Abstract
There is growing concern that global environmental change might exacerbate the ecological impacts of invasive species by increasing their per capita effects on native species. However, the mechanisms underlying such shifts in interaction strength are poorly understood. Here, we test whether ocean acidification, driven by elevated seawater pCO2, increases the susceptibility of native Olympia oysters to predation by invasive snails. Oysters raised under elevated pCO2 experienced a 20% increase in drilling predation. When presented alongside control oysters in a choice experiment, 48% more high-CO2 oysters were consumed. The invasive snails were tolerant of elevated CO2 with no change in feeding behaviour. Oysters raised under acidified conditions did not have thinner shells, but were 29–40% smaller than control oysters, and these smaller individuals were consumed at disproportionately greater rates. Reduction in prey size is a common response to environmental stress that may drive increasing per capita effects of stress-tolerant invasive predators.
Keywords: climate change, carbon dioxide, invasive species, multiple stressors, ocean acidification, predator–prey interaction
1. Introduction
Ecosystems are subjected to a growing number of anthropogenic impacts, creating a pressing need for studies that consider the interactive effects of these stressors [1]. In particular, there is growing concern that global environmental change might exacerbate the ecological influence of invasive species by increasing their competitive or predatory effects on native species [2–4]. Such effects could take at least two forms. Climate change could drive density-mediated effects, whereby the abundance of invasive competitors or predators increase, with detrimental consequences for native communities [2,5,6]. Alternatively, functional changes might arise (i.e. interaction modification effects; sensu [7]), where the per capita effect of an invasive competitor or predator is increased by changes in the physical environment. For example, small increases in river temperatures are predicted to increase the per capita effects of invasive piscivores on salmon and other native fishes in North America [3,8]. A recent meta-analysis found that increasing temperature and carbon dioxide (CO2) generally have negative impacts on the performance of native species in aquatic systems, with less impact on non-native species [6]. These differential responses might increase the susceptibility of native species to stress-tolerant, invasive consumers. Although interactive effects between global environmental change and invasive species are plausible, these effects have received relatively little empirical attention, and thus mechanisms underlying potential shifts in interaction strength remain poorly understood [9].
Oysters provide key ecological services in estuaries (e.g. ecosystem engineering, nutrient cycling, reduction of anthropogenic eutrophication [10]), but have been devastated in many regions of the world [11]. Such declines have been attributed to a combination of stressors, including overfishing, disease, habitat loss, pollution and hypoxia [10,11]. In estuaries along the west coast of North America, invasive predatory snails also threaten native oysters by drilling through oyster shells, often with strong impacts on local oyster populations [12–14].
Ocean acidification has recently been identified as an additional, growing threat to oysters and other bivalves [15–18]. As human activities have increased the atmospheric concentrations of CO2, roughly one-third of this anthropogenic CO2 has been absorbed by the oceans, driving reductions in pH and carbonate ion concentrations [19]. These changes make it more difficult for many marine calcifiers, including various bivalve species, to build calcium carbonate shells [20]. As a result, ocean acidification may reduce bivalve growth and survival, and can lead to individuals with smaller and/or thinner shells [15–18]. Although the direct effects of changing carbonate chemistry have received considerable attention [20], relatively little is known about how ocean acidification might alter species interactions [21–24].
Given potential interactions between abiotic and biotic stressors, it is possible that ocean acidification could increase the vulnerability of native oysters to invasive predators. Starting in the 1800s, human exploitation reduced remarkably the abundance of native Olympia oysters (Ostrea lurida) in estuaries along the west coast of North America [25], and populations may be further threatened by invasive predators, including an invasive snail, the Atlantic oyster drill Urosalpinx cinerea [12–14]. Olympia oysters rarely co-occur with native predatory snails, and therefore drilling predation represents a relatively novel stressor for this species [12–14]. Recent studies also indicate that ocean acidification can negatively affect the early life stages of Olympia oysters, with exposure to elevated CO2 resulting in substantially reduced juvenile growth [18,26].
Here, we investigate whether ocean acidification and invasive oyster drills have synergistic, negative impacts on oysters native to California estuaries. Specifically, we tested two hypotheses. First, we explored whether elevated CO2 would lead to oysters that had thinner shells, which could be drilled at higher rates by invasive snails. Second, because some snails that prey on bivalves are known to selectively attack thin-shelled individuals [27], we examined whether snails provided with a choice of prey raised under ambient versus elevated CO2 might selectively drill oysters that had been raised under acidified conditions.
2. Material and methods
(a). Study system
Both Olympia oysters (Ostrea lurida) and Atlantic oyster drills (U. cinerea) are reproductive during the summer months in California estuaries. Female Olympia oysters brood developing larvae for approximately two weeks before releasing larvae that complete development and settle on the shore after 10–20 days in the plankton [25]. Atlantic oyster drills produce benthic egg capsules, and crawl-away juveniles emerge within 8–10 weeks. In Tomales Bay, CA, USA, where animals for this study were collected, Atlantic oyster drills emerge from capsules in large numbers during the season of peak oyster settlement (August–October). Hatchling snails prey heavily on newly settled oysters and barnacles by means of acid secretions and a file-like radula with which they drill through the shells of prey. Given that the early phase of benthic life can be a critical population bottleneck for Olympia oysters [25], in this study, we focused on the interaction between juvenile oysters and recently hatched invasive drills.
(b). Larval and juvenile prey culturing
Olympia oysters were collected in early October 2010 from an intertidal site on the eastern shore of Tomales Bay (38°06′58″ N, 122°51′16″ W), and were transported to Bodega Marine Laboratory. Oysters released brooded larvae in the laboratory and replicate cultures were established, each containing approximately 1000 larvae in a 4.5 l glass jar filled with 2 l of filtered seawater (FSW). Oysters were raised through the larval phase and into early juvenile life at either an ambient partial pressure of carbon dioxide (pCO2; targeted at 500 µatm) or at an elevated level (targeted at 1000 µatm; n = 18 jars per level). The ambient level approximated a typical pCO2 concentration that occurs presently during the summer months in Tomales Bay [18], whereas the elevated treatment was based on an increase of 500 µatm, within the range of shifts predicted to occur by the end of this century [28]. The pCO2 levels were established by filling the culture jars with FSW that had been held in 20 l carboys bubbled for 2–3 days with gas mixtures containing fixed CO2 concentrations (traceable to the US National Institute of Standards and Technology). To minimize off-gassing and maintain the desired pCO2 within the culture jars, the same mixed gases were added continuously to the sealed air spaces over the jars (for additional description of the culturing system see [18]). Every other day, 90% of the seawater in each jar was removed via reverse-filtration through 125 μm mesh, and jars were refilled with carboy water pre-equilibrated to the appropriate pCO2 level. Immediately after each water change, cultured microalgae (Isochrysis galbana) were added to each jar to create a food concentration of approximately 100 000 cells ml–1. Larval cultures were maintained at a constant 20°C (±0.02°C).
In preparation for larval settlement, larvae were transferred on day 9 to new jars that had been prepared by removing and replacing the bases with roughened PVC sheets (5 mm thick). Most larvae settled on the PVC bases on days 13 and 14. Metamorphosed juveniles were maintained under the same pCO2 levels, with water changes and feeding continuing on the same schedule as during the larval phase. On day 20, approximately one week after larval settlement, the PVC bases were gently removed from the jars. The bases had been previously scored on the underside, so that each base could be easily separated into four wedge-shaped tiles with juvenile oysters attached. The predation experiments required only half of the available tiles, so the two tiles from each jar that had the most oysters present were selected (mean ± s.e. = 128.0 ± 5.7 oysters per tile, n = 72 tiles).
(c). Predator rearing
In preparation for the predation experiments, four sets of egg capsules of U. cinerea were collected in early October 2010 from an intertidal cobble beach in Tomales Bay, just north of Chicken Ranch Beach (38°06′42″ N, 122°52′00″ W). Each set was placed in a separate mesh-sided container and maintained in ambient flow-through seawater at Bodega Marine Laboratory. Juvenile snails hatched within 10 days and were fed small barnacles (Chthamalus dalli) attached to pieces of mussel shell. All snails were acclimated to the pCO2 level that they subsequently experienced during the predation experiments. Immediately prior to the start of the predation trials, snails from each set of egg capsules were divided into two groups that were held for 13 days in carboys continuously bubbled at a pCO2 level of either 500 or 1000 µatm. Snails were starved for the final 7 days of this period.
(d). Predation experiments
The wedge-shaped tiles with oysters were distributed into new 4.5 l glass jars for two concurrent predation experiments (see the electronic supplementary material, figure S1). The first was a non-choice experiment, in which two tiles were placed side-by-side on the bottom of each jar. Oysters on both tiles had been raised continuously in the same culturing jar at either 500 or 1000 µatm (n = 9 jars per pCO2 level). Snails were added to each jar, and predation was quantified at the same pCO2 level under which the oysters and snails had been held. This design tested whether oysters raised and maintained under different CO2 levels experienced different predation rates.
We also conducted a choice experiment to test whether snails differentially selected oysters raised under one of the pCO2 levels. Two tiles were again placed side-by-side in each jar. However, in this case, oysters on one tile had been raised at 500 µatm, and oysters on the other tile had been raised at 1000 µatm. The mean number of oysters present did not differ between tiles from the two pCO2 levels (t-test, t34 = 0.214, p = 0.832; mean ± s.e. = 118.0 ± 6.5 versus 116.28 ± 4.8 oysters, for 500 versus 1000 µatm tiles, respectively). The choice experiment was conducted with half of the sets of oysters and snails immersed in ambient pCO2 seawater, and the other half immersed in elevated pCO2 (n = 9 jars per level).
After the tiles were distributed, four pre-acclimated juvenile snails (one from each set of egg capsules) were added to each jar, and the jars were assigned randomly to spaces in the culturing system where they were maintained at the appropriate pCO2 level. All snails had a shell length of 1.6–2.5 mm, and the lengths of snails did not differ between CO2 levels in either experiment (t-tests; non-choice: t12 = 1.149, p = 0.273; choice: t9 = 1.696, p = 0.124). Snails were placed at the junction between the two adjacent tiles in the jar so that they had equal access to both tiles and could crawl freely between them during the experiment. The duration of the trial was set to 48 h to ensure that depletion of oysters did not influence prey choice. On average, only 20.6 ± 0.82% (mean ± s.e.) of the oysters in each jar was consumed (range = 10–30%).
(e). Water chemistry
During water changes conducted every other day, samples of jar water (drained from the jars) and carboy water (refilling the jars) were collected for in situ and laboratory analyses. Seawater pH (US National Bureau of Standards scale) and temperature were quantified using a pH/temperature meter (Accumet Excel XL60) equipped with glass double-junction electrodes calibrated in low-ionic strength certified buffers. Salinity was determined using a YSI 6600V2 multi-parameter probe. Total alkalinity (AT) was measured using automated Gran titration (Metrohm 809), and standardized using certified reference material (A. Dickson, Scripps Institution of Oceanography). Accepted methods for sampling and standardization were used throughout the study [29]. Additional carbonate system parameters were then calculated using the software program, CO2SYS [30], using pHNBS and AT as the primary input variables, equilibrium constants K1 and K2, and KSO4 (as described in [18,26]).
(f). Predation rates and prey size
At the conclusion of the 48 h experiments, predation in each replicate jar was assessed under a dissecting microscope by counting the number of drilled oysters on each tile (see the electronic supplementary material, figure S2a). No mortality was observed in oysters without drill holes. For both predation experiments, we also tested whether the size of drilled oysters differed between the two CO2 levels. We photographed the top valve of all drilled oysters and used image analysis software (ImageJ v. 1.37, National Institutes of Health) to determine the projected area of the valve. To assess whether snails selectively consumed oysters of a particular size, we photographed and measured 25 non-drilled oysters from each tile as an indicator of prey sizes available. Snails preyed on oysters in localized sections of the tiles, and so we were able to sample randomly from those areas of the tile that contained no drilled oysters (i.e. where size frequencies had not been influenced by predation).
(g). Shell thickness of drilled oysters
We used scanning electron microscopy (SEM) to quantify the thickness of drilled shells in the ambient (n = 22) and elevated pCO2 treatments (n = 20) of the non-choice experiment. For each group, we haphazardly selected drilled oysters from seven to nine tiles to represent the full range of oyster sizes (shell areas) present. For each oyster, the drilled top valve was cross-sectioned through the centre of the drill hole, perpendicular to the anterior–posterior axis of the shell (see the electronic supplementary material, figure S2a). SEM images were collected using a Philips XL30 Turbo-Molecular Pump scanning microscope (FEI Company, Hillsboro, OR, USA), and shell thickness in the region that had been drilled was estimated using image analysis software (see the electronic supplementary material, figure S2b).
(h). Statistical analyses
For all analyses, assumptions of normality and homogeneity of variance were assessed using Shapiro–Wilk and Levene's tests, respectively. For the cases where these assumptions were not satisfied by the raw data, data were log-transformed prior to analysis as specified below. Water chemistry was analysed separately for two periods: the culturing phase (days 1–20) and the subsequent 48 h predation experiments. Photosynthesis of the algal food and organism respiration probably contributed to the relatively minor changes observed in water chemistry within the culture jars during the 2 days separating water changes (see the electronic supplementary material, table S1). To compare typical conditions under the two pCO2 levels, seawater parameters within each jar were first estimated by averaging all measurements of water entering and exiting that jar during that phase. These jar averages were then compared using Student's t-tests to evaluate whether seawater parameters differed between the two pCO2 levels.
In the non-choice experiment, the numbers of oysters drilled per tile were analysed using a mixed-model ANOVA (proc mixed in SAS, v. 9.3). The rearing condition of the oysters, i.e. Oyster CO2 (ambient, high), was treated as a fixed effect, and Jar[Oyster CO2] and Tile[Oyster CO2 × Jar] were incorporated as random effects. The covariance structure was chosen, so that the jars were assumed to be independent of each other, but the two tiles within a jar were not assumed to be independent (because predation on the two tiles could be negatively correlated). The log-transformed sizes of oysters (shell area) were analysed using a separate mixed-model ANOVA. Oyster CO2 (ambient, high) and Oyster Status (drilled, available) were treated as fixed effects, and Jar[Oyster CO2] was included as a random effect.
For the non-choice predation experiment, we also tested whether the thickness of drill holes differed between the two CO2 levels using analysis of covariance (ANCOVA), with Oyster CO2 as the main effect and Oyster Size (shell area) as the covariate.
Analyses of the choice experiment paralleled those of the non-choice experiment, with the exception that, because predation was tested under two CO2 levels (ambient, high), Jar CO2 was included as an additional fixed effect in the mixed-model ANOVAs used to analyse the number of oysters drilled and the log-transformed sizes of oysters.
We also tested whether predators preferentially selected high-CO2 oysters over ambient-CO2 oysters when they were presented together in the choice experiment. In such a scenario, predation on tiles with high-CO2 oysters would be greater in the choice experiment than in the non-choice experiment. Conversely, predation would be lower on ambient-CO2 oysters in the choice versus non-choice experiments. To test this hypothesis, we analysed numbers of oysters drilled per tile in both experiments in a single mixed-model ANOVA (proc glm in SAS, v. 9.3). Oyster CO2 (ambient, high) and Experiment Type (non-choice, choice) were treated as fixed effects, and Jar[Oyster CO2 × Experiment Type] was included as a random effect. A significant interaction (Oyster CO2 × Experiment Type) would be expected if snails consumed high-CO2 oysters in the choice experiment at a greater rate than when high-CO2 oysters were offered alone in the non-choice experiment.
3. Results
(a). Water chemistry
Seawater properties remained relatively stable throughout the study with minor variation among replicate jars within the two pH levels (table 1). As expected, pH levels differed significantly between the two experimental pCO2 levels during the initial rearing phase (t-test, t34 = 221.5, p < 0.001), the non-choice predation experiment (t-test, t16 = 32.6, p < 0.001) and the choice experiment (t-test, t16 = 39.4, p < 0.001). During the rearing phase, total alkalinity (AT) was slightly higher in the elevated pCO2 treatment (t34 = −6.77, p < 0.001), but mean AT differed by only approximately 10 μmol kg−1 of seawater (table 1). AT did not differ between pCO2 levels during the two predation experiments (t-tests, p > 0.20).
Table 1.
Seawater properties during the larval and juvenile rearing phase (day 1–20, i.e. ending approx. 7 days post-settlement), and the two subsequent 48 h predation experiments. (Values are means ± s.d. computed from the jar replicates. Temperature, salinity, total alkalinity and pHNBS are measured values, and pCO2calc, Ωcalcite and Ωaragonite are values calculated using the carbonate system analysis software program, CO2SYS. Asterisks indicate seawater parameters that differed significantly between the two pCO2 levels (t-tests, p < 0.05).)
| larval and juvenile rearing | ambient pCO2 | high pCO2 |
|---|---|---|
| temperature (°C) | 19.37 ± 0.028* | 19.35 ± 0.019* |
| salinity | 34.1 ± 0.004 | 34.1 ± 0.003 |
| total alkalinity (μmol kg−1sw) | 2238 ± 5* | 2248 ± 4* |
| pHNBS | 8.09 ± 0.004* | 7.80 ± 0.004* |
| pCO2calc | 500 ± 6* | 1047 ± 9* |
| Ωcalcite | 3.54 ± 0.024* | 2.00 ± 0.017* |
| Ωaragonite | 2.30 ± 0.016* | 1.30 ± 0.011* |
| predation exp. (non-choice) | ambient pCO2 | high pCO2 |
| temperature (°C) | 19.31 ± 0.100* | 19.46 ± 0.096* |
| salinity | 34.3 ± 0.00 | 34.3 ± 0.00 |
| total alkalinity (μmol kg−1sw) | 2245 ± 2 | 2247 ± 5 |
| pHNBS | 8.08 ± 0.020* | 7.82 ± 0.013* |
| pCO2calc | 500 ± 34* | 959 ± 22* |
| Ωcalcite | 3.56 ± 0.174* | 2.16 ± 0.044* |
| Ωaragonite | 2.31 ± 0.113* | 1.40 ± 0.028* |
| predation exp. (choice) | ambient pCO2 | high pCO2 |
| temperature (°C) | 19.36 ± 0.077* | 19.46 ± 0.085* |
| salinity | 34.3 ± 0.00 | 34.3 ± 0.00 |
| total alkalinity (μmol kg−1sw) | 2245 ± 4 | 2245 ± 10 |
| pHNBS | 8.07 ± 0.016* | 7.82 ± 0.009* |
| pCO2calc | 520 ± 32* | 966 ± 29* |
| Ωcalcite | 3.45 ± 0.150* | 2.14 ± 0.052* |
| Ωaragonite | 2.24 ± 0.098* | 1.39 ± 0.034* |
(b). Non-choice predation experiment
Snails drilled 20.2% more oysters in the elevated-CO2 jars than in the ambient-CO2 jars (figure 1a; mixed-model ANOVA, Oyster CO2, F1,16 = 8.22, p = 0.011). Oysters that had been raised under elevated CO2 were smaller than those raised under ambient conditions (mixed-model ANOVA, Oyster CO2, F1,16 = 43.02, p < 0.0001). Drilled oysters in the high-CO2 treatment were 29.2% smaller than oysters drilled under ambient conditions (figure 1b). At both CO2 levels, drilled oysters were on average smaller than the mean size available (Oyster Status, F1,1767 = 53.70, p < 0.0001; electronic supplementary material, figures S3 and S4). This selection of smaller oysters was accentuated under high-CO2 conditions (mixed-model ANOVA, Oyster Status × Oyster CO2, F1,1767 = 9.33, p = 0.002).
Figure 1.
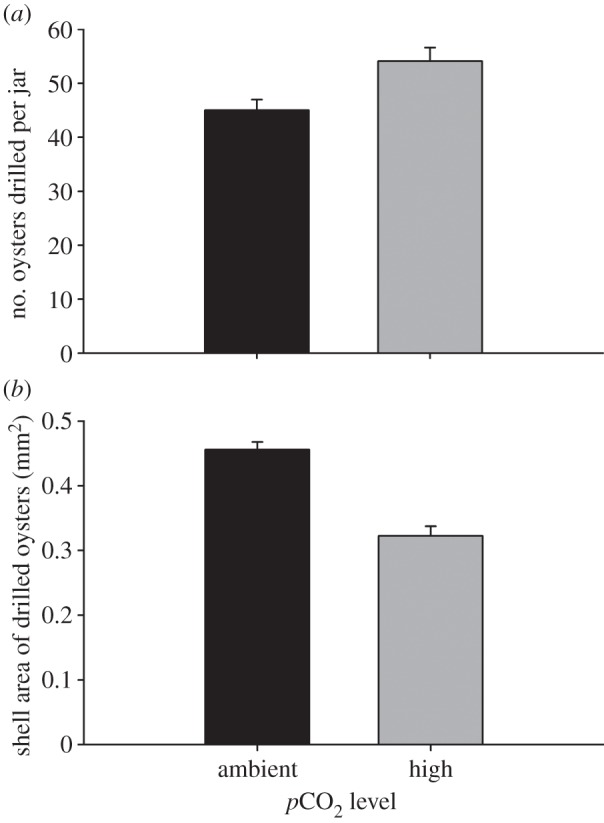
Results of non-choice experiment quantifying predation by Atlantic oyster drills upon native oysters cultured under either ambient (500 µatm) or high (1000 µatm) pCO2 levels. (a) Mean predation (+s.e.) during the 48 h experiment (n = 9 jars per pCO2 level). (b) Mean shell size (area + s.e.) of all drilled oysters in replicate jars (n = 9 jars per pCO2 level). Drills consumed 20.2% more oysters under high pCO2 conditions, and these oysters were smaller in size (t-test, t16 = 7.01, p < 0.0001).
The thickness of drill holes was positively correlated with the size of the juvenile oyster, but did not differ between CO2 levels (figure 2; ANCOVA, Oyster Size, F1,39 = 87.93, p < 0.0001; Oyster CO2, F1,39 = 0.61, p = 0.439; Oyster Size × Oyster CO2, F1,38 = 0.23, p = 0.632).
Figure 2.
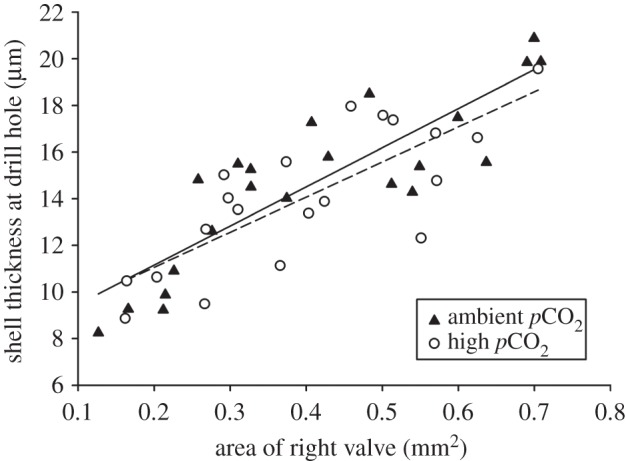
Shell thickness versus shell size (projected area) of juvenile oysters cultured under ambient (500 µatm) and elevated (1000 µatm) pCO2 levels in the non-choice experiment. We selected drilled oysters from the range of sizes present within the ambient (n = 22) and high pCO2 treatment (n = 20). Cross-sectional shell thickness at the drill hole was quantified using scanning electron microscopy. Separate linear regressions are plotted for the ambient (solid line) and high pCO2 treatments (dashed line). Larger oysters had thicker shells, but the thickness of drill holes in shells of a given size did not differ between pCO2 levels (see Results).
(c). Choice predation experiment
In a side-by-side choice, predation on oysters that had been raised under elevated CO2 conditions was greater than predation on control oysters (figure 3; mixed-model ANOVA, Oyster CO2, F1,16 = 4.86, p = 0.043). This effect was consistent whether the choice trials were run under high CO2 or ambient conditions (figure 3; Oyster CO2 × Jar CO2, F1,16 = 0.10, p = 0.754). On average, snails consumed 47.7% more high-CO2 oysters than control oysters.
Figure 3.
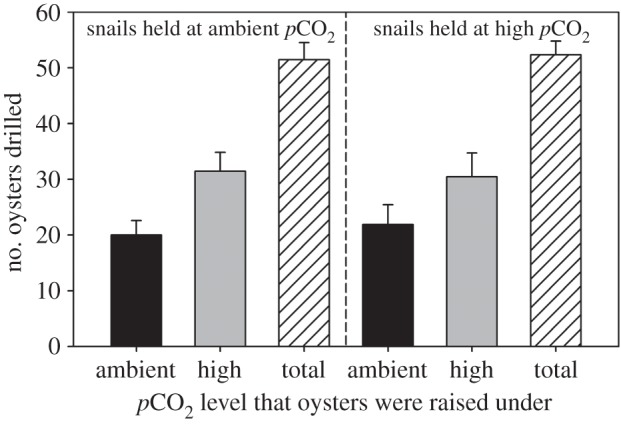
Results of choice experiment quantifying predation by Atlantic oyster drills offered a side-by-side choice of oysters cultured under both ambient (500 µatm) and high (1000 µatm) pCO2 levels. The experiment was conducted with drills held under both ambient and elevated pCO2 levels (n = 9 jars per pCO2 level). Bars are the mean number (+s.e.) of oysters drilled during the 48 h experiment: control oysters (black bars), high-pCO2 oysters (grey bars) and total (cross-hatched bars). Overall, drills consumed 48% more high-pCO2 oysters than control oysters.
As in the non-choice experiment, oysters raised under elevated CO2 were smaller than those raised under control conditions (Oyster CO2, F1,32 = 127.95, p < 0.0001). Drilled oysters that had been raised under high CO2 were 39.9% smaller than the drilled oysters that had been raised under ambient conditions (figure 4). As in the non-choice experiment, predators in the choice experiment drilled oysters that were on average smaller than the mean size available (mixed-model ANOVA, Oyster Status, F1,1700 = 91.92, p < 0.0001; electronic supplementary material, figures S5 and S6). This tendency to select smaller oysters relative to the sizes available occurred, regardless of the CO2 level that the oysters were raised under (Oyster Status × Oyster CO2, F1,1700 = 0.89, p = 0.347), or the CO2 level during the predation trial (Oyster Status × Jar CO2, F1,1700 = 0.04, p = 0.841).
Figure 4.
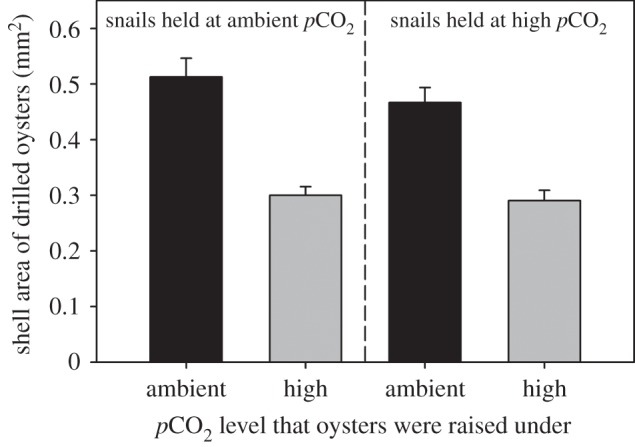
Size (area + s.e.) of oysters drilled in the ambient (500 µatm, black bars) and high (1000 µatm, grey bars) pCO2 groups during the choice experiment. The experiment was conducted with drills held under both ambient and high-pCO2 levels (n = 9 jars per level). Drilled oysters from the high-pCO2 group were significantly smaller than control oysters (paired t-test, t16 = 7.39, p < 0.0001).
We also tested whether predators preferentially selected high-CO2 oysters in the choice experiment at rates that were greater than predicted from the non-choice experiment. As expected, there was higher predation on high-CO2 oysters than on ambient-CO2 oysters in the choice experiment (mixed-model ANOVA, Oyster CO2, F1,10 = 19.81, p = 0.001). There was suggestive evidence that predators consumed more high-CO2 oysters per tile and fewer ambient-CO2 oysters per tile in the choice experiment than in the non-choice experiment, but this interaction was not significant statistically (Experiment Type × Oyster CO2, F1,7 = 3.04, p = 0.123).
4. Discussion
There is growing concern that global environmental change might exacerbate the impacts of invasive species on native communities [2,4]. To date, most attention has focused on the potential for environmental change to alter the growth, survival and fecundity of native versus non-native species. A recent meta-analysis found that increasing temperature and CO2 generally have negative impacts on the performance of native species in aquatic systems, with less impact on non-native species [6]. The greater resilience of invasive species to environmental change might arise if the introduction process itself has selected for species with broad tolerances and high phenotypic plasticity [6,9]. Through effects on performance, increasing temperature, CO2 and anthropogenic nutrient inputs might, in turn, alter competitive interactions between native and non-native species [2], although these effects have rarely been quantified experimentally (but see [31,32]).
The potential for environmental change to increase the consumptive effects of invaders on native species has received even less attention [3]. These impacts could arise via numerical effects, where environmental change increases the abundance of invasive consumers [33]. Alternatively, environmental change might increase the per capita effect of an invasive predator. Such interaction modification effects [7] could occur, for example, if warmer temperatures increased the consumption rates of the predator [3,33], through effects on its activity and physiology. Although seldom considered, the per capita effects of an invasive predator might also be increased if environmental stress causes native prey to become more vulnerable to predation, for example as a result of weakened defences.
We found that ocean acidification led to a striking 20–48% increase in predation by invasive snails on native oysters. Increased per capita effects under elevated CO2 did not appear to arise from a thinning of oyster shells under acidified conditions or from physiological effects on predators. Rather, oysters raised under elevated CO2 appeared to be handled and consumed more quickly because of their smaller size. We discuss these findings in more detail in the following sections.
(a). Effects of elevated CO2 on prey
Relative to ambient conditions, oysters of a given size did not secrete thinner shells when raised under elevated CO2. The lack of an effect of CO2 on shell thickness contrasts with a study of the Atlantic oyster, Crassostrea virginica [16]. In that study, juvenile oysters exposed for 20 weeks to extreme pCO2 levels (3500 µatm) had less shell mass, but similar shell area, compared with oysters reared in ambient conditions. Because shell area did not differ with CO2 level, those results indicated that oysters secreted thinner shells, at least under strongly acidified conditions. Similarly, adult mussels were found to grow thinner shells in association with low pH at hydrothermal vents [34], and the shells of larval bivalves can be thinner when raised under elevated CO2 [15,17].
However, shell thickness in bivalves is not always reduced under experimentally elevated CO2. Rather, faced with increased costs of calcification, some species appear to allocate energy towards maintaining shell thickness at the expense of reduced shell growth [35]. To the extent that shell thickness influences susceptibility to predation and physical disturbance [17,36], there may be selection to maintain shell thickness—even if it comes at a cost of reduced growth in overall shell area. Our results are consistent with such a trade-off. Although shell thickness was not reduced under elevated CO2, the mean size of oysters drilled under these conditions was 29–40% smaller than under ambient conditions. Given the relationship between oyster size and shell thickness (figure 2), these smaller oysters had approximately 14–20% thinner shells than the average oyster drilled in the controls, and thus probably took less time to drill through, as has been shown in other predatory snails [36]. In addition, smaller oysters probably contained less tissue that was consumed more quickly and yielded smaller caloric rewards. Prey size is known to have a strong effect on predator functional responses and predator–prey dynamics [37–39]. Smaller prey often take less time to subdue, consume and digest, and these effects all decrease handling time and thus increase per capita rates of predation [37].
We cannot rule out the possibility that in addition to decreasing oyster size, elevated CO2 may have led to shifts in shell composition and reduced hardness [16] that also reduced handling time. Previous work documented that Sydney rock oysters from sites with strongly acidified waters (pH < 7.0, driven by the run-off of acid sulfate soils) were consumed more quickly by predatory snails than oysters from control sites [40]. Although shell thickness and growth were not quantified in this particular study, oysters from the acidified sites had weaker shells that were crushed more easily.
(b). Effect of elevated CO2 on predators
The very similar predation rates observed in the choice experiment under the two CO2 levels (figure 3) suggests that any physiological effects of elevated CO2 on the snails did not influence their feeding behaviour, in contrast to effects seen in some coral reef fishes [24]. Predatory snails drill through the shell of their prey using their radula to slowly rasp away shell material that is softened by acidic secretions produced by a specialized boring organ. Radular teeth are chitinous, rather than calcareous, and thus the drilling apparatus itself may not be influenced by decreased pH [40]. Although snails in our study were acclimated to the two CO2 levels for two weeks, they were not raised for extended periods under these conditions. We selected snails that were all within a narrow size range for these experiments and did not test whether long-term exposure to elevated CO2 might impact the growth or performance of these calcifying predators [41,42]. Many predator–prey interactions are size-structured, and reductions in the growth rate of predators might have important consequences for prey population dynamics [36,39,43].
Our results provided some support for the hypothesis that snails preferentially consume oysters raised under elevated CO2 when offered alongside control oysters. Relative to ambient-CO2 oysters, snails consumed about 20% more high-CO2 oysters in the non-choice experiment (figure 1), but 48% more high-CO2 oysters in the choice experiment (figure 3). Statistical evidence for preferential consumption of high-CO2 oysters in the choice experiment was equivocal, and follow-up experiments are needed. A trend towards preferential consumption of high-CO2 oysters might arise if predators were attracted to oysters of smaller size, which were more common on the high-CO2 tiles. Alternatively, snails might have been attracted by chemical cues released by oysters raised under elevated CO2. Interestingly, previous work indicated that U. cinerea were attracted to faster growing oysters that had higher metabolic rates [44]. Juvenile Atlantic oysters exposed to elevated CO2 were found to have higher metabolic rates, perhaps reflecting higher energetic costs of homeostasis [16]. Although we did not quantify metabolic rates in this study, it is possible that oyster drills are attracted to juvenile oysters raised under elevated CO2 because they have persistently higher metabolic rates. More detailed experiments are needed to investigate this hypothesis.
(c). Global environmental change and invasive predators
Our results suggest that ocean acidification may exacerbate the impacts of invasive predators on native Olympia oyster populations. Estuaries along the west coast of North America already experience periods of low pH, and ocean acidification is expected to lead to further reductions in estuarine pH [45]. The synergistic effects of ocean acidification and invasive predators may thus hinder efforts to restore native oyster populations in California estuaries, especially in combination with other anthropogenic stressors facing native oysters (e.g. habitat loss and pollution). Other climatic changes expected on the California coast during this century, including increases in air temperature [46], may generate complex interactive effects [47] that also influence intertidal oysters and their predators. Predicting the net effect of environmental changes on the dynamics of a predator–prey interaction is complex, because both species might be impacted [21,22,42]. For example, lowered pH can alter both prey detection and predator avoidance in coral reef fishes through effects on olfactory pathways [23,24,48]. However, if invasive species tend to be more tolerant of environmental change [6,9], then the per capita effects of invasive consumers might be increased as a consequence of stress experienced by native prey species.
A growing body of evidence suggests that increased stress associated with global environmental change is reducing body size in multiple taxa [20,49]. Reductions in prey size may result from the direct effects of environmental stress, phenological shifts or trade-offs in allocation of limited energy (e.g. to stress responses, defence against predation and/or growth), with important consequences for predator–prey dynamics [43]. For example, thermal stress and exposure to toxins can reduce the size of zooplankton in lakes, with rippling effects on fish populations and ecosystem function [50]. These complex effects of environmental change may be more common than is generally appreciated. Predicting the interactive effects of global environmental change and invasive consumers on native assemblages will thus benefit from addressing how stress alters resource allocation to prey defences and growth, and how shifts in these traits ultimately influence predator–prey dynamics.
Acknowledgements
We thank Kirk Sato, Olivia Turnross, Megan Young and Jessica Hosfelt for assistance with data collection, Ann Russell for advice regarding water chemistry, Greg Baxter and Patricia Kysar for their help with shell preparation and electron microscopy, and Neil Willits for assistance with statistical analyses. This manuscript was improved by helpful feedback from Jill Bible, Kristy Kroeker, and Jackie Sones.
Funding statement
This research was supported by NSF grants OCE-0927255, OCE-1041089 and OCE-1220648, and support from the University of California Multicampus Research Programmes and Initiatives. K.M. was supported by BML's NSF-sponsored REU programme (grant no. DBI-0753226). This publication is a contribution of the Bodega Marine Laboratory, University of California, Davis, CA, USA.
References
- 1.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315 (doi:10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 2.Dukes JS, Mooney HA. 1999. Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139 (doi:10.1016/S0169-5347(98)01554-7) [DOI] [PubMed] [Google Scholar]
- 3.Rahel FJ, Bierwagen B, Taniguchi Y. 2008. Managing aquatic species of conservation concern in the face of climate change and invasive species. Conserv. Biol. 22, 551–561 (doi:10.1111/j.1523-1739.2008.00953.x) [DOI] [PubMed] [Google Scholar]
- 4.Diez JM, et al. 2012. Will extreme climatic events facilitate biological invasions? Front. Ecol. Environ. 10, 249–257 (doi:10.1890/110137) [Google Scholar]
- 5.Sorte CJB, Williams SL, Zerebecki RA. 2010. Ocean warming increases threat of invasive species in a marine fouling community. Ecology 91, 2198–2204 (doi:10.1890/10-0238.1) [DOI] [PubMed] [Google Scholar]
- 6.Sorte CJB, et al. 2012. Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 16, 261–270 (doi:10.1111/ele.12017). [DOI] [PubMed] [Google Scholar]
- 7.Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 22, 489–496 (doi:10.1016/j.tree.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 8.Petersen JH, Kitchell JF. 2001. Climate regimes and water temperature changes in the Columbia River: bioenergetic implications for predators of juvenile salmon. Can. J. Fish. Aquat. Sci. 58, 1831–1841 (doi:10.1139/f01-111) [Google Scholar]
- 9.Chown SL, Slabber S, McGeoch MA, Janion C, Leinaas HP. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537 (doi:10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilberg MJ, Livings ME, Barkman JS, Morris BT, Robinson JM. 2011. Overfishing, disease, habitat loss, and potential extirpation of oysters in upper Chesapeake Bay. Mar. Ecol. Prog. Ser. 436, 131–144 (doi:10.3354/meps09161) [Google Scholar]
- 11.Breitburg DL, Riedel GF. 2005. Multiple stressors in marine systems. In Marine conservation biology: the science of maintaining the sea‘s biodiversity (eds Norse EA, Crowder LE.), pp. 167–182 Washington, DC: Island Press [Google Scholar]
- 12.Buhle ER, Ruesink JL. 2009. Impacts of invasive oyster drills on Olympia oyster (Ostrea lurida Carpenter 1864) recovery in Willapa Bay, Washington, United States. J. Shellfish Res. 28, 87–96 (doi:10.2983/035.028.0115) [Google Scholar]
- 13.Kimbro DL, Grosholz ED, Baukus AJ, Nesbitt NJ, Travis NM, Attoe S, Coleman-Hulbert C. 2009. Invasive species cause large-scale loss of native California oyster habitat by disrupting trophic cascades. Oecologia 160, 563–575 (doi:10.1007/s00442-009-1322-0) [DOI] [PubMed] [Google Scholar]
- 14.Koeppel JA. 2011. High predation may hinder native oyster (Ostrea lurida Carpenter, 1864) restoration in North Humboldt Bay, California. Master's thesis, Humboldt State University, Arcata, CA, USA [Google Scholar]
- 15.Talmage SC, Gobler CJ. 2009. The effects of elevated carbon dioxide concentrations on the metamorphosis, size and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnol. Oceanogr. 54, 2072–2080 (doi:10.4319/lo.2009.54.6.2072) [Google Scholar]
- 16.Beniash E, Ivanina A, Lieb NS, Kurochkin I, Sokolova IM. 2010. Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar. Ecol. Prog. Ser. 419, 95–108 (doi:10.3354/meps08841) [Google Scholar]
- 17.Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, Sato KN, Russell AD, Hettinger A. 2011. Functional impacts of ocean acidification in an ecologically critical foundation species. J. Exp. Biol. 214, 2586–2594 (doi:10.1242/jeb.055939) [DOI] [PubMed] [Google Scholar]
- 18.Hettinger A, Sanford E, Hill TM, Russell AD, Sato KN, Hoey J, Forsch M, Page HN, Gaylord B. 2012. Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology 93, 2758–2768 (doi:10.1890/12-0567.1) [DOI] [PubMed] [Google Scholar]
- 19.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 20.Kroeker KJ, Kordas RL, Crim RN, Hendriks IE, Ramajo L, Singh GG, Duarte CM, Gattuso JP. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interactions with warming. Glob. Change Biol. 19, 1884–1896 (doi:10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J. 2007. Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol. Lett. 3, 699–701 (doi:10.1098/rsbl.2007.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landes A, Zimmer M. 2012. Acidification and warming affect both a calcifying predator and prey, but not their interaction. Mar. Ecol. Prog. Ser. 450, 1–10 (doi:10.3354/meps09666) [Google Scholar]
- 23.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 (doi:10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cripps IL, Munday PL, McCormick MI. 2011. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 6, e22736 (doi:10.1371/journal.pone.0022736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker P. 1995. Review of ecology and fishery of the Olympia oyster, Ostrea lurida with annotated bibliography. J. Shellfish Res. 14, 501–518 [Google Scholar]
- 26.Hettinger A, Sanford E, Hill TM, Lenz EA, Russell AD, Gaylord B. 2013. Larval carry-over effects from ocean acidification persist in the natural environment. Glob. Change Biol. 19, 3317–3326 [DOI] [PubMed] [Google Scholar]
- 27.Grey M, Lelievre PG, Boulding EG. 2007. Selection for prey shell thickness by the Naticid gastropod Euspira lewisii (Naticidae) on the bivalve Protothaca staminea (Veneridae). Veliger 48, 317–322 [Google Scholar]
- 28.Moss RH, et al. 2010. The next generation of scenarios for climate change research and assessment. Nature 463, 747–756 (doi:10.1038/nature08823) [DOI] [PubMed] [Google Scholar]
- 29.Dickson AG, Sabine CL, Christian JR. 2007. Guide to best practices for ocean CO2 measurements. Sidney, Canada: PICES Special Publication 3 [Google Scholar]
- 30.Lewis E, Wallace D. 1998. Program developed for CO2 system calculations. ORNL/CIAC-105. Oak Ridge, TN: Oak Ridge National Laboratory, US Department of Energy [Google Scholar]
- 31.Dukes JS, Chiariello NR, Loarie SR, Field CB. 2011. Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes. Ecol. Appl. 21, 1887–1894 (doi:10.1890/11-0111.1) [DOI] [PubMed] [Google Scholar]
- 32.Gennaro P, Piazzi L. 2011. Synergism between two anthropic impacts: Caulerpa racemosa var. cylindracea invasion and seawater nutrient enrichment. Mar. Ecol. Prog. Ser. 427, 59–70 (doi:10.3354/meps09053) [Google Scholar]
- 33.Côté IM, Green SJ. 2012. Potential effects of climate change on a marine invasion: the importance of current context. Curr. Zool. 58, 1–8 [Google Scholar]
- 34.Tunnicliffe V, Davies KTA, Butterfield DA, Embley RW, Rose JM, Chadwick WW., Jr 2009. Survival of mussels in extremely acidic waters on a submarine volcano. Nat. Geosci. 2, 344–348 (doi:10.1038/ngeo500) [Google Scholar]
- 35.Thomsen J, et al. 2010. Calcifying invertebrates succeed in a naturally CO2 enriched coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7, 3879–3891 (doi:10.5194/bg-7-3879-2010) [Google Scholar]
- 36.Hughes RN, Dunkin S.de.B. 1984. Behavioural components of prey selection by dogwhelks, Nucella lapillus (L.), feeding on mussels, Mytilus edulis, L., in the laboratory. J. Exp. Mar. Biol. Ecol. 77, 45–68 (doi:10.1016/0022-0981(84)90050-9) [Google Scholar]
- 37.Thompson DJ. 1975. Towards a predator–prey model incorporating age structure: the effects of predator and prey size on the predation of Daphnia magna by Ischnura elegans. J. Anim. Ecol. 44, 907–916 (doi:10.2307/3727) [Google Scholar]
- 38.Rudolf VHW. 2008. Consequences of size structure in the prey for predator–prey dynamics: the composite functional response. J. Anim. Ecol. 77, 520–528 (doi:10.1111/j.1365-2656.2008.01368.x) [DOI] [PubMed] [Google Scholar]
- 39.Miller TEX, Rudolf VHW. 2011. Thinking inside the box: community-level consequences of stage-structured populations. Trends Ecol. Evol. 26, 457–466 (doi:10.1016/j.tree.2011.05.005) [DOI] [PubMed] [Google Scholar]
- 40.Amaral V, Cabral HN, Bishop MJ. 2012. Effects of estuarine acidification on predator–prey interactions. Mar. Ecol. Prog. Ser. 445, 117–127 (doi:10.3354/meps09487) [Google Scholar]
- 41.Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134 (doi:10.1130/G30210A.1) [Google Scholar]
- 42.Russell BD, Connell SD, Findlay HS, Tait K, Widdicombe S, Mieszkowska N. 2013. Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Phil. Trans. R. Soc. B 368, 20120438 (doi:10.1098/rstb.2012.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang LH, Rudolf VHW. 2010. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett. 13, 1–10 (doi:10.1111/j.1461-0248.2009.01402.x) [DOI] [PubMed] [Google Scholar]
- 44.Blake JW. 1960. Oxygen consumption of bivalve prey and their attractiveness to the gastropod, Urosalpinx cinerea. Limnol. Oceanogr. 5, 273–280 (doi:10.4319/lo.1960.5.3.0273) [Google Scholar]
- 45.Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C. 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Est. Coast. Shelf Sci. 88, 442–449 (doi:10.1016/j.ecss.2010.05.004) [Google Scholar]
- 46.Cayan DR, Maurer EP, Dettinger MD, Tyree M, Hayhoe K. 2008. Climate change scenarios for the California region. Clim. Change 87, S21–S42 (doi:10.1007/s10584-007-9377-6) [Google Scholar]
- 47.Byrne M, Przeslawski R. 2013. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 53, 582–596 (doi:10.1093/icb/ict049) [DOI] [PubMed] [Google Scholar]
- 48.Ferrari MC, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt Ö, Chivers DP. 2011. Putting prey and predator into the CO2 equation: qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol. Lett. 14, 1143–1148 (doi:10.1111/j.1461-0248.2011.01683.x) [DOI] [PubMed] [Google Scholar]
- 49.Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406 (doi:10.1038/nclimate1259) [Google Scholar]
- 50.Moore M, Folt C. 1993. Zooplankton body size and community structure: effects of thermal and toxicant stress. Trends Ecol. Evol. 8, 178–183 (doi:10.1016/0169-5347(93)90144-E) [DOI] [PubMed] [Google Scholar]


