Abstract
The evolutionary potential of populations is mainly determined by population size and available genetic variance. However, the adaptability of spatially structured populations may also be affected by dispersal: positively by spreading beneficial mutations across sub-populations, but negatively by moving locally adapted alleles between demes. We develop an individual-based, two-patch, allelic model to investigate the balance between these opposing effects on a population's evolutionary response to rapid climate change. Individual fitness is controlled by two polygenic traits coding for local adaptation either to the environment or to climate. Under conditions of selection that favour the evolution of a generalist phenotype (i.e. weak divergent selection between patches) dispersal has an overall positive effect on the persistence of the population. However, when selection favours locally adapted specialists, the beneficial effects of dispersal outweigh the associated increase in maladaptation for a narrow range of parameter space only (intermediate selection strength and low linkage among loci), where the spread of beneficial climate alleles is not strongly hampered by selection against non-specialists. Given that local selection across heterogeneous and fragmented landscapes is common, the complex effect of dispersal that we describe will play an important role in determining the evolutionary dynamics of many species under rapidly changing climate.
Keywords: allelic model, gene flow, linkage, local adaptation, meta-populations
1. Introduction
Current predictions of future climate change stand at a 0.3–4.8°C rise in temperature by the end of the twenty-first century (based on the projected change in global mean surface air temperature relative to 1986–2005) [1]. Such unprecedented rates of change will place populations under a greater risk of extinction, particularly in increasingly fragmented landscapes [2]. The specific response of populations exposed to environmental change will depend upon the existence and interplay of a suite of evolutionary and demographic processes. Foremost among these, in situ adaptation and migration (i.e. biogeographic range shifting) will both play key roles in determining the likelihood of population persistence during and following environmental change [3]. The capacity and speed by which a population can respond to change will therefore be affected by evolutionary dynamics. Specifically, a population's level of additive genetic variance [4,5] can directly influence evolutionary outcomes in response to environmental change by providing the necessary genetic variation upon which selection can act [6,7]. While plasticity may facilitate changes in phenotype in response to new climatic conditions (e.g. [8]), there is likely to be a point where plasticity alone becomes insufficient [9]: genetic responses will then be critical for population survival.
The important role of genetic adaptation in species response to changing climate has already been demonstrated for a range of taxa [10–14], including the rapid adaptation of both dispersal traits and thermal tolerance [15]. The dispersal characteristics of a species, combined with such exogenous factors as landscape structure [16] and endogenous factors including a sub-population's local adaptation to climate [17], immediately impact the ability of populations to track a changing environment by migration.
The overall effect of dispersal on rapid adaptation is not easy to predict, in part owing to a complicated balance between the positive and negative effects of dispersal [18,19]. Gene flow can lead to greater evolutionary potential by promoting the pool of genetic variation within a population [7,20]. In addition, dispersal into new populations can both aid the purging of maladaptive alleles (if these are selected against in new environments) and increase the rate at which any beneficial alleles are shared between sub-populations [6,7]. However, theoretical and empirical studies have also demonstrated negative effects of gene flow on adaptation, impairing local fitness and slowing adaptation to alternative conditions, particularly in cases of local adaptation (e.g. [21–23]). During centre-to-range-edge migration, recipient populations swamped by alleles that are locally less fit [24,25] experience a reduction in performance owing to migration load (e.g. [21]). Not only is the average fitness of individuals reduced, but also locally adapted variants in recipient populations are diluted in number, leading to the loss of local adaptation and niche evolution [17,20,25], which in some cases can set the limits to a species's range [26,27]. In the case of spatially structured and locally adapted populations, dispersal therefore plays an indirect but important role in determining a population's evolutionary response to climatic change.
While there exists a large body of literature describing the effect of dispersal on local adaptation in temporally stable environments [24,26–31], fewer studies have sought to explore the interplay of both dispersal and local adaptation in determining the evolutionary potential of populations under temporally changing conditions (but see [23,32]). Where temporal changes are considered, many studies have described the dynamics of single panmictic populations [33–36]. Yet subdivided populations change the arrangement and availability of the genetic pool [19,37] with the potential to alter the adaptive response to environmental change [23,38,39]. The genetic architecture of individuals also strongly modifies responses to selection. Linkage between quantitative trait loci under antagonistic selection can slow the rate of adaptation by restricting the fixation of beneficial mutations in a population, and decreasing the effective population size [40–42].
Given that a genetic response to environmental pressure may be vital for the persistence of many species, and that empirical observations support the role of rapid adaptation in some situations, an important question arises: what are the conditions that favour rapid adaptation, and which exogeneous and endogeneous factors may place limits on this?
In this contribution, we explore how gene flow influences the response of a spatially structured population to climate change, where differential levels of local selection also operate. We use a simple, individual-based model to develop some initial theory on how the interplay between the strength of adaptation to the local environment, dispersal rate and the genetic architecture of loci under selection govern the likelihood that evolution can rescue a population from rapidly changing global environmental conditions. We first establish how, under stable climatic conditions, overall population fitness varies according to the level of divergent selection between two sub-populations. Subsequently, we investigate the effect of local selection, dispersal and genetic architecture on evolutionary potential, following a directional temporal change in the environment (i.e. an increase in the optimal temperature at a given location).
2. Material and methods
An allelic, individual-based simulation model was implemented to assess the effects of local adaptation and dispersal on the adaptation potential of an annual species inhabiting two spatially segregated patches (e.g. separate alpine meadows). The two sub-populations, connected by dispersal, are exposed to local differences in environmental conditions (e.g. soil heavy metal content). Climatic conditions may change temporally, but are assumed to be equal in both patches at any point in time. Individuals carry two sets of genes, one conferring adaptation to local environmental conditions, and the other adaptation to climate. An individual's fitness is determined by its level of adaptation to both the environmental and climatic conditions, and the strength of penalty for maladaptation (i.e. selection strength) [33]. Each patch supports a given number of individuals, determined by the local carrying capacity. Generations are assumed to be discrete and non-overlapping. Every generation undergoes the following steps: (i) reproduction and recombination, (ii) mutation, (iii) offspring dispersal, (iv) selection based on an individual's degree of adaptation, and finally (v) density-dependent mortality (see electronic supplementary material, figure S1).
(a). Genetic architecture
Adaptation to both the local patch (the non-climatic environment of each sub-population) and climate was simulated using a multi-locus system, reflecting the important role of polygenic traits in determining an individual's fitness response to its environment [43,44]. Diploid individuals each have 20 loci, 10 of which code for adaptation to the patch-specific environmental conditions while the other half code for adaptation to climate. The position of the patch-specific and climate loci relative to one another is determined at random. The degree of linkage between loci is varied by altering the probability that, at meiosis, a gamete's next allele does not come from the same chromosome copy as the previous one (i.e. a crossover event). This probability, pC, can vary between 0.0 (i.e. full linkage) and 0.5 (no linkage). The two alleles at each locus take continuous values and are assumed to be co-dominant, with no epistatic or pleiotropic effects. Heritability is assumed to be 1; thus the value of the alleles directly determines an individual's phenotype, each making a small and equal contribution (as in [45,46]).
(b). Life cycle
(i). Reproduction, recombination and mutation
Offspring are generated by the mating of two individuals selected from the same patch, with a small probability of self-fertilization (1/local sub-population size). The number of offspring produced by each mated pair is drawn randomly from a Poisson distribution with mean R. An offspring's genotype is composed of a maternal and a paternal gamete, each of which is produced following recombination (outlined above). Mutation is applied during reproduction, where at each locus (for a haploid gamete) there is a probability μ that a mutation occurs in the allele carried. The magnitude of a mutation is drawn from a zero-mean normal distribution with standard deviation α and is added to the initial value of the allele. After reproduction, the parental generation dies.
(ii). Dispersal
Each offspring moves from its natal patch to the other patch with probability d, simulating dispersal.
(iii). Selection
Before density-dependence (see below), we apply hard selection, where a juvenile's survival probability depends upon its genotype in relation to both patch and climatic conditions. The survival probability W of an individual is the product of its response to both local patch conditions WP and climate WC. Both WP and WC follow normal distributions (bounded by a minimum of 0.0 and a maximum of 1.0) and variance  , so that W is calculated according to
, so that W is calculated according to
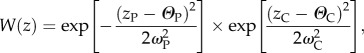 |
with z being the overall phenotype of the individual and ΘP,C the optimal phenotype with respect to local patch conditions or current climate (following [28]). As both the patch and climatic phenotypes are polygenic, the specific phenotype of an individual for each trait (zP and zC) is determined by the mean allelic value for all relevant trait loci. ω2 is the strength of selection applied against departure from non-optimal allelic values, thus determining an individual's fitness for a given departure of the phenotype from the optimum. The absolute effect of ω2 can be regarded as the inverse of the value it takes (i.e. lower values of ω2 represent stronger selection against maladapted alleles). Thus, individual fitness is calculated as the product of the differences between the optimal and the realized phenotype (calculated across all alleles for that trait), conditional on the degree of divergent selection operating between patches  and the strength of selection induced by climatic conditions
and the strength of selection induced by climatic conditions 
(iv). Density-dependent mortality
When the sub-population size following selection exceeds carrying capacity K, each individual has a risk of mortality that is independent of its genotype. The probability of surviving density-dependent mortality is calculated as the quotient of the carrying capacity K and the present sub-population size, where the sub-population size exceeds K: 1 − K/N. On average, this results in the sub-population size being reduced to K.
(c). Simulations
Simulations were run to investigate the interactive effects of local adaptation, dispersal and linkage on a sub-structured population experiencing climate change (see the electronic supplementary material, table S1 for model parameters and values). Each sub-population initially comprised 100 individuals (50% of the patch carrying capacity), all optimally adapted to both local environment and climate (with patch allele optima of 10 and 20, respectively). The final level of local adaptation evolved from the balance between dispersal between patches and the strength by which the two sub-populations experienced antagonistic selection between the alleles coding for each patch. We modified the strength of selection against deviation from the optimum by the parameter  , which ranged from 1 (strong selection against one maladapted allele) to 8192 (alleles effectively neutral). Climate selection
, which ranged from 1 (strong selection against one maladapted allele) to 8192 (alleles effectively neutral). Climate selection  was set to 2, and the rate of climate change v set to a 0.0375°C increase per generation (for 200 generations), unless otherwise specified. This generated a moderately high level of selection against maladaptive climate alleles (see sensitivity analysis in electronic supplementary material, appendix S1). Individual dispersal rates d were varied between 0 and 0.2, and the level of linkage pC varied between 0 and 0.5. Unless otherwise stated, all other parameters were kept constant (see electronic supplementary material, table S1). Details on sensitivity analyses varying
was set to 2, and the rate of climate change v set to a 0.0375°C increase per generation (for 200 generations), unless otherwise specified. This generated a moderately high level of selection against maladaptive climate alleles (see sensitivity analysis in electronic supplementary material, appendix S1). Individual dispersal rates d were varied between 0 and 0.2, and the level of linkage pC varied between 0 and 0.5. Unless otherwise stated, all other parameters were kept constant (see electronic supplementary material, table S1). Details on sensitivity analyses varying  and v, and mutation standard deviation α with patch selection strength
and v, and mutation standard deviation α with patch selection strength  , for different conditions of dispersal rate d and linkage (as pC) are given in the electronic supplementary material (appendix S1 and table S2). Additional analyses on rates of climate change are given in the electronic supplementary material, appendix S2. Reported values for the strength of environmentally induced selection in the literature vary according to the system studied and the fitness traits measured (e.g. [47–49]). Our approach allows for a wide range of realized selection values, ranging from nil/weak (reflecting observations of [47]) to a strong selective force (such as in predator-driven selection of morph types [50]).
, for different conditions of dispersal rate d and linkage (as pC) are given in the electronic supplementary material (appendix S1 and table S2). Additional analyses on rates of climate change are given in the electronic supplementary material, appendix S2. Reported values for the strength of environmentally induced selection in the literature vary according to the system studied and the fitness traits measured (e.g. [47–49]). Our approach allows for a wide range of realized selection values, ranging from nil/weak (reflecting observations of [47]) to a strong selective force (such as in predator-driven selection of morph types [50]).
Except in a single set of diagnostic simulations (see below), we ran between 20 and 100 replicates, recording at population level the mean population fitness  and population size over time. The percentage of population extinctions (as global extinction, of both sub-populations), and the number of extinctions in which only one sub-population survived (i.e. only one of the patches), was also determined across a broad range of parameter space. Below we describe variations in parameters for each simulation.
and population size over time. The percentage of population extinctions (as global extinction, of both sub-populations), and the number of extinctions in which only one sub-population survived (i.e. only one of the patches), was also determined across a broad range of parameter space. Below we describe variations in parameters for each simulation.
(i). Population response under stable climate
Twenty replicate runs were made for simulations assuming stable climatic conditions. Initial simulations over 5000 generations indicated that 500 generations were sufficient to reach population equilibrium.
(ii). Population response under climate change
Following 500 generations to allow the population to obtain quasi-equilibrium, climate change was implemented as a gradual increase from 15°C over 200 generations, with rate v. For an annual species, the rate of climate change assumed is within the range of likely values projected by recent global climate models (see [1]).
The resultant phenotypic distributions from the above simulations are given in the electronic supplementary material (appendix S4 and figures S12–S15).
(iii). Population response to varied rates of climate change
The rate of climate change v was altered as the magnitude of change per generation, with an increase ranging between 0.0225 and 0.06°C per generation, with all other parameters as before (see electronic supplementary material, table S1). Simulations were run for 1500 generations unless the population became extinct.
Finally, in a set of diagnostic simulations to support interpretation of the simulation results, we explored the fate of single beneficial alleles that were introduced into a population that had experienced a recent change in climate. The probability of the mutation surviving and establishing within each sub-population was followed under a range of conditions (see electronic supplementary material, appendix S5).
3. Results
(a). Population response under stable climate
During a stable climate, the interplay between patch selection strength 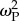 and dispersal d affected an individual's survival probability W and mean population fitness
and dispersal d affected an individual's survival probability W and mean population fitness  (see electronic supplementary material, figure S2), and the point at which specialists or generalists emerged (see below).
(see electronic supplementary material, figure S2), and the point at which specialists or generalists emerged (see below).
Under strong antagonistic patch selection  , individuals maladapted to the alternative environment suffered high mortality. Here, mean population fitness
, individuals maladapted to the alternative environment suffered high mortality. Here, mean population fitness  was unaffected by migration load as strongly maladapted alleles were quickly purged and a high average sub-population fitness was maintained, selecting strongly for well-adapted specialists (see electronic supplementary material, figures S2 and S12). Declines in mean fitness of the population surviving density-dependent selection were observed only over a small range of patch selection values
was unaffected by migration load as strongly maladapted alleles were quickly purged and a high average sub-population fitness was maintained, selecting strongly for well-adapted specialists (see electronic supplementary material, figures S2 and S12). Declines in mean fitness of the population surviving density-dependent selection were observed only over a small range of patch selection values  At ω = 4, specialists were selected for, with dispersed poorly adapted specialists moderately purged, resulting in a small reduction in average fitness of the remaining population. At ω = 8, generalists were selected for, which, while maladapted in both patches, could still survive, causing high genetic load (observed as the greatest dip in patch fitness; electronic supplementary material, figure S2). From ω > 8, population fitness increased because maladaptation was less strongly punished, with population size remaining high and density-dependent selection operating to maintain sub-populations at K.
At ω = 4, specialists were selected for, with dispersed poorly adapted specialists moderately purged, resulting in a small reduction in average fitness of the remaining population. At ω = 8, generalists were selected for, which, while maladapted in both patches, could still survive, causing high genetic load (observed as the greatest dip in patch fitness; electronic supplementary material, figure S2). From ω > 8, population fitness increased because maladaptation was less strongly punished, with population size remaining high and density-dependent selection operating to maintain sub-populations at K.
(b). Population response to climate change
Introducing climate change led to a rapid and significant decrease in mean population fitness (figure 1). Generally, initial levels of mean fitness were regained following a period of steady climate. Where dispersal was permitted (and linkage between loci strong), a persistent reduction in the post-selection mean fitness resulted only for 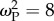 (figure 1). This result is logical because this is the highest value of selection strength that still allows the development of generalists, thereby leading to mutation load (see electronic supplementary material, figure S2) and a slowing of final fitness recovery. Under conditions of no dispersal (see electronic supplementary material, figure S3) fitness initially declined but subsequently improved similarly for all levels of patch selection
(figure 1). This result is logical because this is the highest value of selection strength that still allows the development of generalists, thereby leading to mutation load (see electronic supplementary material, figure S2) and a slowing of final fitness recovery. Under conditions of no dispersal (see electronic supplementary material, figure S3) fitness initially declined but subsequently improved similarly for all levels of patch selection 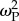 because individuals remain in their optimal patch, avoiding a genetic load.
because individuals remain in their optimal patch, avoiding a genetic load.
Figure 1.
Mean fitness under climate change scenarios and moderate dispersal. (a) Mean population fitness (extant runs out of 20 replicates) for six values of patch selection strength  and three values of crossover probability pC. There was no further change in results for
and three values of crossover probability pC. There was no further change in results for 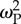 greater than 32. (b) Mean population fitness for extant (black) and extinct (grey) runs out of 20 replicates under the scenario of strong local adaptation and low dispersal (
greater than 32. (b) Mean population fitness for extant (black) and extinct (grey) runs out of 20 replicates under the scenario of strong local adaptation and low dispersal ( , d = 0.05) for three values of crossover probability pC. For all runs: d = 0.05, R = 2.5, K = 200 and ν = 0.0375°C generation−1. Standard errors were negligible and so error bars are left out for clarity. See electronic supplementary material, appendix S1 and figure S3 for results with d = 0.
, d = 0.05) for three values of crossover probability pC. For all runs: d = 0.05, R = 2.5, K = 200 and ν = 0.0375°C generation−1. Standard errors were negligible and so error bars are left out for clarity. See electronic supplementary material, appendix S1 and figure S3 for results with d = 0.
Although post-selection mean fitness recovered at population level, locally adapted (specialist) populations 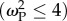 suffered declines in population size that persisted beyond the period of climate change (figure 2a). For those populations remaining extant, recovery was only partial; the mean population size over both patches is almost halved following climate change, and full recovery is only observed when
suffered declines in population size that persisted beyond the period of climate change (figure 2a). For those populations remaining extant, recovery was only partial; the mean population size over both patches is almost halved following climate change, and full recovery is only observed when  is 8 or above (figure 2a).
is 8 or above (figure 2a).
Figure 2.
(a) Mean overall population size under climate change scenarios (extant runs out of 20 replicates) for six values of patch selection strength  and three values of crossover probability pC. For all runs: d = 0.05, R = 2.5, K = 200 and ν = 0.0375°C generation−1. (b) Histograms illustrate that the reduction in mean population size (as observed in panel (a)) following climate change (i.e. partial rescue) is due to a proportion of simulations in which a population only successfully adapts to climate change in one of the two patches. The percentage of simulations in which both patches go extinct (black bars), one goes extinct (grey bars) and for which both survive (white bars) is shown for combinations of dispersal, linkage and patch selection strength. For these runs, R = 2.5, K = 200 and ν = 0.0375°C generation−1.
and three values of crossover probability pC. For all runs: d = 0.05, R = 2.5, K = 200 and ν = 0.0375°C generation−1. (b) Histograms illustrate that the reduction in mean population size (as observed in panel (a)) following climate change (i.e. partial rescue) is due to a proportion of simulations in which a population only successfully adapts to climate change in one of the two patches. The percentage of simulations in which both patches go extinct (black bars), one goes extinct (grey bars) and for which both survive (white bars) is shown for combinations of dispersal, linkage and patch selection strength. For these runs, R = 2.5, K = 200 and ν = 0.0375°C generation−1.
Importantly, the reduction in mean population sizes in surviving systems by approximately 50% was due mainly to the loss of a sub-population in one of the patches (figure 2b). In these cases, there was the complete loss of phenotypes, conferring adaptation to one of the two patches during the episode of climate change, and this local adaptation to patch conditions was not recovered after climate change (electronic supplementary material, appendix S4 and figures S12–S15). For highly locally adapted populations  , at least one patch went extinct in 80% of runs (figure 2b), and both went extinct in at least 40% of runs. For the less strongly locally adapted populations
, at least one patch went extinct in 80% of runs (figure 2b), and both went extinct in at least 40% of runs. For the less strongly locally adapted populations  , almost full recovery was possible, but only when linkage allowed recombination of beneficial climatic alleles and alleles coding for adaptation to one of the two habitats (pC ≥ 0.1). Increasing dispersal from d = 0 to d ≥ 0.05 marked an important transition (figure 2b). Where d = 0 and for all levels of selection, only in roughly 15–20% of the runs did both patches survive. As soon as dispersal was permitted the probability of each patch surviving was increased, as long as local adaptation was not too strong
, almost full recovery was possible, but only when linkage allowed recombination of beneficial climatic alleles and alleles coding for adaptation to one of the two habitats (pC ≥ 0.1). Increasing dispersal from d = 0 to d ≥ 0.05 marked an important transition (figure 2b). Where d = 0 and for all levels of selection, only in roughly 15–20% of the runs did both patches survive. As soon as dispersal was permitted the probability of each patch surviving was increased, as long as local adaptation was not too strong  and only moderate linkage was permitted (pC = 0.1 or 0.5).
and only moderate linkage was permitted (pC = 0.1 or 0.5).
Increasing linkage strongly reduced the likelihood that a population survived climate change (figures 2 and 3), and broadened the range of patch selection under which one of the patches became extinct (figure 2b). The negative effect of linkage was mediated by the reduction of the effective distribution of beneficial climate alleles owing to their association with maladaptive patch alleles (see below), resulting in an increased probability of population extinction during a shift in climate. For example, for  and d = 0.05, the chance of global population survival decreased from 70 to only 15% as recombination (pC) was reduced from 0.5 to 0.01 (figure 1). Under the case of zero dispersal, survival decreases even further, from 70 to 10%, even though migration load is not generated (see electronic supplementary material, figure S3).
and d = 0.05, the chance of global population survival decreased from 70 to only 15% as recombination (pC) was reduced from 0.5 to 0.01 (figure 1). Under the case of zero dispersal, survival decreases even further, from 70 to 10%, even though migration load is not generated (see electronic supplementary material, figure S3).
Figure 3.
Percentage of global population extinctions (over both patches) following climate change, depending on the dispersal probability d and the strength of patch selection  , for four different levels of crossover probability pC. All the parameter combinations were run for 100 replicates with R = 2.5, K = 200,
, for four different levels of crossover probability pC. All the parameter combinations were run for 100 replicates with R = 2.5, K = 200,  and ν = 0.0375°C generation−1 (see electronic supplementary material, appendix S2, and figures S8 and S9 for results with alternative rates of climate change, and appendix S3 and figure S10 for results with K = 400).
and ν = 0.0375°C generation−1 (see electronic supplementary material, appendix S2, and figures S8 and S9 for results with alternative rates of climate change, and appendix S3 and figure S10 for results with K = 400).
(c). Conditions favouring the spread of beneficial alleles
Having established that local adaptation can reduce population survival under climate change, we explored how selection against maladapted patch alleles might hamper the spread and establishment of beneficial climate alleles (see electronic supplementary material, appendix 5, and figures S16 and S17). Only strong local selection  significantly reduced the probability that a beneficial climate allele would establish in the other sub-population, from a 5–10% chance to zero. Where the penalty for maladaptation was less strong
significantly reduced the probability that a beneficial climate allele would establish in the other sub-population, from a 5–10% chance to zero. Where the penalty for maladaptation was less strong  , both potentially beneficial alleles and those causing a migration load could persist together. These results are consistent for all dispersal rates tested, indicating that only a moderate level of dispersal is required to ensure the spread of beneficial alleles, and that as long as local patch selection is below a threshold
, both potentially beneficial alleles and those causing a migration load could persist together. These results are consistent for all dispersal rates tested, indicating that only a moderate level of dispersal is required to ensure the spread of beneficial alleles, and that as long as local patch selection is below a threshold  and pC ≥ 0.01 beneficial alleles generally have a similar probability of establishment in both patches regardless of where they originated. Although the absolute level of linkage on the probability of establishment is relatively weak, tighter linkage slows the speed at which beneficial alleles establish.
and pC ≥ 0.01 beneficial alleles generally have a similar probability of establishment in both patches regardless of where they originated. Although the absolute level of linkage on the probability of establishment is relatively weak, tighter linkage slows the speed at which beneficial alleles establish.
(d). Rate of climate change
For the single rate of climate change implemented in the main simulations, both population extinction and evolutionary rescue were possible, depending upon the level of genetic selection for patch and climate adaptation, the dispersal rate, and the level of linkage. However, the rate of climate change also strongly modulated the overall population response. Faster rates of climate change exacerbated population extinction, with extinction probability increasing rapidly over a narrow range of climate change rates (see electronic supplementary material, figures S7–S9), but also shifted the point at which dispersal provided a positive effect on population persistence and adaptation, dispersal becoming more positive as rate of climate change increased, where recombination was less restrictive (pC > 0.01; see electronic supplementary material, appendix S2).
4. Discussion
In a recent empirical study, Bell & Gonzalez [51] demonstrated the positive effect of local dispersal on evolutionary rescue using an experimental meta-population of yeast. In their study, the probability of population survival depended crucially on the spread of beneficial mutations via gene flow. This empirical observation was consistent with earlier theoretical results predicting beneficial effects of dispersal with source populations acting as both genetic and demographic donors of individuals (e.g. [7]). However, as is suggested by the recent work of North et al. [52] and Schiffers et al. [23], the positive effect of dispersal might, under some conditions of habitat heterogeneity, be outweighed by the increasing maladaptation that dispersal generates. The results of our study indicate that the potential negative effects associated with dispersal generally only dominate the opposing positive effects of gene flow when conditions of patch selection lead to locally adapted phenotypes. In these cases, our results described a high likelihood of patch extinction for locally adapted sub-populations, with dispersal facilitating population rescue only when selection for specialists was relatively weak and recombination partially decoupled local adaptation from adaptation to changing climate. The positive role of dispersal increased from the point where, owing to weaker divergent selection between patches, conditions moved from local adaptation and the maintenance of specialists to the favouring of generalist genotypes performing well enough in both patches to maintain viable populations. This transition marks a vital point in determining whether a patch will experience partial or full extinction as opposed to rescue. Given that local adaptation is widespread in nature (e.g. [14,53]), our results suggest that sub-structured populations, despite being well adapted to their current environments, may have a higher probability of extinction at a local level than expected from overall population size and available genetic variation [53], as long as localized selection pressures reduce effective dispersal and if other factors (such as demographic factors of R and K, and genetic factors, such as µ) cannot provide sufficient alternative sources of genetic variation.
The point at which dispersal switched from having positive to negative effects in our model was also affected by the rate of climate change: dispersal was generally negative or neutral during lower rates of climate change (see electronic supplementary material, figure S11), worsening with increased local adaptation (where beneficial climate alleles linked with highly maladaptive local patch alleles are purged). As the rate of climate change increased, the effects of dispersal became increasingly positive, providing local selection was not extreme. Where linkage and selection were strong, beneficial alleles could not spread to aid in climate adaptation and the positive effect of dispersal was not realized. However, as long as recombination was permitted, dispersal was found to be positive, even at levels of relatively strong local selection. These findings are in agreement with predictions of an analytical model developed by Blanquart & Gandon [32]: they showed that when temporal environmental change is rapid, faster rates of dispersal are favoured, as long as migration is not costly, where instead the highest emigration rate evolves at an intermediate rate of environmental change.
An important implication of our findings is that even without direct genetic constraints, adaptation to a patch-specific environmental factor may affect a population's evolutionary response to another globally changing factor, such as climate. This indirect link is mediated via the purging of locally maladapted individuals that might at the same time carry beneficial mutations with respect to the globally changing factor. In our study, because single individuals—constituting the units of dispersal in our simulations—carry the genes for adaptation to both factors, the spread of a mutation that is beneficial under changed climatic conditions can be limited owing to their linkage to alleles coding for local adaptation (see electronic supplementary material, appendix S5, and figures S16 and S17).
The counteracting effects of gene flow for evolutionary rescue were also studied in related work by Schiffers et al. [23]. Although the same processes govern the impact of dispersal in both approaches, the balance between the positive and negative effects was shifted considerably towards the positive side in our study. Three factors are likely to cause this difference. First, in this study, we investigated a much wider range of selection strengths, resulting in a large proportion of scenarios where selection did not lead to local adaptation and migration load consequently did not occur. By contrast, in the work by Schiffers et al. [23], selection strength was much stronger  , probably leading to strong local adaptation. Second, we did not consider a single spatially continuous habitat with varying local conditions, but two discrete patches within which individuals mix freely, representing habitats with a clearly fragmented structure (e.g. separated meadows). Whereas in the continuous habitat model the carrying capacity of each location was restricted to five individuals, both of our two patches could hold up to 200 individuals (K = 400 for limited runs; electronic supplementary material, appendix S3). This refers to an ecological setting where a coarser habitat/patch classification is suitable for the organism considered. The resulting higher number of individuals occupying one of two patches in our model may reduce the level of migration load compared with the smaller number experiencing greater heterogeneity in the model of Schiffers et al. [23]. In addition, the greater population size under the same selective conditions and in the same pool for mating in our model would increase the probability that beneficial mutations are spread between more individuals on a local scale, thereby increasing the probability of establishment elsewhere. Consequently, dispersal in our two-patch model could have led to a greater capacity for adaptation to climate. Third, for Schiffers et al. [23], the impact of linkage between the quantitative trait loci was considered in a much simpler way, and the interpretation of results was mainly based on full linkage scenarios. Linkage, however, is known to play an important role in the evolutionary response of species [40,41], and our more detailed analysis of the role of linkage illustrates clearly the strong negative impact it can have on the survival probability of populations. Beneficial mutations with respect to climate that arise at certain locations across the genome cannot be inherited independently of locally adapted alleles under full linkage. Thus, the benefit of dispersal in providing the necessary variation in climate alleles for adaptation is considerably higher when relaxing the assumption of linkage between loci. Our findings reflect recent empirical observations on the constraining effect of linkage between traits on the magnitude of response to various stresses [54,55], highlighting the importance of genetic architecture in determining the capacity of populations to respond to environmental change (e.g. [5,14]).
, probably leading to strong local adaptation. Second, we did not consider a single spatially continuous habitat with varying local conditions, but two discrete patches within which individuals mix freely, representing habitats with a clearly fragmented structure (e.g. separated meadows). Whereas in the continuous habitat model the carrying capacity of each location was restricted to five individuals, both of our two patches could hold up to 200 individuals (K = 400 for limited runs; electronic supplementary material, appendix S3). This refers to an ecological setting where a coarser habitat/patch classification is suitable for the organism considered. The resulting higher number of individuals occupying one of two patches in our model may reduce the level of migration load compared with the smaller number experiencing greater heterogeneity in the model of Schiffers et al. [23]. In addition, the greater population size under the same selective conditions and in the same pool for mating in our model would increase the probability that beneficial mutations are spread between more individuals on a local scale, thereby increasing the probability of establishment elsewhere. Consequently, dispersal in our two-patch model could have led to a greater capacity for adaptation to climate. Third, for Schiffers et al. [23], the impact of linkage between the quantitative trait loci was considered in a much simpler way, and the interpretation of results was mainly based on full linkage scenarios. Linkage, however, is known to play an important role in the evolutionary response of species [40,41], and our more detailed analysis of the role of linkage illustrates clearly the strong negative impact it can have on the survival probability of populations. Beneficial mutations with respect to climate that arise at certain locations across the genome cannot be inherited independently of locally adapted alleles under full linkage. Thus, the benefit of dispersal in providing the necessary variation in climate alleles for adaptation is considerably higher when relaxing the assumption of linkage between loci. Our findings reflect recent empirical observations on the constraining effect of linkage between traits on the magnitude of response to various stresses [54,55], highlighting the importance of genetic architecture in determining the capacity of populations to respond to environmental change (e.g. [5,14]).
An interesting outcome of the comparison of our model results with those of Schiffers et al. [23] is the observation in both studies of partial evolutionary rescue, despite the differences in modelling approaches. In our two-patch model, partial rescue often occurred as an approximate halving of the initial population size that resulted from the complete loss of local adaptation to one of the two patches, while in the spatially explicit model, population size was more variable and corresponded to the particular spatial constellation of the location where mutations occurred. These independent observations of partial rescue suggest the importance of this result, and point towards a need for further investigations into its causes and consequences. Decreased population size may have the indirect effect of a further reduction in genetic diversity through drift [56], with the potential to increase inbreeding and with possible ecological consequences such as changes in pollinator–plant dynamics or breeding systems [57,58]. In our model, patch selection strongly influenced the recovery potential of the global population, and suggested that local adaptation is likely to seriously constrain sub-population survival during periods of climate change. Given the ubiquity of local adaptation, these findings have wide implications for spatially structured populations [53].
Our results, emerging from a relatively simple modelling exercise, reveal a complex interaction of differential selection resulting from independent exogenous pressures, underlying genetic architecture and the degree of dispersal across a sub-structured population. There are many interesting potential extensions. For example, the degree of dispersal symmetry between patches plays an important role in restricting evolutionary potential by constraining niche evolution [7,24,59]. Relaxing the assumption of symmetric dispersal would represent an obvious and important future study. Exploring the consequences of some of the underlying genetic and phenotypic assumptions of our model would also be of interest. First, the mutation variance could alter the probability of successful adaptation to changing climate: a greater mutation variance might reduce the probability of extinction for a given rate of climate change and level of local adaptation; initial simulations (see electronic supplementary material, appendix S1, and figures S5 and S6) pointed to this possibility. Second, our assumption that the genotype fully determines the phenotype (i.e. a heritability of 1) is relatively simplistic; a population's adaptability will clearly be modulated by the relative contribution of plasticity in mediating shorter-term responses (e.g. [8,9,12]), and genetic factors such as dominance and epistasis will reduce genetic heritability, altering the nature of genetic adaptation (e.g. [60]). As the relative roles of plasticity versus adaptive responses to environmental change are still not clear [8,9], exploring the effects of these parameters in allelic models such as ours will allow the generation of hypotheses that can then be tested with empirical studies.
Finally, dispersal may itself be subject to evolution, as the optimal level of dispersal varies depending on a number of population characteristics and environmental parameters. For example, dispersal is expected to be selected against in small populations, where migration is associated with increased mortality (e.g. [61–63]). Yet increasing dispersal ability has been shown for many taxa during range expansion [64–68]. There is therefore a need for existing modelling approaches that explore dispersal evolution (e.g. [69–71]) to incorporate the effects of both local adaptation and climatic adaptation.
5. Conclusion
Our model combines key features of genetic architecture involved in adaptation, in the context of a spatially structured population. It is also one of the first studies to address the role of local adaptation in shaping the evolutionary potential of species under environmental change that incorporates more than one trait under selection.
The results demonstrate how local adaptation and dispersal can interact to affect the adaptive response of populations to the selective forces expected to be imposed by environmental change, and reveal the important role of recombination in modulating this response. The positive effects of gene flow—in increasing effective population size and sharing beneficial climate alleles—were strongly reduced when dispersal occurred under strong local adaptation, but were not counteracted when generalist phenotypes evolved as a result of weak divergent selection between patches. The sensitivity of population fitness and persistence to both the degree of divergent selection to local conditions and to dispersal highlights that predictions of whether a species will adapt, migrate or become extinct in response to environmental change need to take into account the effects of habitat heterogeneity and local adaptation.
Acknowledgements
We would like to thank two anonymous referees for their thorough review of earlier versions of the manuscript and the very constructive comments.
Funding statement
The work was supported by the Macaulay Development Trust (PhD funding to E.C.B.) and the European Research Council under the European Community's Seven Framework Programme FP7/2007–2013 (IEF Marie-Curie Fellowship 252811 and Grant Agreement no. 281422 (TEEMBIO) to K.S.). The work of R.J.P. and R.W.B. is funded by the Scottish Government's Rural and Environment Sciences and Analytical Services (RESAS) Division. J.M.J.T. was supported by NERC.
References
- 1.IPCC 2013. Summary for policymakers. In Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Navels A, Xia Y, Bex V, Midgley PM.), pp. 1–27 Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Jump AS, Penuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 (doi:10.1111/j.1461-0248.2005.00796.x) [DOI] [PubMed] [Google Scholar]
- 3.Berg MP, Kiers ET, Driessen G, van der Heiden M, Kooi BW, Kuenen F, Liefting M, Verhoeff HA, Ellers J. 2010. Adapt or disperse: understanding species persistence in a changing world. Glob. Change Biol. 16, 587–598 (doi:10.1111/j.1365-2486.2009.02014.x) [Google Scholar]
- 4.Fisher R. 1930. Genetics, mathematics, and natural selection. Nature 126, 805–806 (doi:10.1038/126805a0) [Google Scholar]
- 5.Etterson JR, Shaw RG. 2001. Constraint to adaptive evolution in response to global warming. Science 294, 151–154 (doi:10.1126/science.1063656) [DOI] [PubMed] [Google Scholar]
- 6.Lande R, Shannon S. 1996. The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50, 434–437 (doi:10.2307/2410812) [DOI] [PubMed] [Google Scholar]
- 7.Gomulkiewicz R, Holt RD, Barfield M. 1999. The effects of density dependence and immigration on local adaptation and niche evolution in a black-hole sink environment. Theor. Popul. Biol. 55, 283–296 (doi:10.1006/tpbi.1998.1405) [DOI] [PubMed] [Google Scholar]
- 8.Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate. Proc. R. Soc. B 279, 3843–3852 (doi:10.1098/rspb.2012.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw WE, Holzapfel CM. 2001. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA 98, 14 509–14 511 (doi:10.1073/pnas.241391498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Réale D, McAdam AG, Boutin S, Berteaux D. 2003. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. Lond. B 270, 591–596 (doi:10.1098/rspb.2002.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 13.Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA 104, 1278–1282 (doi:10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colautti RI, Barrett CH. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342, 364–366 (doi:10.1126/science.1242121) [DOI] [PubMed] [Google Scholar]
- 15.Reusch T, Wood T. 2007. Molecular ecology of global change. Mol. Ecol. 16, 3973–3992 (doi:10.1111/j.1365-294X.2007.03454.x) [DOI] [PubMed] [Google Scholar]
- 16.McInerny G, Travis JMJ, Dytham C. 2007. Range shifting on a fragmented landscape. Ecol. Inform. 2, 1–8 (doi:10.1016/j.ecoinf.2006.12.001) [Google Scholar]
- 17.Atkins KE, Travis JMJ. 2010. Local adaptation and the evolution of species’ ranges under climate change. J. Theor. Biol. 266, 449–457 (doi:10.1016/j.jtbi.2010.07.014) [DOI] [PubMed] [Google Scholar]
- 18.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189 (doi:10.1016/S0169-5347(02)02497-7) [Google Scholar]
- 19.Garant D, Forde SE, Henry GHR. 2007. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 21, 434–443 (doi:10.1111/j.1365-2435.2006.01228.x) [Google Scholar]
- 20.Alleaume-Benharira M, Pen IR, Ronce O. 2006. Geographic patterns of adaptation within a species’ range: interactions between drift and gene flow. J. Evol. Biol. 19, 203–215 (doi:10.1111/j.1420-9101.2005.00976.x) [DOI] [PubMed] [Google Scholar]
- 21.Bolnick DI, Nosil P. 2007. Natural selection in populations subject to a migration load. Evolution 61, 2229–2243 (doi:10.1111/j.1558-5646.2007.00179.x) [DOI] [PubMed] [Google Scholar]
- 22.Lopez S, Rousset F, Shaw FH, Shaw RG, Ronce O. 2008. Migration load in plants: role of pollen and seed dispersal in heterogeneous landscapes. J. Evol. Biol. 21, 294–309 (doi:10.1111/j.1420-9101.2007.01442.x) [DOI] [PubMed] [Google Scholar]
- 23.Schiffers K, Bourne EC, Lavergne S, Thuiller W, Travis JMJ. 2013. Limited evolutionary rescue of locally adapted populations facing climate change. Phil. Trans. R. Soc. B 368, 20120083 (doi:10.1098/rstb.2012.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Ramos G, Kirkpatrick M. 1997. Genetic models of adaptation and gene flow in peripheral populations. Evolution 51, 21–28 (doi:10.2307/2410956) [DOI] [PubMed] [Google Scholar]
- 25.Kirkpatrick M, Barton N. 1997. Evolution of a species’ range. Am. Nat. 150, 1–23 (doi:10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 26.Bridle JR, Vines TH. 2007. Limits to evolution at range margins: when and why does adaptation fail? Trends. Ecol. Evol. 22, 140–147 (doi:10.1016/j.tree.2006.11.002) [DOI] [PubMed] [Google Scholar]
- 27.Kawecki TJ. 2008. Adaptation to marginal habitats. Ann. Rev. Ecol. Evol. Syst. 39, 321–342 (doi:10.1146/annurev.ecolsys.38.091206.095622) [Google Scholar]
- 28.Ronce O, Kirkpatrick M. 2001. When sources become sinks: migrational meltdown in heterogenous habitats. Evolution 55, 1520–1531 (doi:10.1111/j.0014-3820.2001.tb00672.x) [DOI] [PubMed] [Google Scholar]
- 29.Hedrick PW, Ginevan ME, Ewing EP. 1976. Genetic polymorphism in heterogeneous environments. Annu. Rev. Ecol. Syst. 7, 1–32 (doi:10.1146/annurev.es.07.110176.000245) [Google Scholar]
- 30.Haldane JBS. 1957. The cost of natural selection. J. Genet. 55, 511–524 (doi:10.1007/BF02984069) [Google Scholar]
- 31.Bulmer MG. 1972. Multiple niche polymorphism. Am. Nat. 106, 254–257 (doi:10.1086/282765) [Google Scholar]
- 32.Blanquart F, Gandon S. 2011. Evolution of migration in a periodically changing environment. Am. Nat. 177, 188–201 (doi:10.1086/657953) [DOI] [PubMed] [Google Scholar]
- 33.Pease CM, Lande R, Bull JJ. 1989. A model of population growth, dispersal and evolution in a changing environment. Ecology 70, 1657–1664 (doi:10.2307/1938100) [Google Scholar]
- 34.Burger R, Lynch M. 1995. Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution 49, 151–163 (doi:10.2307/2410301) [DOI] [PubMed] [Google Scholar]
- 35.Kopp M, Hermisson J. 2009. The genetics of phenotypic adaptation I: fixation of beneficial mutations in the moving optimum model. Genetics 182, 233–249 (doi:10.1534/genetics.108.099820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polechová J, Barton N, Marion G. 2009. Species’ range: adaptation in space and time. Am. Nat. 174, E186–E204 (doi:10.1086/605958) [DOI] [PubMed] [Google Scholar]
- 37.Higgins K, Lynch M. 2001. Metapopulation extinction caused by mutation accumulation. Proc. Natl Acad. Sci. USA 98, 2928–2933 (doi:10.1073/pnas.031358898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etterson JR. 2004. Evolutionary potential of Chamaecrista fasiculata in relation to climate change. II. Genetic architecture of three populations reciprocally planted along an environmental gradient in the Great Plains. Evolution 58, 1459–1471 (doi:10.1554/04-053) [DOI] [PubMed] [Google Scholar]
- 39.Jacquemyn H, De Meester L, Jongejans E, Honnay O. 2012. Evolutionary changes in plant reproductive traits following habitat fragmentation and their consequences for population fitness. J. Ecol. 100, 76–87 (doi:10.1111/j.1365-2745.2011.01919.x) [Google Scholar]
- 40.Birky CW, Walsh JB. 1988. Effects of linkage on molecular rates of evolution. Proc. Natl Acad. Sci. USA 85, 6414–6418 (doi:10.1073/pnas.85.17.6414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williford A, Comeron JM. 2010. Local effects of limited recombination: historical perspective and consequences for population estimates of adaptive evolution. J. Hered. 101(Suppl. 1), S127–S134 (doi:10.1093/jhered/esq012) [DOI] [PubMed] [Google Scholar]
- 42.Barton NH. 1995. Linkage and the limits to natural selection. Genetics 140, 821–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM. 2011. A map of local adaptation in Arabidopsis thaliana. Science 334, 86–89 (doi:10.1126/science.1209271) [DOI] [PubMed] [Google Scholar]
- 44.Jermstad K, Bassoni D, Wheeler N, Anekonda T, Aitken S, Adams W, Neale D. 2001. Mapping of quantitative trait loci controlling adaptive traits in coastal Douglas-fir. II. Spring and fall cold-hardiness. Theor. Appl. Gen. 102, 1152–1158 (doi:10.1007/s001220000506) [Google Scholar]
- 45.Kawecki TJ, Holt RD. 2002. Evolutionary consequences of asymmetric dispersal rates. Am. Nat. 160, 333–347 (doi:10.1086/341519) [DOI] [PubMed] [Google Scholar]
- 46.Holt RD, Gomulkiewicz R, Barfield M. 2003. The phenomenology of niche evolution via quantitative traits in a ‘black-hole’ sink. Proc. R. Soc. Lond. B 270, 215–224 (doi:10.1098/rspb.2002.2219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kingsolver JG, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gilbert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 (doi:10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 48.Siepielski AM, DiBattista JD, Evans JA, Carlson SM. 2011. Differences in the temporal dynamics of phenotypic selection among fitness components in the wild. Proc. R. Soc. B 278, 1572–1580 (doi:10.1098/rspb.2010.1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. 2012. Synthetic analysis of phenotypic selection in natural populations: lessons, limitations and future directions. Evol. Ecol. 26, 1101–1118 (doi:10.1007/s10682-012-9563-5) [Google Scholar]
- 50.Nosil P. 2009. Adaptive population divergence in cryptic color-pattern following a reduction in gene flow. Evolution 63, 1902–1912 (doi:10.1111/j.1558-5646.2009.00671.x) [DOI] [PubMed] [Google Scholar]
- 51.Bell G, Gonzalez A. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330 (doi:10.1126/science.1203105) [DOI] [PubMed] [Google Scholar]
- 52.North A, Pennanen J, Ovaskainen O, Laine AL. 2011. Local adaptation in a changing world: the roles of gene-flow, mutation, and sexual reproduction. Evolution 65, 79–89 (doi:10.1111/j.1558-5646.2010.01107.x) [DOI] [PubMed] [Google Scholar]
- 53.Leimu R, Fischer M. 2008. A meta-analysis of local adaptation in plants. PLoS ONE. 3, e4010 (doi:10.137/journal.pone.0004010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianoli E, Palacio-López K. 2009. Phenotypic integration may constrain phenotypic plasticity in plants. Oikos 118, 1924–1928 (doi:10.1111/j.1600-0706.2009.17884.x) [Google Scholar]
- 55.Betancourt AJ, Presgraves DC. 2002. Linkage limits the power of natural selection in Drosophila. Proc. Natl Acad. Sci. USA 99, 13 616–13 620 (doi:10.1073/pnas.212277199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens PA, Sutherland WJ. 1999. Consequences of the Allee effect for behavior, ecology and conservation. Trends Ecol. Evol. 14, 401–405 (doi:10.1016/S0169-5347(99)01684-5) [DOI] [PubMed] [Google Scholar]
- 57.Eckert CG, et al. 2010. Plant mating systems in a changing world. Trends Ecol. Evol. 25, 35–43 (doi:10.1016/j.tree.2009.06.013) [DOI] [PubMed] [Google Scholar]
- 58.Harder LD, Aizen MA. 2010. Floral adaptation and diversification under pollen limitation. Phil. Trans. R. Soc. B 365, 529–543 (doi:10.1098/rstb.2009.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt RD, Gaines MS. 1992. The analysis of adaptation in heterogenous landscapes: implications for the evolution of fundamental niches. Evol. Ecol. 6, 433–447 (doi:10.1007/BF02270702) [Google Scholar]
- 60.Latta RG, Gardner KM, Staples DA. 2010. Quantitative trait locus mapping of genes under selection across multiple years and sites in Avena barbata: epistasis, pleiotropy, and genotype-by-environment interactions. Genetics 185, 375–385 (doi:10.1534/genetics.110.114389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799 (doi:10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riba M, et al. 2009. Darwin's wind hypothesis: does it work for plant dispersal in fragmented habitats? New Phytol. 183, 667–677 (doi:10.1111/j.1469-8137.2009.02948.x) [DOI] [PubMed] [Google Scholar]
- 63.Bartoń KA, Phillips BL, Morales JM, Travis JMJ. 2009. The evolution of an ‘intelligent’ dispersal strategy: biased, correlated random walks in patchy landscapes. Oikos 118, 309–319 (doi:10.1111/j.1600-0706.2008.16936.x) [Google Scholar]
- 64.Cwynar LC, MacDonald GM. 1987. Geographical variation of lodgepole pine in relation to population history. Am. Nat. 129, 463–469 (doi:10.1086/284651) [Google Scholar]
- 65.Simmons AD, Thomas CD. 2004. Changes in dispersal during species’ range expansions. Am. Nat. 164, 378–395 (doi:10.1086/423430) [DOI] [PubMed] [Google Scholar]
- 66.Darling E, Samis KE, Eckert CG. 2008. Increased seed dispersal potential towards geographic range limits in a Pacific coast dune plant. New Phytol. 178, 424–435 (doi:10.1111/j.1469-8137.2007.02349.x) [DOI] [PubMed] [Google Scholar]
- 67.Phillips BL, Brown GP, Travis JMJ, Shine R. 2008. Reid's paradox revisited: the evolution of dispersal kernels during range expansion. Am. Nat. 172, S34–S38 (doi:10.1086/588255) [DOI] [PubMed] [Google Scholar]
- 68.Léotard G, Debout G, Dalecky A, Guillot S, Gaume L, Mckey D, Kjellberg F. 2009. Range expansion drives dispersal evolution in an equatorial three-species symbiosis. PLoS ONE 4, e5377 (doi:10.1371/journal.pone.0005377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Travis JMJ, Mustin K, Benton TG, Dytham C. 2009. Accelerating invasion rates result from the evolution of density-dependent dispersal. J. Theor. Biol. 259, 151–158 (doi:10.1016/j.jtbi.2009.03.008) [DOI] [PubMed] [Google Scholar]
- 70.Burton OJ, Phillips BL, Travis JMJ. 2010. Trade-offs and the evolution of life-histories during range expansion. Ecol. Lett. 13, 1210–1220 (doi:10.1111/j.1461-0248.2010.01505.x) [DOI] [PubMed] [Google Scholar]
- 71.Kubisch A, Thomas H, Poethke H-J. 2010. On the elasticity of range limits during periods of expansion. Ecology 91, 3094–3099 (doi:10.1890/09–2022.1) [DOI] [PubMed] [Google Scholar]





