Abstract
Animal populations are frequently limited by the availability of food or of habitat. In central-place foragers, the cost of accessing these resources is distance-dependent rather than uniform in space. However, in seabirds, a widely studied exemplar of this paradigm, empirical population models have hitherto ignored this cost. In part, this is because non-independence among colonies makes it difficult to define population units. Here, we model the effects of both resource availability and accessibility on populations of a wide-ranging, pelagic seabird, the black-browed albatross Thalassarche melanophris. Adopting a multi-scale approach, we define regional populations objectively as spatial clusters of colonies. We consider two readily quantifiable proxies of resource availability: the extent of neritic waters (the preferred foraging habitat) and net primary production (NPP). We show that the size of regional albatross populations has a strong dependence, after weighting for accessibility, on habitat availability and to a lesser extent, NPP. Our results provide indirect support for the hypothesis that seabird populations are regulated from the bottom-up by food availability during the breeding season, and also suggest that the spatio-temporal predictability of food may be limiting. Moreover, we demonstrate a straightforward, widely applicable method for estimating resource limitation in populations of central-place foragers.
Keywords: population regulation, seabirds, density dependence, habitat preference, net primary production, spatial segregation
1. Introduction
A fundamental question posed by ecologists is: what factors regulate population growth? Often, food availability is assumed to be limiting [1]. However, many mobile species, including all birds and many insects and mammals, not only gather food, but also return it to a breeding site or refuge. Foraging costs therefore increase as a function of distance from that central place [2]. Hence, not only resource availability but distance-dependent accessibility (sensu Matthiopoulos [3]) may be hypothesized to limit population size. However, population studies on colonial central-place foragers, such as seabirds, bats and hirundines, frequently make the implicit assumption that resources are equally accessible [4–8], with the potential consequence that an important limitation on population size is ignored.
Seabirds have long been used to test theories on population regulation [5,9–11]. Latterly, however, the need to understand their population dynamics has become more than academic, because many species are endangered by fisheries bycatch, over-fishing, invasive species and climate change [12–14]. Such anthropogenic factors are additive to natural limits on population growth [13], so in order to understand their impacts, it is necessary to first quantify the processes that naturally limit seabird populations. Food availability is usually considered to be a key driver of population change [9,10], but parasites, disease and nesting habitat availability can also have negative, density-dependent effects [15–17], whereas episodic environmental perturbations may also limit some populations [18]. By contrast, natural predation has a minor impact on most species, and adult survival is high compared with other avian groups [19]. As such, the natural regulation of seabird populations is primarily thought to be a bottom-up process, driven by environmental regulation of food supply [14].
Owing to spatio-temporal variability in physical forcing in the marine environment, seabird prey are patchily distributed [20]. In order to meet the energetic demands associated with self-maintenance and provisioning of offspring, pelagic seabirds range widely (10–1000s km) from their colonies during breeding. Consequently, seabird populations are hypothesized to be limited by the distribution of food relative to their colonies. However, most studies of regional population limitation have made the implicit assumption that prey within potential foraging range are uniformly distributed and equally accessible [4–6,10,19]. This assumption is perhaps expedient, because the distribution of seabird prey cannot be measured simultaneously at wide scales [21]. However, proxies of food availability may be more easily surveyed. For example, it is assumed that foraging birds preferentially select profitable habitats [22]. Hence, habitat preference, which can be defined using indices measurable at ocean-wide scales (e.g. bathymetry, thermal gradients, ocean colour, etc. [21,23,24]), may reflect prey availability. However, habitat preference is not only dependent on food abundance. For example, in a patchy, dynamic environment, it could also reflect prey predictability [25]. Hence, preferred foraging habitat may be more usefully regarded as a limiting resource in itself, rather than as a proxy for food availability. An alternative proxy, which can be estimated from remotely-sensed data [26], is net primary production (NPP). Primary production is spatio-temporally patchy but integrated over time and space it limits the distribution of mid-trophic level species (fish, squid and crustaceans) [27,28], which in turn support pelagic seabirds. However, owing to the complexities of trophic transfer and interspecific competition [29], the food available to foraging seabirds may not be directly proportional to primary production.
The principal mechanism through which food availability is hypothesized to limit seabird populations is density-dependent competition during the breeding season [9]. As individual colonies grow in size, prey in surrounding waters are depleted or disturbed in widening halos. Consequently, birds must travel ever further to provision their young, such that the net delivery rate decreases, eventually becoming unsustainable. This is supported by evidence of prey depletion around colonies [30], correlations between foraging range and colony size [11], home range area and colony size [31], and breeding success and proxies of food abundance [14,19,32]. However, the situation becomes more complex in areas where adjacent colonies are very large or located relatively close together. Here, density-dependent intraspecific competition between birds from adjacent colonies can result in segregation of foraging areas [31]. Hence, even apparently distinct colonies may not be functionally independent [5,31], potentially confounding the relationship between regional population size and food availability. If neighbouring colonies compete for the same resources, then it has been predicted that colony size will correlate negatively with the number of neighbouring conspecifics within the potential foraging range [5]. However, if colony size is limited largely by regional food availability, these two factors would be positively correlated.
Black-browed albatrosses Thalassarche melanophris are very wide-ranging pelagic seabirds (max. foraging range approx. 3000 km [23]) and probably the world's most abundant albatross species (approx. 600 000 breeding pairs; electronic supplementary material, table S1). They breed in archipelagos and on isolated islands throughout the sub-Antarctic (figure 1). Black-browed albatrosses are useful model colonial central-place foragers, because all of their major colonies have been censused in recent years, and their habitat use and preferences quantified [23,24,33]. Moreover, there is an urgent need to understand natural population regulation in this species, because it is currently threatened by bycatch in longline and trawl fisheries [13].
Figure 1.
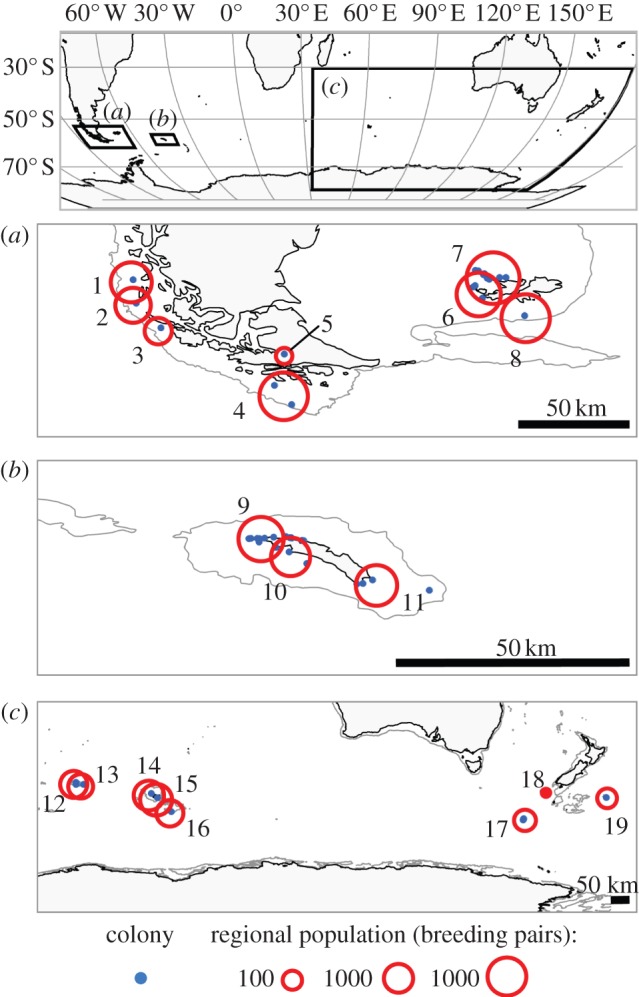
Location and size of black-browed albatross colonies. All known colonies (n = 48) were grouped into regional populations by cluster analysis such that the maximum distance by sea between colonies within regional populations di,j was ≤50, 100, 200, 400 and 800 km. For illustration, open circles indicate the number of pairs of birds breeding annually in regional populations defined by di,j ≤ 100 km (population numbers correspond to those in the 100 km column; electronic supplementary material, table S2). (Online version in colour.)
Black-browed albatrosses prefer to forage in neritic waters, which are markedly more productive than oceanic waters (e.g. fish production is an order of magnitude higher in shelf waters [27]). However, the oceanographic regime in waters around their colonies varies considerably by region (reviewed by [34]). Black-browed albatross demographic parameters have been linked to oceanographic variability [13,32] and population size to regional resource availability [19]. We therefore hypothesized that regional albatross population size is limited principally by the availability and accessibility of foraging resources during the breeding season (we use the term foraging resources advisedly to indicate foraging habitat or NPP, which we consider indirect proxies of either food availability or predictability). Owing to environmental stochasticity, equilibrium population size is generally lower than the theoretical carrying capacity of the environment [35]. Therefore, although we analysed factors limiting observed population size, we assume that these also limit carrying capacity. We define population units objectively as spatial clusters of colonies and test whether habitat, NPP or both together best describe observed population size. By repeating our analysis at several spatial scales, we provide strong evidence that regional albatross populations are limited by both the availability and accessibility of foraging habitat during the breeding season. We suggest that the causal mechanism is density-dependent competition for food. Our intuitive models of resource limitation are applicable to other colonial central-place foragers, including seabirds, pinnipeds, hirundines and bats.
2. Material and methods
(a). Defining regional populations
We collated colony size estimates (breeding pairs) for all extant black-browed albatross colonies (see the electronic supplementary material, table S1). If data were available for more than one breeding season, then we selected estimates that coincided with NPP data coverage (see below). Using cluster analysis [36], we grouped adjacent colonies into nominal regional populations according to the intervening distances. Because albatrosses avoid crossing large land masses, we used the minimum great-circle distance by sea d throughout our analysis. On a 1/6° regular grid, we calculated minimum distances di,x from the ith colony to each grid cell x at sea as well as the distances di,j between all pairs of colonies i and j. We then used a complete-linkage clustering algorithm, implemented with the function ‘hclust’ in the R package ‘cluster’ [37], to group adjacent colonies hierarchically by distance into regional populations. Colony clusters were determined at five spatial scales (di,j = 50, 100, 200, 400 and 800 km). At each scale, we then defined regional population size N as the number of pairs of albatrosses breeding annually within each cluster. Scales were chosen to group colonies with potentially overlapping foraging ranges likely to be subject to the same meso- to macroscale habitat availability and oceanographic regimes. Oceanographic variability at finer scales could not be resolved using available NPP data, and larger scale clustering would have resulted in too few clusters for meaningful analysis. Because potential competition between seabirds from adjacent colonies is thought to be density-dependent [31], our results are likely to be sensitive to assumed di,j at smaller rather than at larger distances. For computational efficiency, we therefore specified a logarithmic range of values of di,j.
(b). Resource availability and accessibility
We used gridded proxies of foraging resource abundance to explore the factors limiting population size. We deemed grid cells accessible from each colony to be those within the maximum foraging range of breeding adults dmax. This we assumed to be 3200 km (1.1 × the maximum observed foraging range of 163 black-browed albatrosses satellite-tracked from eight colonies throughout the breeding distribution [23]). In order to characterize the habitat type, we obtained bathymetric data from the ETOPO2 Global Relief 2v2 dataset (US Department of Commerce, National Oceanic and Atmospheric Administration, National Geophysical Data Center, 2006), and re-sampled these on a 1/6° grid. On the basis of previous research [23], we defined the extent of preferred foraging habitat H as waters < 500 m deep (i.e. neritic waters). Accordingly, cells were flagged as preferred, if the depth zx was less than zp = 500 m. H, the total area of preferred habitat available to birds from the ith regional population, is then
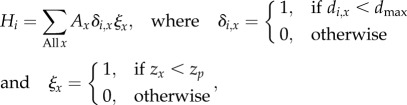 |
2.1 |
where Ax is the area of the xth grid cell. The relative accessibility of grid cells is assumed to be inversely proportional to colony distance [23] and is defined as
| 2.2 |
The total preferred accessibility-weighted (hereafter α-weighted) habitat available to each population is then
| 2.3 |
Monthly NPP climatology data (mg carbon (C) m−2 d−1) for the period 1998–2007 were supplied by Robert O'Malley and the Ocean Productivity website (http://science.oregonstate.edu/ocean.productivity; 2009) on a 1/6° grid for the black-browed albatross breeding period (September–April). NPP estimates were generated using a vertically generalized production model [26]. In order to describe regional variation in the rate of NPP, we calculated the decadal mean of the monthly median NPP in waters accessible to each population. We next quantified the total amount of NPP available to each population, in each month:
| 2.4 |
and the total available α-weighted NPP is
| 2.5 |
(c). Breeding success
Estimates of the annual breeding success (proportion of eggs laid that resulted in a fledged chick) of black-browed albatrosses were made available from a long-term study colony on Bird Island, South Georgia (54°00′ S, 38°03′ W). Each year during laying, daily visits are made to the colony, and all nests with an egg are marked. Thereafter, breeding outcome is monitored in weekly visits, until the time of fledging.
(d). Statistical analysis
In order to test whether adjacent colonies have a limiting effect on one another, following Furness & Birkhead [5], we calculated the Pearson correlation coefficient between colony size and the square-root of the number of pairs breeding at neighbouring colonies. Neighbouring colonies were defined as those within putative foraging range, which was successively assumed to be 50, 100, 200, 400, 800, 1600 or 3200 km. Colony size, which in seabirds is generally lognormally distributed [38], was loge-transformed to improve normality.
In order to determine whether resource availability limits albatross population size, we first modelled N as a function of H or P using linear and quadratic regression. We then assessed whether resource accessibility was also limiting by modelling N as a function of H′ or P′ and comparing relative model fit using second-order Akaike's information criterion (AICc) and R2. The same procedure was used to assess seasonal variation in the dependence of population size on primary production. Similarly, in order to test whether monthly P or P′ limits breeding success, and therefore population size (through the putative mechanism of food limitation), we tested for correlations between breeding success at Bird Island and these covariates.
Having determined whether it was appropriate to weight H and P by accessibility, we used likelihood ratio tests and AICc to test whether these covariates acted in concert or alone to limit population size. We proceeded, by backward selection, to consider models of N containing additive and multiplicative combinations of H′ and P′. We checked for collinearity between explanatory variables using variance inflation factors (VIFs). Models with VIFs exceeding 10 were regarded as unreliable [39]. We repeated model selection procedures at each regional population scale. To improve normality, assessed using Anderson–Darling tests, all covariates were loge-transformed. We assessed heteroscedasticity and spatial autocorrelation using residual plots and semivariograms [40]. Throughout our analysis, we considered p-values to be significant at the 0.05 level.
3. Results
Colony size was lognormally distributed (Anderson–Darling test loge colony size, A = 0.25, p = 0.711, n = 48). Depending on the clustering scale, our analysis identified between eight and 27 regional populations (see the electronic supplementary material, figures S1–S2 and table S2). At the largest scale considered (di,j = 800 km), regional populations coincided with readily identifiable isolated islands or island groups: the coastal islands of Chile; the Falkland Islands; South Georgia; Crozet Island; Kerguelen; Heard, Bishop and Clerk Islands; Snares Island; and Bollons Island). At the scale di,j = 50 km, regional populations tended to comprise individual island colonies in the Chilean and Indian Ocean regions, and subgroups of colonies within the Falkland and South Georgia archipelagos. Note that at the smallest scale (di,j = 0), regional populations are synonymous with individual colonies.
At scales of di,j ≥ 50 km, regional population size (N) was positively correlated with the total availability of neritic habitat (H) within the maximum foraging range (figure 2a and table 1) and α-weighted habitat availability (H′; figure 2b). However, at all clustering scales considered, H′ was a better predictor of N than H (table 1). This pattern was clearest when colonies were clustered at scales of 50 km or more (at di,j = 50 km, logeN = −28.6 + 2.4 × loge H′, F1,22 = 55.79, p < 0.001). At this scale and above, up to 88% of the variability in N was explained by H′, whereas H explained at most 64%.
Figure 2.
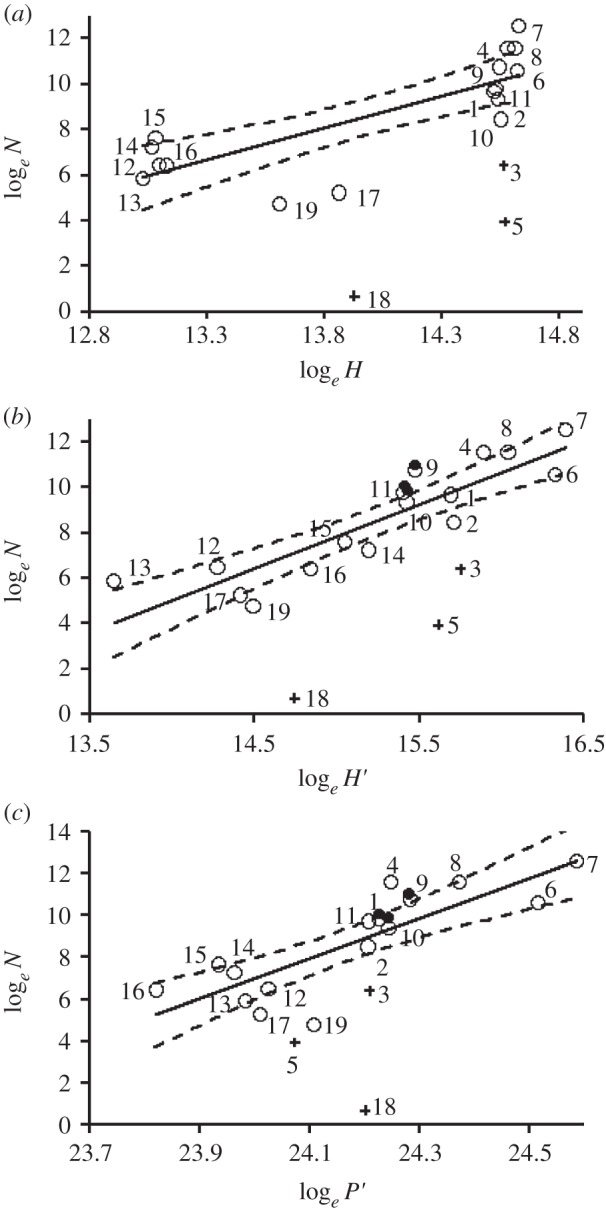
Black-browed albatross population size varies with foraging resource availability and accessibility (n = 45). (a) H, the total area (km2) of preferred habitat (waters <500 m deep) within maximum foraging range (3200 km) explains regional population size (N, breeding pairs) poorly in comparison to (b) H′, the total α-weighted preferred habitat and (c) P′, the total α-weighted daily NPP during January (Gg C d−1). α = maximum foraging range/colony distance. Colonies were grouped at di,j ≤ 100 km, but the trend was similar at all clustering scales. Open circles indicate populations used to fit models, closed circles the South Georgia population censused in 1986, and crosses known outliers (three Islote Leonard, five Islote Albatros and 18 Snares Island). Population numbers refer to figure 1 and the electronic supplementary material, table S1. Dotted lines indicate estimated 95% CIs.
Table 1.
Goodness of fit of linear models of loge regional black-browed albatross population size as a function of the availability and accessibility of foraging habitat and primary production. (Values in italics indicate minimum AICc for each clustering scale. H, total preferred habitat; H′, total accessibility-weighted preferred habitat; P, total net primary production in January; P′, total accessibility-weighted net primary production in January (all covariates loge-transformed). n.s., not significant. *p < 0.05, **p < 0.01, ***p < 0.001.)
| scale (km)a | n | AICc, R2 |
|||
|---|---|---|---|---|---|
| H | H′ | P | P′ | ||
| 0b | 48 | 216.4, 0.13* | 204.2, 0.33*** | 218.5, 0.09* | 212.9, 0.19** |
| 50 | 24 | 89.3, 0.55*** | 78.3, 0.72*** | 95.3, 0.42** | 90.2, 0.54*** |
| 100 | 16 | 61.8, 0.64*** | 52.8, 0.79*** | 64.7, 0.57** | 59.6, 0.68*** |
| 200 | 11 | 47.6, 0.61** | 35.0, 0.88*** | 48.9, 0.56** | 45.4, 0.68** |
| 400 | 9 | 42.3, 0.58* | 31.3, 0.87*** | 42.8, 0.55* | 39.7, 0.68** |
| 800 | 7 | 36.8, 0.55 n.s. | 27.9, 0.88** | 35.3, 0.64* | 28.2, 0.87** |
aMaximum distance di,j between colonies within clusters comprising regional populations.
bAt this scale, regional populations correspond to individual colonies.
The mean rate of NPP in the study area varied seasonally, peaking in December–January (figure 3a), which corresponds to the brood–guard stage. The decadal (1998–2007) mean rate of NPP in water accessible to albatrosses varied markedly among regions (see the electronic supplementary material, figure S3). At the scale di,j = 100 km, the mean rate was highest in waters accessible to birds from colonies in the Falkland Islands, South Georgia and western Australasia (range 402–408 mg C m−2 d−1); it was intermediate in waters accessible to Chilean and eastern Australasian breeders (379–385 mg C m−2 d−1); and lowest around Indian Ocean colonies (312–325 mg C m−2 d−1). This pattern was similar at all regional population scales under consideration (see the electronic supplementary material, figure S3).
Figure 3.
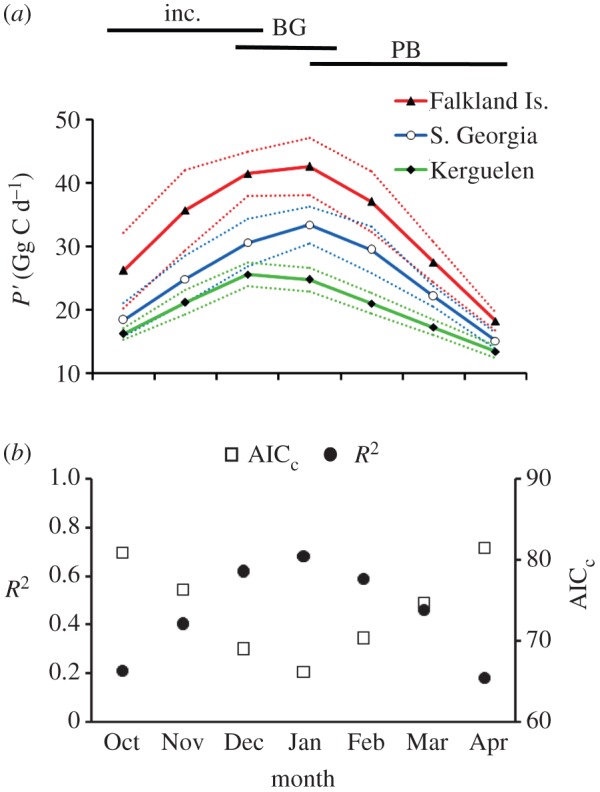
Monthly variation in total net primary production (NPP) and goodness of fit of population models. (a) P′ (accessibility-weighted NPP, in Gg C d−1) available to breeding albatrosses. Dashed lines indicate 95% CIs. (b) The goodness of fit of models of regional population size as a function of P′ (monthly averages, 1997–2007). Horizontal bars indicate the temporal extent of incubation (inc.), brood–guard (BG) and post-brood chick-rearing (PB) periods. Colonies were grouped at di,j ≤ 100 km, but the trend was similar at all clustering scales. (Online version in colour.)
N was positively correlated both with total available NPP (P) and α-weighted NPP (P′) during austral midsummer (table 1 and figure 2c). The strength of this relationship peaked in January (see the electronic supplementary material, table S3 and figure 3b). At all scales, P′ proved a better predictor of N than P (table 1 and figure 2b,c). January P and P′ explained up to 64% and 87% of the variation in N, respectively. There was no significant correlation between the breeding success of black-browed albatrosses at South Georgia and January P (r = −0.04, n = 10, p = 0.917). However, January P′ correlated positively with breeding success (r = 0.65, n = 10, p = 0.041; electronic supplementary material, figure S4). Variance inflation factors associated with multiplicative models of N as a function of H′ and P′ showed that these two covariates were highly co-linear (see the electronic supplementary material, table S4; correlation between H′ and January P′ at di,j = 100 km: r = 0.80, n = 19, p < 0.001). However, at all scales, the most parsimonious models of N contained only one explanatory covariate, H′ (see the electronic supplementary material, table S4).
H′ and P′ explained N more effectively when colonies were clustered into regional populations (up to 88% and 87% of the variance, respectively) than when individual colonies were regarded as population units (33% and 19% of population size explained, respectively). Regardless of putative foraging range, we found no evidence of a negative relationship between colony size and the number of pairs of birds breeding at other colonies within the potential foraging range (table 2). On the contrary, at putative foraging ranges of 100 km and above, there was a significant positive correlation between these factors.
Table 2.
Correlations between black-browed albatross colony size and number of pairs breeding at other colonies within a given range (n = 48).
| putative foraging range (km) | mean no. of conspecifics in range | mean no. of colonies in range | correlation r | p |
|---|---|---|---|---|
| 50 | 36 639 | 5.2 | 0.22 | 0.133 |
| 100 | 78 472 | 8.1 | 0.35 | 0.015 |
| 200 | 102 890 | 11.1 | 0.43 | 0.002 |
| 400 | 129 439 | 12.2 | 0.47 | 0.001 |
| 800 | 180 120 | 21.8 | 0.59 | <0.001 |
| 1600 | 316 712 | 31.2 | 0.57 | <0.001 |
| 3200 | 471 875 | 34.2 | 0.44 | 0.002 |
4. Discussion
Our results clearly indicate that the size of regional black-browed albatross populations (N) is limited by the availability of foraging resources (preferred habitat or NPP). Weighting either resource by accessibility (the inverse of distance to breeding colonies) significantly improved our models, supporting the hypothesis that resource accessibility limits the population sizes of colonial central-place foragers. Although the most parsimonious model of N did not include P′, annual breeding success at Bird Island was correlated with January P′. Below, we consider the implications of these findings in more detail and the rationale for our methods, which are potentially applicable to other species.
Black-browed albatross colonies are clustered in space; larger colonies often have smaller satellite colonies in their immediate vicinity (figure 1 and the electronic supplementary material, figure S2). In these cases, neighbouring birds potentially compete for the same resources, so colonies may not be independent in terms of density-dependent population-regulation [5,6,31]. Depending on breeding stage, the foraging utilization distributions (UDs) of albatrosses from adjacent colonies are either overlapping or mutually exclusive [23,24]. The extent of colony UDs is likely to depend on colony locations, sizes and the distribution of resources [31]. By regarding regional populations as comprising all birds breeding within a particular area, we aimed to delineate population units whose potential resource use is largely independent. We defined regional populations as clusters of colonies with potentially overlapping ranges (specified by the maximum distance between colonies within clusters, di,j). Importantly, this definition is based on the potential rather than the realized UD of each population. An alternative would be to define populations, using tracking data, as groups of colonies with overlapping realized foraging UDs. However, this approach would be inappropriate in the context of our analysis, because the extent of realized foraging UDs is likely to be proportional to colony size [31], leading to the circular argument that population size is dependent on habitat availability, which is dependent on population size.
The scale at which clusters of seabird colonies occur is limited by the spatial distribution of suitable sites [15]. However, there is no consensus on whether this or other factors are the key constraints on distribution. Furthermore, although we assumed that regional populations were spaced sufficiently far apart that the potential for interpopulation competition was negligible, the minimum between-cluster distance to independence is unknown. We therefore conducted our analysis at multiple spatial scales (by specifying values of di,j ranging from 50 to 800 km), allowing us to determine whether varying di,j affected our results. It is possible that albatrosses from populations more than 800 km apart compete for the same resources. However, habitat use tends to decline as an inverse function of colony distance [23], and competition among seabirds is likely to be density-dependent [9,11,31]. Hence, among-population competition is only expected to be significant between relatively close regional populations.
Adult black-browed albatrosses have very high survival rates [41]. However, over the past two decades, major declines have occurred in some populations owing to incidental mortality in human fisheries [13]. In particular, the South Georgia population declined by ca 30% over this period [42], and the Falkland Islands population by 1% per annum between 2000 and 2005 [43]. Conversely, by providing supplementary food, fishery waste may have enhanced breeding success in some areas [13]. As a consequence, N may differ from its natural equilibrium. However, albatross colony sizes are lognormally distributed and larger populations are those most affected by fisheries. Hence, the relationship between population size and resource availability is rather insensitive to proportionately slight changes in loge N (cf. loge N for South Georgia in 1984 and 2004; figure 2b,c). The large amount of variation in N explained by habitat availability and accessibility (up to 88%; table 1) indicates that factors such as fisheries mortality accounted for relatively little regional variation in population size. Nonetheless, global mortality owing to fisheries is high [13], and the relative effects of this and natural population limitation should be investigated further. This could be achieved by modelling N as a function of both foraging resources and spatial proxies of potential fisheries–bird interaction rates (e.g. by integrating bird distribution and fishing effort [44]). Fisheries mortality occurs predominantly outside the breeding season [13,45], so bird–fishery encounter rates in areas used by juveniles, pre-breeders, and wintering adults would probably prove most informative [13]. Unfortunately, the distributions of many populations during these life-history stages remain poorly understood. Filling these knowledge gaps should therefore be a priority [12].
In order to interpret our results further, it is prudent to consider what mechanisms might link N to H′ or P′. Although the hypothesis remains difficult to test directly, seabird populations are generally considered to be regulated from the bottom-up, by food availability during the breeding season [9,10,19]. Black-browed albatrosses predominantly consume middle-to-upper trophic level prey, such as nototheniid and channichthyid fish, Antarctic krill Euphasia superba and ommastrephid squid [46]. These species are typically an order of magnitude more abundant in neritic than oceanic waters [27]. Like most pelagic seabirds, black-browed albatrosses are highly philopatric, so the number of recruits to a colony depends largely on its breeding output in previous years [41]. Hence, H′ could limit population size simply by limiting food availability and therefore chick provisioning rates. However, because the distribution of prey exploited by seabirds is characteristically patchy in time and space [20], its predictability might also determine habitat preferences [25]. At the coarse to mesoscale, the distribution of biological production in shelf seas is strongly tied to bathymetry [47], which is largely fixed over the lifespan of seabirds. Hence, the preference of birds for neritic habitats may reflect the cost of locating prey as well as its abundance. For example, foraging black-browed albatrosses from South Georgia target waters overlying shallow submarine banks, which cause spatially predictable aggregations of E. superba and mesotrophic fishes [33]. Because chick provisioning rates, and thus N, may therefore be limited by food variability, as well as abundance [32], foraging habitat may be regarded as a resource in itself, rather than as a proxy for prey.
Black-browed albatrosses travel 100–1000s of km from the colony during breeding, with birds from some populations foraging in oceanic as well as neritic waters [23]. At this scale, the abundance of mesotrophic organisms in both bathymetric regimes is determined largely by NPP [27,28]. The physical drivers that determine NPP vary markedly in type and intensity across the breeding range of albatrosses. Estimated NPP is highest in waters accessible to birds from Chile, the Falkland Islands and South Georgia (see the electronic supplementary material, figure S3), where the oceanographic regimes are dominated by three very different but productive features: the Humboldt current, a large-scale upwelling system; the Patagonian shelf, an extensive shelf sea; and the Antarctic polar front [34]. These three regions hold 99% of the world population of black-browed albatrosses. By contrast, total NPP in waters accessible to birds from the relatively small colonies in the Indian Ocean is low owing to a largely oceanic regime. Primary production in the Southern Ocean peaks from December to February (figure 3a). We found that α-weighted NPP in these months was the best predictor of regional population size (figure 3b). This corresponds to late incubation and early chick-rearing, when albatross breeding attempts at Kerguelen and South Georgia are most likely to fail [32,41]. Variability in prey abundance during this period, when energetic demand probably peaks [48], offers a mechanism linking the availability of NPP to population size. This is further supported by a positive correlation between the breeding success of black-browed albatrosses at South Georgia and January P′ (see the electronic supplementary material, figure S4).
As noted above, neritic waters are markedly more productive than oceanic waters. Hence, P′ is inevitably collinear with H′. Nevertheless, we found that models of N as an additive function of H′ and P′ were less parsimonious than single covariate models (table 2) and that P′ explained less variation in N than H′ (table 1). There are a number of reasons why NPP may be less closely correlated with N than habitat availability: first, the dependence of N on P′ may be an artefact of the strong relationship between depth and NPP. This is improbable however, because the positive correlation between breeding success at South Georgia and P′ is unlikely to have arisen by chance. Moreover, breeding success at Kerguelen is correlated with local environmental indices, with the causative mechanism thought to be bottom-up regulation of prey availability [13,32]. Second, variation in prey-taxis, advection, growth rates and phenology can cause spatio-temporal lags of the order of days to years and 100–1000s of km between primary producers and higher trophic level organisms [29,47]. However, the mean abundance of E. superba at South Georgia reaches a maximum in the last week of January [49], the month in which NPP peaks (figure 3b), so trophic lags in this ecosystem are apparently short. Third, interspecific competition could reduce the availability of mesotrophic biomass resulting from primary production. The black-browed albatross belongs to a guild of epipelagic consumers of swarming nekton and macro-zooplankton that includes many fishes, whales, sympatrically breeding seabirds and pinnipeds [50]. It would be difficult to test this hypothesis because of the lack of data on the degree of interspecific competition. However, niche partitioning between heterospecifics is greater than that between conspecifics [51], suggesting a stronger limiting effect of intra- than interspecific competition.
We found that the availability of H′ and P′ explained population size increasingly well as the scale (di,j) at which regional populations were defined was increased (table 1). Conversely, these factors explained variation in individual colony size poorly. This may be owing simply to the higher natural variability likely to exist in disaggregated (i.e. individual colony) data. However, it has been hypothesized that competition among seabirds from neighbouring colonies limits colony size, leading to the prediction that colony size correlates negatively with the number of conspecifics in neighbouring colonies [5]. We found no evidence of this effect (table 2). On the contrary, these covariates were positively correlated, and the relationship was strongest when neighbouring colonies were defined as those within 800 km (table 2). This implies that mean colony size varies by region. Only 33% of variation in colony size was explained by resource availability and accessibility (table 1), suggesting that other factors must be limiting at this scale. These may include parasites and disease [16,17], but two other possibilities are suggested by outliers in our data. At clustering scales of less than or equal to 100 km, the sizes of regional populations 3, 5 and 18 lie well below those predicted by either H′ or P′ (figure 2b,c). The first two of these are small islets (Islote Leonard and Islote Albatros, approx. areas 0.15 and less than 0.5 km2, respectively) which provide limited areas of nesting habitat. Indeed, as albatrosses require steep slopes or cliffs for take-off on calm days, the availability of suitable breeding habitat may also be limited on some of the larger islands. The remaining outlier, in the Snares Islands, is thought to be a new colony in a region that has only recently been colonized by this species [52]. As such, it may not yet have approached its equilibrium size, which our best estimate suggests is approximately 880 pairs (95% CIs 350–2220). N could be further regulated by natural processes occurring outside of the breeding season [13], but it is unlikely that these would impact colonies within the same regional population differentially.
On balance, our results provide indirect support for the hypothesis that albatross populations are limited by prey availability during the breeding season [9], with the caveat that prey predictability may also be limiting [32]. We found that weighting measures of resource availability by accessibility improved their explanatory power (table 1), implying that existing population and demographic models for other central-place foragers may be improved simply by weighting resource availability by accessibility. The mechanism linking albatross population size to resource accessibility seems clear: central-place theory predicts that the total cost, in time or energy, of accessing resources increases as a function of distance from the central place [2]. Hence, albatross chick provisioning rates, and ultimately colony size, will be lower if birds have to travel further to find food [9]. Importantly, this implies that population limitation in albatrosses and other central-place foragers will fluctuate with resource accessibility. For example, recent studies have shown that penguin breeding success declines when icebergs reduce habitat accessibility [18], and albatross breeding success improves in response to wind-mediated increases in habitat accessibility [53]. In the coming years, climate change is expected to cause shifts in the distribution of marine productivity [27,28]. Similarly, in the terrestrial sphere, human development has already caused widespread habitat fragmentation. By increasing the distance between available breeding locations or refuges and foraging resources, these changes may result in declines in populations of a diverse range of central-place foragers. It is important therefore that the relative effects of accessibility and other limiting factors are better understood. In the case of some seabird species, habitat preferences, and therefore resource availability may be difficult to quantify because they forage in association with dynamic or ephemeral features [21]. However, proxies of forging resource availability have been identified in many other groups of colonial central-place foragers, including bats, hirundines and pinnipeds [7,8,54]. In principle, our methodology may therefore be used to test whether population limitation owing to resource availability and accessibility occurs more widely. Similarly, if the future distribution of resources such as NPP or foraging habitat can be predicted, then the potential effects of environmental change on central-place forager populations may be investigated.
Acknowledgements
We thank Robert O'Malley for supplying NPP data; the many researchers who censused the world's black-browed albatross colonies; fieldworkers at Bird Island; John Croxall for supporting the long-term studies there; Simeon Hill and Jaume Forcada for statistical advice; and two anonymous referees for helpful comments on an earlier draft. This study represents a contribution to the Ecosystems Programme of the British Antarctic Survey.
Funding statement
This work was supported by the UK Natural Environment Research Council (NER/S/A/2005/13648).
References
- 1.Berryman AA. 1999. Principles of population dynamics and their application. Cheltenham, UK: Stanley Thornes Ltd [Google Scholar]
- 2.Orians GH, Pearson NE. 1979. On the theory of central place foraging. In Analysis of ecological systems (eds Horn DJ, Stairs GR, Mitchelle RG.), pp. 155–177 Columbus, OH: Ohio State University Press [Google Scholar]
- 3.Matthiopoulos J. 2003. The use of space by animals as a function of accessibility and preference. Ecol. Model. 159, 239–268 (doi:10.1016/S0304-3800(02)00293-4) [Google Scholar]
- 4.Laidre KL, Heide-Jorgensen MP, Nyeland J, Mosbech A, Boertmann D. 2008. Latitudinal gradients in sea ice and primary production determine Arctic seabird colony size in Greenland. Proc. R. Soc. B 275, 2695–2702 (doi:10.1098/rspb.2008.0874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furness RW, Birkhead TR. 1984. Seabird colony distributions suggest competition for food supplies during the breeding season. Nature 311, 655–656 (doi:10.1038/311655a0) [Google Scholar]
- 6.Cairns DK. 1989. The regulation of seabird colony size: a hinterland model. Am. Nat. 134, 141–146 (doi:10.1086/284970) [Google Scholar]
- 7.Petit S, Pors L. 1996. Survey of columnar cacti and carrying capacity for nectar feeding bats on Curacao. Conserv. Biol. 10, 769–775 (doi:10.1046/j.1523-1739.1996.10030769.x) [Google Scholar]
- 8.Brown CR, Sas CM, Brown MB. 2002. Colony choice in cliff swallows: effects of heterogeneity in foraging habitat. Auk 119, 446–460 [Google Scholar]
- 9.Ashmole NP. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103, 458–473 [Google Scholar]
- 10.Diamond AW. 1978. Feeding strategies and population-size in tropical seabirds. Am. Nat. 112, 215–223 (doi:10.1086/283261) [Google Scholar]
- 11.Lewis S, Sherratt TN, Hamer KC, Wanless S. 2001. Evidence of intra-specific competition for food in a pelagic seabird. Nature 412, 816–819 (doi:10.1038/35090566) [DOI] [PubMed] [Google Scholar]
- 12.Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan BJ, Symes A, Taylor P. 2012. Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv. Int. 22, 1–34 (doi:10.1017/S0959270912000020) [Google Scholar]
- 13.Rolland V, Barbraud C, Weimerskirch H. 2008. Combined effects of fisheries and climate on a migratory long-lived marine predator. J. Appl. Ecol. 45, 4–13 (doi:10.1111/j.1365-2664.2007.01360.x) [Google Scholar]
- 14.Cury PM, et al. 2011. Global seabird response to forage fish depletion: one-third for the birds. Science 334, 1703–1706 (doi:10.1126/science.1212928) [DOI] [PubMed] [Google Scholar]
- 15.Forbes LS, Jajam M, Kaiser GW. 2000. Habitat constraints and spatial bias in seabird colony distributions. Ecography 23, 575–578 (doi:10.1034/j.1600-0587.2000.230508.x) [Google Scholar]
- 16.Duffy DC. 1983. The ecology of tick parasitism on densely nesting peruvian seabirds. Ecology 64, 110–119 (doi:10.2307/1937334) [Google Scholar]
- 17.Weimerskirch H. 2004. Diseases threaten Southern Ocean albatrosses. Polar Biol. 27, 374–379 (doi:10.1007/s00300-004-0600-x) [Google Scholar]
- 18.Kooyman GL, Ainley DG, Ballard G, Ponganis PJ. 2007. Effects of giant icebergs on two emperor penguin colonies in the Ross Sea, Antarctica. Antarctic Sci. 19, 31–38 (doi:10.1017/S0954102007000065) [Google Scholar]
- 19.Weimerskirch H. 2002. Seabird demography and its relationship with the marine environment. In Biology of marine birds (eds Schreiber EA, Burger J.), pp. 115–135 Boca Raton, FL: CRC Press [Google Scholar]
- 20.Fauchald P, Erikstad KE, Skarsfjord H. 2000. Scale-dependent predator–prey interactions: the hierarchical spatial distribution of seabirds and prey. Ecology 81, 773–783 [Google Scholar]
- 21.Wakefield ED, Phillips RA, Matthiopoulos J. 2009. Quantifying the habitat use and preference of pelagic seabirds using individual movement data: a review. Mar. Ecol. Prog. Ser. 391, 165–182 (doi:10.3354/meps08203) [Google Scholar]
- 22.Rosenzweig ML. 1981. A theory of habitat selection. Ecology 62, 327–335 (doi:10.2307/1936707) [Google Scholar]
- 23.Wakefield ED, et al. 2011. Accessibility, habitat preference and conspecific competition limit the global distribution of breeding albatrosses. Ecol. Monogr. 81, 141–167 (doi:10.1890/09-0763.1) [Google Scholar]
- 24.Catry P, Lemos RT, Brickle P, Phillips RA, Matias R, Granadeiro JP. 2013. Predicting the distribution of a threatened albatross: the importance of competition, fisheries and annual variability. Prog. Oceanogr. 110, 1–10 (doi:10.1016/j.pocean.2013.01.005) [Google Scholar]
- 25.Weimerskirch H. 2007. Are seabirds foraging for unpredictable resources? Deep-Sea Res. II, Top. Stud. Oceanogr. 54, 211–223 (doi:10.1016/j.dsr2.2006.11.013) [Google Scholar]
- 26.Behrenfeld MJ, et al. 2006. Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (doi:10.1038/nature05317) [DOI] [PubMed] [Google Scholar]
- 27.Jennings S, Melin F, Blanchard JL, Forster RM, Dulvy NK, Wilson RW. 2008. Global-scale predictions of community and ecosystem properties from simple ecological theory. Proc. R. Soc. B 275, 1375–1383 (doi:10.1098/rspb.2008.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chassot E, Bonhommeau S, Dulvy NK, Melin F, Watson R, Gascuel D, Le Pape O. 2010. Global marine primary production constrains fisheries catches. Ecol. Lett. 13, 495–505 (doi:10.1111/j.1461-0248.2010.01443.x) [DOI] [PubMed] [Google Scholar]
- 29.Rice J. 2001. Implications of variability on many time scales for scientific advice on sustainable management of living marine resources. Prog. Oceanogr. 49, 189–209 (doi:10.1016/S0079-6611(01)00022-2) [Google Scholar]
- 30.Birt VL, Birt TP, Goulet D, Cairns DK, Montevecchi WA. 1987. Ashmole halo-direct evidence for prey depletion by a seabird. Mar. Ecol. Prog. Ser. 40, 205–208 (doi:10.3354/meps040205) [Google Scholar]
- 31.Wakefield ED, et al. 2013. Space partitioning without territoriality in gannets. Science 341, 68–70 (doi:10.1126/science.1236077) [DOI] [PubMed] [Google Scholar]
- 32.Pinaud D, Weimerskirch H. 2002. Ultimate and proximate factors affecting the breeding performance of a marine top-predator. Oikos 99, 141–150 (doi:10.1034/j.1600-0706.2002.990114.x) [Google Scholar]
- 33.Wakefield ED, Phillips RA, Belchier M. 2012. Foraging black-browed albatrosses target waters overlaying moraine banks: a consequence of upward benthic-pelagic coupling? Antarctic Sci. 24, 269–280 (doi:10.1017/S0954102012000132) [Google Scholar]
- 34.Longhurst AL. 1998. Ecological geography of the sea, p. 398 San Diego, CA: Academic Press [Google Scholar]
- 35.Hui C. 2006. Carrying capacity, population equilibrium, and environment's maximal load. Ecol. Model. 192, 317–320 (doi:10.1016/j.ecolmodel.2005.07.001) [Google Scholar]
- 36.Kauffman L, Rousseeuw PJ. 2005. Finding groups in data: an introduction to cluster analysis, p. 342 Hoboken, NJ: John Wiley & Sons [Google Scholar]
- 37.Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. 2012. cluster: cluster analysis basics and extensions. R package version 1.14.2
- 38.Jovani R, Mavor R, Oro D. 2008. Hidden patterns of colony size variation in seabirds: a logarithmic point of view. Oikos 117, 1774–1781 (doi:10.1111/j.1600-0706.2008.17065.x) [Google Scholar]
- 39.Heiberger RM, Holland B. 2004. Statistical analysis and data display: an intermediate course with examples in S-Plus, R, and SAS. New York, NY: Springer [Google Scholar]
- 40.Cliff AD, Ord JK. 1981. Spatial processes: models and applications, p. 260 London, UK: Pion [Google Scholar]
- 41.Prince PA, Rothery P, Croxall JP, Wood AG. 1994. Population-dynamics of black-browed and gray-headed albatrosses Diomedea melanophris and D. chrysostoma at Bird Island, South Georgia. Ibis 136, 50–71 (doi:10.1111/j.1474-919X.1994.tb08131.x) [Google Scholar]
- 42.Poncet S, Robertson G, Phillips RA, Lawton K, Phalan B, Trathan PN, Croxall JP. 2006. Status and distribution of wandering, black-browed and grey-headed albatrosses breeding at South Georgia. Polar Biol. 29, 772–781 (doi:10.1007/s00300-006-0114-9) [Google Scholar]
- 43.Huin N, Reid T. 2006. Census of the black-browed albatross population of the Falkland Islands 2000 and 2005, p. 37 Stanley, Falkland Islands: Falklands Conservation [Google Scholar]
- 44.Tuck GN, Phillips RA, Small C, Thomson RB, Klaer NL, Taylor F, Wanless RM, Arrizabalaga H. 2011. An assessment of seabird-fishery interactions in the Atlantic Ocean. ICES J. Mar. Sci. 68, 1628–1637 (doi:10.1093/icesjms/fsr118) [Google Scholar]
- 45.Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Bennett VJ. 2005. Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology 86, 2386–2396 (doi:10.1890/04-1885) [Google Scholar]
- 46.Cherel Y, Klages NTW. 1998. A review of the food of albatrosses. In Albatross biology and conservation (eds Robertson G, Gales R.), pp. 113–136 Chipping Norton, UK: Surrey Beatty & Sons [Google Scholar]
- 47.Mann KH, Lazier JRN. 2006. Dynamics of marine ecosystems, 3 edn., p. 496 Malden, MA: Blackwell Publishing [Google Scholar]
- 48.Salamolard M, Weimerskirch H. 1993. Relationship between foraging effort and energy requirement throughout the breeding-season in the wandering albatross. Funct. Ecol. 7, 643–652 (doi:10.2307/2390184) [Google Scholar]
- 49.Saunders RA, Brierley AS, Watkins JL, Reid K, Murphy EJ, Enderlein P, Bone DG. 2007. Intra-annual variability in the density of Antarctic krill (Euphausia superba) at South Georgia, 2002–2005: within-year variation provides a new framework for interpreting previous ‘annual’ estimates of krill density. CCAMLR Sci. 14, 27–41 [Google Scholar]
- 50.Croxall JP, Prince ED. 1980. Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol. J. Linn. Soc. 14, 103–131 (doi:10.1111/j.1095-8312.1980.tb00101.x) [Google Scholar]
- 51.Armstrong RA, McGehee R. 1980. Competitive exclusion. Am. Nat. 115, 151–170 (doi:10.1086/283553) [Google Scholar]
- 52.Moore PJ, Burg TM, Taylor GA, Millar CD. 2001. Provenance and sex ratio of black-browed albatross, Thalassarche melanophrys, breeding on Campbell Island, New Zealand. Emu 101, 329–334 (doi:10.1071/MU00074) [Google Scholar]
- 53.Weimerskirch H, Louzao M, de Grissac S, Delord K. 2012. Changes in wind pattern alter albatross distribution and life-history traits. Science 335, 211–214 (doi:10.1126/science.1210270) [DOI] [PubMed] [Google Scholar]
- 54.Aarts G, MacKenzie M, McConnell B, Fedak M, Matthiopoulos J. 2008. Estimating space-use and habitat preference from wildlife telemetry data. Ecography 31, 140–160 (doi:10.1111/j.2007.0906-7590.05236.x) [Google Scholar]


