Abstract
The mechanisms regulating sexual behaviours in female vertebrates are still poorly understood, mainly because in most species sexual displays in females are more subtle and less frequent than displays in males. In a sex-role reversed population of a teleost fish, the peacock blenny Salaria pavo, an external fertilizer, females are the courting sex and their sexual displays are conspicuous and unambiguous. We took advantage of this to investigate the role of ovarian-synthesized hormones in the induction of sexual displays in females. In particular, the effects of the sex steroids oestradiol (E2) and testosterone (T) and of the prostaglandin F2α (PGF2α) were tested. Females were ovariectomized and their sexual behaviour tested 7 days (sex steroids and PGF2α) and 14 days (sex steroids) after ovariectomy by presenting females to an established nesting male. Ovariectomy reduced the expression of sexual behaviours, although a significant proportion of females still courted the male 14 days after the ovary removal. Administration of PGF2α to ovariectomized females recovered the frequency of approaches to the male's nest and of courtship displays towards the nesting male. However, E2 also had a positive effect on sexual behaviour, particularly on the frequency of approaches to the male's nest. T administration failed to recover sexual behaviours in ovariectomized females. These results suggest that the increase in E2 levels postulated to occur during the breeding season facilitates female mate-searching and assessment behaviours, whereas PGF2α acts as a short-latency endogenous signal informing the brain that oocytes are mature and ready to be spawned. In the light of these results, the classical view for female fishes, that sex steroids maintain sexual behaviour in internal fertilizers and that prostaglandins activate spawning behaviours in external fertilizers, needs to be reviewed.
Keywords: sex steroids, prostaglandins, oestradiol, testosterone, PGF2α, Salaria pavo
1. Introduction
Female mate choice is a major selective force in evolution. For many years, females were assumed to play a passive role during mating sequences, with sexual behaviour in females being limited to receptive displays (e.g. the lordosis reflex in rodents). However, detailed behavioural analysis of mating sequences reveals, in most species, a dynamic interaction between female and male sexual displays with females actively controlling the pacing and outcome of sexual interactions [1]. Nevertheless, the physiological mechanisms regulating sexual behaviour in females are still poorly understood probably because, as for other sexually selected traits, sexual displays by females are less conspicuous and less frequent than displays by males in most species. Sex-role reversed species in which females are the more actively courting sex and thus express conspicuous, stereotyped sexual behaviour patterns, are the model of choice for studying the mechanisms underlying sexual behaviour in females. This is paradoxical, because, in sex-role reversed species, mate choice is often reversed, but the insights into the mechanisms underlying female decision-making gained from this approach can then be compared with mechanisms underlying male decision-making in the context of mate choice. With this in mind, we studied the endocrine mechanisms regulating sexual behaviour in females from a sex-role reversed population of a teleost fish, the peacock blenny, Salaria pavo.
This species occurs along the rocky shores of the Mediterranean and adjacent Atlantic coasts and presents a breeding pattern similar to that of most blenniids: males defend a nest in a rock cavity from which they attract females for spawning. Females attach the eggs to the nest walls and then leave, whereas males provide sole parental care to the eggs until hatching [2,3]. In lagoon populations, however, there is a scarcity of nesting substrates, and females strongly compete for the access to the few available nesting males. This leads to the reversal of the sex roles, with females taking the initiative in courtship and displaying courtship behaviours much more frequently than males, which, in turn, assume a non-courting role during mating sequences and often reject females [4–6]. In addition, female courtship displays towards males are extremely stereotyped in this species, consisting of quick pectoral fin beatings accompanied with synchronous mouth opening-and-closing and body quivering while displaying a typical nuptial coloration characterized by an alternating pattern of vertical dark and light bars in the head and anterior portion of the body ([3] and the electronic supplementary material, video S1). This species thus presents two attributes, sex-role reversal and unambiguous female courtship displays that render it ideal for identifying the endocrine mechanisms driving sexual behaviour in females.
Sexual behaviour in females is likely to be modulated by hormones produced in the female reproductive tract because, in species with sexual reproduction, gamete maturity and sexual behaviour must be synchronized. In addition to synchronizing sexual behaviour with oocyte maturation, female sex hormones can be released externally and stimulate male sexual behaviour, thus synchronizing male and female sexual activity (for a review, see [1]).
Following detailed work in rodents, it was possible to establish that sexual behaviours of female mammals are promoted by oestrogens and progestogens synthesized in the ovary, in particular oestradiol and progesterone, respectively [1]. Likewise, these hormones have been associated with the synchronization of oocyte maturation and sexual behaviour in female birds [7] and reptiles [8]. Fewer data are available for amphibians, but oestradiol treatment in combination with the neuropeptide arginine vasotocin (AVT) recovers sexual behaviour in ovariectomized female rough-skinned newts (Taricha granulosa) [9] and oestradiol alone promotes female phonotaxy to male calls in Túngara frogs (Physalaemus pustulosus) [10]. In fishes, the regulation of sexual behaviour in females has been hypothesized to be determined by the mode of reproduction used [11]. In internal fertilizers, sexual behaviour and fertilization are temporally dissociated. By contrast, in external fertilizers, the oocytes must be fertilized soon after ovulation if viability is to be ensured. Thus, sex steroids have been implicated in the regulation of sexual behaviour in female internal fertilizers (e.g. in the guppy, Poecilia reticulata [12]) as is comparable with typical internally fertilizing tetrapods, whereas in external fertilizers, this regulatory role has been attributed to prostaglandins (e.g. the goldfish Carassius auratus, reviewed in [13]).
In externally fertilizing fish species, female reproductive behaviour is mostly restricted to the process of oviposition, which can be regarded as homologous to parturition in mammals and oviposition in reptiles and birds. In fact, prostaglandins, which are known to induce uterine contractions in mammals [14], have also been associated with the induction of contractile stimulation of the theca muscles leading to oviposition in fishes [15]. In the goldfish, the best studied species to date, the movement of ovulated eggs into the oviduct induces synthesis of the prostaglandin F2α (PGF2α) that, in addition to stimulating oviposition, is thought to also trigger female spawning behaviour in the brain [16,17]. This mechanism ensures that females are motivated to spawn soon after ovulation, at the time of maximum viability of the ovulated eggs, and to continue to spawn until all ovulated eggs have been shed [18]. In addition, in the goldfish, prostaglandins released by females into the water act as pheromones and activate male sexual behaviour, thus synchronizing male and female sexual activity with precision [19]. Similar results have been reported for amphibians with external fertilization where prostaglandins were shown to induce receptive behaviours in females [20]. In sex-role reversed species, however, sexual behaviour in females has an anticipatory component, because females need to court males and be accepted by them before oviposition; just as in species with traditional sex roles where male sexual displays precede copulation. Therefore, sex steroids, in particular oestrogens produced during follicular development, may promote sexual behaviours in females in anticipation of spawning, as has been suggested for internal fertilizers, both in fishes and across other vertebrate classes [21].
It should be highlighted that the apparent dichotomy in the endocrine mechanisms regulating sexual behaviour in female teleosts (i.e. sex steroids in internal fertilizers versus prostaglandins in external fertilizers) stems from the seminal research conducted mostly in two species, the goldfish and the guppy. However, the extraordinary diversity of mating systems, reproductive behaviours and the remarkable plasticity in sexual displays that can be found across teleosts is likely to be paralleled by similar diversity in the neuroendocrine mechanisms regulating sexual behaviour in females. In addition, the role of these two classes of hormones, prostaglandins and sex steroids, in the regulation of sexual behaviour in fishes has seldom been tested in the same species.
We tested the effect of the sex steroids oestradiol (E2) and testosterone (T) and of the PGF2α on the sexual behaviour of female S. pavo. In particular, we tested the effects of ovariectomy on sexual behaviours of females and of administration of these hormones on recovering these behaviours.
2. Material and methods
(a). Experimental animals
Males and females of S. pavo were collected during the breeding season in June and July 2007, 2009 and 2010 at Culatra Island (36°59′ N, 7°51′ W, Algarve, Southern Portugal). Fish were captured with hand nets during low tide from artificial hard substrates such as tiles and bricks that exist in the area to delimit the borders of clam culture fields, and transported to a field station located on the island. Females were individually isolated in 10 l tanks containing a cylindrical PVC tube (10 cm wide and 2 cm in diameter and closed at one of the ends with an opaque plastic sheet) to be used as shelter. Nesting males were kept in stock tanks (80 l) in the presence of females and provided with abundant nests (PVC tubes similar to female shelters but 15 cm wide and 3 cm in diameter), so that they were defending eggs before the experiment began. Animals were kept on a ‘long day’ photoperiod (14 h light : 10 h dark) and at 24±2°C. These conditions are similar to those found in the field during the breeding season. Animals were fed ad libitum with frozen cockles.
(b). Ovariectomy
Females were anaesthetized with MS222 (tricaine methanesulfonate, dilution 1 : 10 000, Sigma) measured and weighed. A small incision (approx. 1 cm) was made in the abdominal cavity under a stereomicroscope. Ovaries were removed by gently cutting the tissue between the ovarian sac and the abdominal wall as well as by sectioning the oviduct. The ovarian sac could generally be removed as a whole without the loss of oocytes into the abdominal cavity and without visible bleeding. In females treated with steroids (see below), implants were inserted into the abdominal cavity after ovariectomy. Fish from the sham group also had their abdomen opened and gonads manipulated with blunted tweezers, but were not ovariectomized or implanted. The wound was sutured with Surgicryl polyglycolic acid thread, USP 6/0 (SMI, St Vith, Belgium). Females were immediately moved to a small tank (3 l) with full aeration and then transferred to their isolation tank after recovery from anaesthesia. Isolation tanks were then treated with the antibiotic flumequine at 50 ppm to prevent post-surgical infections.
(c). Steroid implants
Females were randomly assigned to one of four treatments (table 1): (i) non-ovariectomized controls (sham); (ii) ovariectomized and implanted with castor oil, the vehicle substance in which steroids were dissolved (OV); (iii) ovariectomized and implanted with testosterone plus the aromatase inhibitor fadrozole (OV + T + F); (iv) ovariectomized and implanted with oestradiol (OV + E2). Fadrozole was administered in combination with T to block the conversion by aromatase of T into E2: it does not block conversion of T to 11-ketotestosterone. The aromatase inhibitor was donated by Novartis Pharma AG (Basel, Switzerland). T and E2 were purchased from Steraloids (Newport, RI, USA). Substances were administered via silastic implants (internal diameter = 1.7 mm, external diameter = 1.96 mm). The length of each implant was adjusted to the female's body mass to keep the hormonal dosage per unit body mass equal for all the fish. The hormonal dosage was based on previous studies in fishes [22–25] and was 1 µg g−1 for T, 40 µg g−1 for fadrozole and 5 µg g−1 for E2. Steroids and fadrozole were thoroughly dissolved in castor oil overnight on a magnetic stirrer at 40°C. In a previous study, it was shown that this concentration and mode of administration of fadrozole successfully reduces brain aromatization [22]. The standard length of fish from the different treatments did not differ (one-way ANOVA: F4,40 = 0.25, p = 0.91). Females were tested for sexual behaviour 7 and 14 days after being implanted. This period was considered sufficient for steroid-induced behavioural changes to take place, because, in previous experiments with S. pavo, steroid implants were showing induced behavioural changes after 7 days [22,26]. We decided to test females at both time periods, because, in another study with male S. pavo, we showed that the temporal pattern of T and E2 release from silastic implants differs, with T levels declining from day 7 to 14 and E2 having a more stable pattern (D. Gonçalves, M. C. Teles, R. F. Oliveira 2012, unpublished data).
Table 1.
Description of the experimental groups used in the steroid hormone manipulation experiment.
| treatment | ovariectomy | implant | n |
|---|---|---|---|
| sham | no | no implant | 15 |
| OV | yes | vehicle (castor oil) | 13 |
| OV + T + F | yes | testosterone + fadrozole | 7 |
| OV + E2 | yes | 17β-oestradiol | 11 |
(d). Prostaglandin F2α
Females were assigned to one of three experimental groups (table 2): (i) non-ovariectomized controls intraperitoneally injected with teleost Ringer's solution, the vehicle substance in which PGF2α was dissolved (sham); (ii) ovariectomized and injected with Ringer's solution (OV); (iii) ovariectomized and injected with PGF2α (OV + PGF2α). Following other studies in fishes [27,28], the concentration of PGF2α injections was of 0.5 µg g−1, and a solution of 0.125 µg µl−1 was used. Females were ovariectomized and left to recover in isolation tanks for 7 days, as previously described. On experimental day 7, females were either injected with Ringer's solution or with PGF2α, returned to their isolation tank and tested for sexual behaviour 45 min after the injection [27,28]. The standard length of fish from the different treatments did not differ (one-way ANOVA: F2,28 = 0.22, p = 0.80).
Table 2.
Description of the experimental groups used in the prostaglandin manipulation experiment.
| treatment | ovariectomy | injection | n |
|---|---|---|---|
| sham | no | teleost Ringer's solution | 9 |
| OV | yes | teleost Ringer's solution | 10 |
| OV + PGF2α | yes | prostaglandin F2α | 10 |
(e). Behavioural test
A behavioural test was used to assess whether sexual behaviour was altered by ovariectomy and consequently restored by hormone administration. A nesting male defending eggs was removed from the stock tank with its nest and placed in an experimental tank (70 × 30 × 40 cm) approximately 30 min before the behavioural observations. All males kept guarding the eggs and seldom left the nest while in the test tank, a typical pattern for nesting males of this species. Females were gently introduced, coaxed into the shelter present in their isolation tank (if not already inside), and moved to the test tank with the shelter. The shelter was placed facing the male's nest with its entrance approximately 40 cm from the nest's opening. Observations began immediately, and the following variables were recorded: frequency of approaches to the male's nest (within one body length), frequency of courtship displays directed towards the male and time spent displaying nuptial coloration, behaviours that always precede female spawning (D. Gonçalves 1998, personal observations). Observations lasted 15 min, and behaviours were quantified using JWatcher software v. 0.9 [29]. Observers did not know to which experimental treatment the focal animal belonged. A total of five different nesting males were used, four for the steroid hormone experiment and one for the PGF2α experiment. Within each experiment, the testing order of females from the different treatments was randomized, and the same male was presented to the same number of females from the various treatments. Within the same day, the same male was tested with a maximum of eight females from different treatments with an interval between trials of approximately 20 min. For the steroid hormone experiment, each female was tested with the same male on days 7 and 14.
(f). Blood sampling and hormone analysis
Immediately after being tested, females from the steroid hormone experiment were anaesthetized as previously described. Blood was collected from the caudal vein with a 27G heparinized syringe, centrifuged and the plasma kept at −20°C for hormone analyses by radioimmunoassay. The abdominal cavity was also inspected under a stereomicroscope for the presence of the implant and for the development of regenerating ovarian tissue. Ovariectomized females did not present any visible gonadal tissue, and there was no implant loss.
The free steroid fraction was extracted using previously described methodology [30,31]. Steroid residues were dissolved in phosphate buffer 0.1 M, pH 7.6, containing gelatin (1 g l−1) and stored again at −20°C until assayed for T and E2. The E2 antibody (reference: 20-ER06) was purchased from Interchim (Montluçon, France) and the cross-reactivity measured by the supplier was 1% for oestrone and 0% for androstenedione. The T antibody (reference: RDI-TRK2T2) was purchased from Research Diagnostics Inc. (Concord, USA), and the cross-reactivity measured by the supplier was 16% for 5-alpha-dihydrotestosterone and less than 0.01% for E2. For each hormone, circulating plasma levels from all animals were measured within the same assay. Intra-assay coefficients of variation for the T and E2 assays were 9.5% and 5.5%, respectively.
(g). Statistics
A priori planned contrast tests on the results from ANOVAs were used to determine differences between experimental groups at an alpha level of 0.05. When necessary, data were square root- or log-transformed to comply with heteroscedasticity and normality assumptions. In the PGF2α experiment, data relative to the time spent displaying the nuptial coloration still failed to comply with parametric assumptions after transformation and a non-parametric alternative, Kruskal–Wallis test followed by Dunn's test, was applied.
3. Results
(a). Effect of ovariectomy and steroid implants on plasma levels of oestradiol and testosterone
Circulating levels of E2 and T were analysed on experimental day 14 of the steroid implant experiment. There were overall differences between groups both for T and E2 (E2: F3 = 14.28, p < 0.001; T: F3 = 5.00, p = 0.005; table 3). As expected, ovariectomy reduced the circulating levels of both steroids. All ovariectomized groups not implanted with E2 had lower E2 levels when compared with the sham group. The OV + E2 implanted animals had marginally higher E2 levels than the sham group (table 3). For T, ovariectomized groups not implanted with T had lower levels of this hormone when compared with the sham group. These differences were significant for the sham versus OV + E2 comparison but non-significant for the sham versus OV comparison. Levels of T also did not differ between the sham and the T-implanted (OV + T + F) group (table 3). In general, the expected effect of ovariectomy in reducing steroid levels and of E2 and T implants in restoring the physiological values of these hormones was confirmed.
Table 3.
Mean ± s.e. plasma levels of oestradiol (E2) and testosterone (T) measured at day 14 of the steroid hormone manipulation experiment. (p-values relative to the planned comparisons between the ‘sham’ and the remaining groups are indicated. OV, ovariectomized; F, fadrozole.)
| treatment | n | E2 (ng ml−1) | p-value | T (ng ml−1) | p-value |
|---|---|---|---|---|---|
| sham | 15 | 7.83 ± 1.56 | — | 0.87 ± 0.18 | — |
| OV | 12a | 2.07 ± 0.65 | <0.001 | 0.43 ± 0.09 | 0.103 |
| OV + T + F | 7 | 2.01 ± 0.64 | <0.001 | 1.36 ± 0.47 | 0.426 |
| OV + E2 | 7 | 13.41 ± 2.55 | 0.057 | 0.13 ± 0.05 | 0.003 |
an = 11 for T.
(b). Effect of ovariectomy and steroid implants on sexual behaviour in females
Overall, ovariectomy significantly reduced the expression of sexual display behaviours in females. When compared with intact (sham) females, ovariectomized females (OV) approached the nest less (figure 1a), courted the male less (figure 1b) and spent less time displaying nuptial coloration (figure 1c). Nevertheless, the proportion of ovariectomized females (OV group) that courted the male at least once was only significantly lower than the proportion of sham females at day 14 (table 4). A similar comparison for nuptial coloration gave comparable results (table 4).
Figure 1.
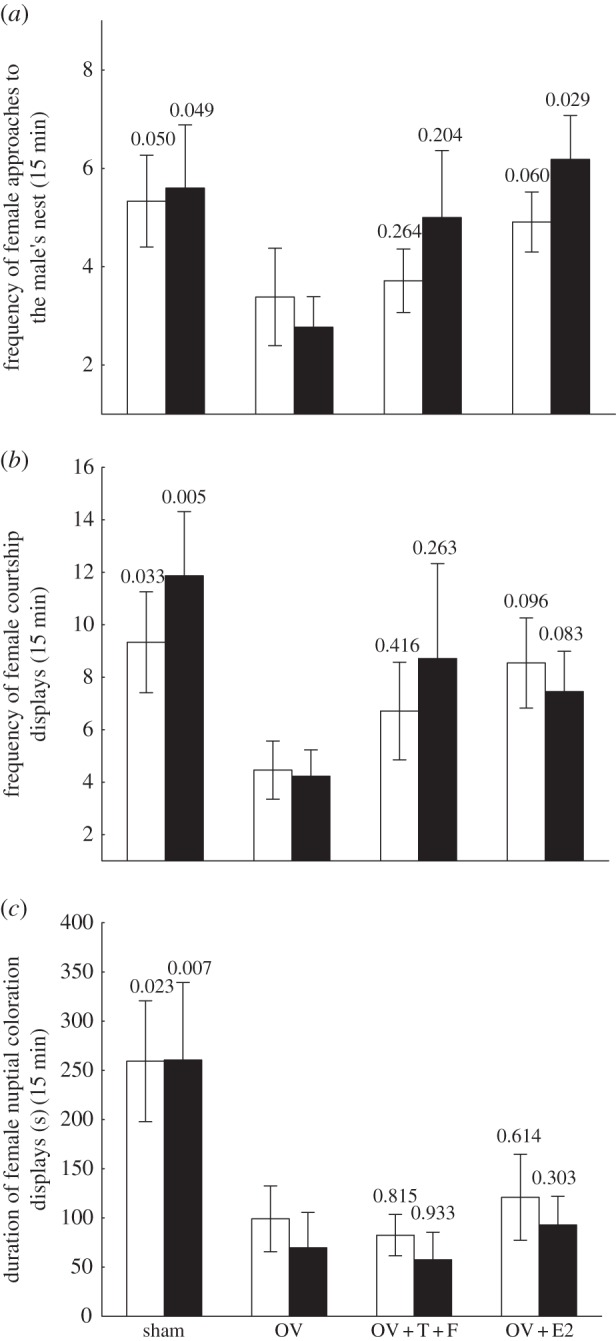
Behavioural results from the steroid hormone manipulation experiment. (a) Frequency of female approaches to the male's nest; (b) frequency of female courtship towards the nesting male; and (c) duration of nuptial coloration displays. p-values relative to the planned comparisons between the ovariectomized (OV) and the remaining groups are indicated above the error bars. OV + T + F, ovariectomized + testosterone + fadrozole, OV + E2, ovariectomized + E2. Sample sizes = 7–15 per treatment. (a–c) Open bar, days 7; filled bar, days 14.
Table 4.
Number (and percentage) of females that courted the male and displayed nuptial coloration at least once on days 7 and 14 in the steroid manipulation experiment. (The proportion of females displaying each behaviour in the ovariectomized (OV) treatment was compared with each of the remaining groups by χ2 tests. E2, oestradiol; F, fadrozole; T, testosterone.)
| treatment | courtship towards male |
nuptial coloration |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| day 7 |
day 14 |
day 7 |
day 14 |
|||||||||
| no of females (%) | χ21 | p-value | no. of females (%) | χ21 | p-value | no. of females (%) | χ21 | p-value | no. of females (%) | χ21 | p-value | |
| OV (n = 13) | 9 (69.2) | — | — | 9 (69.2) | — | — | 8 (61.5) | — | — | 6 (46.2) | — | — |
| sham (n = 15) | 14 (93.3) | 2.76 | 0.097 | 15 (100) | 5.38 | 0.020 | 13 (86.7) | 2.35 | 0.126 | 13 (86.7) | 5.24 | 0.022 |
| OV + T + F (n = 7) | 7 (100) | 2.69 | 0.101 | 6 (85.7) | 0.66 | 0.417 | 6 (85.7) | 1.26 | 0.261 | 4 (57.1) | 0.22 | 0.639 |
| OV + E2 (n = 11) | 10 (90.9) | 1.70 | 0.192 | 11 (100) | 4.06 | 0.044 | 10 (90.9) | 2.74 | 0.098 | 10 (90.9) | 5.37 | 0.020 |
The hypothesis that sex steroids recover sexual behaviour was tested by comparing the steroid-implanted groups (OV + E2 and OV + T + F) with the ovariectomized and vehicle-implanted group (OV). E2 administration recovered female approach to the male's nest as E2-implanted females approached the nest significantly more than ovariectomized females, although results were only significant for day 14 (figure 1a). In addition, there was a marginally non-significant trend for E2 administration to recover the frequency of courtship displays (figure 1b). At day 7, 10 of 11 E2-implanted females courted the male at least once and on day 14 all females in this group courted the male (table 4). These proportions differed from the OV group on day 14 (table 4). E2 administration failed to recover the time females spent displaying nuptial coloration (figure 1c). However, on both day 7 and 14, only one female in the E2 group did not display nuptial coloration at least once, and this proportion of displaying females was significantly higher than the proportion for ovariectomized females on day 14 (table 4).
T administration was not effective in recovering sexual behaviour in females. In spite of higher mean frequency of approaches to the male's nest and courtship displays (figure 1a,b), no statistically significant differences in these variables or in the time spent displaying nuptial coloration (figure 1c) was detected, either on day 7 or day 14, between T-implanted (OV + T + F) and ovariectomized (OV) females. In addition, there was no difference in the proportion of females courting the nesting male or turning on nuptial coloration at least once, both for day 7 and day 14, between the OV and the OV + T + F group (table 4).
(c). Effect of prostaglandin F2α on sexual behaviour in females
Similar to the above results, in this second experiment, ovariectomized females (OV) also had a lower frequency of approaches to the nest (figure 2a), frequency of courtship displays (figure 2b) and marginally lower time spent displaying nuptial coloration (figure 2c) when compared with intact (sham) females. We note that only one of 10 ovariectomized females displayed nuptial coloration at least once as compared with six of nine sham-operated females (χ21 = 6.54, p = 0.010). On the contrary, all females in all treatments courted the male at least once.
Figure 2.
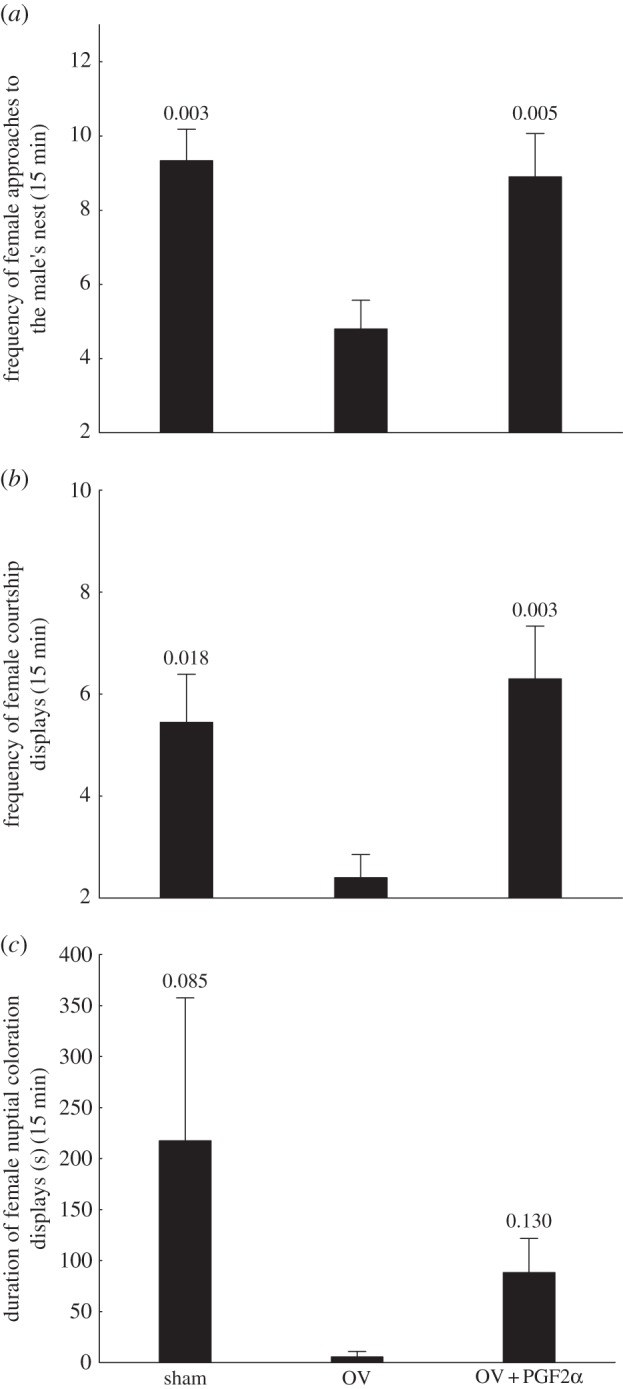
Behavioural results from the prostaglandin F2α (PGF2α) manipulation experiment. (a) Frequency of female approaches to the male's nest; (b) frequency of female courtship towards the nesting male; and (c) duration of nuptial coloration displays. p-values relative to the planned comparisons between the ovariectomized (OV) and the remaining groups are indicated above the error bars. Sample sizes = 9–10 per treatment.
PGF2α administration recovered the frequency of approaches to the male's nest (figure 2a) and courtship displays (figure 2b), but not time spent displaying nuptial coloration (figure 2c). However, the proportion of females, six of 10, turning on nuptial coloration in the PGF2α group was significantly higher than in the OV group (χ21 = 5.49, p = 0.019).
4. Discussion
This study showed that two reproductive hormones from different chemical classes, PGF2α and E2, stimulate sexual displays in females of a single fish species. PGF2α, in particular, was very effective in recovering sexual behaviours after ovariectomy. The injection of this hormone 45 min before testing into females ovariectomized 7 days before, recovered the frequency of both female approaches to the male's nest and courtship displays towards the nesting male to levels similar to those of intact sham females. The positive effect of this hormone on nuptial coloration was, however, less pronounced. The role of prostaglandins in the induction of sexual behaviour in females has been demonstrated in several fishes with external fertilization: paradise fish, Macropodus opercularis [32], black acara Cichlasoma bimaculatum [33] and barb Puntius gonionotus [34]. Interestingly, administration of PGF2α to non-receptive females of the anuran Bufo americanus induced female phonotaxis behaviour towards male calls [35,36] and another prostaglandin, PGE2, induced female receptivity in African clawed frogs, Xenopus laevis [20], both external fertilizers. However, the best studied example to date is the goldfish, C. auratus [16,27]. In this species, when ovulated oocytes are in the oviduct, the plasma concentration of PGF2α increases dramatically [17]. Ovulated eggs within the lumen appear to induce spawning by stimulating PGF2α synthesis, because the effect of ovulated eggs or artificial eggs is blocked by the prostaglandin synthesis inhibitor indomethacin and restored in indomethacin-treated fish by PGF2α injection [16,17]. PGF2α-mediated mechanisms synchronizing sexual behaviour in females with ovulation are thus probably widespread among oviparous fishes (reviewed in [13]). In goldfish, PGF2α appears to act within the brain [27] independently of sex steroids, because behavioural response is unaffected by ovariectomy or by steroid-replacement therapy [28]. The brain mechanism underlying PGF2α-induced sexual behaviour in female goldfish is functional in juveniles and may be modulated by gonadotropin-releasing hormone (reviewed in [13]), although the central site of action of this hormone in the stimulation of sexual displays remains unknown. The existing evidence thus points to PGF2α functioning in S. pavo, as in other external fertilizing teleost species and probably also amphibians, as a short-latency, short-duration hormone that acts as an endogenous messenger to synchronize sexual behaviour with the presence of mature oocytes in the ovaries.
E2, however, also had a positive role in the recovery of sexual display behaviours in ovariectomized S. pavo females. Interestingly, E2 was particularly effective in recovering more anticipatory behaviours, namely the frequency of approaches to the male's nest, whereas it was less effective in recovering behaviours that immediately precede mating, i.e. courtship and nuptial coloration displays. Similar results were found for anurans with external fertilization, where E2 mainly promoted female phonotaxis towards male calls [10]. These results suggest that E2 promotes female mate-searching and/or mate-assessment behaviours but has a less significant role in more consummatory phases of the mating cycle. The argument for oestrogens having a minor role in the activation of sexual displays in females of species with external fertilization [11] is partly based on the fact that, for many species, plasma E2 levels peak during vitellogenesis and decrease during the second phase of the ovulatory cycle [37–39]. However, this relationship seems to depend on the pattern of oocyte development and spawning. In a study comparing three closely related cyprinid species, it was found that E2 levels peaked before the breeding season and decreased throughout the reproductive period in a species that lays a single egg clutch per season, whereas in two species that lay multiple egg clutches, E2 levels remained elevated throughout the reproductive period [40]. Although there are no detailed data regarding seasonal variation of E2 levels in female S. pavo, this species is a multiple spawner, suggesting that high levels of E2 are maintained throughout the breeding season. Thus, high E2 levels may be needed throughout the reproductive period to motivate mate searching in multiple spawners.
In most species of teleost fishes, and in contrast with other vertebrates, T levels are similar in females and males [41], although in the peacock blenny nesting males have higher T levels than females [42]. Nevertheless, in fishes, T has seldom been implicated in the regulation of sexual display behaviours in females. Indeed, a stimulatory effect of T on sexual behaviour of females has been reported for masu salmon [43] but not for any other fish species (reviewed in [44]). In this study, the sexual behaviour of females implanted with T did not differ from the behaviour of control females, showing that this androgen did not promote sexual displays in females. A previous study with this species reported differences in circulating T levels between females from two populations with divergent expression of sexual behaviour [45]. Females from the sex-role reversed population focused on this study have lower T levels than females from a population in the Adriatic Sea where there is an abundance of nesting males and where females seldom court males [45]. One hypothesis to explain these results is that the postulated environmental modulation of sexual displays by females is translated by T, with higher levels of this hormone inhibiting female displays. Although our results are not consistent with this hypothesis, because T administration to ovariectomized females did not further decrease sexual behaviours, the results should be interpreted with caution as it is still possible that in intact females T will decrease the expression of sexual displays.
This study also shows that complete removal of cues from the ovaries does not abolish sexual behaviour in females. Fourteen days after ovariectomy, a significant proportion of females (approx. 70%) still approached the nest and courted the male at least once. Similar results have been reported for other fish species. For example, in the bluehead wrasse Thalassoma bifasciatum, female-to-male behavioural sex change can occur in the absence of gonads [46]. This raises the question of why ovariectomized females still had the motivation to approach and display courtship behaviour towards males. One hypothesis is that sexual displays exhibited by females of S. pavo have a dual function. Besides its classic role during mating sequences, female displays are occasionally used outside a mating context, apparently as submissive behaviours that seem to lower male's aggression (D. Gonçalves 2003, personal observations). In the field, females visit several nesting males before spawning and often display courtship behaviours towards the males, even to those males where no attempt to enter the nest is made [47], probably to be able to approach nests and assess mating partners without receiving aggressive charges. If this is the case, female visits to nests and courtship displays may be exhibited not just when females are ready to spawn but throughout the breeding season for the purpose of assessing potential mating partners. Nevertheless, some signal from the gonads should inform the brain that females are in reproductive condition and activate mate-searching behaviours. It is possible that the timeframe used in our experiment was insufficient to reset gonadal hormones to basal levels. This seems unlikely for PGF2α, thought to act as a short-term endocrine signal. E2, although significantly reduced by ovariectomy, was still present at detectable levels in the plasma with a mean of 2.1 ng ml−1 after 14 days. In the sham group, the mean E2 level was 7.8 ng ml−1, with a range of 1.9–26.4 ng ml−1, values that are similar to those previously described for females captured in the field during the reproductive period [42]. In addition, the mean E2 level of ovariectomized females fell within the normal range of values for females of other fish species in reproductive conditions (e.g. 2–3 ng ml−1 for the cyprinids Rutilus rutilus, Alburnus alburnus and Blicca bjoerkna [40]; 3.6 ng ml−1 for the midshipman Porichthys notatus [48]). Thus, considering that E2 seems to have a positive effect on sexual behaviours in female S. pavo, it seems possible that the E2 levels observed in ovariectomized females allowed the maintenance of a certain level of sexual behaviour. The central mechanism through which E2 may be exerting these effects in S. pavo is for now unclear. One possibility is that E2 produced in the ovaries interacts with neurons that produce AVT. In a previous study, it was shown that AVT promotes sexual displays in female S. pavo [49] and in birds, oestrogens increase the expression of AVT in brain areas related to reproduction [50]. In amphibians, AVT induces female egg-laying behaviour but gonadal steroids are needed to maintain this behavioural action of AVT (reviewed in [51]). Thus, the possibility that E2 promotes sexual display behaviours in female S. pavo by stimulating the AVT system deserves further consideration.
Ovariectomy was particularly efficient in abolishing nuptial coloration displays. Both steroid and prostaglandin treatments failed to recover this display in an effective way, suggesting that other endocrine signals of ovarian origin may be implicated in its expression. Corroborating this idea, in the two-spotted goby (Gobiusculus flavescens) female nuptial coloration is influenced by the hormones prolactin, alpha-melanocyte stimulating hormone, melatonin and noradrenaline but not by sex steroids [52]. This suggests that multiple endocrine messengers are implicated in the different aspects of female courtship behaviour in S. pavo.
Taken together, the results from this study indicate that the endocrine modulation of sexual display behaviour in female fishes is more complex than the previously suggested simple dichotomy of oestrogens-internal fertilizers/prostaglandins-external fertilizers. By taking advantage of a sex-role reversed species with unambiguous sexual displays, we showed that both E2 and PGF2α influence sexual behaviours in females. The results further suggest that while E2 may be more related with the activation of anticipatory sexual behaviours, such as mate-searching and mate assessment, PGF2α acts as a short-term internal messenger informing the brain that oocytes are ready to be released and triggering behavioural displays leading to spawning sequences.
Acknowledgements
We thank two anonymous reviewers and Dr Rosemary Knapp for providing useful comments on a previous version of the manuscript.
During the experimental procedures, the ‘ASAB Guidelines for the Use of Animals in Research’ [53] were strictly applied. All animal protocols were performed under a ‘Group-1’ license from the Direcção-Geral de Veterinária, Ministério da Agricultura, do Desenvolvimento Rural e das Pescas, Portugal.
Funding statement
This study was supported by the research grants nos. POCTI/BSE/38395/2001 and PTDC/MAR/69749/2006 and the R&D units Plurianual Programme (R&D unit no. 331/2001) from the Portuguese Foundation for Science and Technology (FCT) and from grant no. 012/2012/A1 from the Macao Science and Technology Development Fund (FDCT). During the course of this project S.S.C. was being supported by an FCT fellowship (SFRH/BPD/30367/2006).
References
- 1.McCarthy MM, Becker JB. 2002. Neuroendocrinology of sexual behavior in the female. In Behavioral endocrinology (eds Becker JB, Breedlove SM, Crews D, McCarthy MM.), pp. 117–151, 2nd edn. Cambridge, MA: The MIT Press [Google Scholar]
- 2.Fishelson L. 1963. Observations on littoral fishes of Israel I. Behaviour of Blennius pavo Risso (Teleostei, Blenniidae). Isr. J. Zool. 12, 67–91 [Google Scholar]
- 3.Patzner RA, Seiwald M, Adlgasser M, Kaurin G. 1986. The reproduction of Blennius pavo (Teleostei, Blenniidae). V. Reproductive behavior in natural environment. Zool. Anz. 216, 338–350 [Google Scholar]
- 4.Almada VC, Gonçalves EJ, Oliveira RF, Santos AJ. 1995. Courting females: ecological constraints affect sex roles in a natural population of the blenniid fish Salaria pavo. Anim. Behav. 49, 1125–1127 (doi:10.1006/anbe.1995.0142) [Google Scholar]
- 5.Saraiva J, Pignolo G, Gonçalves D, Oliveira RF. 2012. Interpopulational variation of the mating system in the peacock blenny Salaria pavo. Acta Ethol. 15, 25–31 (doi:10.1007/s10211-011-0104-y) [Google Scholar]
- 6.Almada VC, Gonçalves EJ, Santos AJ, Baptista C. 1994. Breeding ecology and nest aggregations in a population of Salaria pavo (Pisces, Blenniidae) in an area where nest sites are very scarce. J. Fish Biol. 45, 819–830 (doi:10.1111/j.1095-8649.1994.tb00947.x) [Google Scholar]
- 7.Ball GF, Balthazart J. 2009. Neuroendocrine regulation of reproductive behavior in birds. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT.), pp. 856–895, 2nd edn San Diego, CA: Academic Press [Google Scholar]
- 8.Whittier JM, Tokarz R. 1992. Physiological regulation of sexual behavior in female reptiles. In Hormones, brain and behavior: biology of the reptilia, vol. 18 (eds Gans C, Crews D.), pp. 24–69 Chicago, IL: University of Chicago Press [Google Scholar]
- 9.Moore FL, Wood RE, Boyd SK. 1992. Sex steroids and vasotocin interact in a female amphibian (Taricha granulosa) to elicit female-like egg-laying behavior or male-like courtship. Horm. Behav. 26, 156–166 (doi:10.1016/0018-506X(92)90039-X) [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty M, Burmeister SS. 2009. Estradiol induces sexual behavior in female tungara frogs. Horm. Behav. 55, 106–112 (doi:10.1016/j.yhbeh.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 11.Stacey NE. 1981. Hormonal regulation of female reproductive behavior in fish. Am. Zool. 21, 305–316 [Google Scholar]
- 12.Liley NR. 1972. The effects of estrogens and other steroids on the sexual behavior of the female guppy, Poecilia reticulata. Gen. Comp. Endocrinol. 3, 542–552 (doi:10.1016/0016-6480(72)90185-2) [Google Scholar]
- 13.Kobayashi M, Sorensen MW, Stacey NE. 2002. Hormonal and pheromonal control of spawning behavior in the goldfish. Fish Physiol. Biochem. 26, 71–84 (doi:10.1023/A:1023375931734) [Google Scholar]
- 14.Thorburn GD. 1991. The placenta, prostaglandins and parturition: a review. Reprod. Fertil. Dev. 3, 277–294 (doi:10.1071/RD9910277) [DOI] [PubMed] [Google Scholar]
- 15.Jalabert B, Szollosi D. 1975. In vitro ovulation of trout oocytes: effect of prostaglandins on smooth muscle-like cells of the theca. Prostaglandins 9, 765–779 (doi:10.1016/0090-6980(75)90113-6) [DOI] [PubMed] [Google Scholar]
- 16.Stacey NE. 1976. Effects of indomethacin and prostaglandins on spawning behavior of female goldfish. Prostaglandins 12, 113–126 (doi:10.1016/S0090-6980(76)80010-X) [DOI] [PubMed] [Google Scholar]
- 17.Sorensen PW, Brash AR, Goetz FW, Kellner RG, Bowdin L, Vrieze LA. 1995. Origins and functions of F prostaglandins as hormones and pheromones in the goldfish. In Fish symposium 95, pp. 244–248 University of Texas at Austin [Google Scholar]
- 18.Stacey NE. 1983. Hormones and reproductive behaviour in teleosts. In Control processes in fish physiology (eds Rankin JC, Pitcher TJ, Duggan R.), pp. 117–129 London, UK: Croom Helm [Google Scholar]
- 19.Sorensen PW, Hara TJ, Stacey NE, Goetz FW. 1988. F prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol. Reprod. 39, 1039–1050 (doi:10.1095/biolreprod39.5.1039) [DOI] [PubMed] [Google Scholar]
- 20.Weintraub AS, Kelley DB, Bockman RS. 1985. Prostaglandin E2 induces receptive behaviors in female Xenopus laevis. Horm. Behav. 19, 386–399 (doi:10.1016/0018-506X(85)90036-4) [DOI] [PubMed] [Google Scholar]
- 21.Wilczynski W, Lynch KS. 2011. Female sexual arousal in amphibians. Horm. Behav. 59, 630–636 (doi:10.1016/j.yhbeh.2010.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonçalves D, Alpedrinha J, Teles M, Oliveira RF. 2007. Endocrine control of sexual behavior in sneaker males of the peacock blenny Salaria pavo: effects of castration, aromatase inhibition, testosterone and estradiol. Horm. Behav. 51, 534–541 (doi:10.1016/j.yhbeh.2007.02.003) [DOI] [PubMed] [Google Scholar]
- 23.Modesto T, Canário AVM. 2003. Hormonal control of swimbladder sonic muscle dimorphism in the Lusitanian toadfish. J. Exp. Biol. 206, 3467–3477 (doi:10.1242/jeb.00581) [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi S, Kagawa H, Gen K, Okuzawa K, Matsuyama M. 2004. Silicone implants for delivery of estradiol-17 β and 11-ketotestosterone to red seabream Pagrus major. Aquaculture 239, 485–496 (doi:10.1016/j.aquaculture.2004.05.031) [Google Scholar]
- 25.Ros AF, Bruintjes R, Santos RS, Canario AV, Oliveira RF. 2004. The role of androgens in the trade-off between territorial and parental behavior in the Azorean rock-pool blenny, Parablennius parvicornis. Horm. Behav. 46, 491–497 (doi:10.1016/j.yhbeh.2004.04.007) [DOI] [PubMed] [Google Scholar]
- 26.Oliveira RF, Carneiro LA, Goncalves DM, Canario AV, Grober MS. 2001. 11-Ketotestosterone inhibits the alternative mating tactic in sneaker males of the peacock blenny, Salaria pavo. Brain Behav. Evol. 58, 28–37 (doi:10.1159/000047259) [DOI] [PubMed] [Google Scholar]
- 27.Stacey NE, Peter RE. 1979. Central action of prostaglandins in spawning behaviour of female goldfish. Physiol. Behav. 22, 1191–1196 (doi:10.1016/0031-9384(79)90275-0) [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Stacey N. 1993. Prostaglandin-induced female spawning behavior in goldfish (Carassius auratus) appears independent of ovarian influence. Horm. Behav. 27, 38–55 (doi:10.1006/hbeh.1993.1004) [DOI] [PubMed] [Google Scholar]
- 29.Blumstein DT, Evans CS, Daniel JC. 2000 JWatcher 0.9. An introductory user's guide. See http://wwwjwatcheruclaedu . [Google Scholar]
- 30.Canário AVM, Scott AP. 1989. Synthesis of 20α-hydroxylated steroids by ovaries of the dab (Limanda limanda). Gen. Comp. Endocrinol. 76, 147–158 (doi:10.1016/0016-6480(89)90041-5) [DOI] [PubMed] [Google Scholar]
- 31.Scott AP, Canario AVM. 1992. 17a,20ß-Dihydroxy-4-pregnen-3-one 20-sulphate: a major new metabolite of the teleost oocyte maturation-inducing steroid. Gen. Comp. Endocrinol. 85, 91–100 (doi:10.1016/0016-6480(92)90176-K) [DOI] [PubMed] [Google Scholar]
- 32.Villars TA, Hale N, Chapnick D. 1985. Prostaglandin-F2α stimulates reproductive behavior of female paradise fish (Macropodus opercularis). Horm. Behav. 19, 21–35 (doi:10.1016/0018-506X(85)90003-0) [DOI] [PubMed] [Google Scholar]
- 33.Cole KS, Stacey NE. 1984. Prostaglandin induction of spawning behavior in Cichlasoma bimaculatum (Pisces cichlidae). Horm. Behav. 18, 235–248 (doi:10.1016/0018-506X(84)90013-8) [DOI] [PubMed] [Google Scholar]
- 34.Liley NR, Tan ESP. 1985. The induction of spawning behaviour in Puntius gonionotus (Bleeker) by treatment with prostaglandin PGF2α. J. Fish Biol. 26, 491–502 (doi:10.1111/j.1095-8649.1985.tb04289.x) [Google Scholar]
- 35.Schmidt RS. 1984. Mating call phonotaxis in the female American toad: induction by hormones. Gen. Comp. Endocrinol. 55, 150–156 (doi:10.1016/0016-6480(84)90139-4)) [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RS. 1985. Mating call phonotaxis in female American toad: induction by intracerebroventricular prostaglandin. Copeia 2, 490–492 (doi:10.2307/1444863) [Google Scholar]
- 37.Kobayashi M, Aida K, Hanyu I. 1988. Hormone changes during the ovulatory cycle in goldfish. Gen. Comp. Endocrinol. 69, 301–307 (doi:10.1016/0016-6480(88)90018-4) [DOI] [PubMed] [Google Scholar]
- 38.Munakata A, Amano M, Ikuta K, Kitamura S, Aida K. 2001. The involvement of sex steroid hormones in downstream and upstream migratory behavior of masu salmon. Comp. Biochem. Phys. B 129, 661–669 (doi:10.1016/S1096-4959(01)00365-7) [DOI] [PubMed] [Google Scholar]
- 39.Ramsey ME, Wong RY, Cummings ME. 2011. Estradiol, reproductive cycle and preference behaviour in a northern swordtail. Gen. Comp. Endocrinol. 170, 381–390 (doi:10.1016/j.ygcen.2010.10.012) [DOI] [PubMed] [Google Scholar]
- 40.Rinchard J, Kestemont P, Heine R. 1997. Comparative study of reproductive biology in single and multiple-spawner cyprinid fish. II. Sex steroid and plasma protein phosphorus concentrations. J. Fish Biol. 50, 169–180 (doi:10.1111/j.1095-8649.1997.tb01349.x) [Google Scholar]
- 41.Oliveira RF, Gonçalves D. 2008. Hormones and social behaviour of teleost fish. In Fish behaviour (eds Magnhagen C, Braithwaite VA, Forsgren E, Kapoor BG.), pp. 61–125 Enfield, NH: Science Publishers [Google Scholar]
- 42.Gonçalves D, Teles M, Alpedrinha J, Oliveira RF. 2008. Brain and gonadal aromatase activity and steroid hormone levels in female and polymorphic males of the peacock blenny Salaria pavo. Horm. Behav. 54, 717–725 (doi:10.1016/j.yhbeh.2008.07.014) [DOI] [PubMed] [Google Scholar]
- 43.Munakata A, Amano M, Ikuta K, Kitamura H, Aida K. 2001. Sex steroids control migration of masu salmon. Fisheries Sci. 68, 49–52 [Google Scholar]
- 44.Gonçalves D, Oliveira RF. 2010. Hormones and sexual behavior of teleost fishes. In Hormones and reproduction of vertebrates (eds Norris D, Lopez KH.), pp. 119–147 San Diego, CA: Academic Press [Google Scholar]
- 45.Saraiva J, Gonçalves D, Oliveira RF. 2010. Environmental modulation of androgen levels and secondary sex characters in two populations of the peacock blenny Salaria pavo. Horm. Behav. 57, 192–197 (doi:10.1016/j.yhbeh.2009.10.013) [DOI] [PubMed] [Google Scholar]
- 46.Godwin J, Crews D, Warner RR. 1996. Behavioural sex change in the absence of gonads in a coral reef fish. Proc. R. Soc. Lond. B 263, 1683–1688 (doi:10.1098/rspb.1996.0246) [DOI] [PubMed] [Google Scholar]
- 47.Fagundes T, Gonçalves D, Oliveira RF. 2007. Female mate choice and mate search tactics in a sex role reversed population of the peacock blenny, Salaria pavo (Risso, 1810). J. Fish Biol. 71, 77–89 (doi:10.1111/j.1095-8649.2007.01466.x) [Google Scholar]
- 48.Sisneros JA, Forlano PM, Knapp R, Bass AH. 2004. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen. Comp. Endocrinol. 136, 101–116 (doi:10.1016/j.ygcen.2003.12.007) [DOI] [PubMed] [Google Scholar]
- 49.Carneiro L, Oliveira R, Canário AVM, Grober M. 2003. The effect of arginine vasotocin on courtship behaviour in a blenniid fish with alternative reproductive tactics. Fish Physiol. Biochem. 28, 241–243 (doi:10.1023/B:FISH.0000030542.31395.8a) [Google Scholar]
- 50.Seth R, Kohler A, Grossmann R, Chaturvedi CM. 2004. Expression of hypothalamic arginine vasotocin gene in response to water deprivation and sex steroid administration in female Japanese quail. J. Exp. Biol. 207, 3025–3033 (doi:10.1242/jeb.01118) [DOI] [PubMed] [Google Scholar]
- 51.Moore FL. 1992. Evolutionary precedents for behavioral actions of oxytocin and vasopressin. Ann. N.Y. Acad. Sci. 652, 156–165 (doi:10.1111/j.1749-6632.1992.tb34352.x) [DOI] [PubMed] [Google Scholar]
- 52.Skold HN, Amundsen T, Svensson PA, Mayer I, Bjelvenmark J, Forsgren E. 2008. Hormonal regulation of female nuptial coloration in a fish. Horm. Behav. 54, 549–556 (doi:10.1016/j.yhbeh.2008.05.018) [DOI] [PubMed] [Google Scholar]
- 53.1991. Guidelines for the use of animals in research. Anim. Behav. 41, 183–186 (doi:10.1016/S0003-3472(05)80519-6) [Google Scholar]


