Abstract
Arboreal herbivory is rare among mammals. The few species with this lifestyle possess unique adaptions to overcome size-related constraints on nutritional energetics. Sloths are folivores that spend most of their time resting or eating in the forest canopy. A three-toed sloth will, however, descend its tree weekly to defecate, which is risky, energetically costly and, until now, inexplicable. We hypothesized that this behaviour sustains an ecosystem in the fur of sloths, which confers cryptic nutritional benefits to sloths. We found that the more specialized three-toed sloths harboured more phoretic moths, greater concentrations of inorganic nitrogen and higher algal biomass than the generalist two-toed sloths. Moth density was positively related to inorganic nitrogen concentration and algal biomass in the fur. We discovered that sloths consumed algae from their fur, which was highly digestible and lipid-rich. By descending a tree to defecate, sloths transport moths to their oviposition sites in sloth dung, which facilitates moth colonization of sloth fur. Moths are portals for nutrients, increasing nitrogen levels in sloth fur, which fuels algal growth. Sloths consume these algae-gardens, presumably to augment their limited diet. These linked mutualisms between moths, sloths and algae appear to aid the sloth in overcoming a highly constrained lifestyle.
Keywords: commensalism, Costa Rica, Cryptoses, Trichophilus
1. Introduction
While herbivory is the predominant foraging strategy among mammals, arboreal herbivores are exceedingly rare. Indeed, less than 4% of all mammalian genera contain species that are, to some extent, arboreal and herbivorous, and only 10 species of mammals (or less than 0.2% of mammalian diversity) are considered specialized arboreal herbivores [1]. Species that forage on plant matter in trees possess a highly constrained lifestyle. On one hand, they must be small and light to be supported in the canopy; on the other hand, small body size limits digestive capacity, especially for processing plant matter, which is rich in fibre but low in digestible nutrients. So, although the evolution towards arboreal herbivory is found in taxonomically disparate mammalian groups, including primates, tree sloths and marsupials, all weigh between 1 and 14 kg [2]. Thus, the rarity of this lifestyle and convergence of body size among herbivorous and arboreal mammals appears to reflect constraints of nutritional energetics on body size [3]. To overcome such constraints, arboreal herbivores have evolved dramatic anatomical (e.g. ruminant-like pregastric digestive organs), physiological (e.g. depressed metabolic rates) and behavioural (e.g. strict dietary preferences) adaptations.
Sloths, or los perezosos (‘the lazies’) in Spanish, are slow-moving Neotropical mammals. The two phylogenetic groups, two- (Choloepus spp.) and three-toed (Bradypus spp.) sloths (figure 1a,b), diverged around 40 Ma [4] and are ecologically quite different. Although both are mid-sized foregut fermenting arboreal mammals [2], two-toed sloths possess relatively large home-ranges (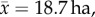 but up to 140 ha) [5] and a comparatively diverse diet of animal matter, fruits and leaves, whereas three-toed sloths have highly restricted home-ranges (
but up to 140 ha) [5] and a comparatively diverse diet of animal matter, fruits and leaves, whereas three-toed sloths have highly restricted home-ranges (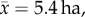 range = 0.3–15.0 ha) [6] and are regarded as strict folivores [7]. Furthermore, individual three-toed sloths are specialists, roosting and consuming leaves from only a few tree species within the forest [6,7]. Because of their nutritionally poor and toxic diet, three-toed sloths possess the slowest rate of digestion for any mammal [8,9]. To account for this low-energy accrual, three-toed sloths possess an exceedingly low metabolic rate, less than half of that expected for their mass [3,10].
range = 0.3–15.0 ha) [6] and are regarded as strict folivores [7]. Furthermore, individual three-toed sloths are specialists, roosting and consuming leaves from only a few tree species within the forest [6,7]. Because of their nutritionally poor and toxic diet, three-toed sloths possess the slowest rate of digestion for any mammal [8,9]. To account for this low-energy accrual, three-toed sloths possess an exceedingly low metabolic rate, less than half of that expected for their mass [3,10].
Figure 1.
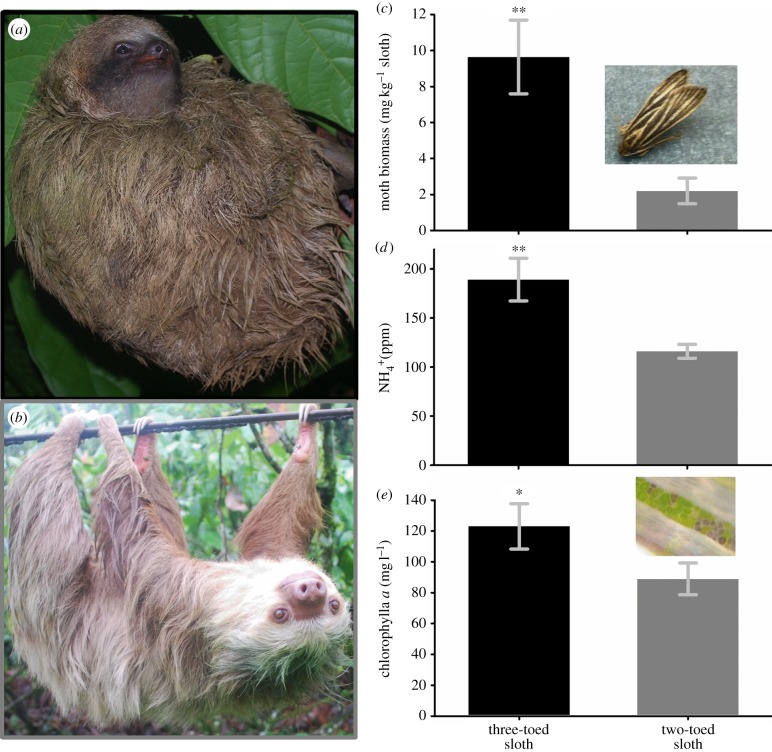
Both (a) three- and (b) two-toed sloths harbour a diverse ecosystem in their fur. (c–e) The more sedentary three-toed sloths (black bars) possessed (c) a greater number of moths, (d) more inorganic nitrogen in the form of 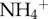 and (e) greater algal biomass on their fur compared with two-toed sloths (grey bars). Error bars represent ±1 s.e.; *p < 0.05, **p < 0.001.
and (e) greater algal biomass on their fur compared with two-toed sloths (grey bars). Error bars represent ±1 s.e.; *p < 0.05, **p < 0.001.
About once a week, three-toed sloths descend from the canopy to the base of their modal tree, where they create a depression in the ground with their vestigial tail, and deposit their dung. After defecation, sloths cover their latrine with leaf litter and ascend to the canopy [7]. Two-toed sloths defecate from the canopy or on the ground, especially when switching trees (which they do frequently) [7], and their routine, in terms of both frequency and site fidelity, is far less constrained [11]. Descending a tree is both risky and energetically costly for any sloth. Indeed, it is the leading cause of mortality for a sloth; more than one-half of all adult sloth mortalities we have documented were depredation events when sloths were at or near the ground [12]. Furthermore, we estimate that the average cost of descending from the canopy to defecate constitutes approximately 8% of a sloth's daily energetic budget (see the electronic supplementary material for details). Given the heightened risk and energetic cost for a sloth to defecate on the forest floor, one would expect it to be an important fitness-enhancing behaviour. Suggested benefits of this behaviour to three-toed sloths include fertilizing their preferred trees, communicating with other sloths via latrines or avoiding detection from predators [13]. Given the nutritional constraints imposed by the lifestyle of tree sloths, we hypothesized that this behaviour could be driven by a cryptic, yet important, nutritional input.
Both species of sloths harbour a diverse assemblage of symbiotic microorganisms in their fur, including species of algae, arthropods and detritivorous fungi, many of which only exist within the phoretic ecosystem residing in sloth fur. Green algae (Trichophilus spp.) are especially abundant [14]. Individual hairs of three-toed sloths possess unique transverse cracks, which allow the hair shaft to become saturated with rainwater, and which algae then colonize and grow hydroponically [15]. A commensal relationship has also been ascribed to sloths and pyralid moths (Cryptoses spp.) [16], in which moths require the association (+), but because they do not feed on sloths, impose no consequence (0) on their host [17]. When a sloth descends a tree and defecates, gravid female moths leave the sloth and oviposit in the fresh excrement. Larvae are copraphagous, developing entirely within the dung, and adults emerge and fly to the canopy to seek their mating grounds in sloth fur to continue their life cycle. Although three-toed sloths regularly autogroom [18], they are ineffective in removing sloth moths [19]. Because the life cycle of pyralid moths is entirely dependent on these otherwise inexplicable behaviours in three-toed sloths, we posited that the moth–sloth interaction might actually be an important mutualism, where sloths are also benefiting by virtue of their association (+/+).
Mutualisms—jointly beneficial interactions between members of different species—are ubiquitous in nature, and among the most important of all ecological interactions [20]. Ranging from diffuse and indirect to tightly coevolved direct interactions among multiple species [20,21], mutualisms have previously been invoked to account for otherwise unexplained behaviours, such as ‘cleaner fish’ removing ectoparasites from client reef fish [22], or ants defending acacia trees [23], and as a mechanism by which nutritionally limited organisms cultivate and maintain a food source, like those observed in fungicultural systems of leaf cutter ants [24]. Given the ostensibly unrewarded risks the sloth appears to be enduring on behalf of the moths, we hypothesized that the phoretic symbionts, previously believed to possess a commensal relationship with sloths, were in fact reinforcing this relationship by providing nutritional inputs to their hosts.
To explore the relationship of sloths with their phoretic symbionts, we captured adult two- and three-toed sloths and quantified the number of pyralid moths infesting each individual, as well as other important ecosystem components within sloth fur, including the concentration of inorganic nitrogen and phosphorus, and algal biomass on their fur. We also collected digesta from the forestomach of sloths to determine whether community members within the fur were being consumed. We predicted that the rigid behaviour observed in three-toed sloths promoted moth infestation, and moth density would be greater compared with two-toed sloths. We further predicted that, because moths are one of the only portals of exogenous organic material to this ecosystem, increasing moth density would promote nutrient availability and productivity within sloth fur, and potentially provide nutritional inputs to ease some of the constraints faced by this specialized arboreal herbivore.
2. Material and methods
(a). Describing the ecosystem on a sloth
We conducted fieldwork approximately 85 km northeast of San José, Costa Rica (10.32° N, −83.59° W). Both brown-throated three-toed sloths (Bradypus variegatus) and Hoffmann's two-toed sloths (Choloepus hoffmanni) are relatively abundant across our study site. Fieldwork was conducted as stipulated and authorized by IACUC protocol A01424 by the University of Wisconsin-Madison, and adhered to the guidelines for the use of mammals in research set forth by the American Society of Mammalogists. Access was granted by the private landowner, and our project and sample collection was approved by the Ministerio de Ambiente, Energia y Telecomunicaciones, Sistema Nacional de Áreas de Conservación, Costa Rica. All samples were imported to the United States with CITES and United States Fish and Wildlife Service approval. To document the moth and algal community, as well as quantify levels of inorganic nitrogen and phosphorus in sloth fur, we captured previously marked adult two- (n = 14) and three-toed (n = 19) sloths following standard procedures [5,6] in August 2012. Because young sloths are often devoid of algae and appear to acquire their algal community from their mother [14], we did not include juveniles in our analyses. We cut a lock of hair from the dorsum of each sloth and collected all moths from the sloth with an invertebrate vacuum. On average, we collected 15.2 (±2.9; range = 4–39) and 4.5 (±1.3; range = 0–21) moths from three- and two-toed sloths, respectively. To quantify the concentrations of inorganic nitrogen and phosphorus, we sampled (0.1 g) of sloth fur, and washed it with 15 ml of de-ionized water for 15 min. The wash was filtered through a 0.45 µm syringe membrane filter, and analysed for 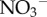 and
and 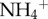 using a flow injection analyser (Quickchem 8000 FIA, Lachat Instruments) and total phosphorus using an ICP/OES (Iris Advantage, Thermo-Fisher). Moths were identified (Cryptoses choloepi Dyar; Pyralidae: Chrysauginae) at the Systematic Entomology Laboratories (USDA, Agricultural Research Service). We weighed each moth (±0.1 μg), and divided the total biomass of moth infestation by the mass of the sloth (±0.1 kg) to account for individual differences in body size; even without scaling, however, we detected a significant relationship between
using a flow injection analyser (Quickchem 8000 FIA, Lachat Instruments) and total phosphorus using an ICP/OES (Iris Advantage, Thermo-Fisher). Moths were identified (Cryptoses choloepi Dyar; Pyralidae: Chrysauginae) at the Systematic Entomology Laboratories (USDA, Agricultural Research Service). We weighed each moth (±0.1 μg), and divided the total biomass of moth infestation by the mass of the sloth (±0.1 kg) to account for individual differences in body size; even without scaling, however, we detected a significant relationship between 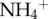 and both number of moths and total moth biomass (see electronic supplementary material, figure S1).
and both number of moths and total moth biomass (see electronic supplementary material, figure S1).
We measured the chlorophyll a concentration of the microbial biomass in the fur of each sloth via fluorometry. Briefly, 0.01 g of fur from each sloth was sonicated in ddH2O 1× and methanol 3× (for approx. 30 min each) to separate algal cells from the fur (see electronic supplementary material, figure S2); the filtrate from the wash was centrifuged and measured on a fluorometer to calculate the concentration of chlorophyll. Our estimates of algal biomass were corroborated by the change in mass of the fur after sonication (i.e. the decrease in fur mass after algal removal was related to chlorophyll a concentration). We used that change in mass of the fur following sonication to approximate the biomass of algae in each sample. We then scaled our estimate of algal biomass from the sample to approximate the total mass of algae on the entire sloth by assuming that 20% of the sloth's body mass was fur [9] and that the observed percentage change in fur samples from cleaning was constant across the animal's surface area. We compared differences between two- and three-toed sloths in scaled moth biomass, 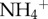 concentrations and algal biomass with t-tests, and explored the relationship between moth biomass and
concentrations and algal biomass with t-tests, and explored the relationship between moth biomass and 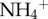 , and between
, and between 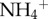 with species as a categorical variable. Because the interaction for species and the predictor variable were non-significant for both moth × species (t = 1.09, p = 0.28) and
with species as a categorical variable. Because the interaction for species and the predictor variable were non-significant for both moth × species (t = 1.09, p = 0.28) and 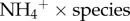 (t = 1.40, p = 0.17), we reported the simple linear regression model with the continuous variable (e.g. moth biomass or
(t = 1.40, p = 0.17), we reported the simple linear regression model with the continuous variable (e.g. moth biomass or 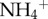 ).
).
(b). In vitro fermentation experiments
To quantify the digestibility of the algae in the fur to organic acids from pregastric microbial fermentation, in vitro fermentations were conducted using a ruminal inoculum, a readily available microbial community with the capacity to degrade a wide variety of plant components. The inoculum was composited from two Holstein dairy cows (Bos taurus) and prepared as described previously [25], except that the squeezed ruminal fluid was diluted to an OD525 of 5.0 using reduced buffer [26] that contained 1 g trypticase peptone per litre. Fermentations were conducted in 5 ml glass serum vials (Wheaton Scientific) that contained 150 mg of air-dried sloth fur from two- (n = 10) and three-toed sloths (n = 10). To assess the contribution of algae and organic matter to the fermentation, we included vials of fur from the same sloths, but which had been washed and sonicated in methanol to remove algae and other associated organic matter. Four blank vials with only ruminal inoculum and reduced buffer were also analysed. Vials first were gassed vigorously with CO2 for 2 min, and then sealed tightly with #00 butyl rubber stoppers. Diluted ruminal inoculum (2.00 ml) was added under CO2 gassing, and the vial was sealed with a flanged butyl rubber stopper and secured with an aluminium crimp seal; these inoculations were performed in a 39°C room after temperature equilibration of the diluted ruminal fluid. Except for vigorous hand shaking at approximately 0, 1, 2, 4 and 16 h, vials were incubated in an upright position without shaking. After 24 h of incubation, vials were uncapped and 1.00 ml of deionized water was added. The liquid contents were mixed several times with a micropipetter, and 1.00 ml of the liquid was removed for microcentrifugation (12 000×g, 10 min, 4°C). The supernatant was analysed for organic acids by HPLC [27]. Net production of individual and total volatile fatty acids (VFA; after subtraction of concentrations in blanks) was analysed by a mixed model in SAS v. 9.2 with sloth species and fur treatment (washed versus unwashed) as class variables, a species × treatment interaction, and with individual animal as random variable. The data (see electronic supplementary material, table S1) revealed that C2–C5 straight-chain VFA (acetic through valeric) derived primarily from carbohydrate fermentation were produced in higher amounts from unwashed fur than from washed fur, and in fur from three-toed sloths than from two-toed sloths. By contrast, the production of branched-chain VFA (isobutyric, 2-methylbutyric and isovaleric), which are uniquely derived from amino acid fermentations (specifically, the branched-chain amino acids leucine, isoleucine and valine), was low and did not differ between species or with treatment (p > 0.30), suggesting minimal capacity for pregastric fermentation of algal protein.
(c). Compositional analysis of algae and plants
We conducted compositional analyses for carbohydrates, proteins and lipids of algal samples extracted from the fur of two- (n = 10) and three-toed (n = 10) sloths, as well as leaf samples from the six most commonly consumed plant species as percentage of dry matter content. For carbohydrate and protein analysis, samples (1–7 mg, weighed to 0.001 mg) were suspended in 200–600 μl of 0.2 M NaOH, heated at 80°C for 40 min with frequent mixing by inversion, and cooled to room temperature. After neutralization with 0.38 volumes of 10% (v/v) glacial acetic acid, protein was assayed by the Bradford method [28] using Coomassie Plus reagent (BioRad) with lysozyme as standard; carbohydrates were analysed by the phenol-sulfuric acid method [29], using glucose as standard. For lipid extractions [30], air-dried (60°C) algae (approx. 50 mg) and leaves (100–200 mg) were suspended in 2 ml CHCl3, 2 ml methanol and 1 ml H2O in screw-cap tubes with Teflon liners. After vortexing for 2 min, the tubes were centrifuged (2500×g, 10 min, room temperature) and the chloroform phase recovered. The remaining material was extracted three additional times, each with 2 ml chloroform. The four chloroform extracts were pooled and treated with 3 ml of saturated NaCl in water. After the final centrifugation, the chloroform phase was recovered, and evaporated to approximately 0.5 ml volume under N2. The concentrated extracts were quantitatively transferred with CHCl3 washes to preweighed 1.5 ml microfuge tubes and the CHCl3 evaporated. The microfuge tubes were then air-dried overnight at 60°C prior to weighing. Blank tubes were used to correct for weight loss of empty microfuge tubes on drying.
(d). Identifying algae in the digesta of sloths
We collected digesta from the forestomach of two- (n = 16) and three-toed sloths (n = 12) via gastric gavage to determine whether algae had been consumed by sloths. We filtered a 2 ml aliquot of digesta through a 60 μm sieve to exclude large particles. We then prepared microscope slides using 30 μl of filtered digesta, and viewed each slide with a compound light microscope at 400× magnification. We counted 100 cells of algal or cyanobacterial material for each slide, and photographed each cell detected. Algae and cyanobacteria were identified to the highest taxonomic resolution possible, and representative algal and cyanobacterial groups were photographed (see electronic supplementary material, figure S3).
We compared the algal community detected in the digesta to that on the fur of two- (n = 5) and three-toed (n = 5) sloths. Fur from these individuals were placed in a microcentrifuge tube containing 1 ml of ddH2O and soaked for 1 h, agitated every 15 min for 5 min. Fur was removed from vials while the supernatant was used for microscope mounts. Microscope slides were prepared using 30 μl of supernatant, viewed using a compound light microscope at 400× magnification and identified as described above. Photographs of 100 algal and cyanobacterial cells from each slide were collected.
3. Results and discussion
As predicted, three-toed sloths harboured more moths (figure 1c), as well as greater concentrations of 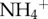 (figure 1d) and increased biomass of algae (figure 1e) in their fur than two-toed sloths. We found a similar trend, but did not detect a significant (p > 0.05) difference, in
(figure 1d) and increased biomass of algae (figure 1e) in their fur than two-toed sloths. We found a similar trend, but did not detect a significant (p > 0.05) difference, in 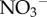 or total phosphorus (principally in the form of
or total phosphorus (principally in the form of 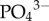 ) between the species (see electronic supplementary material, figure S4). However, as commonly observed in soils, these nutrients are likely to be rapidly acquired by photosynthetic organisms or leached during rain showers [31]. Regardless of sloth species,
) between the species (see electronic supplementary material, figure S4). However, as commonly observed in soils, these nutrients are likely to be rapidly acquired by photosynthetic organisms or leached during rain showers [31]. Regardless of sloth species, 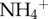 concentration was positively related to the number of pyralid moths in the fur (figure 2a), and the biomass of algae also increased with the concentration of
concentration was positively related to the number of pyralid moths in the fur (figure 2a), and the biomass of algae also increased with the concentration of 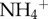 in the fur of sloths (figure 2b).
in the fur of sloths (figure 2b).
Figure 2.

Within the fur of sloths, an increasing number of moths led to greater concentration of nitrogen, which is related to the biomass of algae. Relationship between (a) pyralid moth infestation and 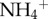 concentration, and (b) amount of
concentration, and (b) amount of  and algal biomass, in the fur of two- (grey) and three-toed (black) sloths.
and algal biomass, in the fur of two- (grey) and three-toed (black) sloths.
We estimate that the sloths on average harbour 125.5 g (±14.8 g, ±1 s.e.) of microbial biomass (principally algae) in their fur, which translates into approximately 2.6% (±0.2%) of their body mass. Our in vitro fermentation experiments revealed that algae in sloth fur are also highly digestible, that VFA production from algal digestion is primarily associated with carbohydrate fermentation, and that fur of three-toed sloths contains organic material sufficient to yield 24.4 mg of VFAs·(g fur−1) from pregastric fermentation (see electronic supplementary material, table S1), nearly twice the amount compared with two-toed sloths (p < 0.001). Compositional analysis of algae and leaves of plant species preferred by sloths revealed that both items were rich in carbohydrates (25.7% ± 1.4 for algae versus 42.4% ± 3.5 for plants; see electronic supplementary material, table S2), and possessed equivalent amounts of protein (5.0% ± 0.39). Compared with plant leaves, however, microalgae were three to five times richer in lipid content—algae from two- and three-toed sloths were 45.2% (±4.0) and 27.4% (±0.8) lipid, respectively (see electronic supplementary material, table S2). Lipid content of microalgae is inversely related to inorganic nitrogen levels [32], which could explain the difference in lipid content of algae between sloth species. Regardless, a food item with this high lipid composition would provide an especially rich (over twice that compared with protein or carbohydrate per gram) and rapid source of energy to sloths, as lipids would typically bypass the pregastric fermentation process.
Unsurprisingly, the same species of alga occurred in the fur and digesta of both two- and three-toed sloths. Specifically, we identified Trichophilus spp. in the digesta of two of the three-toed sloths (or 17%) and six of the two-toed sloths sampled (or 38%)—this symbiotic alga is only known to inhabit the fur of sloths (see electronic supplementary material, figure S3) [14]. The fact that the algae are readily digestible yet were detected in our limited sample size suggests that the frequency of ingested algae is likely to be high.
4. Conclusion
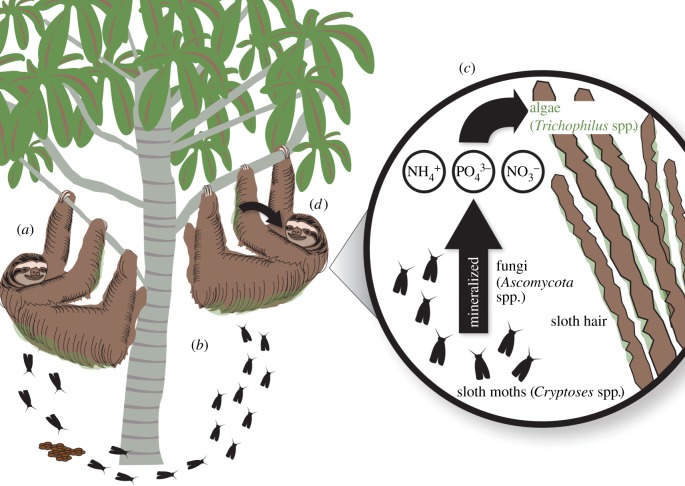
Our data suggest that a series of linked mutualisms occurs between sloths, moths and algae (figure 3). Specifically, sloths appear to promote pyralid moth infestation by descending to the base of the tree to defecate and assisting the life cycle of moths [16,19], even in the face of heightened predation risk and significant energetic costs [12]. Moths in the fur of sloths, in turn, act as a portal for nutrients, linking the ecosystem within sloth fur to the surrounding environment. Within the sloth’s ecosystem, fungi are common [14] and we postulate that moths are being mineralized by this abundant community of decomposers. Alternatively, moths could be directly transporting organic waste from the dung pile to the fur. Regardless of the mechanism, increasing moth biomass increased inorganic nitrogen levels, which appeared to augment the growth of algal communities on sloth fur. Sloths consume algae, presumably via autogrooming, for nutritional benefit. Our VFA and compositional data suggest that algae on the fur of sloths are especially rich in digestible carbohydrates and lipids. In short, we propose that sloths are grazing the ‘algae-gardens’ they have derived from a three-way mutualism (figure 3).
Figure 3.
Postulated linked mutualisms (+) among sloths, moths and algae: (a) sloths descend their tree to defecate, and deliver gravid female sloth moths (+) to oviposition sites in their dung; (b) larval moths are copraphagous and as adults seek sloths in the canopy; (c) moths represent portals for nutrients, and via decomposition and mineralization by detritivores increase inorganic nitrogen levels in sloth fur, which fuels algal (+) growth, and (d) sloths (+) then consume these algae-gardens, presumably to augment their limited diet.
In addition to providing nutrition, it is possible that algal cultivation enhances sloth survival via camouflage reducing mortality from aerial predators [13]. These two ultimate mechanisms of algal cultivation are not mutually exclusive, but we speculate that the camouflage provided by algae is secondary to nutritional supplementation. First, the advantage of increased concealment within the canopy would have to be very strong to offset the high predation rates encountered when descending the tree to defecate, yet the algae–sloth symbiosis appears unrelated to the distribution of the primary aerial predator of sloths, the harpy eagle (Harpia harpyja). Second, previously constructed energy budgets for three-toed sloths suggests that daily energy expenditure can actually exceed intake [3], which might be from computational error [10] or because a cryptic food item, like algae, has been missed. An unaccounted food source would help to explain why three-toed sloths are difficult to keep well nourished in sanitized captive facilities [33]. Finally, the mutualisms associated with the two-toed sloth, which is the more vagile and less restricted forager, were more equivocal. Two-toed sloths possessed significantly fewer moths, and less inorganic nitrogen and algae, even though they presumably face similar predation pressure in the forest canopy. Indeed, two- and three-toed sloths from the same geographical area harbour phylogenetically distinct groups of Trichophilus spp., suggesting a long coevolutionary relationship between sloths and their algal community [14].
Whatever advantage algae confer to sloths, this complex syndrome of mutualisms—among moths, sloths and algae—appears to have locked three-toed sloths into an evolutionary trade-off that requires it to face increased predation risk in order to preserve linked mutualisms. Supporting the life cycle of moths may explain why three-toed sloths possess a high fidelity to only a few modal trees, and a marked willingness to defecate in what is, for a sloth, the most dangerous part of the forest. These mutualisms could also contribute to the sloth's success as an arboreal herbivore, one of the most constrained and rarest foraging strategies among vertebrates [1]. Our study is the first to suggest that unique ecological interactions, in addition to physiological and anatomical adaptations, may foster an arboreal and herbivorous lifestyle; future experiments that test the mechanistic linkages and putative benefits of the interactions between sloths, moths and algae will help tease apart the exact nature of these linkages.
Acknowledgements
Many thanks to E. Stanley and B. Zuckerberg for helpful discussions and comments on the manuscript, and to A. Solis for moth identifications.
Funding statement
Funding was provided by the National Science Foundation (DEB-1257535), the Milwaukee Public Museum, the University of Wisconsin–Madison and the American Society of Mammalogists.
References
- 1.Eisenberg JF. 1978. The evolution of arboreal herbivores in the class mammalia. In The ecology of arboreal folivores (ed. Montgomery GG.), pp. 135–152 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 2.Cork SJ, Foley WJ. 1991. Digestive and metabolic strategies of arboreal folivores in relation to chemical defences in temperate and tropical forests. In Plant defenses against mammalian herbivory (eds Palo RT, Robbins CT.), pp. 166–175 Boca Raton, FL: CRC Press [Google Scholar]
- 3.McNab BK. 1978. Energetics of arboreal folivores: physiological problems and ecological consequences of feeding on an ubiquitous food supply. In The ecology of arboreal folivores (ed. Montgomery GG.), pp. 153–162 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 4.Gaudin TJ. 2004. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zool. J. Linnean Soc. 140, 255–305 (doi:10.1111/j.1096-3642.2003.00100.x) [Google Scholar]
- 5.Peery MZ, Pauli JN. 2012. The mating system of a ‘lazy’ mammal, Hoffmann's two-toed sloth. Anim. Behav. 84, 555–562 (doi:10.1016/j.anbehav.2012.06.007) [Google Scholar]
- 6.Pauli JN, Peery MZ. 2012. Unexpected strong polygyny in the brown-throated three-toed sloth. PLoS ONE 7, e51389 (doi:10.1371/journal.pone.0051389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery GG, Sunquist ME. 1978. Habitat selection and use by two-toed and three-toed sloths. In The ecology of arboreal folivores (ed. Montgomery GG.), pp. 329–359 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 8.Foley WJ, Engelhardt WV, Charles-Dominique P. 1995. The passage of digesta, particle size, and in vitro fermentation rate in the three-toed sloth Bradypus tridactylus (Edentata: Bradypodidae). J. Zool. 236, 681–696 (doi:10.1111/j.1469-7998.1995.tb02739.x) [Google Scholar]
- 9.Britton SW. 1941. Form and function in the sloth (concluded). Q. Rev. Biol. 16, 190–207 (doi:10.1086/394628) [Google Scholar]
- 10.Nagy KA, Montgomery GG. 1980. Field metabolic rate, water flux, and food consumption in three-toed sloths (Bradypus variegatus). J. Mammal. 61, 465–472 (doi:10.2307/1379840) [Google Scholar]
- 11.Goffart M. 1971. Function and form in the sloth. Oxford, UK: Pergamon Press [Google Scholar]
- 12.Peery MZ, Pauli JN. In press Shade-grown cacao supports a self-sustaining population of two-toed but not three-toed sloths. J. Appl. Ecol. (doi:10.1111/1365-2664.12182) [Google Scholar]
- 13.Chiarello AG. 2008. Sloth ecology: an overview of field studies. In The biology of the Xenarthra (eds Vizcaino SF, Loughry WJ.), pp. 269–280 Gainesville, FL: University Press of Florida [Google Scholar]
- 14.Suutari M, Majaneva M, Fewer DP, Voirin B, Aiello A, Friedl T, Chiarello AG, Blomster J. 2010. Molecular evidence for a diverse green algal community growing in the hair of sloths and a specific association with Trichophilus welckeri (Chlorophyta, Ulvophyceae). BMC Evol. Biol. 10, e86 (doi:10.1186/1471-2148-10-86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiello A. 1985. Sloth hair: unanswered questions. In The evolution and ecology of armadillos, sloths, and vermilinguas (ed. Montgomery GG.), pp. 213–218 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 16.Waage JK, Montgomery GG. 1976. Cryptoses choloepi: a coprophagous moth that lives on a sloth. Science 193, 157–158 (doi:10.1126/science.193.4248.157) [DOI] [PubMed] [Google Scholar]
- 17.Odum E. 1953. Fundamentals of ecology. Philadelphia, PA: WB Saunders Company [Google Scholar]
- 18.Chiarello AG. 1998. Activity budgets and ranging patterns of the Atlantic forest maned sloth Bradypus torquatus (Xenarthra: Bradypodidae). J. Zool. 246, 1–10 (doi:10.1111/j.1469-7998.1998.tb00126.x) [Google Scholar]
- 19.Waage JK, Best RC. 1985. Arthropod associates of sloths. In The evolution and ecology of armadillos, sloths, and vermilinguas (ed. Montgomery GG.), pp. 297–311 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 20.Herre EA, Knowlton N, Mueller UG, Rehner SA. 1999. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 14, 49–53 (doi:10.1016/S0169-5347(98)01529-8) [DOI] [PubMed] [Google Scholar]
- 21.Kiers ET, Palmer TD, Ives AR, Bruno JF, Bronstein JL. 2010. Mutualisms in a changing world: an evolutionary perspective. Ecol. Lett. 13, 1459–1474 (doi:10.1111/j.1461-0248.2010.01538.x) [DOI] [PubMed] [Google Scholar]
- 22.Bshary R, Grutter AS, Willener AST, Leimar O. 2008. Pairs of cooperating cleaner fish provide better service quality than singletons. Nature 455, 964–966 (doi:10.1038/nature07184) [DOI] [PubMed] [Google Scholar]
- 23.Janzen DH. 1966. Coevolution of mutualism between ants and acacias in Central America. Evolution 20, 249–275 (doi:10.2307/2406628) [DOI] [PubMed] [Google Scholar]
- 24.Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA 96, 7998–8002 (doi:10.1073/pnas.96.14.7998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weimer PJ, Dien BS, Springer TL, Vogel KP. 2005. In vitro gas production as a surrogate measurement of the fermentability of cellulosic biomass to ethanol. Appl. Microbiol. Biotechnol. 67, 52–58 (doi:10.1007/s00253-004-1844-7) [DOI] [PubMed] [Google Scholar]
- 26.Goering HK, Van Soest PJ. 1970. Forage fiber analysis: apparatus, reagents, procedures, and some applications. Agricultural Handbook No. 379 Washington, DC: US Department of Agriculture [Google Scholar]
- 27.Weimer PJ, Shi Y, Odt CL. 1991. A segmented gas/liquid delivery system for continuous culture of microorganisms on insoluble substrates and its use for growth of Ruminococcus flavefaciens on cellulose. Appl. Microbiol. Biotechnol. 36, 178–183 (doi:10.1007/BF00164416) [Google Scholar]
- 28.Bradford MM. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (doi:10.1016/0003-2697(76)90527-3) [DOI] [PubMed] [Google Scholar]
- 29.Dubois M, Gilles KE, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (doi:10.1021/ac60111a017) [Google Scholar]
- 30.Huang G-H, Chen G, Chen F. 2009. Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass Bioenergy 33, 1386–1392 (doi:10.1016/j.biombioe.2009.05.022) [Google Scholar]
- 31.Nadelhoffer KJ, Aber JD, Melillo JM. 1984. Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant Soil 80, 321–335 (doi:10.1007/BF02140039) [Google Scholar]
- 32.Williams PJ le B, Laurens LML. 2010. Microalgae as biodiesel and biomass feedstocks: review and analysis of the biochemistry, energetics and economics. Energy Environ. Sci. 3, 554–590 (doi:10.1039/b924978h) [Google Scholar]
- 33.Diniz LS, Oliveira PM. 1999. Clinical problems of sloths (Bradypus sp. and Choloepus sp.) in captivity. J. Zoo Wildl. Med. 30, 76–80 [PubMed] [Google Scholar]