Abstract
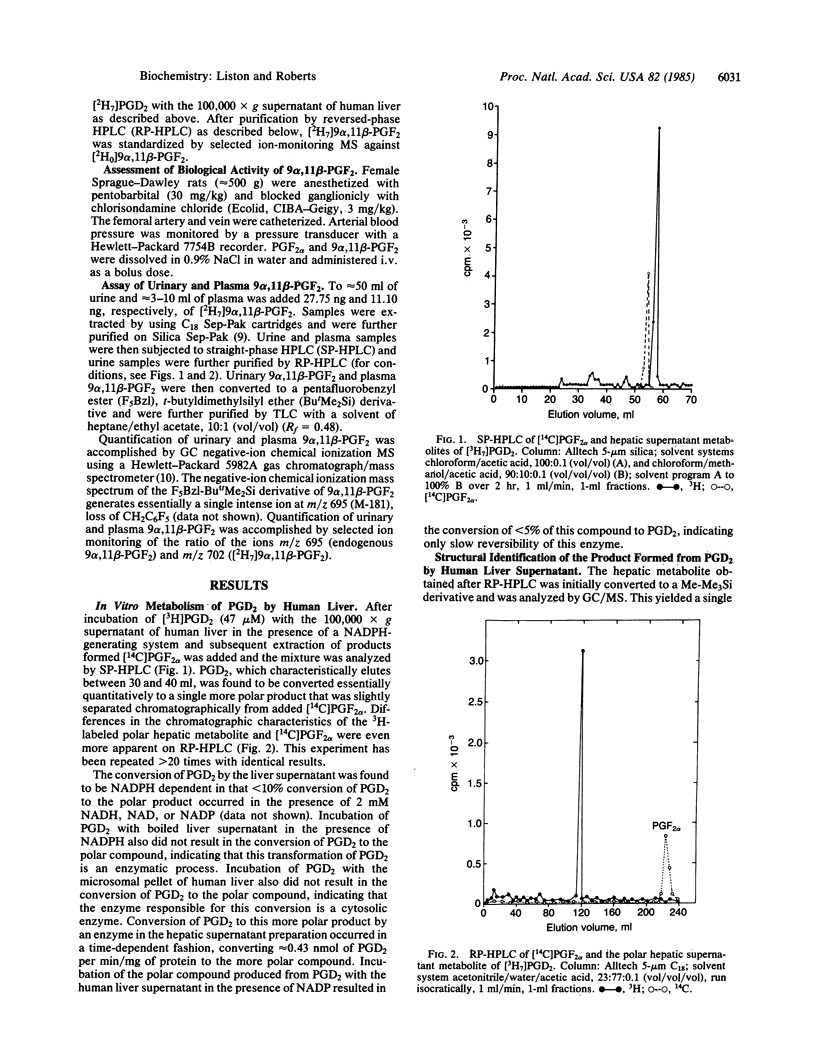
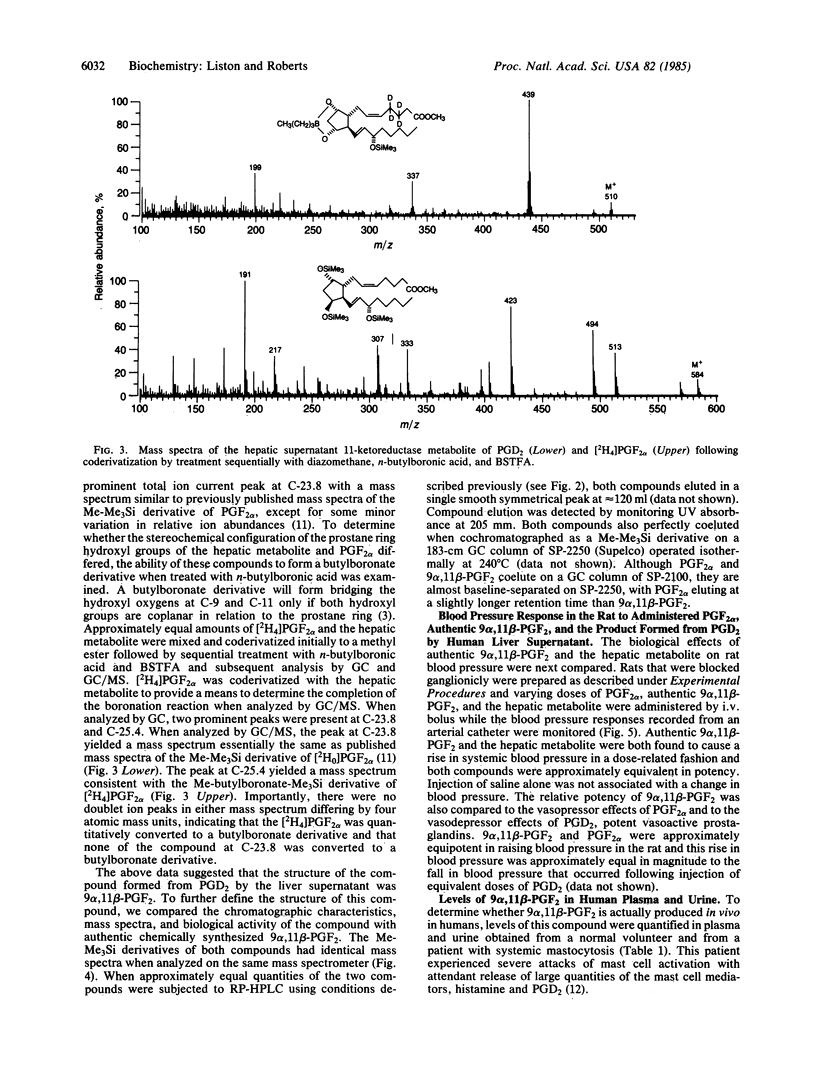
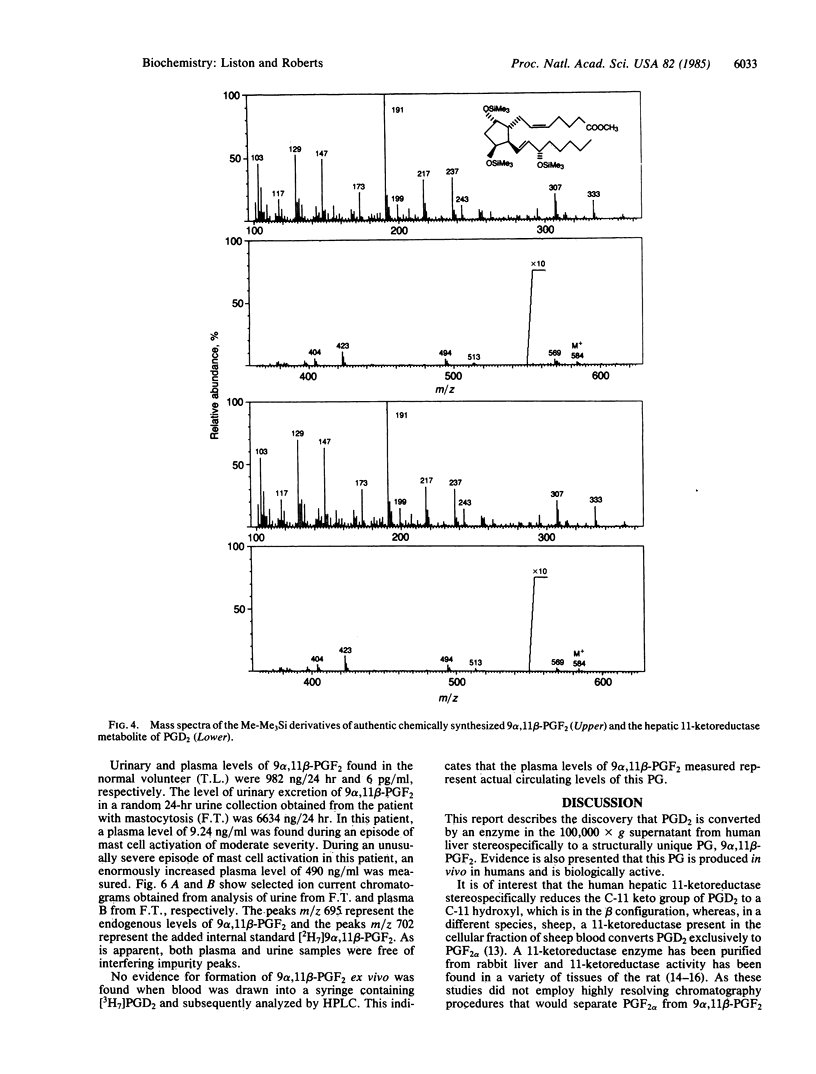
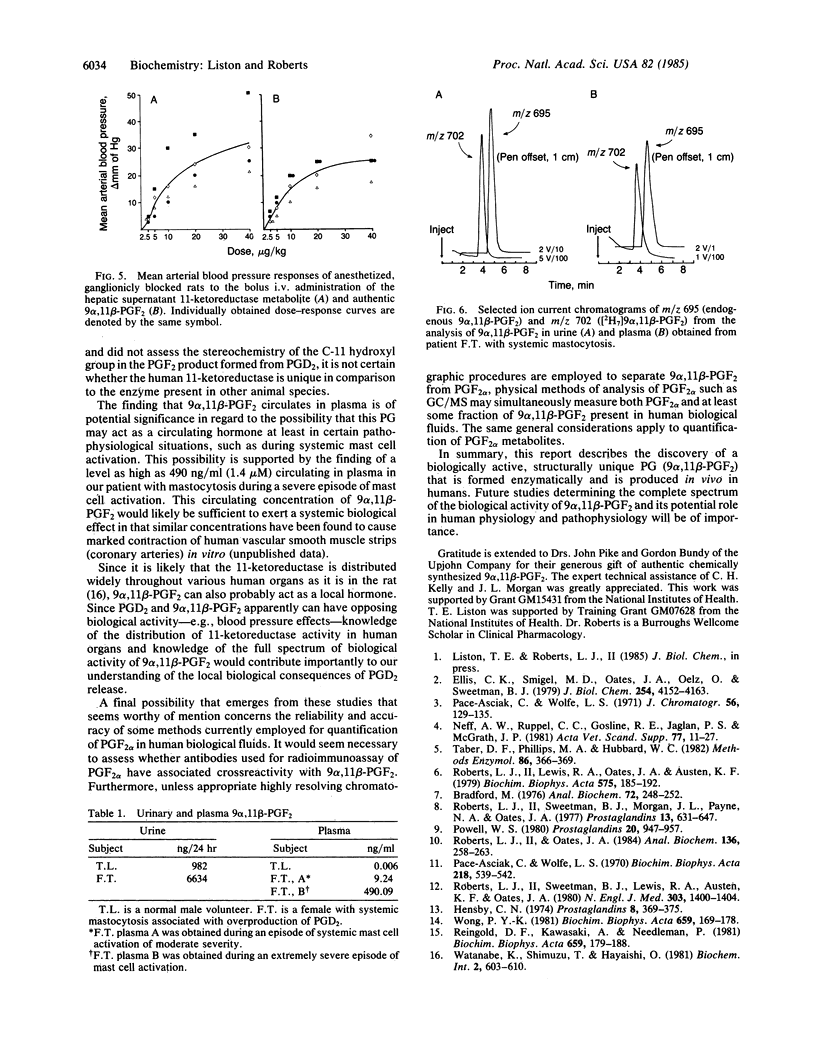
In vitro studies examining the metabolic transformation of prostaglandin D2 (PGD2) by human liver were conducted. PGD2 was found to be converted by a NADPH-dependent enzyme in the 100,000 X g supernatant of human liver exclusively to a single more polar compound that had a mass spectrum essentially the same as that of prostaglandin F2 alpha (PGF2 alpha). However, this compound could be chromatographically separated from PGF2 alpha and failed to form a butylboronate derivative. The structure of this compound was established as 9 alpha, 11 beta-(15S)-trihydroxyprosta-(5Z, 13E)-dien-1-oic acid (9 alpha, 11 beta-PGF2) by comparison of its chromatographic and mass spectral characteristics with authentic 9 alpha, 11 beta-PGF2. This compound was found to be biologically active by demonstrating increases in blood pressure in rats in a dose-related fashion following intravenous administration. By using a mass spectrometric assay, levels of this compound in plasma and urine from a normal volunteer were 6 pg/ml and 982 ng/24 hr, respectively. In a patient with systemic mastocytosis associated with overproduction of PGD2, urinary excretion of 9 alpha, 11 beta-PGF 2 was 6634 ng/24 hr and a circulating plasma level as high as 490 ng/ml was found during a severe episode of systemic mast cell activation. 9 alpha, 11 beta-PGF2 is structurally a unique prostaglandin, is enzymatically formed, is produced in vivo in humans, and is biologically active.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ellis C. K., Smigel M. D., Oates J. A., Oelz O., Sweetman B. J. Metabolism of prostaglandin D2 in the monkey. J Biol Chem. 1979 May 25;254(10):4152–4163. [PubMed] [Google Scholar]
- Hensby C. N. The enzymatic conversion of prostaglandin D2 to prostaglandin F2 alpha. Prostaglandins. 1974 Dec 10;8(5):369–375. doi: 10.1016/0090-6980(74)90111-7. [DOI] [PubMed] [Google Scholar]
- Neff A. W., Ruppel C. C., Gosline R. E., Jaglan P. S., McGrath J. P. PGF2 alpha residue studies in beef and dairy cattle. Acta Vet Scand Suppl. 1981;77:11–27. [PubMed] [Google Scholar]
- Pace-Asciak C., Wolfe L. S. Biosynthesis of prostaglandins E2 and F2-alpha from tritium-labelled arachidonic acid by rat stomach homogenates. Biochim Biophys Acta. 1970 Dec 15;218(3):539–542. doi: 10.1016/0005-2760(70)90017-2. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C., Wolfe L. S. N-butylboronate derivatives of the F prostaglandins. Resolution of prostaglandins of the E and F series by gas-liquid chromatography. J Chromatogr. 1971 Mar 24;56(1):129–133. doi: 10.1016/s0021-9673(00)97786-0. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of oxygenated metabolites of arachidonic acid from biological samples using octadecylsilyl silica. Prostaglandins. 1980 Nov;20(5):947–957. doi: 10.1016/0090-6980(80)90144-6. [DOI] [PubMed] [Google Scholar]
- Reingold D. F., Kawasaki A., Needleman P. A novel prostaglandin 11-keto reductase found in rabbit liver. Biochim Biophys Acta. 1981 May 14;659(1):179–188. doi: 10.1016/0005-2744(81)90282-5. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Lewis R. A., Oates J. A., Austen K. F. Prostaglandin thromboxane, and 12-hydroxy-5,8,10,14-eicosatetraenoic acid production by ionophore-stimulated rat serosal mast cells. Biochim Biophys Acta. 1979 Nov 21;575(2):185–192. doi: 10.1016/0005-2760(79)90020-1. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Oates J. A. Accurate and efficient method for quantification of urinary histamine by gas chromatography negative ion chemical ionization mass spectrometry. Anal Biochem. 1984 Jan;136(1):258–263. doi: 10.1016/0003-2697(84)90333-6. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Sweetman B. J., Lewis R. A., Austen K. F., Oates J. A. Increased production of prostaglandin D2 in patients with systemic mastocytosis. N Engl J Med. 1980 Dec 11;303(24):1400–1404. doi: 10.1056/NEJM198012113032405. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Sweetman B. J., Morgan J. L., Payne N. A., Oates J. A. Identification of the major urinary metabolite of thromboxane B2 in the monkey. Prostaglandins. 1977 Apr;13(4):631–647. doi: 10.1016/0090-6980(77)90234-9. [DOI] [PubMed] [Google Scholar]
- Wong P. Y. Purification and partial characterization of prostaglandin D2 11-keto reductase in rabbit liver. Biochim Biophys Acta. 1981 May 14;659(1):169–178. doi: 10.1016/0005-2744(81)90281-3. [DOI] [PubMed] [Google Scholar]



