NG. Docosahexaenoic acid signaling modulates cell survival in experimental ischemic stroke penumbra and initiates long-term repair in young and aged rats. PLoS One. 2012;7:e46151. doi: 10.1371/journal.pone.0046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinto L, Marracci G, Bumgarner L, Yadav V. The effects of omega-3 fatty acids on matrix metalloproteinase-9 production and cell migration in human immune cells: Implications for multiple sclerosis. Autoimmune Dis. 2011;2011:134592. doi: 10.4061/2011/134592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 62.Sifringer M, Stefovska V, Zentner I, Hansen B, Stepulak A, Knaute C, Marzahn J, Ikonomidou C. The role of matrix metalloproteinases in infant traumatic brain injury. Neurobiol Dis. 2007;25:526–535. doi: 10.1016/j.nbd.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006;96:130–133. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- 64.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: A possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Svedin P, Hagberg H, Savman K, Zhu C, Mallard C. Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J Neurosci. 2007;27:1511–1518. doi: 10.1523/JNEUROSCI.4391-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harkness KA, Adamson P, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Dexamethasone regulation of matrix metalloproteinase expression in cns vascular endothelium. Brain. 2000;123(Pt 4):698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- 68.Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995:184–196. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Ogawa K, Chen F, Kuang C, Chen Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappab site. Biochem J. 2004;381:413–422. doi: 10.1042/BJ20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuniga J, Cancino M, Medina F, Varela P, Vargas R, Tapia G, Videla LA, Fernandez V. N-3 pufa supplementation triggers ppar-alpha activation and ppar-alpha/ nf-kappab interaction: Anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS One. 2011;6:e28502. doi: 10.1371/journal.pone.0028502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. Nf-kappa b inhibition by omega-3 fatty acids modulates lps-stimulated macrophage tnf-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.