Abstract
The order of species arrival during community assembly can greatly affect species coexistence, but the strength of these effects, known as priority effects, appears highly variable across species and ecosystems. Furthermore, the causes of this variation remain unclear despite their fundamental importance in understanding species coexistence. Here, we show that one potential cause is environmental variability. In laboratory experiments using nectar-inhabiting microorganisms as a model system, we manipulated spatial and temporal variability of temperature, and examined consequences for priority effects. If species arrived sequentially, multiple species coexisted under variable temperature, but not under constant temperature. Temperature variability prevented extinction of late-arriving species that would have been excluded owing to priority effects if temperature had been constant. By contrast, if species arrived simultaneously, species coexisted under both variable and constant temperatures. We propose possible mechanisms underlying these results using a mathematical model that incorporates contrasting effects of microbial species on nectar pH and amino acids. Overall, our findings suggest that understanding consequences of priority effects for species coexistence requires explicit consideration of environmental variability.
Keywords: community assembly, environmental variability, meta-community, nectar-inhabiting bacteria and yeast, arrival order
1. Introduction
It is now widely recognized that variation in the order of species arrival among sites can drive local communities to divergent successional trajectories, thereby affecting the coexistence of species—the phenomenon known as priority effects (e.g. [1–6]). However, studies of community assembly have yielded variable results as to the importance of priority effects [4], and identifying the causes of this variation remains an active area of research. Although many potential causes have been considered (e.g. [4,7–9]), one likely cause, environmental variability, has received little experimental attention despite the theoretical interest it has long received as a general factor affecting species coexistence (e.g. [10–13]). The dearth of such research creates a major knowledge gap not only for the basic understanding of species coexistence, but also for the application of this understanding to environmental issues. For example, recent studies have suggested that consequences of climate change for ecological communities will depend on the extent of variability in temperature, rainfall and other climatic conditions [14,15], but these studies rarely consider how priority effects may be modified by climate variability.
In theory, environmental variability can affect the strength of priority effects by changing species growth rates [16]. Priority effects are expected to be strong when early-arriving species have high growth rates because they are then likely to pre-empt resources or modify habitats rapidly enough to hinder or facilitate the establishment of late-arriving species [17–19]. If environmental variability makes growth rates temporally variable, it can in turn result in overall reduction of growth rates and therefore priority effects. This reduction occurs because the growth rate of a species averaged over time is represented as the geometric, rather than arithmetic, mean (i.e. Jensen's inequality) [12,20,21]. Consequently, priority effects may be weakened by environmental variability.
Predictions are not necessarily straightforward, however, because whether priority effects are weakened or strengthened may depend on the relative nonlinear response of different species to environmental conditions. For example, imagine a situation where priority effects are weak in a constant environment, with one species (species A) always outcompeting another (species B) regardless of arrival order. If species B is less sensitive to environmental variability (e.g. because of a storage effect [20]), showing a lesser decline in growth rate than species A, the two species may become competitively similar in a variable environment, making the outcome of competition dependent on arrival order. Thus, priority effects would, in this case, be strengthened by environmental variability.
Despite the potential of these theoretical ideas to explain when priority effects should be stronger or weaker, the ideas remain largely untested. In this paper, we experimentally test the hypothesis that environmental variability alters the influence of priority effects on community assembly. To this end, we conducted a series of laboratory experiments using the communities of yeast and bacterial species that inhabit the floral nectar of a hummingbird-pollinated shrub in California as a model system [22]. Our choice of a microbial system was motivated by the logistical advantages in testing general hypotheses regarding community assembly (reviewed in [23–25]). In particular, short generation times and small habitat sizes of microbial species allow community dynamics to be observed for many generations under rigorous experimental control over environmental conditions and species arrival history. In addition, rapidly accumulating knowledge on the natural history of nectar-inhabiting microorganisms [22,26–29] enables one to design experiments in which ecological drivers experienced by these species in the field can be simulated and manipulated in the laboratory. Evidence indicates the presence of inhibitive priority effects among some of these nectar-inhabiting species [30] and high sensitivity of these species to ambient temperature [29]. It has also been suggested that microbial species affect one another via pre-emption of resources (amino acids and sugars) in nectar [30], and that species vary in the extent to which they alter and tolerate nectar pH [31]. Based on these previous studies, we hypothesized that temperature variability would alter the strength of priority effects by influencing the extent of resource pre-emption and pH modification by different species. Our results support this hypothesis and provide the first empirical evidence, to our knowledge, that the effect of arrival order on species coexistence depends on at least one type of environmental variability.
2. Material and methods
(a). Study organisms
Our experiments involved yeast and bacterial species isolated from nectar samples collected from flowers of Mimulus aurantiacus at the Jasper Ridge Biological Preserve (JRBP) in the Santa Cruz Mountains of California [22]. At JRBP, ambient temperature is highly variable on a daily basis in both space and time (see electronic supplementary material, figure S1A). A field survey of M. aurantiacus nectar at JRBP indicated that species diversity was low, with on average about one yeast species per flower, most commonly Metschnikowia reukaufii [22]. Individuals belonging to the genera of acetic acid bacteria such as Gluconobacter were some of the most common bacterial species found in M. aurantiacus nectar [31]. Although less common, many other species have also been found in M. aurantiacus nectar at JRBP, including another yeast species, Starmerella bombicola [22], and a bacterial species, Asaia sp. (T. Fukami, M.-P. L. Gauthier & R. L. Vannette 2013, unpublished data). Strains of these species were collected at or near JRBP and stored at −80°C in 20% glycerol. The strains were freshly streaked on yeast–malt agar (YMA; Difco, Sparks, MD) 2–4 days before the experiment described below.
Our study was designed as an initial attempt to experimentally determine whether environmental variability can influence the strength of priority effects. For this reason, we chose to use species that were likely to compete strongly [30,32] and had differing responses to temperature [29]. Future research should expand to consider more interacting species in order to understand how generally the influence of environmental variability on priority effects may be observed across many species.
(b). Experimental flowers
We used 200 µl round-topped PCR tubes, each intended to mimic a M. aurantiacus flower, in which a local community of nectar microbes was assembled. Two identical tubes were paired as an experimental unit, to form a metacommunity of the microbes. To each tube, we added 10 µl of artificial nectar, which was within the range of the amount of nectar contained by a single flower [30]. The artificial nectar was prepared by filter-sterilizing 15% w/v sucrose solution supplemented with 0.32 mM amino acids from digested casein, to mimic levels of sugar and amino acids of M. aurantiacus nectar in the field [31].
(c). Experimental design
We used a two-way full factorial design, with three different orders of species introductions and four different types of temperature variability. Introduction treatment groups included: (i) simultaneous introductions of two yeast species, Metschnikowia reukaufii and S. bombicola, and two bacterial species, Gluconobacter sp. and Asaia sp., to the artificial nectar placed in the experimental flowers; (ii) ‘yeast-first’ sequential introductions, in which we introduced the two yeast species first and, 48 h later, the two bacterial species; and (iii) ‘bacteria-first’ sequential introductions, in which we introduced the two bacterial species first and, 48 h later, the two yeast species. For brevity, we will refer to the species by their generic names (i.e. Metschnikowia, Starmerella, Gluconobacter, Asaia). For each introduction, we prepared 0.5 µl inoculation solutions by suspending a single colony of each species from YMA agar plates in sterile 15% w/v sucrose solution and diluting this solution to obtain approximately 150–200 cells per species in 0.5 µl.
Temperature treatment groups included: (1) no variability (constant at 15°C); (2) spatial variability (10°C in one of the two local communities in the metacommunity and 20°C in the other community); (3) temporal variability (daily fluctuations, with 5°C as the minimum and 25°C as the maximum, in both local communities); and, (4) both spatial and temporal variability (daily fluctuations, with 0°C as the minimum and 20°C as the maximum in one local community, and 10°C as the minimum and 30°C as the maximum in the other local community; electronic supplementary material, figure S1B). All four treatments shared the same average temperature through time and space for the metacommunity (15°C), and the range of temperature used in these treatments was within the range typically recorded during the M. aurantiacus flowering season at JRBP [22]. Temperature treatments in the microcosm were implemented by holding the PCR tubes in thermal cyclers that were programmed to control temperature as appropriate for each treatment group. Each of the 12 treatments (i.e. three introduction orders × four variability types) was replicated four times.
In the field where our microbial strains were collected, ambient air temperature varied temporally over the flowering season, and plants at different locations differed in the amount of variance in temperatures they experienced (see electronic supplementary material, figure S1A and table S1; it should be noted, however, that temperature may be less variable in nectar within flowers than in the air). We were mainly interested in investigating whether realistic environmental variability, occurring both spatially and temporally, would interact with species arrival order to affect microbial populations. Therefore, we focused on comparing temperature treatments 1 (no variability) and 4 (both spatial and temporal variability). Temperature treatments 2 (spatial variability) and 3 (temporal variability) were used to assess which aspect of variability, spatial or temporal (or both), was responsible for any difference that we might find between temperature treatments 1 and 4.
(d). Dispersal between flowers
Every 96 h throughout the duration of the experiment, beginning at 48 h after introduction of early-arriving species, we vortexed each tube for 30 s and replaced 9 µl of nectar with fresh artificial nectar. In addition, every 96 h, beginning at 48 h after introduction of late-arriving species, we exchanged 0.5 µl of nectar using a sterile pipette between paired tubes that form each metacommunity.
Our intention was to simulate the natural process of flower senescence followed by recolonization of new flowers by yeasts and bacteria that characterizes nectar microbe communities. The exchange of nectar in our experiment could also be considered analogous to nectar feeding by a hummingbird, followed by replenishment with fresh nectar. The frequency at which we exchanged nectar, every 96 h, is a realistic length of time for which an individual flower holds nectar microbes before the flower matures and wilts, as we previously found that M. aurantiacus flowers at JRBP lasted for about a week and that yeasts were detected in the nectar of about 70% of flowers by 3 days after the opening of the flower [30]. This study also suggested that flowers could go a few days between pollinator visits. Thus, 48 h could be a reasonable approximation of pollinator visitation rates and, potentially, microbial immigration rates that might be possible in M. aurantiacus at JRBP.
We repeated the nectar exchange eight times to run the experiment for a total of 32 days, which is similar in duration to a typical length of time individual M. aurantiacus plants bloom during a flowering season at JRBP. Because this schedule of periodic nectar replacement creates a non-equilibrium situation, we will define coexistence as long-term persistence of species in a metacommunity, rather than a more formal definition.
(e). Population abundance estimation
Every 96 h throughout the experiment, we plated 0.01 and 0.001 µl (diluted into a total of 50 µl) of the nectar removed for dispersal onto YMA agar plates. After 4 days of plate incubation at 22°C, we determined the species identity of colonies based on morphology and enumerated colony-forming units (CFU) of each species. Molecular sequencing of colonies, conducted as described by Belisle et al. [22] for yeasts and by Vannette et al. [31] for bacteria, confirmed that colony morphology could be used reliably to identify the four species used in our experiment. Previously, we confirmed that the number of CFU was correlated closely to the number of cells in solution for yeasts [30] and bacteria [31].
(f). Additional experiments
We performed two additional experiments to explore the mechanisms of priority effect that may have operated in our communities. In one experiment, we quantified the effect of the two common species, Metschnikowia and Gluconobacter, on the pH and amino acid concentrations of nectar, because previous work indicated that these chemical properties of nectar might explain how the microbial species affected one another [30,31]. To this end, we grew Metschnikowia and Gluconobacter by introducing 150–200 cells suspended in 0.5 µl of deionized water to 10 µl of the artificial nectar in 370 µl wells of a 96-well microplate and sampled 0.5 µl of nectar after 36 h of incubation at 22°C to measure the pH of nectar using pH indicator strips (colorpHast pH indicator strips by EMD, Darmstadt, Germany). Each of three treatments (introduction of either of the two species or only deionized water as control) was replicated three times. Additionally, we sampled 1 µl from each replicate at 0 and 36 h, replicating each sample three times, to measure amino acid concentrations. Amino acids in each nectar sample were derivatized using an AccQ-Tag Kit (Waters, Milford, MA) following the manufacturer's instructions. Briefly, 1 μl of derivatized solution was injected into an AccQ-Tag Ultra Column (2.1 × 100 mm) at 43°C using a Waters H-Class U-HPLC. Each gradient run was 10 min long, with a flow rate of 700 μl min−1 and began with an aqueous mobile phase with increasing concentration of organics, following the Waters AccQ-Tag Protocol for the H-Class. Derivatized compounds were detected using UV absorbance at 260 nm. Acquired peaks in each sample were identified by comparing each retention time with those generated by known compounds in Waters Hydrolysate standards, and the concentration of each compound was calculated based on a series of external standards.
In the second experiment, we quantified the effect of temperature on the population abundances of Metschnikowia and Gluconobacter. We grew Metschnikowia and Gluconobacter as in the first additional experiment, but under different constant temperatures of 5, 13, 22, 28 and 33°C, each replicated four times. Tubes were incubated for 4 days, after which 50 µl of a 1/10 dilution was plated on YMA agar plates. We then counted the number of CFU for each species in each replicate.
(g). Mathematical model
To further explore possible mechanisms that may explain our experimental results, we used a modification of deFreitas & Frederickson's [17] model to investigate the dynamics of species interactions and the consequences for the likelihood of species coexistence (see electronic supplementary material, appendix S1). This is a consumer–resource model that considers two competing species’ interactions as a result of the consumption of limiting resources and the production of an inhibitory substance. In relation to the results of the additional experiments (detailed in the Results section) and those of Vannette et al. [31], we assumed that one of the species in the model is more efficient at consuming the limiting resource than the other species (as may be the case with Metschnikowia interacting with Gluconobacter). We also assumed that the other species modifies the habitat by producing a larger amount of a growth-inhibiting chemical to which it is less sensitive than the other species (as may be the case with Gluconobacter interacting with Metschnikowia). In this model, priority effects are possible because consumption of a limiting resource by the first species makes it harder for the second species to invade, whereas production of an inhibitory substance by the second species makes it harder for the first species to invade [17]. We solved the equations to obtain zero net growth isoclines, which were used to analyse species coexistence. To examine the effect of temperature variability on priority effects and the implications for species coexistence, the analysis based on zero net growth isoclines was compared under constant versus variable temperature. The purpose of this modelling exercise was to aid clarification of plausible mechanisms, rather than to obtain quantitative predictions.
3. Results
(a). Main experiment
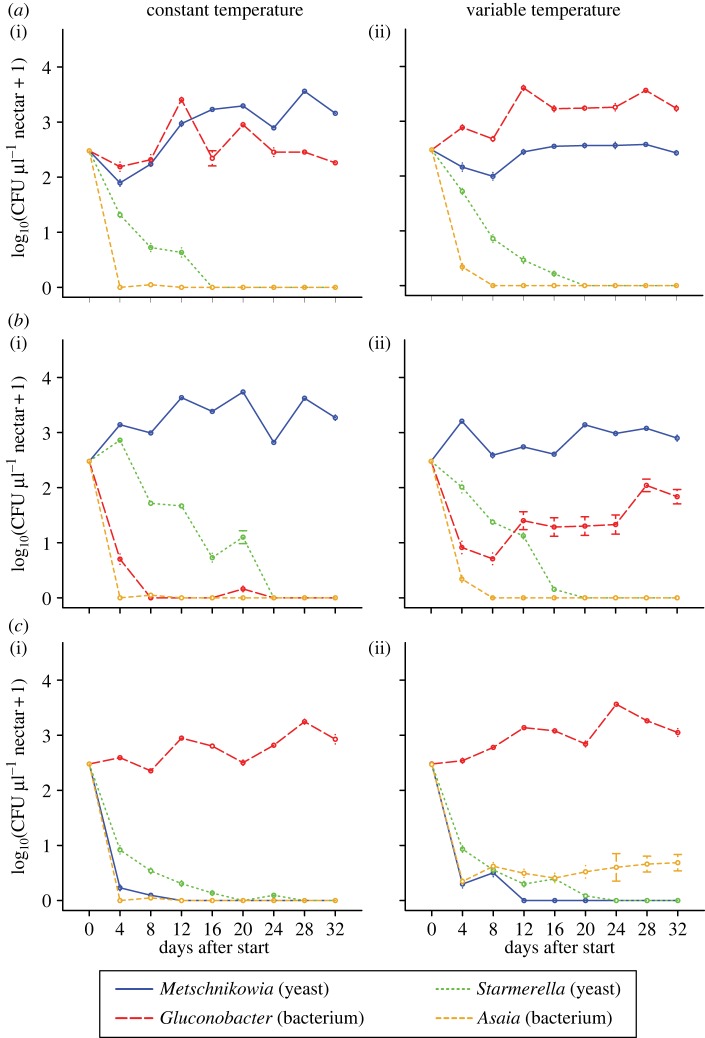
Species abundance was significantly affected by both introduction order and temperature variability, but the effect of the two factors depended on each other (table 1). Specifically, when the yeasts were introduced first, Metschnikowia was the only species that persisted if temperature was constant (figure 1b(i)), whereas Gluconobacter coexisted with Metschnikowia if temperature was spatio-temporally variable (figure 1b(ii)), albeit at a low abundance compared with the simultaneous introduction treatment (figure 1a). Conversely, when the bacteria were introduced first, Gluconobacter was the only species that persisted if temperature was constant (figure 1c(i)), whereas Asaia coexisted with Gluconobacter, though at a low abundance, if temperature was spatio-temporally variable (figure 1c(ii)).
Table 1.
Results from a two-way ANOVA relating the abundance of (a) Metschnikowia, (b) Starmarella, (c) Gluconobacter and (d) Asaia during the main experiment to temperature regime (constant, spatial, temporal or spatio-temporal variation); introduction order (simultaneous, yeast-first or bacteria-first); their interaction and timing of sampling. Bold text shows p-values less than or equal to 0.05.
| (a) Metschnikowia (yeast) |
(b) Starmarella (yeast) |
(c) Gluconobacter (bacteria) |
(d) Asaia (bacteria) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| variable | d.f. | F-value | p-value | d.f. | F-value | p-value | d.f. | F-value | p-value | d.f. | F-value | p-value |
| temperature | 3 | 7.05 | <0.001 | 3 | 2.23 | 0.08 | 3 | 42.69 | <0.001 | 3 | 2.97 | 0.03 |
| introduction order | 2 | 1756.57 | <0.001 | 2 | 38.22 | <0.001 | 2 | 614.68 | <0.001 | 2 | 13.06 | <0.001 |
| temperature × introduction order | 6 | 4.09 | 0.05 | 6 | 6.39 | <0.001 | 6 | 7.48 | <0.001 | 6 | 2.77 | 0.01 |
| time | 1 | 3.72 | <0.001 | 1 | 1628.21 | <0.001 | 1 | 1.03 | 0.309 | 1 | 312.85 | <0.001 |
| R-adj | 0.77 | R-adj | 0.62 | R-adj | 0.57 | R-adj | 0.25 | |||||
Figure 1.
Temporal changes in mean species abundances (±s.e., n = 4 metacommunity replicates), averaged over the paired flowers for each metacommunity, when species were introduced in different timings in a constant or variable environment. Simultaneous introductions were carried out on day 0, sequential introductions on days 0 and 2. (a) Simultaneous arrival of yeasts and bacteria, (b) sequential arrival of yeasts then bacteria, and (c) sequential arrival of bacteria then yeasts. Temperature was either held constant (i) or both spatially and temporally variable (ii).
When all species were introduced simultaneously, Metschnikowia and Gluconobacter persisted throughout the duration of the experiment, whereas Starmerella and Asaia went extinct (figure 1a(i)). Given simultaneous species introductions, temperature variability did not influence the number or identity of persistent species, although their relative abundances were affected, with Metschnikowia and Gluconobacter more abundant under constant (figure 1a(i)) and spatio-temporally variable (figure 1a(ii)) temperature, respectively.
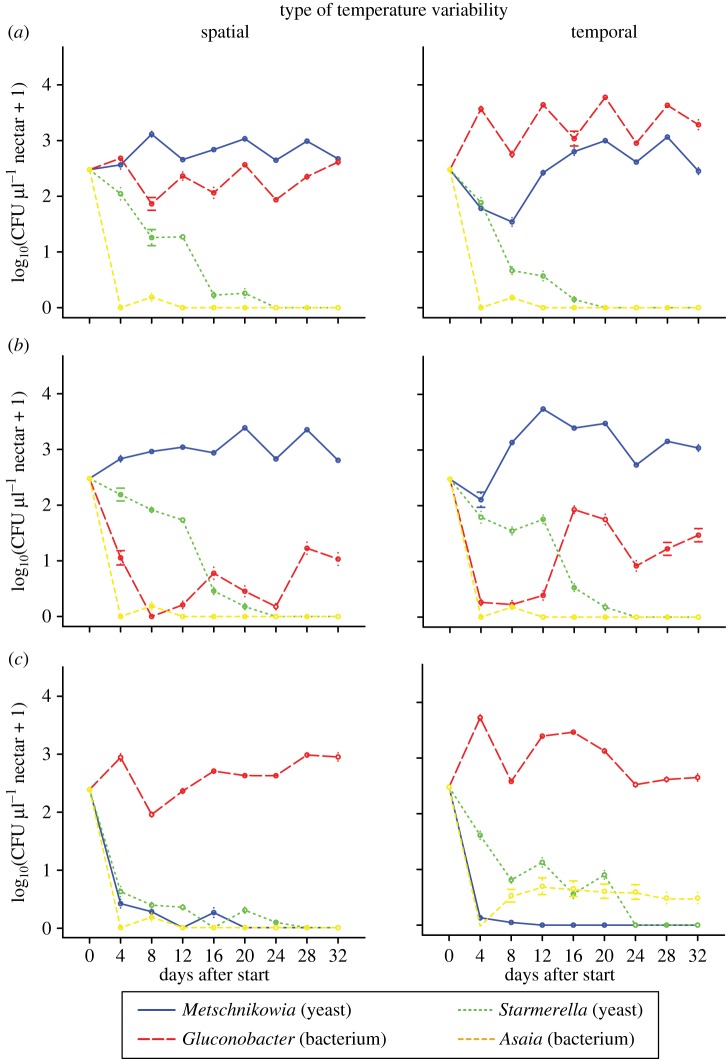
Comparison of the four temperature variability treatments within each introduction order treatment (figures 1 and 2) suggested that temporal, not spatial variability was mainly responsible for the differences observed between constant and spatio-temporally variable treatments (figure 1), particularly in the case of the simultaneous and bacteria-first treatments. For the yeast-first treatments, Gluconobacter populations were quantitatively larger when there was temporal variation in temperature present. Specifically, within the simultaneous introduction treatments, Gluconobacter was more abundant than Metschnikowia in both the spatio-temporally (figure 1a) and temporally (figure 2a) variable environments, but not in the spatially (figure 2a) variable environments. Furthermore, within the yeast-first treatments, Gluconobacter abundance in the spatio-temporally (figure 1b) variable environment was more similar to that in the temporally (figure 2b) than in the spatially (figure 2b) variable environment. Finally, within the bacteria-first treatments, Asaia coexisted with Gluconobacter in both the spatio-temporally (figure 1c) and temporally (figure 2c) variable environments, but not in the spatially (figure 2c) variable environment.
Figure 2.
Temporal changes in mean species abundances when species were introduced in different timings with either spatial or temporal temperature variability. (a) Simultaneous arrival of yeasts and bacteria, (b) sequential arrival of yeasts then bacteria, and (c) sequential arrival of bacteria then yeasts. (Online version in colour.)
(b). Additional experiments
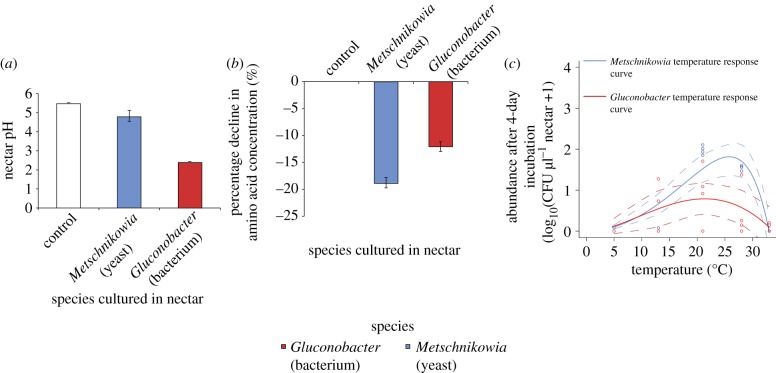
Gluconobacter lowered nectar pH from 5.5 to 2.5 within 36 h, whereas Metschnikowia lowered it only to 5.0 (figure 3a). By contrast, Metschnikowia reduced amino acid concentrations more than Gluconobacter did (figure 3b). Gluconobacter was less sensitive to temperature (either high or low), although it had a lower abundance than Metschnikowia when averaged across all temperatures examined (figure 3c). Four days after introduction, Metschnikowia had a higher abundance than Gluconobacter at moderate temperatures (22 and 28°C), but showed negligible growth at low (5 and 13°C) and high temperatures (33°C).
Figure 3.
Characterization of the common species Metschnikowia reukaufii and Gluconobacter sp. (a) Effect on mean nectar pH after 36 h of growth (±s.e., n = 3). (b) Percentage decline in amino acid concentrations in nectar after 36 h of growth (±s.e., n= 3). (c) Mean abundance attained after 4 days of growth at different temperatures. Data for both species are fitted with a modified Ratkowsky curve [33], dashed lines indicate 95% CI for each curve.
(c). Model analysis
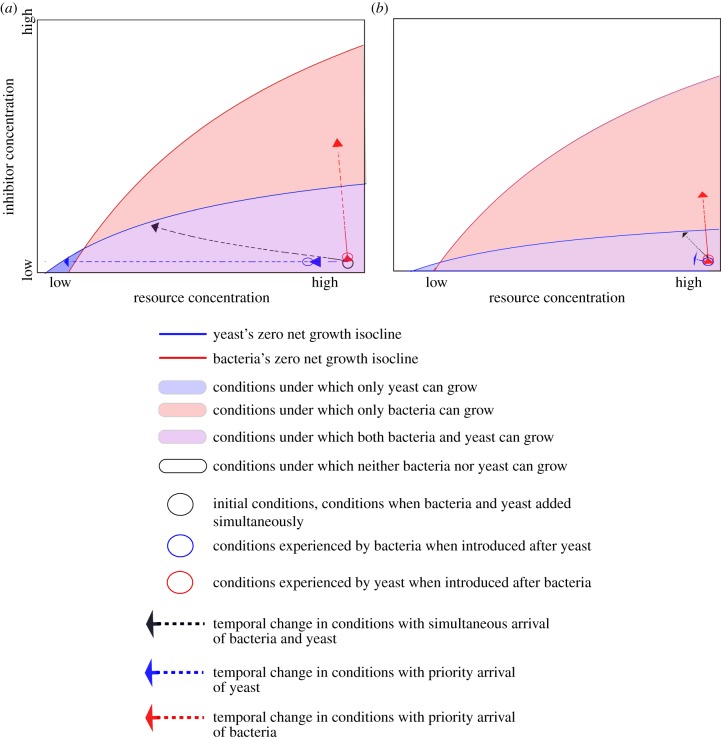
Model analysis (figure 4) suggested that the reduced growth rates owing to temperature variability temper the concentrations of the limiting resource (amino acids) and the inhibiting chemical (pH) that the late-arriving species experiences. Analysis also suggested that changes in the growth rate of the two species as a result of temperature variability shifted the zero net growth isoclines, affecting the range of conditions under which species coexist. As a consequence of these two effects, temperature variability can increase the likelihood of coexistence when the yeasts are introduced first (compare the blue arrows between figure 4a and 4b). Although coexistence in this model is unstable, the high (95%) mortality of the species and addition of fresh nectar every 4 days could prevent the system from reaching a local equilibrium in the experiment.
Figure 4.
Graphical representation of one hypothesis for how environmental variability promotes species coexistence when species arrive sequentially, but not simultaneously, in the experimental system of nectar microbes (see the electronic supplementary material, appendix S1 for model details). Dashed vectors represent the changes in resource concentration and inhibitory substance concentration over 4 days when there is simultaneous arrival (black), yeasts arrive first (blue) or bacteria arrive first (red); the corresponding coloured circles represent the conditions experienced by the late-arriving species. The positions of the zero net growth isoclines, the rate of change of conditions caused by the species and the conditions experienced by the late-arriving species are all different between (a) constant and (b) variable temperatures.
4. Discussion
Taken together, our results suggest that temperature variability can promote the coexistence of nectar-inhabiting microbial species by counteracting priority effects in a metacommunity in which new flowers repeatedly emerge as local habitats for species colonization. Results of our laboratory experiments should not be extrapolated uncritically to explain natural microbial communities in the field. Nonetheless, our data from this model system represent the first experimental evidence, to our knowledge, that the effect of arrival order on species coexistence can depend on at least one type of environmental variability.
We emphasize that our model analysis is merely a tool to visualize possible mechanisms and that the scenario summarized in figure 4 is only one plausible outcome predicted by the model. Although the mechanism underlying priority effects and the dependence of their magnitude on the temperature regime remains unclear, the supplementary experiments (figure 3) and the mathematical modelling (figure 4) suggest one plausible mechanism. The model assumes that one species is a superior resource competitor, via consumption of amino acids in nectar, and the other is a superior habitat modifier (see electronic supplementary material, appendix S1 for model details). This assumption is analogous to Metschnikowia reducing amino acids more rapidly than Gluconobacter [30,31] (figure 3b) and Gluconobacter reducing nectar pH more greatly than Metschnikowia [31] (figure 3a). The model analysis suggested that this contrast between Metschnikowia and Gluconobacter creates opportunities for priority effects under constant temperature, as depicted in figure 4a. The conditions under which both species have a positive growth rate are more restricted under variable temperature than under constant temperature (compare the area shaded in purple between figure 4a and 4b). However, the slow yeast growth under variable temperature allows the bacteria to coexist even when the yeast is introduced earlier (figure 4b), providing a possible explanation for the weaker priority effects under variable temperature (figure 1). In addition, a storage effect [20] may have contributed to the weakening of priority effects by environmental variability. In a temporal storage effect, some species are able to persist because they exhibit dormant stages during unfavourable conditions and achieve high growth rates only at an optimal subset of conditions. Some species of nectar yeast have been indicated to have dormant stages [34].
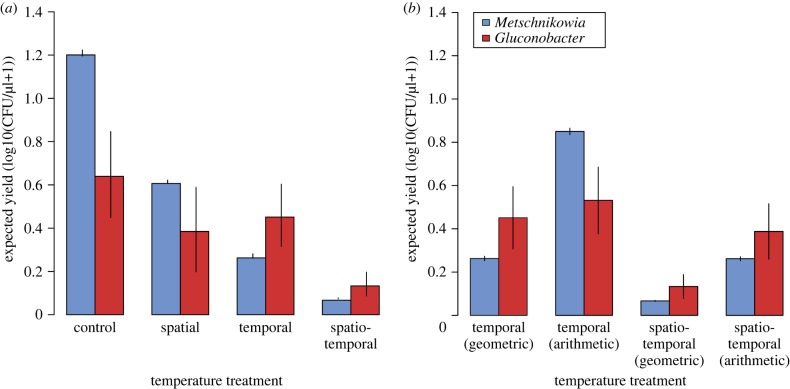
It may be argued that particular temperatures, not temperature variability per se, caused the differences observed in species abundances between the constant and variable temperature treatments. To evaluate this alternative explanation, we calculated the expected abundance of Metschnikowia and Gluconobacter after 4 days of growth in nectar for each temperature treatment, using the observed relationship between abundance and temperature (figure 5). As Gluconobacter is more tolerant of changing temperatures, it changes from having expected abundances less than Metschnikowia under non-variable temperature conditions (spatial and control) to having expected abundances higher than Metschnikowia under the variable temporal and spatio-temporal treatments (figure 5a). Importantly, for the temporal treatment, only with temperature variability (i.e. only for geometric mean and not for arithmetic mean) is Gluconobacter predicted to have a greater abundance than Metschnikowia (figure 5b), suggesting that variability per se contributed to the coexistence of Metschnikowia and Gluconobacter when the yeasts were introduced first under variable temperature. In the case of spatio-temporal variability, both the arithmetic and geometric mean abundances of Gluconobacter are expected to be greater than Metschnikowia, suggesting that both variability and the particular temperatures experienced may have favoured Gluconobacter (figure 5b).
Figure 5.
(a) Expected (i.e. geometric mean) abundances of Metschnikowia and Gluconobacter under each of the four temperature treatments, calculated over the range of experienced temperatures, using the species' observed temperature response curves shown in figure 3. Error bars are estimated by allowing each species's model to vary within the 95% CIs and re-calculating the expected growth rate for each treatment: this process was repeated 1500 times with error bars representing the standard error in the predicted growth rates for each treatment. Temperature treatments that involve temporal variability (spatio-temporal, temporal) are calculated in part or whole with the geometric mean of species growth curves. In (b), we evaluate whether it is this geometric aspect of growth, or simply the presence of a wide range of temperatures in the temporal and spatio-temporal treatments, that is changing species' growth rate. (Online version in colour.)
In conclusion, our results indicate that knowledge of species' tolerance of, and responses to, environmental variability improves our ability to predict how the importance of priority effects may change as the environment becomes more (or less) variable. Such improved understanding [32] may help to predict not only the composition of the assembled communities, but also its broader ecosystem-level consequences [9]. For example, we have recently found that Metschnikowia and Gluconobacter differ in their effects on pollination, probably owing to the contrasting ways in which they modify the chemical properties of nectar and, consequently, the attractiveness of flowers to pollinators [31]. In combination with the results from this study, this finding suggests that priority effects in nectar microbes and the modification of their strength by temperature variability may have consequences for plant–pollinator interactions.
Acknowledgements
We thank Breanna Allen, Melinda Belisle, Nicole Bradon, Daria Hekmat-Scafe and Pat Seawell for laboratory assistance. We are grateful to Marc Cadotte, Rachel Vannette, John Drake and two anonymous reviewers for comments on the manuscript. Rachel Vannette also assisted with quantifying amino acids in nectar. C.M.T. and T.F. conceived the study and designed the experiments. C.M.T. conducted the experiments, analysed the data and developed the model. C.M.T. and T.F. wrote the manuscript.
Funding statement
The Department of Biology and the Terman Fellowship of Stanford University and the National Science Foundation (award no. DEB1149600) funded this research. C.M.T. was supported by a National Science and Engineering Research Council (NSERC) fellowship (CGS-D) and Canada Graduate Scholarships—Michael Smith Foreign Study Supplements.
References
- 1.Sutherland JP. 1974. Multiple stable points in natural communities. Am. Nat. 136, 859–873 [Google Scholar]
- 2.Sutherland JP. 1990. Perturbations, resistance, and alternative views of the existence of multiple stable points in nature. Am. Nat. 136, 270–275 (doi:10.1086/285097) [Google Scholar]
- 3.Drake JA. 1991. Community-assembly mechanics and the structure of an experimental species ensemble. Am. Nat. 137, 1–26 (doi:10.1086/285143) [Google Scholar]
- 4.Chase JM. 2003. Community assembly: when should history matter? Oecologia 136, 489–498 (doi:10.1007/s00442-003-1311-7) [DOI] [PubMed] [Google Scholar]
- 5.Mergeay J, De Meester L, Eggermont H, Verschuren D. 2011. Priority effects and species sorting in a long paleoecological record of repeated community assembly through time. Ecology 92, 2267–2275 (doi:10.1890/10-1645.1) [DOI] [PubMed] [Google Scholar]
- 6.Kennedy PG, Peay KG, Bruns TD. 2009. Root-tip competition among ectomycorrhizal fungi: are priority effects the rule or the exception. Ecology 90, 2098–2107 (doi:10.1890/08-1291.1) [DOI] [PubMed] [Google Scholar]
- 7.Knowlton N. 2004. Multiple ‘stable’ states and the conservation of marine ecosystems. Progr. Oceanogr. 60, 387–396 (doi:10.1016/j.pocean.2004.02.011) [Google Scholar]
- 8.Jiang L, Patel SN. 2008. Community assembly in the presence of disturbance: a microcosm experiment. Ecology 89, 1931–1940 (doi:10.1890/07-1263.1) [DOI] [PubMed] [Google Scholar]
- 9.Fukami T, Dickie IA, Wilkie JP, Paulus BC, Park D, Roberts A, Buchanan PK, Allen RB. 2010. Assembly history dictates ecosystem functioning: evidence from wood decomposer communities. Ecol. Lett. 13, 675–684 (doi:10.1111/j.1461-0248.2010.01465.x) [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson GE. 1961. The paradox of the plankton. Am. Nat. 95, 137–145 (doi:10.1086/282171) [Google Scholar]
- 11.Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol. Rev. 52, 107–145 (doi:10.1111/j.1469-185X.1977.tb01347.x) [Google Scholar]
- 12.Chesson P. 1985. Coexistence of competitors in spatially and temporally varying environments: a look at the combined effects of different sorts of variability. Theor. Popul. Biol. 28, 263–287 (doi:10.1016/0040-5809(85)90030-9) [Google Scholar]
- 13.Snyder RE. 2008. When does environmental variation most influence species coexistence. Theor. Ecol. 1, 129–139 (doi:10.1007/s12080-008-0015-3) [Google Scholar]
- 14.Lloret F, Escudero A, Iriondo JM, Martínez-Vilalta J, Valladares F. 2012. Extreme climatic events and vegetation: the role of stabilizing processes. Glob. Change Biol. 18, 797–805 (doi:10.1111/j.1365-2486.2011.02624.x) [Google Scholar]
- 15.Thompson RM, Beardall J, Beringer J, Grace M, Sardina P. 2013. Means and extremes: building variability into community-level climate change experiments. Ecol. Lett. 16, 799–806 (doi:10.1111/ele.12095) [DOI] [PubMed] [Google Scholar]
- 16.Loeuille N, Leibold MA. 2008. Evolution in metacommunities: on the relative importance of species sorting and monopolization in structuring communities. Am. Nat. 171, 788–799 (doi:10.1086/587745) [DOI] [PubMed] [Google Scholar]
- 17.deFreitas MJ, Frederickson AG. 1978. Inhibition as a factor in the maintenance of the diversity of microbial ecosystems. J. Gen. Microbiol. 106, 307–320 (doi:10.1099/00221287-106-2-307) [Google Scholar]
- 18.Tilman D. 1980. Resources: a graphical-mechanistic approach to competition and predation. Am. Nat. 116, 362–393 (doi:10.1086/283633) [Google Scholar]
- 19.Facelli J, Facelli E. 1993. Interactions after death: plant litter controls priority effects in a successional plant community. Oecologia 95, 277–282 (doi:10.1007/BF00323500) [DOI] [PubMed] [Google Scholar]
- 20.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (doi:10.1146/annurev.ecolsys.31.1.343) [Google Scholar]
- 21.Ruel JJ, Ayres MP. 1999. Jensen's inequality predicts effects of environmental variation. Trends Ecol. Evol. 14, 361–366 (doi:10.1016/S0169-5347(99)01664-X) [DOI] [PubMed] [Google Scholar]
- 22.Belisle M, Peay KG, Fukami T. 2012. Flowers as islands: spatial distribution of nectar-inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird-pollinated shrub. Microb. Ecol. 63, 711–718 (doi:10.1007/s00248-011-9975-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake JA, Huxel GR, Hewitt CL. 1996. Microcosms as models for generating and testing community theory. Ecology 77, 670–677 (doi:10.2307/2265489) [Google Scholar]
- 24.Jessup CM, Kassen R, Forde SE, Kerr B, Buckling A, Rainey PB, Bohannan BJ. 2004. Big questions, small worlds: microbial model systems in ecology. Trends Ecol. Evol. 19, 189–197 (doi:10.1016/j.tree.2004.01.008) [DOI] [PubMed] [Google Scholar]
- 25.Cadotte MW, Drake JA, Fukami T. 2005. Constructing nature: laboratory models as necessary tools for investigating complex ecological communities. Adv. Ecol. Res. 37, 333–353 [Google Scholar]
- 26.Jacquemyn H, Lenaerts M, Brys R, Willems K, Honnay O, Lievens B. 2013. Among-population variation in microbial community structure in the floral nectar of the bee-pollinated forest herb Pulmonaria officinalis L. PLoS ONE 8, e56917 (doi:10.1371/journal.pone.0056917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Perez SC, Herrera CM, de Vega C. 2012. Zooming in on floral nectar: a first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol. Ecol. 80, 591–602 (doi:10.1111/j.1574-6941.2012.01329.x) [DOI] [PubMed] [Google Scholar]
- 28.Herrera CM, Garcia IM, Perez R. 2008. Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89, 2369–2376 (doi:10.1890/08-0241.1) [DOI] [PubMed] [Google Scholar]
- 29.Pozo MI, Lachance MA, Herrera CM. 2012. Nectar yeasts of two southern Spanish plants: the roles of immigration and physiological traits in community assembly. FEMS Microbiol. Ecol. 80, 281–293 (doi:10.1111/j.1574-6941.2011.01286.x) [DOI] [PubMed] [Google Scholar]
- 30.Peay KG, Belisle M, Fukami T. 2012. Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc. R. Soc. B 279, 749–758 (doi:10.1098/rspb.2011.1230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vannette RL, Gauthier M-PL, Fukami T. 2013. Nectar bacteria, but not yeast, weaken a plant-pollinator mutualism. Proc. R. Soc. B 280, 2012–2016 (doi:10.1098/rspb.2012.2601). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vannette RL, Fukami T. 2014. Historical contingency in species interactions: towards niche based predictions. Ecol. Lett. 17, 115–124 (doi:10.1111/ele.12204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwietering M, Jongenburger I, Rombouts F, Van't Riet K. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56, 1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrera CM, Pozo MI, Bazaga P. In press Non-random genotype distribution among floral hosts contributes to local and regional genetic diversity in the nectar-living yeast Metschnikowia reukaufii. FEMS Microbiol. Ecol. (doi:10.1111/1574-6941.12245) [DOI] [PubMed] [Google Scholar]