Abstract
A goal of evolutionary biology is to understand the roles of geography and ecology in speciation. The recent shared ancestry of sister species can leave a major imprint on their geographical and ecological attributes, possibly revealing processes involved in speciation. We examined how ecological similarity, range overlap and range asymmetry are related to time since divergence of 71 sister species pairs in the California Floristic Province (CFP). We found that plants exhibit strikingly different age-range correlation patterns from those found for animals; the latter broadly support allopatric speciation as the primary mode of speciation. By contrast, plant sisters in the CFP were sympatric in 80% of cases and range sizes of sisters differed by a mean of 10-fold. Range overlap and range asymmetry were greatest in younger sisters. These results suggest that speciation mechanisms broadly grouped under ‘budding’ speciation, in which a larger ranged progenitor gives rise to a smaller ranged derivative species, are probably common. The ecological and reproductive similarity of sisters was significantly greater than that of sister–non-sister congeners for every trait assessed. However, shifts in at least one trait were present in 93% of the sister pairs; habitat and soil shifts were especially common. Ecological divergence did not increase with range overlap contrary to expectations under character displacement in sympatry. Our results suggest that vicariant speciation is more ubiquitous in animals than plants, perhaps owing to the sensitivity of plants to fine-scale environmental heterogeneity. Despite high levels of range overlap, ecological shifts in the process of budding speciation may result in low rates of fine-scale spatial co-occurrence. These results have implications for ecological studies of trait evolution and community assembly; despite high levels of sympatry, sister taxa and potentially other close relatives, may be missing from local communities.
Keywords: age-range correlation, allopatric and ecological speciation, niche conservatism, community phylogenetics, ecological niche, sister species
1. Introduction
A major goal of evolutionary biology is to understand the relative roles of geography and ecology in speciation. Historically, geographical isolation has been considered a prerequisite for reproductive isolation [1–4]. For example, ‘Jordan's rule’ states that ‘[g]iven any species in any region, the nearest related species is not likely to be found in the same region nor in a remote region, but in a neighbouring district separated from the first by a barrier of some sort’ [1,2, p. 73; 5]. Analyses of animal clades have largely supported Jordan's assertion: a common geographical mode of speciation for animals appears to be allopatric speciation via vicariance, where the range of an ancestral species is divided to form two new species by a geographical barrier, resulting in equal-sized ranges with no overlap among descendants [6,7]. However, recent work has demonstrated that ecological and parapatric speciation, in which ranges are in close proximity, are more common than formerly appreciated [5,8–10]. The sessile nature of plants may increase the relative importance of ecological speciation, as plants may be more sensitive to fine scale environmental heterogeneity.
The study of closest relatives (i.e. sister species) offers insights into the relative importance of geographical versus ecological segregation in speciation. Sister species are more closely related to each other than to any other species, which can leave a major imprint on their geographical and ecological attributes [11–13]. The current degrees of range overlap and range asymmetry of sister species may provide some ability to infer the geographical mode of speciation [5,14], with the caution that post-speciation range shifts may obscure mechanisms (e.g. allopatric speciation followed by secondary contact may be mistaken for sympatric speciation) [7,14–16]. The importance of ecological segregation in speciation would be highlighted by traits that limit or prevent gene flow among nascent, sympatric lineages (e.g. habitat segregation, pollinator divergence, behavioural changes, phenological shifts and mating system shifts). Young sister species with high degrees of range overlap might also be expected to diverge ecologically. For example, in carnivores, canine size divergence is greater in areas of sympatry than allopatry [17,18].
‘Budding speciation’, defined as when a new species forms within or at the edge of the retained ancestral species, may be especially common among plants [19]. Budding speciation contains the unique signature that early in the speciation process, sisters should have overlapping or adjacent ranges with very different sizes (i.e. asymmetric ranges) [5,20]. This term includes peripatric speciation (also known as ‘peripheral isolate speciation’) [21,22], catastrophic speciation, in which environmental stress causes bottlenecks that result in reproductive isolation from the progenitor species [20,23–25], centrifugal speciation, where mutants are spawned in central areas of the range and subsequently left isolated owing to range contractions of the ancestral species [26], and ecological/sympatric speciation, in which strong divergent selection from adjacent environments favours reproductive isolation [9,27–30].
Mechanisms of reproductive isolation in budding speciation in plants include divergent selection across habitats favouring phenological, pollinator or mating system shifts [31,32], and/or mutations that can result in instantaneous reproductive isolation, for example ploidy shifts [33–35]. In budding speciation, the progenitor species is paraphyletic at first, but it is expected that lineage sorting and extinction will result in monophyly with time [19,36]. For plants, budding speciation may be an especially common mode of speciation [19,20], as high rates of selfing and polyploidization can cause instantaneous reproductive isolation [37–40]; moreover, sessile plants cannot move away from stressful environments once germinated, and thus may be especially exposed to strong divergent selection from fine-scale environmental heterogeneity. For example, serpentine soil-affiliated and non-serpentine sister species co-occur in Layia (Asteraceae), with one species’ range completely subsumed within the other. These species grow within metres of each other, have very different flowers, use contrasting soil types and provide a good example of how close relatives may not co-occur in very local communities, despite high range overlap [41,42].
Here, we examine the geographical range overlap, range asymmetry, and ecological and reproductive similarity of 71 plant sister species from 12 families in the California Floristic Province (CFP), a global biodiversity hotspot. We ask the following questions: first, do patterns of range overlap and asymmetry in sister species suggest budding speciation as an important mode of divergence for plants of the CFP? Second, how ecologically and reproductively similar are sisters? Do sisters show strong niche conservatism, or is there evidence for character divergence in sisters, especially when they have high range overlap? We also discuss the implications of the geographical and ecological characteristics of sister species for studies of ecological community assembly and trait evolution. We define sympatry as overlapping in geographical range. With this definition of sympatry, species can be distributed micro-allopatrically on the landscape, despite being in range-wide sympatry. Allopatric species exhibit no range overlap.
The system. The CFP has more than 5800 plant species and nearly 50% plant endemism [43]. Many plant taxa have narrow geographical ranges and small population sizes [44]. For example, 85% of the plants endemic to California have range sizes that are smaller than 10% of the state [45]. Edaphic and climatic factors have played important roles in the generation and maintenance of species diversity [46–48]. For example, plant species diversity peaks in regions where wet, aseasonal climates overlap with high levels of topographic and edaphic diversity [25,49]. Such high levels of environmental diversity over localized gradients might promote divergent selection and novel adaptations, driving ecological speciation, but also favour the persistence of sympatric ranges among sisters [50]. A recent review of endemism in the CFP concluded that allopatric processes appear to be more associated with animal endemism than with plant endemism [51], but no formal analysis has been conducted. Taking advantage of growing datasets on range and ecological traits, and the increasing numbers of complete phylogenies, we conducted, to our knowledge, the first large-scale analysis of the geographical and ecological attributes of sister species to infer their mode of speciation and to quantify their similarity.
2. Material and methods
We identified species-level phylogenies for all genera in the CFP for which complete or near-complete phylogenies (more than 95% taxa sampled) were available to feel confident that we were examining true sister species (21 genera, 12 families; electronic supplementary material, table S1). For each group, we used the topology that included the most genetic information and excluded morphological information, so that comparison of morphological traits between sisters in our study would not be circular. We kept the sister pairs that met three criteria (rationale described below): (i) spatial occurrences were available for both species, (ii) at least three non-sister congeners could be identified, and (iii) at least one of the sisters had some geographical range in the western United States. We did not include taxa that were part of a polytomy. Our final sample included 71 sister pairs (see the electronic supplementary material, tables S2 and S3 for a list of the sister species attributes).
(a). Do patterns of range overlap and asymmetry in sister species suggest budding speciation as an important mode of divergence for plants of the California Floristic Province?
Our source for range information was georeferenced herbarium specimens. Georeferenced occurrence data were downloaded from both the Global Biodiversity Information Facility, using the ‘gbif’ function in the R library ‘dismo’, and the California Consortium of Herbarium, using the ‘getConsortium’ function in the R library ‘Jepson’. The geographical coordinates were then combined and duplicated records were omitted. Coordinates that lacked subdegree resolution (e.g. 34.000, −121.000) were also excluded. Coordinates for each species were then carefully reviewed to identify and remove erroneous records. In total, 32 415 records remained; the mean number of records per species was 155 (median 90, maximum 1028). We set a minimum threshold of five records per species, but made exceptions for three species, which were known from only one or two sites (Streptanthus vernalis, Sidalcea stipularis and Clarkia lingulata). To estimate the geographical range for each species, we placed a 10 km buffer around each location using the ‘gBuffer’ function of ‘rgeos’ library and merged the overlapping areas between species using the ‘joinPolys’ function of the ‘PBSmapping’ library. This method avoids overestimation of range size and range overlap, relative to minimum convex hulls, which create a single polygon that encloses all known occurrences and assumes that all enclosed habitat is suitable for a given species [52]. As a result, our criteria for sympatry are relatively conservative.
We calculated range overlap as the area occupied by both species divided by the area of the smaller ranged species [14]. The range overlap metric ranges from 0 (no overlap) to 1 (complete overlap). Range overlap is 1 when the ranges of two species are fully syntopic, meaning the two species always co-occur and are never apart, or when a smaller ranged species is fully nested within a larger ranged species. We classified species using the range overlap metric as follows: allopatric means range overlap of 0 and sympatric means range overlap more than 0. We also calculated range asymmetry [14], a key prediction of budding speciation, as the area of the larger ranged species divided by the area of the smaller ranged species.
To generate a comparison group for our observed values of range overlap, range asymmetry and ecological similarity among sisters, we randomly selected three non-sister congeners for comparison with sisters. While the use of ranks can be problematic as congeners differ in age across groups, in every case, all our rank-based comparisons were made within, but not between groups. The same range statistics were calculated for each sister–non-sister congener pair. The mean of the six sister–non-sister congener pairs was used to generate a comparison group for sister pairs. Thus, the overall range overlap of the 71 sister–sister pairs was compared with the overlap of the 71 sister–non-sister congener values, each a mean of six comparisons, using a paired, two-tailed t-test. An analogous test was conducted for range asymmetry.
Age-range correlation analysis, in which time since divergence of sisters is related to range overlap, can provide insights into speciation mode. The slope of the relationship should reflect how range overlap changes with time, given post-speciation range shifts [5].
We estimated the relative ages of each sister pair based on a single ultrametric molecular phylogeny we created for our sister taxa and all of their congeners (n = 464 taxa total). The molecular phylogeny was based on internal transcribed spacer (ITS) sequence data acquired from GenBank and directly from authors (see the electronic supplementary material, table S1). ITS is very widely used in studies of the flora in the CFP and allows us to use the same sequence for comparison across all our taxa. We first used the software program Phylomatic [53] to generate a partially resolved topology that was used as a topological constraint tree in a RAxML [54] analysis. This phylomatic tree was based on a recent Angiosperm Phylogeny Working Group tree (R20100428). Below family-level relationships were manually added to the phylomatic tree to enforce the known sister species relationships; other lower level relationships were not constrained. Sequences were then aligned using MUSCLE [55] and maximum-likelihood analyses were done in RAxML, using the modified phylomatic tree as a topological constraint, a GTRCAT model, and 1000 bootstrap replicates. The resulting RAxML tree was fully dichotomous with branch lengths in substitutions per site. We calibrated these branch lengths by enforcing a molecular clock using the ‘chronos’ function in the R library ‘ape’. A strict molecular clock yielded a lower φIC score than a correlated molecular clock or a relaxed molecular clock and was therefore used for branch length calibration [56]. We extracted the age for each sister pair from the resulting chronogram.
We compared range overlap with age of sister species using linear regression. We also regressed range asymmetry with age to assess whether asymmetry is greater for young sisters; both outcomes would be consistent with ‘budding’ speciation. Given the observed shape of the relationships of range overlap and asymmetry with age, we also fitted a quantile regression model to the data using the ‘rq’ function of the ‘quantreg’ library; a tau value of 0.99 was chosen and the significance of the coefficient was assessed with 1000 bootstrap replications.
We tested the possibility that some genera were overly influential for our range results by using a K-statistic to test for phylogenetic signal in range overlap and range asymmetry [57], where K = 0 when there is no phylogenetic signal and K = 1 for a Brownian motion model (see the electronic supplementary material). The K-values observed for range overlap and range asymmetry were near zero and were not significantly different from expectations drawn from a null model based on randomly shuffling the tips of the phylogeny (range overlap: K = 0.04, p = 0.96; range asymmetry: K = 0.06, p = 0.66).
(b). How ecologically and reproductively similar are sisters?
(i). Ecological attributes
We collected data for six ecological attributes for sisters and their non-sister congeners: habitat, growth form, soil type, altitude in 100 m bands, plant height (cm) and climate niche. The first four attributes were measured as discrete variables and the remaining two were measured as continuous variables.
We scored habitat, soil and growth form using information from online and published sources, especially CalFlora, the Flora of North America and the Jepson Manual II [58], for all sisters and non-sister congeners. Habitat designations were typically based on vegetation type (e.g. chaparral) or natural features (e.g. vernal pools; see the electronic supplementary material, table S2). Soil designations were typically based on parent material (e.g. granitic or serpentine). We note that it is common for two species to share a habitat (e.g. chaparral), but not share a soil type (e.g. sandstone versus serpentine), and vice versa. Thus, we retain both traits in our study. For growth form, three states were possible: annual herb, perennial herb and perennial shrub.
For habitat, when a pair of species shared all habitats, they were scored as 0 for ‘shift absent’; if they shared some habitats, they were scored as 0.5 for ‘partial shift’; when no habitats were shared, they were scored as 1 for ‘shift present’. For example, if one species grew in seeps and the second in chaparral, the pair was scored as ‘shift present’. If one species grew in seeps and the other in riparian areas and seeps, they were scored as ‘partial shift’. Shifts in soil were coded analogously to shifts in habitat. Categorizing habitat and soil shifts from descriptions can be somewhat subjective; therefore, we were conservative in our designation of ‘full shift’ by only including pairs that showed clear differences in affinity (e.g. woodland versus dune). The raw attribute data and pairwise scores are available for review (electronic supplementary material, tables S2 and S3). Shifts in growth form were scored as binary because partial shifts were not possible.
Altitude was recorded in 100 m bands from the Jepson Manual II. If two species occupied exactly the same bands, or if one species was nested within the bands occupied by the other species, they were scored as 0 for ‘no shift’. If they share some, but not all, bands and were not completely nested, they were scored as 0.5 for ‘partial shift’. If they did not share any bands, they were scored as 1 for ‘shift present’.
Shifts in latitude and altitude may be related; for example, sisters sharing a climate niche might express that by occupying divergent altitudes at different latitudes; of interest with respect to sympatry is whether sisters at the same latitude diverge more in altitude. To investigate this further, we examined the relationship between the latitudinal and altitudinal bands occupied by sisters. We found no relationship between altitudinal shifts and latitudinal shifts in sisters in a supplementary analysis (see the electronic supplementary material).
Plant height data were extracted from the Jepson Manual II. Plant height contrasts were calculated as |log(XA) − log(XB)|, where X is the trait value of species A and B in a sister pair.
To estimate climate divergence, we created niche models and compared their predictions. We used a dataset acquired from WorldClim (www.worldclim.org) comprising four climate variables (annual temperature, annual precipitation, seasonality of temperature and seasonality of precipitation) for contemporary conditions (mean 1950–mean 2000) at 1 km2 resolution. For each taxon (including the three non-sister congeners), we fitted a Maxent model using the georeferenced occurrence data and the four climate surfaces; background points were selected randomly. The resulting model was then used to make a predictive surface of climatic suitability with values ranging from 0 to 1. Maxent was run using the default ‘auto features’ mode, allowing the use of linear, quadratic, product, threshold and hinge features. We used Schoener's D as our metric of climatic niche similarity to compare predictive surfaces [59], as implemented in the ‘nicheOverlap’ function of the ‘dismo’ library. We report 1 − Schoener's D to create an index of climate divergence that ranges from 0 (identical climate niches) to 1 (no niche overlap). All scores are provided in the electronic supplementary material, table S2.
(ii). Reproductive attributes
We collected data on three traits that affect the probability of species to cross: flower size (mm), flowering time in months and chromosome number. Flower size was measured as a continuous variable and flowering time and chromosome count were measured as discrete variables.
Because a previous study has shown floral divergence in sympatry in Mimulus sister pairs, and because flower size changes could represent opportunities for reproductive isolation through changes in mating system or pollinators [31,32], we measured flower size for our sister–sister pairs and sister–non-sister congeners. Our metrics of flower size were compiled from two sources. For most genera, flower width data came from the Jepson Manual II, using the mean of the reported flower size range. For five genera (Leptosiphon, Linanthus, Mimulus, Perideridia and Sanicula), floral dimensions were not available in the Jepson Manual II, so flower width on herbarium specimens was measured. The same data source and metric was used for all members of a genus, so any bias in absolute size arising from method is controlled for by within-group comparisons. Floral size contrasts were calculated as |log(XA) − log(XB)|, where X is the trait value of species A and B in a sister pair.
Flowering time data were extracted primarily from the Jepson Manual II using the ‘getJep’ function in the ‘Jepson’ library and secondarily from online sources. If two species flowered in the exact same months, or if one species was nested within the flowering time of the other species, they were scored as 0 for ‘no shift’. If they co-flowered in some, but not all, months and were not completely nested, they were scored as 0.5 for ‘partial shift’. If they did not co-flower in any month, they were scored as 1 for ‘shift present’.
For chromosome counts, we collected information from the online and published sources described above. Counts were available for 65 of the 71 sister pairs and for all non-sister congeners. Shifts in chromosome count were scored as binary because partial shifts were not possible.
(c). Analysis
Values for each sister pair were compared to the mean values expected from non-sister–congener comparisons using paired, two-tailed t-tests. We controlled for multiple testing (11 tests for conservatism in total: nine for traits, one for range overlap and one for range asymmetry) by adjusting our p-values according to the false discovery rate at α-level 0.05 [60]. We report only the adjusted p-values. Smaller divergences among sister–sister pairs than sister–non-sister congener pairs were taken as evidence for niche conservatism.
To test for character displacement among sisters, we examined whether the relationship between range overlap and character divergence was positive (i.e. greater divergence in sympatry) [18,32]. We created models with range overlap as the response variable and one of the traits as the predictor, using Kruskal–Wallis tests for the six discrete traits and Spearmen's r-rank-correlation tests for the three continuous traits. We controlled for multiple testing (nine tests for character displacement) as described above.
We asked whether sister species were characterized by at least one shift in one of the six discrete traits. For this, we analysed partial shifts in two ways: first, partial shifts were scored as 1 for ‘present’; second, partial shifts were scored as 0 for ‘shift absent’. We present results for both.
3. Results
(a). Do patterns of range overlap and asymmetry in sister species suggest budding speciation as an important mode of divergence for plants of the California Floristic Province?
Sister ranges were predominately sympatric (80% sympatric) and range overlap was significantly higher in sister–sister pairs than overlap in sister–non-sister congener pairs using the same sister (table 1). Sisters with the highest amounts of overlap also had the most asymmetric range sizes (range overlap versus range asymmetry: r = 0.44, p < 0.001).
Table 1.
Geographical and niche attributes among sister–sister pairs and sister–non-sister congener pairs. (Ecological and reproductive shifts were scored as 0 for ‘no shift’, 0.5 for ‘partial shift’ and 1.0 for ‘shift present’. Morphological trait contrasts were calculated as |log(XA) − log(XB)|, where X is the trait value of species A and B in a sister pair. Climate divergence is 1 − Schoener's D, estimated based on climatic niche models predictions. ***p < 0.001; **p < 0.01; *p < 0.05.)
| attribute | sister–sister pair | sister–non-sister congener pair | p-valuesa |
|---|---|---|---|
| proportion sympatric | 0.80 | 0.57 | *** |
| range overlap | 0.26 | 0.13 | *** |
| log(range asymmetry) | 1.27 | 1.74 | *** |
| habitat shift | 0.54 | 0.70 | ** |
| soil shift | 0.55 | 0.74 | ** |
| growth form shift | 0.03 | 0.12 | ** |
| altitude shift | 0.20 | 0.26 | * |
| plant height contrast | 0.53 | 0.73 | ** |
| climate divergence | 0.62 | 0.75 | *** |
| flowering time shift | 0.20 | 0.28 | * |
| flower size contrast | 0.41 | 0.56 | ** |
| chromosome count shift | 0.15 | 0.29 | ** |
ap-values corrected for multiple comparisons.
Small ranges were typical within our sample: 89% of the sisters had range sizes that were less than 10% of the area of California [45]; further, 23% of the sisters were listed on the California Native Plant Society's Rare Plant Lists [44].
There was no linear relationship between age and range overlap or range asymmetry between sisters at α-level 0.05; however, by inspection, the range overlap observed in older sisters was much less variable than that observed in younger sisters (figure 1a), and no older sisters had extensive range overlap, resulting in an ‘empty corner’ in the age-overlap relationship (quantile regression, p < 0.01; figure 1a). In other words, high range overlap was only observed between relatively young sisters, whereas low overlap was observed in both young and old sisters. Range asymmetry showed a similar relationship with age using quantile regression (p < 0.01; figure 1b), with asymmetry being greater, and more variable, in younger sisters. There are relatively few older sisters, however, which might account for the lower variance in their overlap and asymmetry. A larger sample of older sister taxa, when more complete phylogenies become available, would help to further clarify the relationships of range overlap and age.
Figure 1.
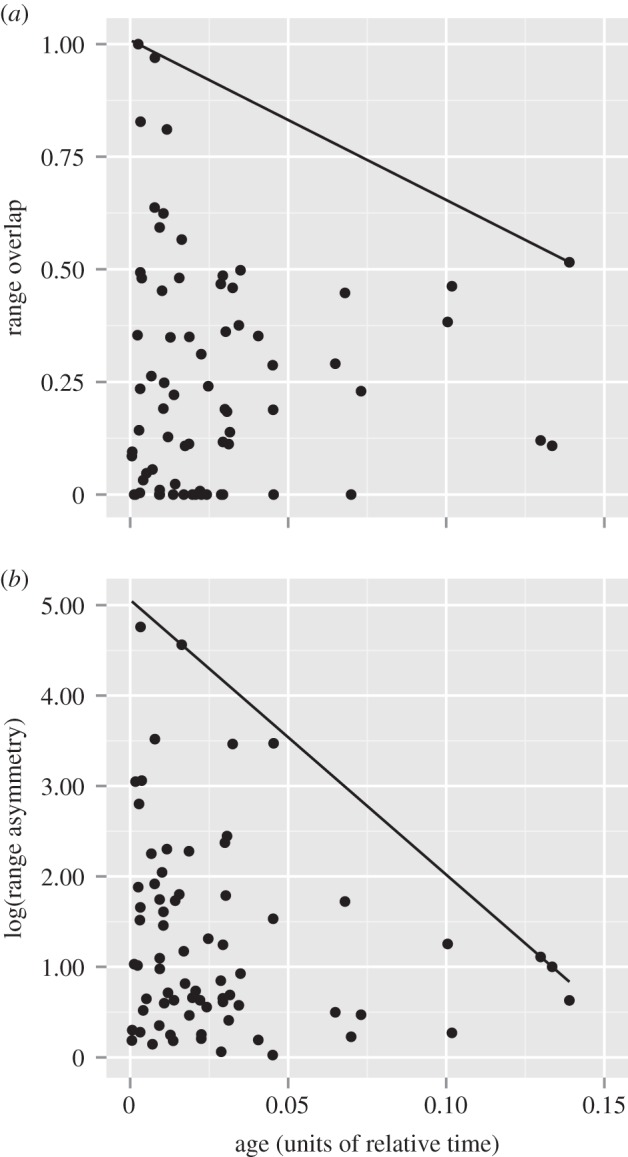
(a) Range overlap and (b) range asymmetry as a function of age. Lines indicate quantile regression model fits.
Together, greater range overlap with greater range asymmetry in younger taxa supports budding speciation as an important process generating diversity in the CFP. Note that there are also young sisters with low range overlap and low range asymmetry; for these cases, we might infer other, more commonly invoked speciation processes, like vicariant speciation.
(b). How ecologically and reproductively similar are sisters?
We found pervasive evidence for niche conservatism among sisters, based on comparison with randomly selected non-sister congeners for all nine attributes: habitat, soil, growth form, altitude, plant height, climate niche, flower size, flowering time and chromosome count (table 1). Although all these attributes showed greater similarity between sister–sister pairs than between sister–non-sister congener pairs, some attributes were more likely to differ between sisters than others (see the electronic supplementary material, figure S1). For example, shifts in growth form were observed in just one case, while partial and full shifts in soil type were common. Considering all six discrete traits (habitat, soil, growth form, altitude, flowering time and chromosome count), 93% of our sisters exhibited partial or complete shifts for at least one attribute, and 65% had a complete shift for at least one attribute. For the subset of discrete traits that could lead to micro-allopatry in range-wide sympatry (habitat, soil, growth form and altitude), 58% had a complete shift for at least one attribute, suggesting that ecological segregation may be important in plant speciation or in allowing ecological coexistence of these species post-reproductive isolation.
We found no evidence for character displacement in flower size or any other trait with increasing range overlap between sisters. Shifts in flower size were large in many cases, however. The maximum flower size contrast size was 2.4, corresponding to a 11-fold difference in flower size among a sister pair in Leptosiphon, a clade well known for reductions in flower size with transitions to selfing [61].
4. Discussion
The observed level of sympatry among sister taxa was remarkably high (80%) and higher than expected based on range overlap of sister–non-sister congener comparisons. Based on the combined result of high range overlap plus high range asymmetry in young sisters of the CFP, we infer budding speciation, where new species form within or peripheral to the range of the retained ancestral species, as an important process generating new species, a result recently supported in analysis of the genus Mimulus [62]. Our results are in striking contrast to the classic model of allopatric speciation by subdivision [22] as well as to results for non-plant clades, where levels of sympatry are uniformly lower (33–45% sympatry in mammalian clades, 50% sympatry in Drosophila, 35% in flycatchers and less than 30% in a group of mostly verterbrate animals), and where age-range analyses find support for primarily allopatric speciation ([5,14,63,64], but see [65]).
The geological complexity of California, the sessile nature of plants and plant tendency towards selfing and polyploidization may all combine to make budding speciation more common in plants than animals. The observation that high range overlap and asymmetry is unique to relatively young species suggests that extinction, hybridization and reticulate evolution, competitive exclusion or range shifts curtail overlap of sister species with time. We note that other speciation mechanisms are also probably important in the CFP, given the high variance in range overlap in young sisters that we observed. For example, there were many young taxa with low range overlap and low range asymmetry, from which we might infer vicariant speciation events. Moreover, it is possible that sister species may have originated in allopatry and later achieved sympatry through secondary contact.
The greater ecological and reproductive similarity of sisters to each other than to non-sister congeners in all traits reflects their recent shared ancestry and phylogenetic niche conservatism, as found in a number of other groups [13,66,67]. This result, coupled with geographically overlapping ranges, suggests that sister species co-occur more often with each other than with their non-sister congeners at regional scales. Their local co-occurrence at small spatial scales, however, might be reduced if sisters hybridize, compete strongly for shared resources or segregate into different microsites (i.e. micro-allopatry in range-wide sympatry) [68–70].
While ecological and reproductive similarity overall was higher for sister–sister pairs than sister–non-sister congener pairs, some attributes were more conserved than others. Of the shifts investigated, growth form was almost completely conserved; sisters typically shared being annual herbs, perennial herbs or shrubs. Shifts in altitude were also rare (full shifts observed for just two sister pairs), suggesting a surprisingly limited role of altitudinal zonation in promoting speciation, unlike findings for tropical vertebrates [71]. A more detailed analysis also failed to show altitudinal shifts, even after accounting for latitude (see the electronic supplemental material); thus, despite the fact that we can often find ecotypes locally adapted to elevation [72], this divergence is not sufficient to be consistently associated with speciation in our study.
While growth form, altitude and flowering time were highly conserved between sisters, soil type was the most labile of the discrete traits (29 full shifts and 20 partial shifts), suggesting that soil heterogeneity in the CFP may play an important role in speciation and ecological segregation [73]. For example, sisters Layia glandulosa and Layia discoidea have a range overlap of 0.65; L. discoidea exists on a single serpentine outcrop in central California, separated by approximately 100 m from populations of its more widely distributed serpentine-intolerant ancestor L. glandulosa [41]. In this case, fine-scale soil heterogeneity may result in strong selection against cross-habitat migrants or hybrids, promoting reproductive isolation [48]. It is important to bear in mind, however, that 22 of the sister species had no shifts in soil type, and shifts for sister–sister pairs were lower than shifts for sister–non-sister congener pairs, so this mechanism is only one of many possible sources of ecological divergence among sister species. Across the discrete traits (habitat, soil, growth form, altitude, flowering time and chromosome count), 93% of sister pairs had at least partial shifts in at least one attribute (65% had complete shifts); including just the ecological traits (i.e. excluding flowering time and chromosome count), 57% had complete shifts, suggesting ecological segregation is common (see the electronic supplementary material, figure S1).
The magnitude of the ecological shifts we found is similar to that reported for the Cape floristic region of South Africa, another biodiversity hotspot with Mediterranean climate, in which 87% of plant sister species show partial or complete ecological shifts (57% have complete shifts; percentages calculated from the electronic supplementary material of van der Niet and Johnson using five of the six discrete traits reported here; note, we were unable to compare chromosome changes between the two studies as this metric was not measured by van der Niet & Johnson [74]). However, an important difference between the CFP and the Cape is that complete shifts in soil affinity were evident in only 17% of Cape pairs versus in 41% of CFP pairs. This difference may reflect the fact that the Cape region has lower levels of topographic and edaphic diversity than the CFP [75,76]. Alternatively, we may have divided our soil affinities more finely than van der Niet and Johnson because there is more soil-specific information for the CFP. We were conservative in our designations by assigning ‘full shift’ only to those pairs that showed clear differences in soil affinity. Regardless, our results point strongly to the importance of soil heterogeneity in generating plant diversity in the CFP hotspot. Analyses of other floras will indicate the degree to which CFP is unique in this regard.
We found no evidence of character displacement in sympatric sisters. Our results contrast with those of a previous study [32], which showed reproductive character displacement in sympatric species of Mimulus, one of the genera included in our study. In Mimulus, sympatric sisters typically include one small-flowered selfing species and one large-flowered outcrossing species. Reductions in flower size reduce the separation between stigmas and anthers, thereby increasing the probability of self-pollination. Across all groups in the CFP we considered, we find an opposing pattern: floral size divergence among sisters is lower than expected when compared with non-sister congeners. However, there are clear examples of mating system shifts in our sample (e.g. Leptosiphon bicolor and Leptosiphon jepsonii). Isolation of sister species may also be caused by post-zygotic mechanisms. For example, the putative progenitor taxon Mimulus guttatus will form hybrids when it receives pollen from putative derivate taxon Mimulus nasutus, but the resulting plants have low rates of germination and survival [77]. Additional post-zygotic mechanisms in Mimulus include male and female sterility [78,79].
The absence of evidence of character displacement in our study is not clear evidence of absence, for several reasons. First, character displacement may operate on multivariate combinations of attributes, in which case there may be no relationship between any single measure and range overlap; similarly, different traits may separate different sisters, a condition that would prevent an overall pattern of trait divergence and range overlap with respect to any single trait. The fact that sisters are diverged in at least one trait in 65% (full shift) or 93% (partial shift) of pairs provides many opportunities for trait divergence and coexistence. Second, character divergence may have evolved in regions of overlap in the past, but subsequent range shifts may result in species that currently show low range overlap but high trait divergence, thereby removing any signature of trait divergence with high overlap. Third, our trait divergence estimates may average over important intraspecific variation; detailed study of populations in close proximity may still find character displacement, despite a lack of difference in species-wide mean values. For example, Geospiza fortis finches exhibit markedly different beak sizes when they co-occur with their congeneric competitor [80] than when they occur alone. Fourth, we may simply have missed an important attribute that reflects the niche in our study.
Co-occurrence across large areas for some sisters can occur without obvious ecological differences. For example, we found little evidence that the species pair of C. lingulata and Clarkia biloba differ in the ecological traits we measured, yet these species have a very high degree of range overlap (0.97) and range asymmetry (34x). This pair of sisters is a well-known example of catastrophic speciation, where C. lingulata formed at the southern edge of C. biloba's range and became reproductively isolated following chromosomal rearrangement [23,24,81–84]. In this mode of speciation, environmental stress at the extreme range boundaries is thought to create population bottlenecks, leading to the formation of a budded, derivative species [22,23,25]. Ecologically very similar species might then coexist through neutral or nearly neutral dynamics that allow long-term coexistence [85,86]. In total, we found chromosomal changes in 10 sister pairs, but these shifts were unrelated to range attributes, and sister–sister chromosome shifts were less common than sister–non-sister shifts. For five of these pairs, including the Clarkia pair, chromosome count was the only trait that had a full shift; the remaining five had a full shift in at least two traits. Thus, while we have identified an overall high level of sympatry and asymmetry in sister taxa, identifying the particular mode of speciation that any ‘budded’ pair of sister species in the CFP has followed (e.g. catastrophic versus polyploid hybrid speciation versus peripheral isolates versus ecological speciation) requires detailed case study [19].
Aside from the implications for speciation, sister species range overlap bears directly on the ecological assembly of communities [68,87,88]. Community ecologists measure traits and assemblages of species in species pools, which represent potential colonists to local communities [89], to understand trait evolution and its role in community assembly [68,90,91]. While our study shows a remarkable level of sympatry among sisters, their ranges are highly asymmetric, meaning that most regions of the CFP will not contain both members of a given sister species pair. Moreover, habitat and soil shifts are common, meaning that many habitat- or soil-specific studies will not contain both members of a given sister species pair. If species pools frequently omit sisters, and perhaps even congeners or other very close relatives [42,68,87], then studies showing a lack of relationship between ecological similarity and relatedness in communities [90] may reflect two contrasting scenarios: (i) high levels of trait divergence among close relatives, or (ii) the lack of coexisting ecologically similar ‘closest relatives’ in local communities. The spatial scale (or habitat types) for which regional species pools are defined will affect these analyses. Thus, both historical and current ecological processes may influence the strength of phylogenetic signal measured in extant ecological communities.
In conclusion, our results provide support for budding speciation, a term that collectively includes speciation modes in which a larger ranged progenitor species buds off smaller ranged derivatives, as an important source of biodiversity in the CFP. The high incidence of soil shifts, the diversity of soils found in the CFP and the sessile nature of plants may all contribute to the prevalence of budding speciation in the CFP. These results for plants are in stark contrast to those found in animal studies within the CFP [51] and elsewhere, most of which support allopatric speciation modes; the commonness of budding speciation in plants may reflect their lower mobility and the importance and prevalence of genetic isolation mechanisms in plants. We show that sister species in the CFP have high degrees of sympatry and sister–sister pairs are more ecologically similar than sister–non-sister congener pairs. Despite these attributes that might lead sisters to co-occur locally, the highly asymmetric ranges and ecological shifts of sisters may prevent their widespread co-occurrence in local communities.
Acknowledgements
We especially thank Dena Grossenbacher and Luke Mahler, as well as Strauss, Stanton and Schmitt laboratories at UC Davis for extensive discussion, Katherine Wood for data collection, and Howard Cornell and Susan Harrison for manuscript comments. Thanks to N.I. Cacho and K. Sytsma for sharing phylogenetic hypotheses for Streptanthoid complex and Clarkia, respectively.
Funding statement
Support was provided by NSF DEB 09-000097 and DEB 11-20387 to S.Y.S.
References
- 1.Jordan DS. 1905. The origin of species through isolation. Science 22, 545–562 (doi:10.1126/science.22.566.545) [DOI] [PubMed] [Google Scholar]
- 2.Jordan DS. 1908. The law of geminate species. Am. Nat. 42, 73–80 (doi:10.1086/278905) [Google Scholar]
- 3.Mayr E. 1959. Isolation as an evolutionary factor. Proc. Am. Philos. Soc. 103, 221–230 [Google Scholar]
- 4.Anderson S, Evensen MK. 1978. Randomness in allopatric speciation. Syst. Biol. 27, 421–430 (doi:10.1093/sysbio/27.4.421) [Google Scholar]
- 5.Fitzpatrick BM, Turelli M. 2006. The geography of mammalian speciation: mixed signals from phylogenies and range maps. Evolution 60, 601–615 [PubMed] [Google Scholar]
- 6.Futuyma DJ. 1986. Evolutionary biology. Sunderland, MA: Sinauer Associates [Google Scholar]
- 7.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates [Google Scholar]
- 8.Berlocher SH, Feder JL. 2002. Sympatric speciation in phytophagous insects: moving beyond controversy? Annu. Rev. Entomol. 47, 773–815 (doi:10.1146/annurev.ento.47.091201.145312) [DOI] [PubMed] [Google Scholar]
- 9.Rundle HD, Nosil P. 2005. Ecological speciation. Ecol. Lett. 8, 336–352 (doi:10.1111/j.1461-0248.2004.00715.x) [Google Scholar]
- 10.Nosil P. 2008. Speciation with gene flow could be common. Mol. Ecol. 17, 2103–2106 (doi:10.1111/j.1365-294X.2008.03715.x) [DOI] [PubMed] [Google Scholar]
- 11.Darwin C. 1859. The origin of species. London, UK: John Murray [Google Scholar]
- 12.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (doi:10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 13.Burns JH, Strauss SY. 2011. More closely related species are more ecologically similar in an experimental test. Proc. Natl Acad. Sci. USA 108, 5302–5307 (doi:10.1073/pnas.1013003108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barraclough TG, Vogler AP. 2000. Detecting the geographical pattern of speciation from species-level phylogenies. Am. Nat. 155, 419–434 (doi:10.1086/303332) [DOI] [PubMed] [Google Scholar]
- 15.Losos JB, Glor RE. 2003. Phylogenetic comparative methods and the geography of speciation. Trends Ecol. Evol. 18, 220–227 (doi:10.1016/S0169-5347(03)00037-5) [Google Scholar]
- 16.Nattier R, Grandcolas P, Elias M, Desutter-Grandcolas L, Jourdan H, Couloux A, Robillard T. 2012. Secondary sympatry caused by range expansion informs on the dynamics of microendemism in a biodiversity hotspot. PLoS ONE 7, e48047 (doi:10.1371/journal.pone.0048047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dayan T, Simberloff D. 2005. Ecological and community-wide character displacement: the next generation. Ecol. Lett. 8, 875–894 (doi:10.1111/j.1461-0248.2005.00791.x) [Google Scholar]
- 18.Davies TJ, Meiri S, Barraclough TG, Gittleman JL. 2007. Species co-existence and character divergence across carnivores. Ecol. Lett. 10, 146–152 (doi:10.1111/j.1461-0248.2006.01005.x) [DOI] [PubMed] [Google Scholar]
- 19.Crawford DJ. 2010. Progenitor-derivative species pairs and plant speciation. Taxon 59, 1413–1423 [Google Scholar]
- 20.Gottlieb LD. 2004. Rethinking classic examples of recent speciation in plants. New Phytol. 161, 71–82 (doi:10.1046/j.1469-8137.2003.00922.x) [Google Scholar]
- 21.Mayr E. 1954. Change of genetic environment and evolution. In Evolution as a process (eds Huxley J, Hardy A, Ford E.), pp. 157–180 London, UK: Allen and Unwin [Google Scholar]
- 22.Grant V. 1981. Plant speciation. New York, NY: Columbia University Press [Google Scholar]
- 23.Lewis H, Roberts MR. 1956. The origin of Clarkia lingulata. Evolution 10, 126–138 (doi:10.2307/2405888) [Google Scholar]
- 24.Lewis H. 1962. Catastrophic selection as a factor in speciation. Evolution 16, 257–271 (doi:10.2307/2406275) [Google Scholar]
- 25.Raven PH, Axelrod DI. 1978. Origin and relationships of the California flora. Berkeley, CA: University of California Press [Google Scholar]
- 26.Brown JWL. 1957. Centrifugal speciation. Q. Rev. Biol. 32, 247–277 (doi:10.1086/401875) [Google Scholar]
- 27.Lande R. 1982. Rapid origin of sexual isolation and character divergence in a cline. Evolution 36, 213–223 (doi:10.2307/2408039) [DOI] [PubMed] [Google Scholar]
- 28.Kawecki TJ. 1997. Sympatric speciation via habitat specialization driven by deleterious mutations. Evolution 51, 1751–1763 (doi:10.2307/2410998) [DOI] [PubMed] [Google Scholar]
- 29.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 30.Fitzpatrick BM, Fordyce JA, Gavrilets S. 2008. What, if anything, is sympatric speciation? J. Evol. Biol. 21, 1452–1459 (doi:10.1111/j.1420-9101.2008.01611.x) [DOI] [PubMed] [Google Scholar]
- 31.Dobzhansky T. 1940. Speciation as a stage in evolutionary divergence. Am. Nat. 74, 312–321 (doi:10.1086/280899) [Google Scholar]
- 32.Grossenbacher DL, Whittall JB. 2011. Increased floral divergence in sympatric monkeyflowers. Evolution 65, 2712–2718 (doi:10.1111/j.1558-5646.2011.01306.x) [DOI] [PubMed] [Google Scholar]
- 33.Baker HG. 1959. Reproductive methods as factors in speciation in flowering plants. Cold Spring Harb. Symp. Quant. Biol. 24, 177–191 (doi:10.1101/SQB.1959.024.01.019) [DOI] [PubMed] [Google Scholar]
- 34.Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 29, 467–501 (doi:10.1146/annurev.ecolsys.29.1.467) [Google Scholar]
- 35.Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. 2009. The frequency of polyploid speciation in vascular plants. Proc. Natl Acad. Sci. USA 106, 13 875–13 879 (doi:10.1073/pnas.0812917106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieseberg LH, Brouillet L. 1994. Are many plant species paraphyletic? Taxon 43, 21–32 (doi:10.2307/1223457) [Google Scholar]
- 37.Antonovics J. 1968. Evolution in closely adjacent plant populations. V. Evolution of self-fertility. Heredity 23, 219–238 (doi:10.1038/hdy.1968.30) [DOI] [PubMed] [Google Scholar]
- 38.Otto SP, Whitton J. 2000. Polyploid incidence and evolution. Annu. Rev. Genet. 34, 401–437 (doi:10.1146/annurev.genet.34.1.401) [DOI] [PubMed] [Google Scholar]
- 39.Abbott RJ, Lowe AJ. 2004. Origins, establishment and evolution of new polyploid species: Senecio cambrensis and S. eboracensis in the British Isles. Biol. J. Linn. Soc. 82, 467–474 (doi:10.1111/j.1095-8312.2004.00333.x) [Google Scholar]
- 40.Soltis DE, Soltis PS, Tate JA. 2004. Advances in the study of polyploidy since plant speciation. New Phytol. 161, 173–191 (doi:10.1046/j.1469-8137.2003.00948.x) [Google Scholar]
- 41.Baldwin BG. 2005. Origin of the serpentine-endemic herb Layia discoidea from the widespread L. glandulosa (Compositae). Evolution 59, 2473–2479 [PubMed] [Google Scholar]
- 42.Runquist RB, Stanton ML. 2013. Asymmetric and frequency-dependent pollinator-mediated interactions may influence competitive displacement in two vernal pool plants. Ecol. Lett. 16, 183–190 (doi:10.1111/ele.12026) [DOI] [PubMed] [Google Scholar]
- 43.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 44.California Native Plant Society 2001. Inventory of rare and endangered plants of California, 6th edn Sacramento, CA: CNPS [Google Scholar]
- 45.Thorne J, Viers J, Price J, Stoms D. 2009. Spatial patterns of endemic plants in California. Nat. Areas J. 29, 344–366 (doi:10.3375/043.029.0402) [Google Scholar]
- 46.Kruckeberg AR. 1991. An essay: geoedaphics and island biogeography for vascular plants. Aliso 13, 225–238 [Google Scholar]
- 47.Anacker BL, Whittall JB, Goldberb EE, Harrison SP. 2011. Origins and consequences of serpentine endemism in the California flora. Evolution 63, 365–376 (doi:10.1111/j.1558-5646.2010.01114.x) [DOI] [PubMed] [Google Scholar]
- 48.Kay KM, Ward KL, Watt LR, Schemske DW. 2011. Plant speciation. In Serpentine: the evolution and ecology of a model system (eds Harrison SP, Rajakaruna N.), pp. 71–96 Berkeley, CA: University of California Press [Google Scholar]
- 49.Lancaster LT, Kay KM. 2013. Origin and diversification of the California flora: re-examining classic hypotheses with molecular phylogenies. Evolution 67, 1041–1054 (doi:10.1111/evo.12016) [DOI] [PubMed] [Google Scholar]
- 50.Stebbins GL, Major J. 1965. Endemism and speciation in the California flora. Ecol. Monogr. 35, 1–35 (doi:10.2307/1942216) [Google Scholar]
- 51.Harrison SP. 2013. Plant and animal endemism in the California Floristic Province. Berkeley, CA: University of California Press [Google Scholar]
- 52.Nakazato T, Warren DL, Moyle LC. 2010. Ecological and geographic modes of species divergence in wild tomatoes. Am. J. Bot. 97, 680–693 (doi:10.3732/ajb.0900216) [DOI] [PubMed] [Google Scholar]
- 53.Webb CO, Donoghue MJ. 2005. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181–183 (doi:10.1111/j.1471-8286.2004.00829.x) [Google Scholar]
- 54.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57, 758–771 (doi:10.1080/10635150802429642) [DOI] [PubMed] [Google Scholar]
- 55.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (doi:10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paradis E. 2013. Molecular dating of phylogenies by likelihood methods: a comparison of models and a new information criterion. Mol. Phylogenet. Evol. 67, 436–444 (doi:10.1016/j.ympev.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 57.Blomberg SP, Garland T, Jr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 [DOI] [PubMed] [Google Scholar]
- 58.Baldwin BC, Goldman D, Keil D, Patterson R, Rosatti T, Wilken D. 2012. The Jepson manual: vascular plants of California, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- 59.Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883 (doi:10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 60.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 [Google Scholar]
- 61.Goodwillie C. 1999. Multiple origins of self-compatibility in Linanthus section Leptosiphon (Polemoniaceae): phylogenetic evidence from internal-transcribed-spacer sequence data. Evolution 53, 1387–1395 (doi:10.2307/2640885) [DOI] [PubMed] [Google Scholar]
- 62.Grossenbacher DL, Veloz SD, Sexton JP. In press Niche and range size patterns suggest that speciation begins in small, ecologically diverged populations in North American monkeyflowers (Mimulus spp.). Evolution. [DOI] [PubMed] [Google Scholar]
- 63.Shaw KL. 2002. Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: what mtDNA reveals and conceals about modes of speciation in Hawaiian crickets. Proc. Natl Acad. Sci. USA 99, 16 122–16 127 (doi:10.1073/pnas.242585899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson NK, Cicero C. 2002. The role of ecologic diversification in sibling speciation of Empidonax flycatchers (Tyrannidae): multigene evidence from mtDNA. Mol. Ecol. 11, 2065–2081 (doi:10.1046/j.1365-294X.2002.01588.x) [DOI] [PubMed] [Google Scholar]
- 65.Turelli M, Lipkowitz JR, Brandvain Y. In press On the Coyne and Orr-igin of species: effects of intrinsic postzygotic isolation, ecological differentiation, X chromosome size, and sympatry on Drosophila speciation. Evolution (doi:10.1111/evo.12330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ackerly DD. 2003. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int. J. Plant Sci. 164, 165–184 (doi:10.1086/368401) [Google Scholar]
- 67.Peterson AT. 2011. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr. 38, 817–827 (doi:10.1111/j.1365-2699.2010.02456.x) [Google Scholar]
- 68.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505 (doi:10.1146/annurev.ecolsys.33.010802.150448) [Google Scholar]
- 69.Sargent RD, Ackerly DD. 2008. Plant–pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23, 123–130 (doi:10.1016/j.tree.2007.11.003) [DOI] [PubMed] [Google Scholar]
- 70.Yost JM, Barry T, Kay KM, Rajakaruna N. 2012. Edaphic adaptation maintains the coexistence of two cryptic species on serpentine soils. Am. J. Bot. 99, 890–897 (doi:10.3732/ajb.1100521) [DOI] [PubMed] [Google Scholar]
- 71.Cadena CD, et al. 2012. Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. R. Soc. B 279, 194–201 (doi:10.1098/rspb.2011.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clausen J, Keck D, Hiesey WM. 1948. Experimental studies on the nature of species. III. Environmental responses of climatic races of Achillea. Washington, DC: Carnegie Institution of Washington [Google Scholar]
- 73.Smith HM. 1965. More evolutionary terms. Syst. Biol. 14, 57–58 [Google Scholar]
- 74.van der Niet T, Johnson SD. 2009. Patterns of plant speciation in the Cape floristic region. Mol. Phylogenet. Evol. 51, 85–93 (doi:10.1016/j.ympev.2008.11.027) [DOI] [PubMed] [Google Scholar]
- 75.Cowling RM, Rundel PW, Lamont BB, Kalin Arroyo M, Arianoutsou M. 1996. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 11, 362–366 (doi:10.1016/0169-5347(96)10044-6) [DOI] [PubMed] [Google Scholar]
- 76.Goldblatt P, Manning JC. 2002. Plant diversity of the Cape region of southern Africa. Ann. Mo. Bot. Gard. 89, 281–302 (doi:10.2307/3298566) [Google Scholar]
- 77.Martin NH, Willis JH. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61, 68–82 (doi:10.1111/j.1558-5646.2007.00006.x) [DOI] [PubMed] [Google Scholar]
- 78.Fishman L, Willis JH. 2001. Evidence for Dobzhansky–Muller incompatiblities contributing to the sterility of hybrids between Mimulus guttatus and M. nasutus. Evolution 55, 1932–1942 [DOI] [PubMed] [Google Scholar]
- 79.Fishman L, Willis JH. 2006. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution 60, 1372–1381 [DOI] [PubMed] [Google Scholar]
- 80.Grant PR, Grant BR. 2007. How and why species multiply: the radiation of Darwin‘s finches. Princeton, NJ: Princeton University Press [Google Scholar]
- 81.Lewis H. 1961. Experimental sympatric populations of Clarkia. Am. Nat. 95, 155–168 (doi:10.1086/282173) [Google Scholar]
- 82.Gottlieb LD. 1974. Genetic confirmation of the origin of Clarkia lingulata. Evolution 28, 244–250 (doi:10.2307/2407325) [DOI] [PubMed] [Google Scholar]
- 83.Sytsma KJ, Gottlieb LD. 1986. Chloroplast DNA evolution and phylogenetic relationships in Clarkia sect. Peripetasma (Onagraceae). Evolution 40, 1248–1261 (doi:10.2307/2408951) [DOI] [PubMed] [Google Scholar]
- 84.Ford VS, Gottlieb LD. 2003. Reassessment of phylogenetic relationships in Clarkia sect. Sympherica. Am. J. Bot. 90, 284–292 (doi:10.3732/ajb.90.2.284) [DOI] [PubMed] [Google Scholar]
- 85.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (doi:10.1146/annurev.ecolsys.31.1.343) [Google Scholar]
- 86.Adler PB, HilleRisLambers J, Levine JM. 2007. A niche for neutrality. Ecol. Lett. 10, 95–104 (doi:10.1111/j.1461-0248.2006.00996.x) [DOI] [PubMed] [Google Scholar]
- 87.Ackerly DD. 2000. Taxon sampling, correlated evolution, and independent contrasts. Evolution 54, 1480–1492 [DOI] [PubMed] [Google Scholar]
- 88.Swenson NG, Enquist BJ, Pither J, Thompson J, Zimmerman JK. 2006. The problem and promise of scale dependency in community phylogenetics. Ecology 87, 2418–2424 (doi:10.1890/0012-9658(2006)87[2418:TPAPOS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 89.Ricklefs RE. 1987. Community diversity: relative roles of local and regional processes. Science 235, 167–171 (doi:10.1126/science.235.4785.167) [DOI] [PubMed] [Google Scholar]
- 90.Cahill JF, Kembel SW, Lamb EG, Keddy PA. 2008. Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspect. Plant Ecol. Evol. Syst. 10, 41–50 (doi:10.1016/j.ppees.2007.10.001) [Google Scholar]
- 91.Donoghue MJ. 2008. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl Acad. Sci. USA 105, 11 549–11 555 (doi:10.1073/pnas.0801962105) [DOI] [PMC free article] [PubMed] [Google Scholar]


