Abstract
Understanding how quickly physiological traits evolve is a topic of great interest, particularly in the context of how organisms can adapt in response to climate warming. Adjustment to novel thermal habitats may occur either through behavioural adjustments, physiological adaptation or both. Here, we test whether rates of evolution differ among physiological traits in the cybotoids, a clade of tropical Anolis lizards distributed in markedly different thermal environments on the Caribbean island of Hispaniola. We find that cold tolerance evolves considerably faster than heat tolerance, a difference that results because behavioural thermoregulation more effectively shields these organisms from selection on upper than lower temperature tolerances. Specifically, because lizards in very different environments behaviourally thermoregulate during the day to similar body temperatures, divergent selection on body temperature and heat tolerance is precluded, whereas night-time temperatures can only be partially buffered by behaviour, thereby exposing organisms to selection on cold tolerance. We discuss how exposure to selection on physiology influences divergence among tropical organisms and its implications for adaptive evolutionary response to climate warming.
Keywords: thermoregulation, thermal physiology, physiological evolution, Anolis lizards, Bogert effect
1. Introduction
Rising temperatures present unique challenges for tropical ectotherms, which already generally function near their upper thermal limits: even small temperature increases can have disproportionately large negative consequences for these organisms [1,2]. Studies assessing tropical ectotherms' vulnerability to climate warming have traditionally focused on predicting where warming will have the most pronounced effects on organismal fitness by correlating physiological traits with environmental data and using these relationships to infer where range shifts and local extinctions will occur [3,4]. However, the evolutionary potential of populations to respond to novel selective pressures imposed by rising temperatures is an equally important and comparatively unexplored aspect of response to climate warming [5].
Behaviour and physiology determine how organisms interact with their thermal environments [6]. Organisms that thermoregulate limit exposure to suboptimal temperatures—a phenomenon commonly referred to as the ‘Bogert effect’ [7,8]. Physiological traits that behavioural thermoregulation can shield from selection should evolve less than traits that cannot be so easily buffered, and thus are exposed to stronger selection. Because many environments are more thermally complex in the day than at night [9,10], thermoregulation should be more effective at shielding diurnal organisms from selection on upper than lower physiological limits and, consequently, tolerance to cold should evolve faster than tolerance to heat.
In this study, we compare rates of physiological evolution in the cybotoids, a tropical clade of Anolis lizards from the Caribbean island of Hispaniola whose members differ extensively in thermal habitat [11,12]. Previous work by Hertz & Huey [13] found similar body temperatures and heat tolerance among three cybotoid species and provided ecological data suggesting that they are good thermoregulators. Our study expands on this work in terms of populations, species and physiological traits examined. The cybotoid clade is unique among Caribbean anoles because its species are found from sea level to almost 3000 m [14]; as a result, the environments they experience pose different selective pressures and provide the opportunity for behavioural and physiological adaptation to different thermal extremes.
We first present a comparative analysis of three ecologically important physiological traits—cold tolerance (CTmin), body temperature (Tb), and heat tolerance (CTmax)—across thermal environments. We then compare rates of evolution among these traits using a likelihood-based approach and test whether behavioural thermoregulation can limit exposure to extreme temperatures using field estimates of basking site selection and night-time temperatures. Finally, we discuss the role of behaviour in setting the pace of physiological evolution in tropical ectotherms and how behaviour influences adaptive evolutionary potential in physiological traits.
2. Material and methods
(a). Study organisms and study sites
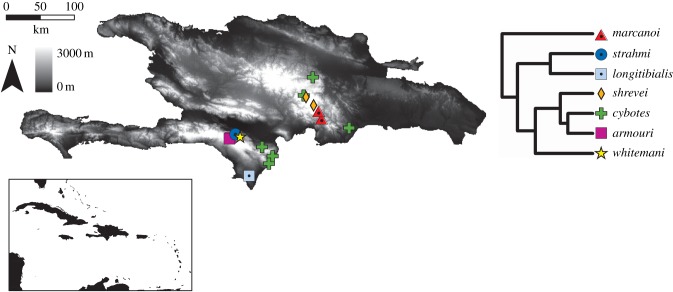
The cybotoid anoles are a clade of nine species from Hispaniola commonly found on trunks or near the ground [11]. Species occupy nearly all available climatic environments from xeric semi-deserts to high elevation mountains, which have been occupied independently by two different lineages [12,15]. Our sampling was conducted in June and July 2011 and focused on the seven cybotoids found in the Dominican Republic (figure 1). The other two species are Anolis breslini, which is restricted to northwestern Haiti and is ecologically quite similar to Anolis whitemani [17], and Anolis haetianus, which is found only in western Haiti and is probably synonymous with Anolis cybotes [12]. Where possible, we sampled several populations that, together, encompassed most of a species’ altitudinal range (figure 1 and table 1). Anolis cybotes is found nearly island-wide; we sampled it at three elevations in each of the two principal mountain chains, the Sierra de Baoruco (SB) and Cordillera Central (CC). In the SB, we sampled the mid-elevation species, Anolis strahmi and A. whitemani, and the high elevation species, Anolis armouri. In the CC, we sampled the mid-elevation species, Anolis marcanoi, and the high elevation species, Anolis shrevei.
Figure 1.
Map showing altitudinal variation and the localities for each cybotoid population sampled in this study. The map inset shows the location of Hispaniola with respect to the other islands in the Caribbean basin. Greyscale indicates elevation and range from dark (low) to light (high) elevation. Species are denoted in different colours and shapes in the phylogeny generated based on Mahler et al. [16] (see Material and methods).
Table 1.
Locality name, species sampled, and altitude (m) are given. (Mean critical thermal minimum (CTmin), body temperature (Tb), midday body temperature (midday Tb), and critical thermal maximum (CTmax) are given for each population. Units for physiological metrics are °C ± 1 s.e.m. and sample size is given in parentheses. For A. cybotes, the mountain chain corresponding to the sampling locality—Cordillera Central (CC) or the Sierra de Baoruco (SB)—is also given.)
| species | locality | alt (m) | CTmin (°C) | Tb (°C) | CTmax (°C) |
|---|---|---|---|---|---|
| wild-measured | |||||
| A. cybotes (SB) | Los Patos | 45 | 11.3 ± 0.4 (16) | 30.1 ± 0.3 (45) | 39.5 ± 0.1 (20) |
| A. cybotes (CC) | San Cristóbal | 56 | 11.4 ± 0.2 (16) | 29.4 ± 0.5 (53) | 39.2 ± 0.2 (16) |
| A. longitibialis | Jaragua | 105 | 12.8 ± 0.2 (18) | 28.9 ± 0.2 (101) | 38.5 ± 0.3 (18) |
| A. whitemani | Puerto Escondido | 411 | 12.2 ± 0.4 (15) | 27.9 ± 1.1 (17) | 38.8 ± 0.3 (15) |
| A. strahmi | Camino Aguacate | 454 | 11.3 ± 0.5 (6) | 26.3 ± 0.7 (7) | 39.2 ± 0.2 (6) |
| A. marcanoi | Ocoa | 458 | 12.6 ± 0.3 (9) | — | 38.2 ± 0.3 (9) |
| A. cybotes (CC) | Jarabacoa | 690 | 10.7 ± 0.2 (18) | 29.0 ± 0.6 (39) | 40.3 ± 0.2 (18) |
| A. cybotes (SB) | Guayuyal | 727 | 10.8 ± 0.4 (15) | 26.6 ± 0.4 (53) | 38.7 ± 0.2 (15) |
| A. marcanoi | La Horma | 879 | 11.3 ± 0.1 (16) | 29.1 ± 0.6 (48) | 38.7 ± 0.2 (16) |
| A. cybotes (CC) | Constanza | 1390 | 10.0 ± 0.5 (11) | 29.2 ± 0.5 (10) | 39.5 ± 0.6 (11) |
| A. cybotes (SB) | La Hoz | 1395 | 8.7 ± 0.4 (9) | 28.6 ± 0.9 (11) | 38.9 ± 0.2 (9) |
| A. shrevei | Valle Nuevo—low | 1950 | 9.6 ± 0.6 (9) | 28.0 ± 1.0 (10) | 39.9 ± 0.3 (9) |
| A. armouri | Loma de Toro—low | 2020 | 8.2 ± 0.6 (9) | — | 39.3 ± 0.3 (9) |
| A. armouri | Loma de Toro—high | 2318 | 7.2 ± 0.4 (12) | 25.9 ± 0.7 (21) | — |
| A. shrevei | Valle Nuevo—high | 2450 | 6.2 ± 0.3 (11) | 27.4 ± 1.0 (20) | 40.4 ± 0.3 (11) |
| cold acclimatized | |||||
| A. cybotes | Los Patos | 45 | 10.6 ± 0.3 (19) | — | — |
| A. armouri | Loma de Toro—high | 2318 | 7.2 ± 0.3 (18) | — | — |
We gathered climatic measurements for each locality by extracting all temperature variables (bio 1—bio 11; electronic supplementary material, table S1) from environmental layers available in the WorldClim dataset (resolved to approx. 1 km2; [18]). These variables summarize seasonal and annual temperature trends. To account for collinearity among thermal variables, we reduced data dimensionality using a principal components (PCs) analysis on the correlation matrix.
(b). Measurement of physiological performance indices
We measured field body temperature, Tb, during one continuous 13 h period (06.00–19.00) at 13 localities from 20 June to 31 July 2011. Owing to logistical constraints, one locality (A. shrevei—1950 m) was sampled from 06.00 to 13.45, but results for that population are consistent with those from other populations (table 1). Following established methods [19,20], we walked slowly through each habitat and used a standard noose to capture adult male lizards, which are more conspicuous and easier to sample than females. For every lizard, we recorded core temperature (Tb) to the nearest 0.1°C using a thermocouple (Type T, Copper-Constantan) inserted approximately 1 cm into the cloaca and connected to a temperature logger (HH603A, Omega). Each lizard was measured only once. Tb generally correlates closely with the optimal performance temperature (Topt, the temperature at which organisms maximally perform a function, for example running) in many diurnal lizards, including tropical anoles [21].
For each lizard captured, we recorded the time, weather conditions (sunny, mixed, or overcast skies) and ‘basking status’ (basking in the full or filtered sun, perching in the shade under sunny or mixed skies or in the shade under overcast skies) following Hertz [20]. For a statistical analysis of basking site choice, we removed observations for lizards captured in overcast conditions, because these lizards did not have the opportunity to choose between perching in the sun or in the shade. We tested for weather-dependent basking choice using a logistic regression model such that individuals’ basking behaviour (perching in shade = 0, perching in sun = 1) was evaluated as a function of elevation, weather (sunny or mixed skies) and an elevation × weather interaction. An effect of elevation alone would indicate that certain basking behaviours are more likely to occur at different elevations, whereas a significant interaction would indicate that active lizards differ in how they exploit weather conditions at different elevations.
We measured the critical thermal minimum (CTmin) and maximum (CTmax), which refer to the low and high temperatures at which an organism loses locomotor function; these are widely used for measuring the tolerance limits of performance in ectotherms [22,23]. CTmin and CTmax are estimated as the lower and upper temperatures at which a lizard fails to right itself when flipped onto its back [22]. After capture, we gave adult male lizards a 24 h rest period in a large, insulated ice chest (Coleman) in which temperature was maintained near 23°C at all sites. To measure core temperature during the tolerance experiments, an Omega temperature probe (Type T, 36 gauge) was placed approximately 1 cm into the cloaca of each lizard and secured to the base of the tail using a small piece of surgical tape, ensuring that tail movement was uncompromised. The temperature probe was connected to a digital temperature logger (HH147U, Omega). The lizard was placed into a perforated plastic container where it could move freely. After the lizard attained ambient temperature, the container was moved to an insulated icebox coated with a layer of crushed ice. Because the rate of temperature change during a tolerance experiment can alter an organism's performance [24], we reduced body temperature at a constant rate of approximately 1°C min−1 for all lizards. To conduct the experiment, we reduced body temperature to 14°C, at which point we flipped the lizard onto its back using a pair of blunt tweezers and stimulated it to flip itself back over by gently probing the base of its tail and pressing its thighs. If the lizard flipped over after 15 s, we then lowered core temperature 0.5°C and repeated this procedure, continuing until a temperature was reached at which the lizard failed to right itself in the allotted time. CTmin was recorded as the temperature at which the righting response was lost.
Animals were given 24 h to rest in the ice chest before CTmax trials. The method for estimating CTmax was similar to that of CTmin except that a 100 W light bulb was suspended approximately 30 cm above the Tupperware container. We placed lizards in the Tupperware container and increased their core temperature at a rate of approximately 1°C min−1 by exposing them to the heat source. We began flipping lizards when they began to cool through panting (i.e. the ‘panting threshold’; [25]) following the procedure described above, and recorded the temperature at which the righting response was lost as CTmax.
Estimation of CTmin and CTmax is potentially confounded by the rate of temperature change, body size and starting conditions [24,26,27]. We performed linear regressions with mean population CTmin and CTmax as the dependent variables against the population means for rate of temperature change, initial experimental temperature and body mass (see the electronic supplementary material, table S2). We conducted separate analyses for each pair of dependent and independent variables, and each regression was weighted by the variance in CTmin or CTmax. Because none of these models were statistically significant (see the electronic supplementary material, table S3), we used raw CTmin and CTmax values in subsequent analyses.
We compared physiological traits to thermal habitat (three PC variables, see Results) using population means and independent contrasts. We calculated standardized independent contrasts (scaled by the expected variance) for the weighted species means of each physiological trait (CTmin, Tb and CTmax) and each of the thermal habitat variables (PC I, PC II and PC III) using the pic function in the APE package [28,29] in R [30]. Although they are not properties of the organisms, environmental traits may reflect underlying ecological traits [31,32]. We used the time-calibrated, majority rule consensus tree of Mahler et al. [16], with the topology generated using Bayesian maximum clade credibility [33]. This consensus tree contains 187 of approximately 375 recognized species of anoles (all but 19 species of Caribbean anoles), including all the species used in this study (figure 1). We used regression through the origin to compare the contrasts for physiological traits with the contrasts for thermal environment traits using the lmorigin function in APE [29].
(c). Measuring rates of physiological trait evolution
To ensure comparability among traits, we used the fitContinuous function in the GEIGER package [34] in R to fit three different models of evolution to each physiological trait. These models were: (i) Brownian Motion, a random walk; (ii) Ornstein–Uhlenbeck, a random walk in which characters tend to return to a single optimum; and (iii) Early Burst, in which the overall rate of evolution exponentially slows through time [35–38]. We calculated the Akaike information criterion corrected for small sample size (AICc; [39]) for each model and compared the fits by examining the Akaike weights [40].
We used Adams’ [41] method to evaluate whether the rate of evolutionary change varied among physiological traits. This method compares a model that allows rates to vary among traits to one in which the rates are constrained to be equal using a likelihood ratio test and AICc. To account for intraspecific measurement error, we incorporated the standard error of the mean in our estimation of rates of evolution. We used the APE library [42,43] and new code supplied by Adams [41] in R.
(d). Cold-acclimatization trials
Because of the large differences in CTmin we discovered among populations (see Results), we conducted an experiment to assess how short-term acclimatization influences variation in this trait. In June 2013, we collected adult male lizards from two populations differing greatly in thermal environment—A. armouri (Loma de Toro, elevation = 2318 m; n = 18) and A. cybotes (Los Patos, elevation = 45 m; n = 19). Kolbe et al. [44] found that a two-week acclimatization at 22.5°C was sufficient to elicit a strong plastic response in CTmin in a lowland population of Anolis cristatellus from Puerto Rico without inducing severe thermal stress. We maintained lizards at 19.4°C (range = 17.4°C–21.9°C) for three weeks and measured CTmin following the procedure described above.
(e). Night-time temperature measurement
We measured night-time operative temperature (Te) in the same two, thermally contrasting, localities. Te refers to an organism's equilibrium temperature in the absence of metabolic heating or evaporative cooling (sensu [45]), which we estimated using replicas made of electroformed copper. These models mimic the thermal properties of a thermoconforming lizard (e.g. colour, shape and size; [46]). We embedded iButton data loggers (DS1921K Maxim) into copper models shaped using a mould of A. cybotes—this new generation of copper models permits automated temperature recording (for details of their construction, see [47]). Methods for calibrating the copper models are given in the electronic supplementary material. We launched these models in Los Patos (12 on trees, 11 on rocks and 11 under rocks; 5–7 June 2013) and in Loma de Toro (11 each on trees, on rocks and under rocks; 14–16 June 2013) with the devices set to automatically record Te at 10 min intervals. We randomly selected perches, orientation and height for model placement on trees following Hertz [20]. We also recorded sleep site selection for lizards at each of these localities during the experimental period.
3. Results
(a). Thermal habitat varies markedly across Hispaniola
For this study, we visited various localities in the Dominican Republic that spanned more than 2400 m in altitude and a variety of habitats ranging from lowland scrub to montane pine forests. Not surprisingly, sites varied considerably in temperature (figure 2). In the PC analysis of the WorldClim thermal variables, we recovered three axes with eigenvalues greater than 1 that together explained 99.6% of the variation in the thermal data (see the electronic supplementary material, tables S4 and S5). PC I (hereafter ‘Thermal PC I’) explained 73.2% of the variation and loaded highly for mean annual temperature, mean temperatures of the wettest and driest quarters, maximum temperature of the warmest month and minimum temperature of the coldest month. PC II (14.2% variation explained; hereafter ‘Range PC II’) loaded highly for daily and annual temperature ranges, and PC III (12.2% variation explained; ‘Seasonality PC III’ axis) loaded with variables related to thermal seasonality.
Figure 2.
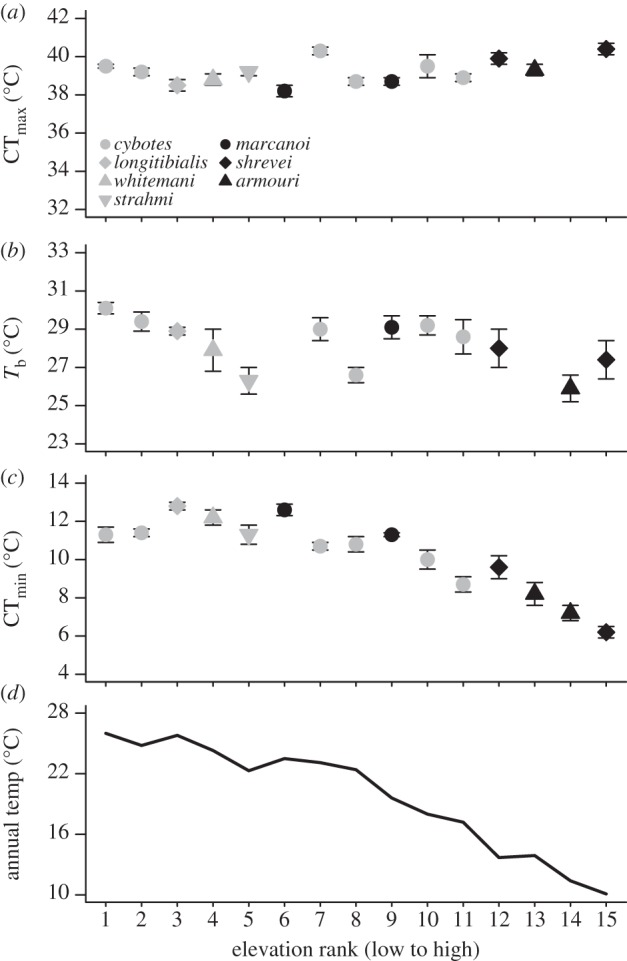
Population means (±1 s.e.m.) are given for critical (a) thermal maximum (CTmax), (b) body temperature (Tb), and (c) critical thermal minimum (CTmin). The mean annual temperature for each locality is provided in (d). The x-axis denotes elevation rank for each population. Species are denoted in different colours and shapes.
(b). Analyses of thermoregulation and physiology
In approximately 164 h of field observations, we collected Tb from 435 lizards and basking site data from 381 lizards. The extent of basking in the sun varied greatly at different elevations (logistic interaction term; χ12 = 4.07, p = 0.044); lizards at higher elevation were more likely to bask, whereas those at lower elevation sought shade. Neither CTmax nor Tb varied significantly with any of the thermal habitat PC variables (table 2). CTmin was positively correlated with Thermal PC I (r = 0.934, p < 0.001), which loaded heavily with mean annual temperature, and this relationship remained significant after phylogenetic correction (table 2).
Table 2.
Results from linear regressions assessing the relationship between physiological traits (critical thermal minimum, CTmin; mean body temperature, Tb, and critical thermal maximum, CTmax) and thermal environment (PC I/mean annual temperature, PC II/temperature range and PC III/temperature seasonality) using population means (a) and independent contrasts of species means (b). (Degrees of freedom are given in parentheses. Correlations among contrasts were measured using the cor.table function in picante [48] in R.)
| (a) populations |
(b) contrasts |
|||||
|---|---|---|---|---|---|---|
| reg. coeff. | Pearson's r | p | reg. coeff. | Pearson's r | p | |
| CTmin (13,5) | ||||||
| PC I/mean annual temperature | 1.87 | 0.934 | <0.001 | 1.56 | 0.973 | 0.001 |
| PC II/temperature range | −0.20 | 0.022 | 0.755 | 0.05 | 0.032 | 0.951 |
| PC III/seasonality | 0.46 | 0.140 | 0.503 | 0.38 | 0.132 | 0.790 |
| Tb (11,5) | ||||||
| PC I/mean annual temperature | 0.52 | 0.363 | 0.142 | 0.78 | 0.637 | 0.143 |
| PC II/temperature range | −0.35 | −0.296 | 0.341 | −0.48 | −0.421 | 0.460 |
| PC III/seasonality | 0.68 | 0.479 | 0.054 | −0.73 | −0.381 | 0.464 |
| CTmax (12,5) | ||||||
| PC I/mean annual temperature | −0.29 | −0.618 | 0.110 | −0.24 | 0.186 | 0.204 |
| PC II/temperature range | −0.16 | −0.228 | 0.300 | 0.13 | 0.881 | 0.672 |
| PC III/seasonality | 0.13 | 0.193 | 0.524 | 0.03 | 0.865 | 0.957 |
(c). Evolutionary analyses of physiology
Brownian motion was the most strongly supported model for all three traits (CTmin, Tb and CTmax)—Akaike weights were more than 0.93 in all cases (see the electronic supplementary material, table S6), allowing for a comparison of evolutionary rates among traits. Likelihood ratio tests indicated that, overall, the three physiological traits evolved at different rates, although the differences were just above the significance threshold (p = 0.06) when intraspecific measurement error was taken into account (table 3). Pairwise comparisons showed that rates of evolution for CTmin were significantly higher than for CTmax, even when intraspecific measurement error was considered. However, differences in rates of evolution between Tb and other traits were not significant in the analysis incorporating intraspecific variation (table 3), either because rates do not actually differ, or because high variance in Tb obscures differences in rates of evolution.
Table 3.
Comparison of evolutionary rates for CTmin, Tb, and CTmax. (a) The full analysis of evoluationary rates (σ2) incorporating covariation among all three traits. One test accounted for intraspecific measurement error (corrected), whereas the other did not (uncorrected). AICC scores for a model that allows rates to vary (observed) among traits and a model that constrains rates of evolution to be equal among traits are given (constrained), and likelihood ratio test results are also given. (b) Likelihood ratio tests for pairwise comparisons of evolutionary rates among traits. As above, the results for models that incorporate intraspecific measurement error (corrected) and for models that do not (uncorrected) are presented.
| trait | σ2 | uncorrected | corrected |
|---|---|---|---|
| (a) full analysis | |||
| CTmin | 10.60 | AICC (OBS) = 167.3 | AICC (OBS) = 171.7 |
| Tb | 6.36 | AICC (CONS) = 175.8 | AICC (CONS) = 173.2 |
| CTmax | 0.78 | LRTd.f. = 2 = 12.56, p = 0.002 | LRTd.f. = 2 = 5.57, p = 0.06 |
| comparison | uncorrected LRTd.f. = 1; p | corrected LRTd.f. = 1; p | |
|---|---|---|---|
| (b) pairwise analysis | |||
| CTmin versus CTmax | 12.40; <0.001 | 7.85; 0.005 | |
| CTmin versus Tb | 0.87; 0.350 | 0.32; 0.574 | |
| CTmax versus Tb | 6.86; 0.009 | −4.70; 1.00 | |
(d). Cold-acclimatization experiment
Mean CTmin was not significantly different between wild-measured (mean = 11.3°C) and cold-acclimatized A. cybotes (mean = 10.6°C) (unpaired t-test: t = 1.53, p = 0.136). Mean CTmin remained the same (7.2°C) between wild-measured and cold-acclimatized A. armouri. CTmin was significantly higher in A. cybotes than in A. armouri in both the wild-measured (unpaired t-test: t = 7.72, p < 0.001) and cold-acclimatized treatments (t = 9.28, p < 0.001).
(e). Night-time temperature experiment
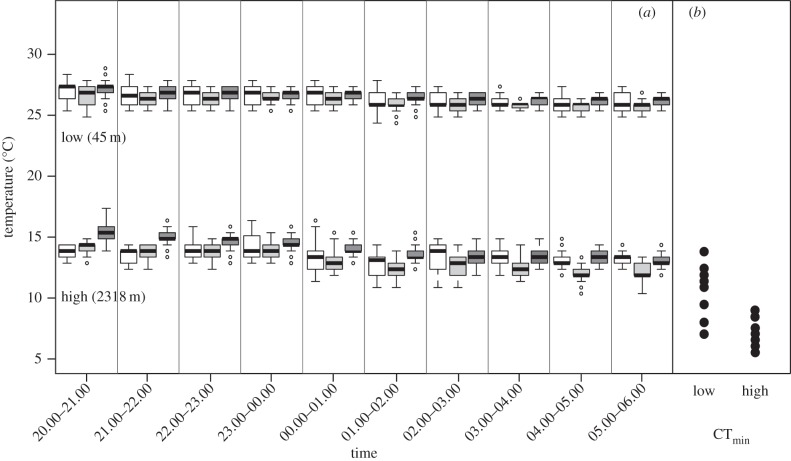
Night-time operative temperatures (Te) showed marked differences between high and low elevation (figure 3). At Los Patos (low elevation), Te ranged from 24.6°C to 29.8°C, whereas at Loma de Toro Te (high elevation), it ranged from 10.9°C to 18.1°C. Te was on average, though not always, somewhat higher under rocks than on top of rocks or on trees (figure 3; electronic supplementary material, figure S1), particularly early in the evening. All lizards at Los Patos were observed sleeping on vegetation (43 observations), whereas lizards at Loma de Toro were observed sleeping on vegetation (14 out of 30) and underneath rocks (16 out of 30) in roughly equal numbers (test for differences in site selection among populations: χ12 = 26.3, p < 0.001).
Figure 3.
(a) Box plots showing the variation in operative temperatures during 1 h time blocks. Each time block summarizes temperatures collected over three consecutive nights in June 2013 at low elevation (45 m) and high elevation (2318 m). Colour denotes the type of perch where the temperature was measured as follows: on a tree, white; on top of a rock, light grey; underneath a rock, dark grey. (b) CTmin measured in individuals of A. cybotes (left) and A. armouri (right) from the same localities in part (a) are given.
4. Discussion
(a). Thermoregulatory behaviour influences the rate of physiological evolution
The question of how behaviour influences patterns of physiological evolution dates back to the middle of the last century [7,49] and has received renewed interest in light of concern about how ectothermic organisms can respond to climate warming [2,50]. We studied a clade of lizards whose species vary markedly in thermal environment from hot semi-deserts to cold montane environments. Despite occurring in environments that differ by as much as 15°C in mean annual temperature, field body temperature and heat tolerance were remarkably similar among populations, indicating that behavioural thermoregulation can be extraordinarily effective in limiting exposure to excessively hot or cold temperatures (i.e. the ‘Bogert effect’; [8]). Our behavioural analysis demonstrates that lowland lizards were more likely to retreat to the shade under sunny conditions, whereas upland lizards were more likely to bask when the sun was out, a result in agreement with previous work on three of these species [13]. Our results are particularly striking given that other anole species exhibit markedly different body temperatures, even when they occur in sympatry [51–54], but see [55,56].
Given the ability of cybotoids to thermoregulate to approximately the same temperature throughout its range, it is not surprising that CTmax also shows very little interspecific variation. However, these lizards have a much more limited ability to thermoregulate at night, particularly at high elevation, where operative temperatures measured on all types of sleep sites were so low that they would incapacitate approximately 80% of lowland lizards (figure 3; electronic supplementary material, figure S1). In the absence of thermal refuges, populations have no option but to adapt physiologically. Indeed, we found that none of the lizards from high elevation experienced night-time temperatures lower than their CTmin (figure 3).
An alternative explanation for this finding is that differences in CTmin represent non-genetic effects of living in different environments. Previous studies suggest that adaptive plasticity is unlikely to account for physiological differences among populations [57]: our data support this view, as cold tolerance exhibits little acclimatization, even less so than in other anoles [44], which suggests that there is probably a genetic basis for the observed variation in CTmin.
The inability of thermoregulation to buffer selection on physiology during the night is an explanation for the fast rate of CTmin evolution in this clade (table 3; [8]). The relative stasis in CTmax documented here aligns with results from recent meta-analyses showing that there is less variation in heat tolerance than in cold tolerance in several ectotherm clades [58,59]. In short, behavioural thermoregulation allows cybotoid species to maintain similarly warm body temperatures during the day, but not during the night, forcing species in montane environments on Hispaniola to adapt to lower temperatures.
(b). What limits heat tolerance evolution?
Behavioural thermoregulation can help explain why CTmax is less variable than CTmin in the cybotoids, but not why the response to different environmental conditions involved behavioural, rather than physiological, change. Given that time spent thermoregulating imposes a cost with regards to other activities such as foraging, predator avoidance and reproduction [19,60,61], it is unclear why selection should favour the maintenance of high body temperatures in montane habitats, instead of physiological adaptation to lower temperatures. One possibility is that behavioural modifications are easier to evolve than changes in physiological tolerances [62,63]. Given that a myriad of physiological processes (e.g. locomotion, digestion, and growth) are sensitive to temperature, the evolution of physiological tolerances may necessitate the concerted evolution of many genes (discussed in [64,65]). By contrast, shifts in basking frequency change seasonally within populations, and so behavioural shifts at different elevations may not require substantial evolutionary change. Moreover, even if evolutionary shifts in behaviour are required, such changes may require fewer genetic changes than shifts in physiology [36].
This ‘evolution along lines of least genetic resistance’ (sensu [66]) explanation suggests that there is no inherent advantage to warmer body temperatures, but an alternative explanation for the lack of evolutionary variability in CTmax revolves around the fitness benefits of high temperatures. Specifically, selection may favour the maintenance of high body temperatures in cold environments because rates of biochemical reactions increase with optimal temperature [65,67,68]. Indeed, warm-adapted ectotherms generally experience higher levels of physiological performance than cold-adapted organisms [69,70]. However, if this ‘hotter is better’ hypothesis is true, it still fails to address why low elevation populations have not evolved even higher heat tolerances.
(c). Impacts of climate change
Climate warming will probably have different effects on cybotoids from lowland and upland habitats. Warming temperatures threaten to make current ranges thermally inhospitable for many cool-adapted montane ectotherms, which may force their ranges upwards [4,71]. By contrast, it is likely that upland cybotoids will benefit, at least in the short term, from climate warming. As the climate warms, environmental temperatures will more often approximate lizards’ preferred temperatures, and thus the time lizards need to spend thermoregulating should decrease and the number of hours available for other activities should increase. By contrast, higher temperatures may allow species from lower elevations to migrate upwards leading to negative interspecific interactions [2].
The challenge facing lowland cybotoids will be to avoid stressfully hot temperatures as habitats continue to warm. Many tropical lizards, particularly those near sea level, are already frequently experiencing temperatures exceeding their preferred ranges [2,3]. As warming continues, lizards in such lowland populations will eventually be unable to maintain temperatures within their preferred range for long enough periods to survive. At that point, lowland populations can only avoid local extinction by shifting their physiology to adapt to these higher environmental temperatures.
Evolutionary stasis in CTmax may suggest a limited ability to evolve and, thus, a heightened vulnerability to environmental warming. Some studies on Drosophila support the idea that heat tolerance evolution is genetically constrained, as the amount of genetic diversity for heat tolerance is limited compared with that for cold tolerance [72–74]. The observation that CTmin evolves readily in cybotoids and in other ectotherms [75,76] would tend to support this hypothesis. Nonetheless, it is hard to construe why diverse physiological systems would be constrained from evolving upper, but not lower tolerances. In fact, experiments on Drosophila [77] and salmon [78] have demonstrated that heat tolerance can increase in response to selection, although there appears to be an upper ceiling on how high heat tolerance can evolve [79]; no similar experiments have ever been conducted on vertebrates. Moreover, although cybotoid anoles show relatively little variation in heat sensitivity, some other anole clades have diversified extensively while adapting to different thermal environments [54]. Looking more broadly, other lizard species possess heat tolerances that approach 50°C (reviewed in [58,59]), suggesting that if genetic constraints exist in lizards, they are phylogenetically localized. Finding an explanation for variation among clades in physiological diversity could aid in assessing ectotherms’ vulnerability to climate warming, but it is a challenge that will require integration of physiological, behavioural, and evolutionary approaches.
Acknowledgements
We thank O. Baez-Landestoy and R. Castañeda for field assistance, the Ministerio de Medio Ambiente y Recursos Naturales and the Museo Nacional de Historia Natural in the Dominican Republic for granting our research permit requests, and T. Espirito, P. Hertz, S. Lambert, J. Lawrence, M. Stute, and Y. Stuart for helpful feedback on this project and manuscript. We thank three reviewers for greatly improving this manuscript.
All work was conducted in accordance with IACUC protocols 26-11 at Harvard University.
Funding statement
Financial support was provided by a National Science Foundation grant (DEB: award no. 0918975) to J.B.L., an NSF Graduate Research Fellowship to M.M.M., a Putnam Expedition grant to M.M.M. and a Craig Family Award to M.A.S.
References
- 1.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Álvarez Pérez HJ, Garland T., Jr 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948 (doi:10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 (doi:10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 4.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 5.Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G. 2008. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6, 2621–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson RD. 1985. The relative importance of behvioural and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 126, 362–386 (doi:10.1086/284423) [Google Scholar]
- 7.Bogert CM. 1949. Thermoregulation in reptiles, a factor in evolution. Evolution 3, 195–211 (doi:10.2307/2405558) [DOI] [PubMed] [Google Scholar]
- 8.Huey RB, Hertz PE, Sinervo B. 2003. Behvioural drive versus behvioural inertia in evolution: a null model approach. Am. Nat. 161, 357–366 (doi:10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 9.Sarmiento G. 1986. Ecological features of climate in high tropical mountains. In High altitude tropical biogeography (eds Vuilleumier F, Monasterio M.), pp. 11–46 New York, NY: Oxford University Press [Google Scholar]
- 10.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 11.Schwartz A. 1989. A review of the cybotoid anoles (Reptilia: Sauria: Iguanidae) from Hispaniola. Milwaukee Public Mus. Contrib. Biol. and Geol. 78, 1–32 [Google Scholar]
- 12.Glor RE, Kolbe JJ, Powell R, Larson A, Losos JB. 2003. Phylogenetic analysis of ecological and morphological diversification in Hispaniolan trunk-ground anoles (Anolis cybotes group). Evolution 57, 2383–2397 (doi:10.1554/02-369) [DOI] [PubMed] [Google Scholar]
- 13.Hertz PE, Huey RB. 1981. Compensation for altitudinal changes in the thermal environment by some Anolis lizards on Hispaniola. Ecology 62, 515–521 (doi:10.2307/1937714) [Google Scholar]
- 14.Henderson RW, Powell R. 2009. Natural history of West Indian reptiles and amphibians. Gainesville, FL: University Press of Florida [Google Scholar]
- 15.Wollenberg KC, Wang IJ, Glor RE, Losos JB. 2013. Determinism in the diversification of Hispaniolan trunk-ground anoles (Anolis cybotes species complex). Evolution 67, 3175–3190 (doi:10.1111/evo.12184) [DOI] [PubMed] [Google Scholar]
- 16.Mahler DL, Revell LJ, Glor RE, Losos JB. 2010. Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean anoles. Evolution 64, 2731–2745 (doi:10.1111/j.1558-5646.2010.01026.x) [DOI] [PubMed] [Google Scholar]
- 17.Williams EE. 1963. Anolis whitemani, new species from Hispaniola (Sauria, Iguanidae). Breviora 197, 1–8 [Google Scholar]
- 18.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (doi:10.1002/joc.1276) [Google Scholar]
- 19.Huey RB. 1974. Behvioural thermoregulation in lizards: importance of associated costs. Science 184, 1001–1003 (doi:10.1126/science.184.4140.1001) [DOI] [PubMed] [Google Scholar]
- 20.Hertz PE. 1992. Temperature regulation in Puerto Rican Anolis lizards: a field test using null hypotheses. Ecology 73, 1405–1417 (doi:10.2307/1940686) [Google Scholar]
- 21.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology, and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679 (doi:10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spellerberg IF. 1972. Temperature tolerances of southeast Australian reptiles examined in relation to reptile thermoregulatory behaviour and distribution. Oecologia 9, 23–46 (doi:10.1007/BF00345241) [DOI] [PubMed] [Google Scholar]
- 23.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574 (doi:10.1139/z97-783) [Google Scholar]
- 24.Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. 2007. Critical thermal limits depend on methodological context. Proc. R. Soc. B 274, 2935–2942 (doi:10.1098/rspb.2007.0985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hertz PE, Arce-Hernandez A, Ramirez-Vazquez J, Tirado-Rivera W, Vazquez-Vives L. 1979. Geographical variation of heat sensitivity and water loss rates in the tropical lizard Anolis gundlachi. Comp. Biochem. Phys. A 62, 947–953 (doi:10.1016/0300-9629(79)90033-1) [Google Scholar]
- 26.Gaston KJ, Spicer JI. 1998. Do upper thermal tolerances differ in geographically separated populations of the beachflea Orchestia gammarelllus (Custacea: Amphipoda)? J. Exp. Mar. Biol. Ecol. 229, 265–276 (doi:10.1016/S0022-0981(98)00057-4) [Google Scholar]
- 27.Chown SL, Jumbam KR, Sørensen JG, Terblanche JS. 2009. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 23, 133–140 (doi:10.1111/j.1365-2435.2008.01481.x) [Google Scholar]
- 28.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- 29.Paradis E. 2006. Analysis of phylogenetics and evolution with R. New York, NY: Springer [Google Scholar]
- 30.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 31.Garland T, Jr, Harvey PH, Ives AR. 1992. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 (doi:10.2307/2992503) [Google Scholar]
- 32.Oufiero CE, Gartner GEA, Adolph SC, Garland T., Jr 2011. Latitudinal and climatic variation in body size and dorsal scale counts in Sceloporus lizards: a phylogenetic perspective. Evolution 65, 3590–3607 (doi:10.1111/j.1558-5646.2011.01405.x) [DOI] [PubMed] [Google Scholar]
- 33.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harmon LJ, Weir JT, Brock C, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131 (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 35.Hansen TF. 1997. Stabilizing selection and the comparative analysis of adaptation. Evolution 51, 1341–1351 (doi:10.2307/2411186) [DOI] [PubMed] [Google Scholar]
- 36.Blomberg SP, Garland T, Jr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behvioural traits are more labile. Evolution 57, 717–745 [DOI] [PubMed] [Google Scholar]
- 37.Butler MA, King AA. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am. Nat. 164, 683–695 (doi:10.1086/426002) [DOI] [PubMed] [Google Scholar]
- 38.Freckleton RP, Harvey PH. 2006. Detecting non-Brownian trait evolution in adaptive radiations. PLoS Biol. 4, e373 (doi:10.1371/journal.pbio.0040373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugihara N. 1978. Further analysis of the data by Akaike's information criterion and the finite corrections. Commun. Stat. Theory Methods A7, 13–26 (doi:10.1080/03610927808827599) [Google Scholar]
- 40.Burnham KP, Anderson DR. 2002. Model selection and multimode inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 41.Adams DC. 2013. Comparing evolutionary rates for different phenotypic traits on a phylogeny using likelihood. Syst. Biol. 62, 181–192 (doi:10.1093/sysbio/sys083) [DOI] [PubMed] [Google Scholar]
- 42.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 43.Paradis E. 2012. Analyses of phylogenetics and evolution with R. New York, NY: Springer [Google Scholar]
- 44.Kolbe JJ, VanMiddlesworth PS, Losin N, Dappen N, Losos JB. 2012. Climatic niche shift predicts thermal trait response in one but not both introductions of the Puerto Rican lizard Anolis cristatellus to Miami, Florida, USA. Ecol. Evol. 2, 1503–1516 (doi:10.1002/ece3.263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakken GS. 1992. Measurement and application of operative and standard operative temperatures in ecology. Am. Zool. 32, 194–216 [Google Scholar]
- 46.Bakken GS, Gates DM. 1975. Heat-transfer analysis of animals: some implications for field ecology, physiology, and evolution. In Perspectives of biophysical ecology (eds Gates DM, Schmerl RB.), pp. 255–290 New York, NY: Springer [Google Scholar]
- 47.Bakken GS, Angilletta MJ., Jr In press How to avoid errors when quantifying thermal environments. Funct. Ecol. (doi:10.1111/1365-2435.12149) [Google Scholar]
- 48.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (doi:10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 49.Cowles RB, Bogert CM. 1944. A preliminary study of the thermal requirements of desert reptiles. Br. Am. Mus. Nat. Hist. 83, 265–296 [Google Scholar]
- 50.Kearney M, Shine R, Porter WP. 2009. The potential for behavioural thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruibal R. 1961. Thermal relations of five species of tropical lizards. Evolution 15, 98–111 (doi:10.2307/2405846) [Google Scholar]
- 52.Rand AS. 1964. Ecological distribution in anoline lizards of Puerto Rico. Ecology 45, 745–752 (doi:10.2307/1934922) [Google Scholar]
- 53.van Berkum FH. 1986. Evolutionary patterns of thermal sensitivity of sprint speed in Anolis lizards. Evolution 40, 594–604 (doi:10.2307/2408580) [DOI] [PubMed] [Google Scholar]
- 54.Hertz PE, Arima Y, Harrison A, Huey RB, Losos JB, Glor RE. 2013. Asynchronous evolution of physiology and morphology in Anolis lizards. Evolution 677, 2101–2113 (doi:10.1111/evo.12072) [DOI] [PubMed] [Google Scholar]
- 55.Huey RB, Webster TP. 1976. Thermal biology of Anolis lizards in a complex fauna: the cristatellus group on Puerto Rico. Ecology 57, 985–994 (doi:10.2307/1941063) [Google Scholar]
- 56.Gunderson AR, Leal M. 2012. Geographic variation in vulnerability to climate warming in a tropical Caribbean lizard. Funct. Ecol. 26, 783–793 (doi:10.1111/j.1365-2435.2012.01987.x) [Google Scholar]
- 57.Janzen DH. 1967. Why are mountain passes higher in the tropics? Am. Nat. 101, 233–249 (doi:10.1086/282487) [Google Scholar]
- 58.Sunday JM, Bates AE, Dulvy NK. 2010. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 (doi:10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219 (doi:10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 60.Grant BW, Dunham AE. 1988. Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology 69, 167–176 (doi:10.2307/1943171) [Google Scholar]
- 61.Adolph SC, Porter WP. 1993. Temperature, activity, and lizard life histories. Am. Nat. 142, 273–295 (doi:10.1086/285538) [DOI] [PubMed] [Google Scholar]
- 62.Mayr E. 1963. Animal species and evolution. Cambridge, MA: The Belknap Press of Harvard University Press [Google Scholar]
- 63.West-Eberhard M. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press [Google Scholar]
- 64.Angilletta MJ., Jr 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press [Google Scholar]
- 65.Huey RB. 2009. Evolutionary physiology of insect thermal adaptation to cold environments. In Low temperature biology of insects (eds Denlinger DL, Lee RE., Jr), pp. 223–241 Cambridge, UK: Cambridge University Press [Google Scholar]
- 66.Schluter D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766–1774 (doi:10.2307/2410734) [DOI] [PubMed] [Google Scholar]
- 67.Bennett AF. 1987. Evolution of the control of body temperature: is warmer better? In Comparative physiology: life in the water and on land (eds Dejours P, Bolis L, Taylor CR, Weibel ER.), pp. 421–431 Padova, Italy: Liviana Press [Google Scholar]
- 68.Huey RB, Kingsolver JG. 1989. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 4, 131–135 (doi:10.1016/0169-5347(89)90211-5) [DOI] [PubMed] [Google Scholar]
- 69.Savage VM, Gillooly JF, Brown JH, West GB, Charnove EL. 2004. Effects of body size and temperature on population growth. Am. Nat 163, 429–441 (doi:10.1086/381872) [DOI] [PubMed] [Google Scholar]
- 70.Angilletta MJ, Jr, Huey RB, Frazier MR. 2010. Thermodynamic effects on organismal performance: is hotter better? Physiol. Biochem. Zool. 83, 197–206 [DOI] [PubMed] [Google Scholar]
- 71.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- 72.Hoffmann AA, Sorensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216 (doi:10.1016/S0306-4565(02)00057-8) [Google Scholar]
- 73.Hoffmann AA, Chown SL, Clusella-Trullas S. 2012. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934–949 (doi:10.1111/j.1365-2435.2012.02036.x) [Google Scholar]
- 74.Ragland GJ, Kinsolver JG. 2008. Evolution of thermotolerance in seasonal environments: the effects of annual temperature variation and life-history timing in Wyeomyia smithii. Evolution 62, 1345–1357 (doi:10.1111/j.1558-5646.2008.00367.x) [DOI] [PubMed] [Google Scholar]
- 75.Barrett RDH, Paccard A, Healy TM, Bergek S, Schulte PM, Schluter D, Rogers SM. 2010. Rapid evolution of cold tolerance in stickleback. Proc. R. Soc. B 278, 233–238 (doi:10.1098/rspb.2010.0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leal M, Gunderson AR. 2012. Rapid change in the thermal tolerance of a tropical lizard. Am. Nat. 180, 815–822 (doi:10.1086/668077) [DOI] [PubMed] [Google Scholar]
- 77.Gilchrist GW, Huey RB. 1999. The direct response of Drosophila melanogaster to selection on knock-down temperature. Heredity 83, 15–29 (doi:10.1038/sj.hdy.6885330) [DOI] [PubMed] [Google Scholar]
- 78.Donaldson LR, Olson PR. 1957. Development of rainbow trout brood stock by selective breeding. Trans. Am. Fish. Soc. 85, 93–101 (doi:10.1577/1548-8659(1955)85[93:DORTBS]2.0.CO;2) [Google Scholar]
- 79.Hamilton WJ., III 1973. Life's color code. New York, NY: McGraw Hill [Google Scholar]