Abstract
Fever is commonly attenuated with antipyretic medication as a means to treat unpleasant symptoms of infectious diseases. We highlight a potentially important negative effect of fever suppression that becomes evident at the population level: reducing fever may increase transmission of associated infections. A higher transmission rate implies that a larger proportion of the population will be infected, so widespread antipyretic drug use is likely to lead to more illness and death than would be expected in a population that was not exposed to antipyretic pharmacotherapies. We assembled the published data available for estimating the magnitudes of these individual effects for seasonal influenza. While the data are incomplete and heterogeneous, they suggest that, overall, fever suppression increases the expected number of influenza cases and deaths in the US: for pandemic influenza with reproduction number 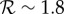 , the estimated increase is 1% (95% CI: 0.0–2.7%), whereas for seasonal influenza with
, the estimated increase is 1% (95% CI: 0.0–2.7%), whereas for seasonal influenza with 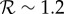 , the estimated increase is 5% (95% CI: 0.2–12.1%).
, the estimated increase is 5% (95% CI: 0.2–12.1%).
Keywords: influenza, transmission, fever, antipyretic drugs
1. Introduction
For millennia, humans have suppressed fevers without understanding the potential effects [1,2] beyond the obvious alleviation of symptoms. Antipyretic drug treatment is extremely prevalent in Western countries—especially by parents [3], and also by healthcare professionals [4–6]. Even when treatment is not aimed at fever specifically, fever is likely to be reduced, because most common drugs that relieve other typical symptoms of infectious diseases also contain an antipyretic component [7].
Previous investigations of the effects of fever suppression have focused on the clinical benefits and costs to the individual [8,9]. The adaptive value of fever [10–13] is well known to immunologists; for example, Janeway's Immunobiology [14, p. 110] notes that ‘At higher temperatures, bacterial and viral replication is less efficient, whereas the adaptive immune response operates more efficiently’. Others argue that the adaptive value of fever arises instead from activation and coordination of the immune response [12]. By contrast, a common view in the medical community, as expressed for example in Harrison's Principles of Internal Medicine, is that the ‘treatment of fever and its symptoms does no harm and does not slow the resolution of common viral and bacterial infections’ [15, p. 107]. Here, we consider some population-level effects of widespread fever suppression, effects that do not appear to have been investigated previously.
An individual whose fever has been reduced is likely to feel better and is therefore more likely to interact with others. In addition, fever suppression may increase both the rate and duration of viral shedding, further increasing the pathogen's transmission rate; this effect has been shown experimentally for influenza in ferrets [16]. A higher transmission rate will in general lead to larger epidemics [17,18] and hence to greater morbidity and mortality. The increase in epidemic size is larger for more weakly transmissible pathogens.
2. Theoretical argument
To make this idea more precise, suppose that (i) a proportion p of infected individuals develop a fever and are treated with antipyretics (the treatment probability) and (ii) on average, antipyretic treatment of febrile individuals increases their probability of infecting others by a factor fi (the individual transmission enhancement factor). The proportion of infected individuals with enhanced transmission is then p, and the proportion without enhanced transmission (i.e. with transmission enhancement factor 1) is 1 − p. Antipyretics therefore increase the overall transmission rate by the factor
| 2.1a |
| 2.1b |
which we call the population transmission enhancement factor. (Note that fp > 1 given that fi > 1 and 0 < p ≤ 1.)
Transmissibility of a pathogen is normally quantified by the basic reproduction number 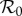 , the average number of secondary cases caused by a primary case in a wholly susceptible population [19]. In a population that is not wholly susceptible—which is always the situation for seasonal influenza—the effective reproduction number is reduced by the proportion susceptible at the start of the epidemic
, the average number of secondary cases caused by a primary case in a wholly susceptible population [19]. In a population that is not wholly susceptible—which is always the situation for seasonal influenza—the effective reproduction number is reduced by the proportion susceptible at the start of the epidemic 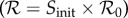 . In these terms, antipyretic use has the effect of increasing the reproduction number
. In these terms, antipyretic use has the effect of increasing the reproduction number
| 2.2 |
We would like to estimate how many additional infections, and correspondingly how many severe illnesses and deaths, can be expected to result from this increase in 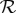 during an influenza epidemic or pandemic [20].
during an influenza epidemic or pandemic [20].
For a very large class of epidemic models, the proportion of the population that is expected to be infected during an epidemic (the expected final size Z) is related to the reproduction number by the classical final size relation [17,18],
| 2.3 |
which can be solved explicitly (see the electronic supplementary material). Note that Z is the final size as a proportion of those who were initially susceptible; if everyone were susceptible initially (Sinit = 1), as is possible in a pandemic, then 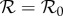 and Z is the proportion of the entire population infected.
and Z is the proportion of the entire population infected.
Figure 1a shows this final size relation, 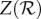 , and figure 1b shows the relative incremental change in final size,
, and figure 1b shows the relative incremental change in final size,
| 2.4 |
as a function of the population transmission enhancement factor fp, for three values of 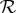 in the plausible range for influenza,
in the plausible range for influenza, 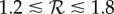 (
(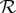 is likely near the lower end of this range for seasonal influenza [21,22] and the higher end for pandemic influenza [23–26]). Because the final size Z is a decelerating function of the reproduction number
is likely near the lower end of this range for seasonal influenza [21,22] and the higher end for pandemic influenza [23–26]). Because the final size Z is a decelerating function of the reproduction number 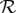 (figure 1a), antipyresis always enhances transmission more for less transmissible diseases (which have smaller
(figure 1a), antipyresis always enhances transmission more for less transmissible diseases (which have smaller 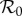 : figure 1b). The precise quantitative predictions in figure 1b depend on our use of the standard final size relation; however, the qualitative conclusions are very general because the expected final size always increases (typically in a decelerating fashion) as
: figure 1b). The precise quantitative predictions in figure 1b depend on our use of the standard final size relation; however, the qualitative conclusions are very general because the expected final size always increases (typically in a decelerating fashion) as 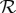 increases [27–31].
increases [27–31].
Figure 1.
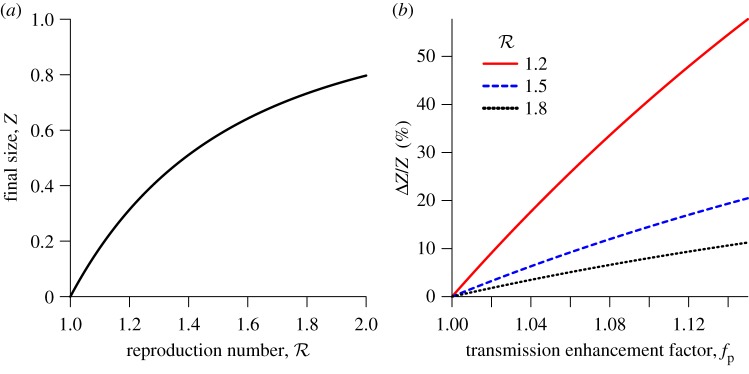
The effects of increases in transmission rate (by the factor fp) on the expected proportion of the initially susceptible population that will be infected in a single influenza epidemic (the final size Z). (a) The standard final size relation (2.3), for the plausible range of (effective) reproduction number for influenza. (b) The relative increase in final size resulting from increasing the transmission rate by the factor fp. For example, a 10% increase in the proportion of individuals infected during an epidemic will arise from a 2% transmission enhancement if 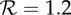 , a 6% enhancement if
, a 6% enhancement if 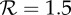 or a 12% enhancement if
or a 12% enhancement if 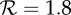 . (Online version in colour.)
. (Online version in colour.)
3. Estimating the effect for influenza
To predict the magnitude of the effect in practice, we need an estimate of the population transmission enhancement factor fp. We have insufficient data to estimate how all the relevant biological mechanisms contribute to increasing fp; in particular, we expect the increase in social interaction owing to reduced symptoms to lead to a major increase in the epidemiological contact rate, but are unable to quantify this. Nevertheless, by focusing on how antipyretics affect individual infectivity, we can at least estimate a lower bound on fp for influenza. Throughout all stages of the calculations described below, we propagate error estimates by randomly sampling 10 000 values from the sampling distribution of each of the estimated parameters (assumed normal unless otherwise specified), computing the relevant metric with each set of parameters in the random sample, and finding the lower 2.5% and upper 97.5% quantiles of the resulting distribution. All computations were done in the R language [32] (see the electronic supplementary material).
The two components of fp in equation (2.1) (p and fi) are independent. Limited information is available concerning the treatment probability p: parents treat febrile children with antipyretics in approximately 90% of cases [3], and nurses treat fever with antipyretics in approximately 70% of cases [5,6]. We know that adults frequently take analgesics that are antipyretic, but we have little influenza-specific information. On these grounds, we propose a broad distribution for p (Beta(4,2)), with mean 0.67 (95% CI: 0.28–0.95). (Substituting a uniform [0,1] distribution for p instead to represent complete uncertainty does not change the results qualitatively; see the electronic supplementary material.) We must also adjust our estimate of p to take into account that only 67% (95% CI: 58–75%) of individuals show symptoms and only 35% (95% CI: 27–44%) develop a fever ([33]; see the electronic supplementary material).
To obtain a lower bound on individual transmission enhancement fi, and hence to complete an estimate of a lower bound on population transmission enhancement fp from equation (2.1), we consider two aspects of infectivity enhancement for which data exist.
First, antipyretics appear to increase viral shedding. To our knowledge, the only published experiment concerning the effects of antipyretic treatment on influenza viral shedding was conducted in ferrets (considered the best animal model for human influenza [34]). The study, conducted by Husseini et al. [16], considered two strains of influenza A/H3N2 that differed in virulence. For both strains, and regardless of whether fever was suppressed by shaving the ferrets or by administration of an antipyretic drug, the authors found that ‘significantly more virus was shed in the nasal washes of ferrets whose febrile response was suppressed and the viral levels decreased less rapidly than in untreated ferrets or in those in which the treatments were ineffective’ [16, p. 520]. This study was prompted by an earlier study from the same group showing that unmedicated ferrets with higher fevers shed less influenza virus [35]. The results are consistent with other studies showing that antipyretic treatment increases viral shedding in human volunteers infected with rhinovirus [36] and lengthens the infectious period in children with chickenpox [37]. Moreover, in a study of human volunteers infected with influenza A, the number of antipyretic doses received was positively correlated with the duration of illness [38]. Some cytokines reduce viral shedding, so a likely mechanism by which antipyresis increases viral shedding is the suppression of temperature-dependent cytokine responses to influenza infection (see the electronic supplementary material, §3.2). Based on these considerations, we assume that the clinical effects of fever suppression on nasal shedding in humans infected with influenza virus are similar to the effects measured in ferrets. Based on inverse-variance weighted mean values for the difference in the logarithm of viral titres between the antipyretic-treated and untreated ferrets, we estimate that antipyretic treatment increases influenza viral titres in nasal droplets by a factor of order 1.78 (95% CI: 1.35–2.35) (see the electronic supplementary material for further details).
Second, greater viral shedding increases infectivity. This is unsurprising, but estimating the strength of the effect is challenging. A recent review [39] describes 30 studies in which human volunteers were given various doses of a variety of influenza viruses. To analyse these data, we used a binomial generalized linear mixed model incorporating random effects of strain and study [40,41] to estimate the relationship between log10(dose) and probability of infection. We conclude (see the electronic supplementary material) that a dose that is larger by a factor 10 (which we assume would arise from an increase in viral titres in nasal droplets by the same factor) yields an increase of 0.28 (95% CI: 0.01–0.54) in the log-odds of infection. (This effect would correspond to an increase of 0.07 (95% CI: 0.004–0.13) in the proportion infected if we started from a baseline infection probability of 0.5.)
In order to infer the overall transmission implications, we need an estimate of the natural infectivity of influenza, i.e. the probability that a susceptible contacted by an (non-antipyretic-using) infectious individual will become infected. We are not aware of direct measurements of this probability, so we use published estimates [42] of the household secondary attack rate (SAR) as a proxy. We used a linear mixed model incorporating variation among strains and among studies to estimate the log-odds of the SAR, based on measurements of antibody response of individuals between the beginning and end of the influenza season. Based on the coefficients of this model, we estimate the expected SAR to be 0.14 (95% CI: 0.07–0.27). As study participants were not prevented from taking antipyretics, the reported SAR likely represents an overestimate of the natural infectivity (which will make our inferences more conservative; see the electronic supplementary material, §5).
Associating proportional changes in the viral titre of nasal washes in the ferret study [16] with proportional changes in viral titres in nasal sprays in the human challenge studies [39], and taking the household SAR to approximate natural infectivity, we estimated the antipyretic-induced individual transmission enhancement factor fi using equation S22 in the electronic supplementary material. We infer a conservative lower bound of 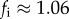 (95% CI: 1.002–1.14).
(95% CI: 1.002–1.14).
Putting together our estimates of the treatment probability p and the individual transmission enhancement factor fi using equation (2.1) (details in the electronic supplementary material), we conclude that the current practice of frequently treating fevers with antipyretic medication has the population-level effect of enhancing the transmission of influenza by at least 1% (95% CI: 0.04–3%) (i.e. fp > 1.01 (95% CI: 1.00–1.03)). This estimate does not take into account the known effect that the infectious period of influenza is also increased by antipyresis [16], nor does it take into account the potentially large effect of increasing the rate of contact among infectious and susceptible individuals because antipyresis makes infectious individuals feel better.
4. Discussion
To put our lower bound for fp into perspective, consider that approximately 41 400 (95% CI: 27 100–55 700) deaths per year are attributed to seasonal influenza epidemics in the United States [43] (and an order of magnitude more worldwide [44]). Taken at face value, our results indicate, for example, that if 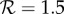 then at least 700 deaths per year (95% CI: 30–2100) (and many more serious illnesses) could be prevented in the US alone by avoiding antipyretic medication for the treatment of influenza (see table 1). While subject to large uncertainty, our estimates in table 1 should be considered conservative, as we have ignored concomitant antipyretic-induced increases in infectious periods and contact rates.
then at least 700 deaths per year (95% CI: 30–2100) (and many more serious illnesses) could be prevented in the US alone by avoiding antipyretic medication for the treatment of influenza (see table 1). While subject to large uncertainty, our estimates in table 1 should be considered conservative, as we have ignored concomitant antipyretic-induced increases in infectious periods and contact rates.
Table 1.
Percentage of influenza deaths attributable to common use of antipyretic medication (for the plausible range of reproduction number for influenza). See the electronic supplementary material for details.
| attributable influenza deaths |
||
|---|---|---|
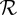 |
estimate (%) | 95% CI |
| 1.2 | 5 | (0.3%, 12.6%) |
| 1.5 | 2 | (0.1%, 4.9%) |
| 1.8 | 1 | (0.1%, 2.8%) |
The population-level effects of antipyretic treatment during influenza pandemics could be especially dramatic. It has been suggested that widespread use of aspirin in 1918 may have increased disease severity, and consequently death rates, during the pandemic [45], and experimental research in humans and other animals suggests that antipyretic use may increase the risk of death from serious infections [10,46]. Even without this individual-level effect, the population transmission-enhancing effect that we have highlighted here could have increased the final size of the 1918 pandemic significantly, suggesting that a non-negligible proportion of the 50–100 million [47] pandemic-related deaths could have been attributable to transmission enhancement from widespread use of antipyretic medication.
While our theoretical argument that links antipyretic treatment with an increase in epidemic size is straightforward, estimation of the magnitude of this effect is necessarily indirect, and our attempt here provides only a crude lower bound. We have been conservative in every step of our estimation of this lower bound, but we have not been able to quantify all potentially contributing factors. One further effect that could be important in principle is transmission of influenza by infected individuals before they show symptoms; however, evidence for this effect—and for asymptomatic transmission in general—is weak [48] and seems likely to be balanced in our calculations by ignoring the known lengthening of the infectious period caused by antipyresis [16]. Another potentially important effect that we have not considered is age-dependent mixing. Exceptionally high rates of antipyretic treatment in children [3] might contribute to the disproportionate role that children play in influenza transmission [26,49]; taking this into account would increase our estimated lower bound. Readers who want to consider the impact of including additional factors, or modifying our estimates, can use figure 1 to approximate the effect of changes to the population transmission enhancement factor fp. Because the estimated absolute magnitude of fp is fairly small, and because the curves in figure 1(b) are close to linear, most effects will be close to linear as well. For example, if amelioration of symptoms led to a lengthening of the infectious period by 20%, the number of estimated attributable cases would increase by 19.2%.
Experiments and observational studies designed specifically to estimate the magnitude of transmission enhancement by antipyresis could give much more precise constraints on the population-level effects of antipyretic use. In particular, randomized trials assigning individuals to antipyretic or placebo treatment could characterize increases in the infectious period and viral shedding owing to antipyretic drugs, while challenge experiments could better characterize the relationship between dosage and infection probability. Increases in contact rates caused by infectious individuals feeling well enough to go to work, school and other gathering places may be even more important in practice. These effects would best be estimated as part of the randomized trials discussed above, but even observational studies that survey individuals' symptoms and behaviour and correlate them with variation in use of medications could be a useful first step; we are beginning pilot studies of this sort.
We have shown that—as is well understood for antibiotics [50]—the use of antipyretics can have subtle and potentially important negative effects at the population level. Any medical intervention that aims to relieve the symptoms of an infectious disease in an individual should also be evaluated in light of potentially harmful effects at the population level. Practices that prevent infection (e.g. vaccination), or increase individual comfort without increasing transmission, are preferable from a population perspective. We hope that our analysis in this paper will spur further research to determine more precise estimates of the effects that we have discussed. Such estimates should assist in the development of evidence-based guidelines for antipyretic treatment practices.
Acknowledgements
We thank Susan Marsh-Rollo and Sangeeta Sutradhar for assistance in digitizing data, and Ali Ashkar, Sigal Balshine, Dawn Bowdish, David Champredon, Ferric Fang, Brian Lichty, Mark Loeb, Chai Molina, Karen Mossman, Marek Smieja, Gerry Wright and Dan Yamin for valuable discussions and correspondence.
Funding statement
D.J.D.E. and B.M.B. were supported by NSERC and the M.G. DeGroote Institute for Infectious Disease Research.
References
- 1.Jack DB. 1997. One hundred years of aspirin. Lancet 350, 437–439 (doi:10.1016/S0140-6736(97)07087-6) [DOI] [PubMed] [Google Scholar]
- 2.Mackowiak PA. 2000. Brief history of antipyretic therapy. Clin. Infect. Dis. 31, S154–S156 (doi:10.1086/317510) [DOI] [PubMed] [Google Scholar]
- 3.Walsh A, Edwards H, Fraser J. 2007. Over-the-counter medication use for childhood fever: a cross-sectional study of Australian parents. J. Paediatr. Child Health 43, 601–606 (doi:10.1111/j.1440-1754.2007.01161.x) [DOI] [PubMed] [Google Scholar]
- 4.Ipp M, Jaffe D. 1993. Physicians’ attitudes toward the diagnosis and management of fever in children 3 months to 2 years of age. Clin. Pediatr. 32, 66–70 (doi:10.1177/000992289303200201) [DOI] [PubMed] [Google Scholar]
- 5.Thomas V, Riegel B, Andrea J, Murray P, Gerhart A, Gocka I. 1994. National survey of pediatric fever management practices among emergency department nurses. J. Emerg. Nurs. 20, 505–510 [PubMed] [Google Scholar]
- 6.Plaisance KI, Mackowiak PA. 2000. Antipyretic therapy. Arch. Intern. Med. 160, 449–456 (doi:10.1001/archinte.160.4.449) [DOI] [PubMed] [Google Scholar]
- 7.Wood DM, et al. 2010. Patient knowledge of the paracetamol content of over-the-counter (OTC) analgesics, cough/cold remedies and prescription medications. Emerg. Med. J. 27, 829–833 (doi:10.1136/emj.2009.085027) [DOI] [PubMed] [Google Scholar]
- 8.Mackowiak PA. 2000. Physiological rationale for suppression of fever. Clin. Infect. Dis. 31, S185–S189 (doi:10.1086/317511) [DOI] [PubMed] [Google Scholar]
- 9.Eyers S, Weatherall M, Shirtcliffe P, Perrin K, Beasley R. 2010. The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analyis. J. R. Soc. Med. 103, 403–411 (doi:10.1258/jrsm.2010.090441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluger MJ. 1991. The adaptive value of fever. In Fever: basic mechanisms and management (ed. Mackowiak PA.), pp. 105–124 New York, NY: Raven Press Ltd [Google Scholar]
- 11.Hasday JD, Fairchild KD, Shanholtz C. 2000. The role of fever in the infected host. Microbes Infect. 2, 1891–1904 (doi:10.1016/S1286-4579(00)01337-X) [DOI] [PubMed] [Google Scholar]
- 12.Blatteis CM. 2003. Fever: pathological or physiological, injurious or beneficial? J. Thermal Biol. 28, 1–13 (doi:10.1016/S0306-4565(02)00034-7) [Google Scholar]
- 13.Simon HB. 2003. Hyperthermia, fever, and fever of undetermined origin In Infectious diseases: the clinician‘s guide to diagnosis, treatment, and prevention. (ed. Dale DC.). New York, NY: WebMD Professional Publishing [Google Scholar]
- 14.Murphy KM. 2011. Janeway‘s immunobiology, 8th edn New York, NY: Garland Science [Google Scholar]
- 15.Dinarello CA, Porat R. 2008. In Harrison's principles of internal medicine (eds Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, Loscalzo J.), 17th edn, p. 2958 New York, NY: McGraw-Hill [Google Scholar]
- 16.Husseini RH, Sweet C, Collie MH, Smith H. 1982. Elevation of nasal viral levels by suppression of fever in ferrets infected with influenza viruses of differing virulence. J. Infect. Dis. 145, 520–524 (doi:10.1093/infdis/145.4.520) [DOI] [PubMed] [Google Scholar]
- 17.Kermack WO, McKendrick AG. 1927. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A 115, 700–721 (doi:10.1098/rspa.1927.0118) [Google Scholar]
- 18.Ma J, Earn DJD. 2006. Generality of the final size formula for an epidemic of a newly invading infectious disease. Bull. Math. Biol. 68, 679–702 (doi:10.1007/s11538-005-9047-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RM, May RM. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press [Google Scholar]
- 20.Earn DJD, Dushoff J, Levin SA. 2002. Ecology and evolution of the flu. Trends Ecol. Evol. 17, 334–340 (doi:10.1016/S0169-5347(02)02502-8) [Google Scholar]
- 21.Chowell G, Miller MA, Viboud C. 2008. Seasonal influenza in the United States, France, and Australia: transmission and prospects for control. Epidemiol. Infect. 136, 852–864 (doi:10.1017/S0950268807009144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowell G, Viboud C, Simonsen L, Miller M, Alonso WJ. 2010. The reproduction number of seasonal influenza epidemics in Brazil, 1996–2006. Proc. R. Soc. B 277, 1857–1866 (doi:10.1098/rspb.2009.1897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuite AR, et al. 2010. Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. Can. Med. Assoc. J. 182, 131–136 (doi:10.1503/cmaj.091807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katriel G, Yaari R, Huppert A, Roll U, Stone L. 2011. Modelling the initial phase of an epidemic using incidence and infection network data: 2009 H1N1 pandemic in Israel as a case study. J. R. Soc. Interface 8, 856–867 (doi:10.1098/rsif.2010.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He D, Dushoff J, Day T, Ma J, Earn DJD. 2011. Mechanistic modelling of the three waves of the 1918 influenza pandemic. Theor. Ecol. 4, 283–288 (doi:10.1007/s12080-011-0123-3) [Google Scholar]
- 26.Earn DJD, et al. 2012. Effects of school closure on incidence of pandemic influenza in Alberta, Canada. Ann. Intern. Med. 156, 173–181 (doi:10.7326/0003-4819-156-3-201202070-00005) [DOI] [PubMed] [Google Scholar]
- 27.Ludwig D. 1975. Final size distribution for epidemics. Math. Biosci. 23, 33–46 (doi:10.1016/0025-5564(75)90119-4) [Google Scholar]
- 28.Drake JM. 2006. Limits to forecasting precision for outbreaks of directly transmitted diseases. PLoS Med. 3, 57–62 (doi:10.1371/journal.pmed.0030003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brauer F. 2008. Age-of-infection and the final size relation. Math. Biosci. Eng. 5, 681–690 [DOI] [PubMed] [Google Scholar]
- 30.Katriel G. 2012. The size of epidemics in populations with heterogeneous susceptibility. J. Math. Biol. 65, 237–262 (doi:10.1007/s00285-011-0460-2) [DOI] [PubMed] [Google Scholar]
- 31.Miller JC, Slim AC, Volz EM. 2012. Edge-based compartmental modelling for infectious disease spread. J. R. Soc. Interface 9, 890–906 (doi:10.1098/rsif.2011.0403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 33.Carrat F, et al. 2008. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am. J. Epidemiol. 167, 775–785 (doi:10.1093/aje/kwm375) [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka Y, Lamirande EW, Subbarao K. 2009. The ferret model for influenza. In Current protocols in microbiology, vol. 13, pp. 15G.2.1–15G.2.29. Hoboken, NJ: John Wiley & Sons [DOI] [PubMed]
- 35.Toms GL, Davies JA, Woodward CG, Sweet C, Smith H. 1977. The relation of pyrexia and nasal inflammatory response to virus levels in nasal washings of ferrets infected with influenza viruses of differing virulence. Br. J. Exp. Pathol. 58, 444–458 [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. 1975. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 231, 1248–1251 (doi:10.1001/jama.1975.03240240018017) [PubMed] [Google Scholar]
- 37.Doran TF, Deangelis C, Baumgardner RA, Mellits ED. 1989. Acetaminophen: more harm than good for chickenpox? J. Pediatr. 114, 1045–1048 (doi:10.1016/S0022-3476(89)80461-5) [DOI] [PubMed] [Google Scholar]
- 38.Plaisance KI, Kudaravalli S, Wasserman SS, Levine MM, Mackowiak PA. 2000. Effect of antipyretic therapy on the duration of illness in experimental influenza A, Shigella sonnei, and Rickettsia rickettsii infections. Pharmacotherapy 20, 1417–1422 (doi:10.1592/phco.20.19.1417.34865) [DOI] [PubMed] [Google Scholar]
- 39.Yezli S, Otter JA. 2011. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ. Virol. 3, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolker BM, et al. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 41.Bates D, Maechler M, Bolker B. 2012. lme4: linear mixed-effects models using S4 classes. R package v. 0.999375–42. See http://CRAN.R-project.org/package=lme4 [Google Scholar]
- 42.Yang Y, et al. 2009. Transmissibility and control of pandemic influenza A (H1N1) virus. Science 326, 729–733 (doi:10.1126/science.1177373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dushoff J, Plotkin JB, Viboud C, Earn DJD, Simonsen L. 2006. Mortality due to influenza in the United States: an annualized regression approach using multiple-cause mortality data. Am. J. Epidemiol. 163, 181–187 (doi:10.1093/aje/kwj024) [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization 2009. Media centre: fact sheet 211. See http://www.who.int/mediacentre/factsheets/fs211/en/ [Google Scholar]
- 45.Starko KM. 2009. Salicylates and pandemic influenza mortality, 1918–1919 pharmacology, pathology, and historic evidence. Clin. Infect. Dis. 49, 1405 (doi:10.1086/606060) [DOI] [PubMed] [Google Scholar]
- 46.Schulman CI, et al. 2005. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg. Infect. 6, 369–375 (doi:10.1089/sur.2005.6.369) [DOI] [PubMed] [Google Scholar]
- 47.Taubenberger JK, Morens DM. 2006. 1918 influenza, the mother of all pandemics. Emerg. Infect. Dis. 12, 15–22 (doi:10.3201/eid1209.05-0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patrozou E, Mermel LA. 2009. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Public Health Rep. 124, 193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loeb M, et al. 2010. Effect of influenza vaccination of children on infection rates in Hutterite communities. JAMA 303, 943–950 (doi:10.1001/jama.2010.250) [DOI] [PubMed] [Google Scholar]
- 50.DiazGranados CA, McGowan JE., Jr 2009. Antimicrobial resistance: an international public health problem. In Antimicrobial drug resistance (ed. Mayers DL.), vol. 2, pp. 1267–1276 New York, NY: Springer Science+Business Media [Google Scholar]


