Abstract
The understanding of physiological and molecular processes underlying the sense of smell has made considerable progress during the past three decades, revealing the cascade of molecular steps that lead to the activation of olfactory receptor (OR) neurons. However, the mode of primary interaction of odorant molecules with the OR proteins within the sensory cells is still enigmatic. Two different concepts try to explain these interactions: the ‘odotope hypothesis’ suggests that OR proteins recognize structural aspects of the odorant molecule, whereas the ‘vibration hypothesis’ proposes that intra-molecular vibrations are the basis for the recognition of the odorant by the receptor protein. The vibration hypothesis predicts that OR proteins should be able to discriminate compounds containing deuterium from their common counterparts which contain hydrogen instead of deuterium. This study tests this prediction in honeybees (Apis mellifera) using the proboscis extension reflex learning in a differential conditioning paradigm. Rewarding one odour (e.g. a deuterated compound) with sucrose and not rewarding the respective analogue (e.g. hydrogen-based odorant) shows that honeybees readily learn to discriminate hydrogen-based odorants from their deuterated counterparts and supports the idea that intra-molecular vibrations may contribute to odour discrimination.
Keywords: vibration hypothesis of olfaction, proboscis extension reflex, differential conditioning
1. Introduction
Our understanding of physiological and molecular processes underlying the sense of smell has made considerable progress during the past 25 years: olfactory receptor (OR) proteins which reside in the membranes of olfactory sensory neurons have been identified and cloned [1] and the transduction cascades of second messenger molecules leading to the activation of olfactory neurons once an odour molecule interacts with the OR protein have been revealed ([2,3]; reviewed by [4]). Differences exist between the OR proteins and their genes in vertebrates and insects [5], but the basic functions are similar, as are the designs of the circuitry underlying primary olfactory processing in the vertebrate olfactory bulb and the insect antennal lobe [6–8].
In vertebrates and insects, the responses of olfactory neurons are influenced by, but cannot be fully explained by ‘simple’ individual chemical characteristics of the respective odorant molecules (such as carbon atom number or functional group type and position; [9,10]). Multidimensional metrics using 32 different molecular properties as predictors result in a better prognosis, but still cannot fully predict neuronal responses [11]. This reflects the fact that we know comparatively little about the important first stage of olfaction, i.e. the interaction of OR proteins within sensory neurons with their ‘substrate’, the odorant molecules. The empirical analysis of molecular characteristics that define the molecular receptive range of an OR protein is difficult and time-consuming as hundreds of compounds have to be tested for each individual OR protein and more than a thousand genes coding for such OR proteins are known in mice alone [12]. Accordingly, this has been done only for a very few identified ORs [9,13]. Insights about the potential mechanisms underlying the interaction and specificity of odorant receptors come from recent advances in our understanding of the interactions of G protein-coupled receptors with their ligands [14], which include hormone and neurotransmitter receptors as well as photoreceptors, taste receptors and ORs. However, the location of the agonist binding sites in these receptors is highly variable [15], and three-dimensional structures and potential binding sites are known only for a few ORs [16], which hampers computational models for determining potential olfactory ligands and specific structural motifs (often referred to as ‘odotopes’) within odorant molecules [17]. Early ideas suggested a key/lock type mechanism between the odorant and the OR protein [18,19], and much research has been performed during the past 50 years to describe the interactions of these odotopes with particular sites of the OR molecules. Our understanding of these interactions has far progressed from the early notion that the overall three-dimensional shape of the odorant molecule ‘unlocks’ the odorant receptor, thus giving rise to a conformational change which triggers the second messenger cascades, but a cohesive and predictive theory of these interactions is still missing (reviewed by [20]).
Despite its general acceptance, there are several aspects of human olfactory perception that do not seem to support the odotope hypothesis. For example, some structurally very different molecules smell the same or similar (e.g. musks and camphoraceous compounds), whereas other molecules that are structurally very similar may have very different smells (reviewed by [21]). Therefore, no current theory can entirely explain how a given molecule results in the perception of a particular smell [22].
An alternative hypothesis, first introduced by Dyson [23], has recently re-emerged, proposing that the interactions between the olfactory proteins and the odour molecules are not strictly chemical in nature but are also based on matching molecular vibration frequency spectra of the odorant molecule and its OR protein target [24]. Briefly, this ‘vibration hypothesis’ suggests that only those odorant molecules with a vibrational energy mode in a particular frequency range, to which a specific OR protein is tuned, would be able to cause electron transfer, thus activating the receptor [25]. While the detail of the vibration hypothesis is beyond the scope of this study, the concept makes testable predictions about the odour sensations evoked by certain molecules. Specifically, the vibration hypothesis suggests that olfactory systems should be able to discriminate common, hydrogen-based odorants from their deuterated counterparts (molecules in which hydrogen has been replaced by deuterium, which is identical with respect to the electrons, which determine chemical properties and bonds, but which contains a proton and is thus twice as heavy as hydrogen). The difference in the mass of these atoms affects their respective molecular vibrations (see examples in the electronic supplementary material, figure S2). By contrast, the odotope hypothesis would predict that such odorant pairs should be indistinguishable, because the atomic sizes and ground state conformations are not altered by introducing isotopes, hence both molecules should interact with the receptor protein in the same way. The resulting suggestion that humans should be able to discriminate deuterated compounds from their hydrogen-based counterparts has been debated [22,26] and rejected for some odorants but appears to hold for large odorant molecules [27].
A recent study on fruit flies [28] suggested that flies do learn to discriminate deuterated compounds from their common, hydrogen-based counterparts when one of the compounds is associated with punishment (mild electrical shocks). These findings are difficult to explain based on the odotope hypothesis and are seen as support for the vibration hypothesis at least in insects, whose OR proteins are slightly different from those of vertebrates (see Discussion). In this study, we test these predictions of the vibration hypothesis using odour learning in honeybees, which allows examining effects at the individual level, as opposed to the population effects examined in studies on fruit flies. Honeybees are well known for their learning and memory and their odour discrimination abilities ([29–31]; reviewed by [32]), and we use a particularly successful learning paradigm, the proboscis extension response learning [33,34]. We follow some of the experiments performed in fruit flies [28], and we show that individual bees can learn to discriminate common (hydrogen-based) odorants from deuterated ones after three to four pairings with sugar water rewards, a finding that supports the vibration hypothesis of olfaction but which conflicts with the odotope hypothesis unless the vibrational frequency of a molecule is considered as another one of the many parameters that may determine an ‘odotope’ [11]. It suggests that considering a quantum mechanics approach may help advance our ideas about receptor protein–odorant interactions in new ways as it has for understanding processes such as vision or photosynthesis in the emerging field of ‘quantum biology’ [35].
2. Methods
(a). Animals
Honeybees (Apis mellifera) were collected at the United States Department of Agriculture Carl Hayden Bee Research Center, in Tucson, AZ, between May 2012 and September 2013. In order to have bees of comparatively similar motivational status and experience, we focused on water foragers, which are most easily identified. Associative learning performance and sucrose responsiveness differ between bees performing different foraging tasks (nectar/pollen/water; [36,37]), and water foragers are most responsive to sugar water [38]. They were collected using an aspirator at a watering site in the bee yard of the Bee Research Center where bees from more than 50 colonies surrounding the water source would gather and forage for water. Hence, every sample presumably comprised water foragers from many colonies. Once a week, about 200–300 bees were caught, and were then kept for up to 4 days in a wire mesh cage in the laboratory where they had access to 15% sucrose solution ad libitum.
(b). Training procedure
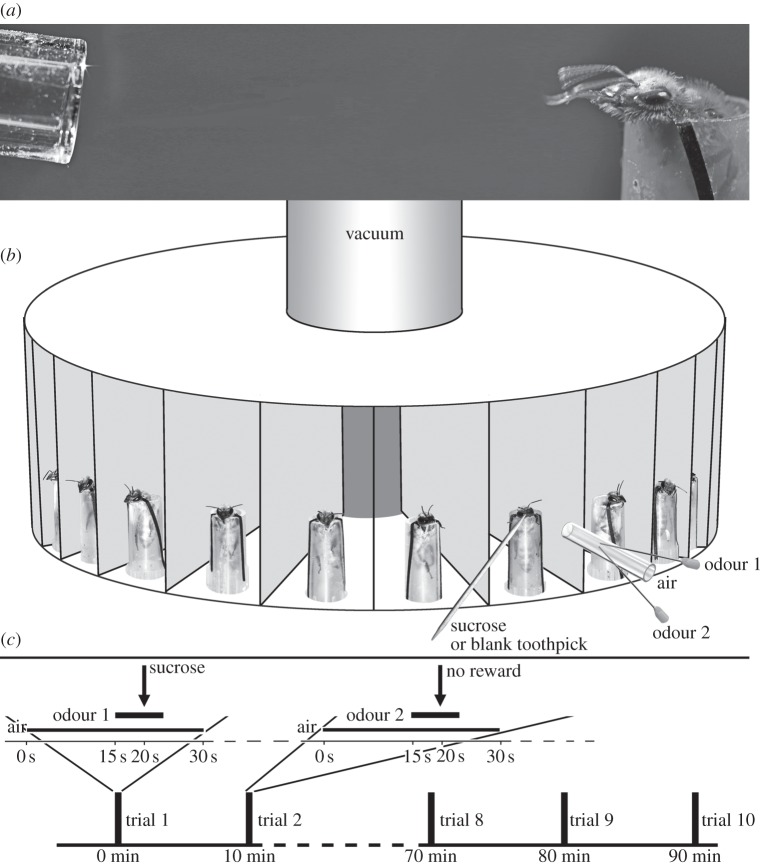
On the morning of the experiment, worker honeybees were chilled on ice and harnessed in plastic tubes as described in [34]. Twenty bees that showed the proboscis extension response upon direct antennal contact with 50% sucrose solution were chosen for each experiment and were mounted in the rotary training apparatus consisting of a drum (35 cm diameter, 10 cm high; figure 1b) divided into 20 individual wedge-shaped chambers and connected at the centre to a weak vacuum line that removed any residual odours.
Figure 1.
Experimental design. (a) A conditioned honeybee responds by proboscis extension to a deuterated acetophenone stimulus (odour not visible). (b) Rotary training set-up; bees are individually treated with an air current into which either odour 1 or odour 2 is injected; then the antennae and proboscis (if extended) are stimulated with sucrose. Alternatively, for unrewarded trials, during the odour stimulus, the antenna is touched with a blank unwetted toothpick. (c) Training paradigm: trials last for 30 s and for each bee are repeated every 10 min. Trials (enlarged) comprise an air current into which odour is injected after 15 s; after 5 s of odour stimulation, the antennae and proboscis are touched with sucrose solution. For absolute conditioning only one odour is used (10 trials); for differential conditioning, two odours are alternated pseudo-randomly every 10 min (20 trials total).
The training began 2 h after mounting. Each bee, in turn, was rotated into the stimulation position. It was then subjected to a non-odorized filtered air current generated by an aquarium pump (ca 2 l min−1 air flow) delivered through a nozzle 5 cm from the bee's head. After 15 s of plain air, an odour current was injected into the outgoing air current through a syringe needle terminating inside the nozzle. The odour current (conditioned stimulus, CS) was generated using an aquarium pump whose air current was directed through a cartridge containing a strip of filter paper (3 cm2) impregnated with 5 μl of the respective pure odorant. The resulting odorized air current was such that a human observer detected an unmistakable odour 5 cm from the nozzle. The additional air flow caused by the odour injection was negligible (less than 2% of the outgoing air current). Five seconds after initializing the odour stream, the antennae were manually touched with a wooden toothpick wetted with a 50% sucrose solution (unconditioned stimulus), upon which the bees reflexively extended their proboscides (unconditioned response) to receive the reward: licking the sugary toothpick for about 3 s, after which the odour was switched off and the toothpick removed. The amount of sugar water the bees imbibed during each trial was relatively small (1–2 μl as measured using calibrated glass capillary pipettes) but sufficient for learning, and ensuring that the bees did not become satiated before the end of the experiment (which included 10 rewarded trials; the crop of honeybees holds over 60 μl [39]). A bee was assumed to have associated the odour with the sugar reward if she extended her proboscis during the 5 s of odour presentation before the sucrose stimulus was offered (figure 1a). Each individual trial took about 30 s (figure 1c), after which the next bee was rotated into the trial position and exposed to the air current.
Trials were repeated every 10 min, and each experiment comprised 10 trials (absolute conditioning) or 20 trials (differential conditioning). Because of the long duration of the experiments, individual bees occasionally failed to show proboscis extension upon sucrose stimulation. Bees that failed to respond to sucrose more than three times (about 20% of the bees) were excluded from the data. Bees that responded to the odour during the first trial (before any sucrose reward; about 5% of the bees) were also excluded as such a spontaneous response precluded the bee from demonstrating signs of true learning. Likewise, experiments in which fewer than 30% of the bees learned the association within 10 trials (about 3% of the experiments) were excluded because they do not represent the learning potential of honeybees as established in other studies [32,34].
(c). Odorants
Two common odorants (hydrogen-based) and their respective deuterated isotopes were used in the experiments: common acetophenone (1-phenylethanone; here referred to as ‘h-ACP’) and fully deuterated acetophenone (‘d8-ACP’) as well as common benzaldehyde (‘h-BNZ’) and fully deuterated benzaldehyde (‘d6-BNZ’). Additional experiments were performed using an odorant pair with a higher number of deuterium atoms: common 1-octanol (‘h-OCT’) and fully deuterated 1-octanol (‘d17-OCT’). Deuterated compounds were purchased from CDN Isotopes Inc., Pointe-Claire, Quebec, Canada (gas-chromatographically analysed purity: 99.4% (d8-ACP), 99.5% (d6-BNZ) and 99.1% (d-OCT)). The purity of the deuterated compounds is represented by the gas-chromotographic analyses provided by the manufacturer (CDN) and shown in the electronic supplementary material, figure S1. Common ACP, BNZ and OCT were from Fluka Analytical (Sigma-Aldrich; analytical standard 99.5% purity).
(d). Conditioning paradigms
Two different sets of experiments were performed.
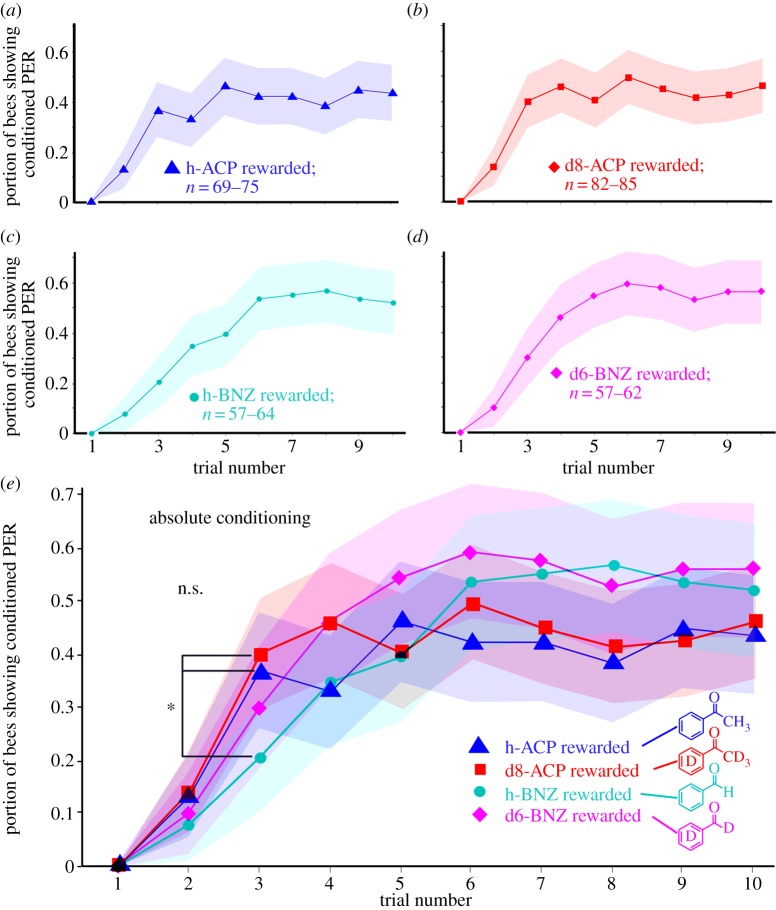
For absolute conditioning (figure 2), half of the 20 bees per experiment were rewarded with one odour (e.g. ‘h-ACP’) and the other half with the complementary odour (e.g. ‘d8-ACP’). Each bee went through 10 training cycles, during each of which the same odour was used as a reward each time. The objective of these experiments was to test whether bees can learn to detect and associate deuterated compounds as well as common, hydrogen-based compounds.
Figure 2.
Learning curves (absolute conditioning; portion of bees showing conditioned proboscis extension response (PER)) for the four aromatic compounds tested: (a) ‘h-ACP’ (triangles), (b) ‘d8-ACP’ (squares), (c) ‘h-BNZ’ (circles), (d) ‘d6-BNZ’ (diamonds), (e) curves a–d overlaid to show similarity. Shaded areas show 95% confidence intervals (CIs); the differences between curves are not significant (F-tests; F = 0.87–1.25; p = 0.18–0.49; d.f. = 60–84); t-tests for individual trials did not show significant differences except for trial no. 3 where response to h-BNZ is different from those to h-ACP and d8-ACP (*p < 0.05; note that these differences are not significant after Bonferroni correction for multiple comparisons). (Online version in colour.)
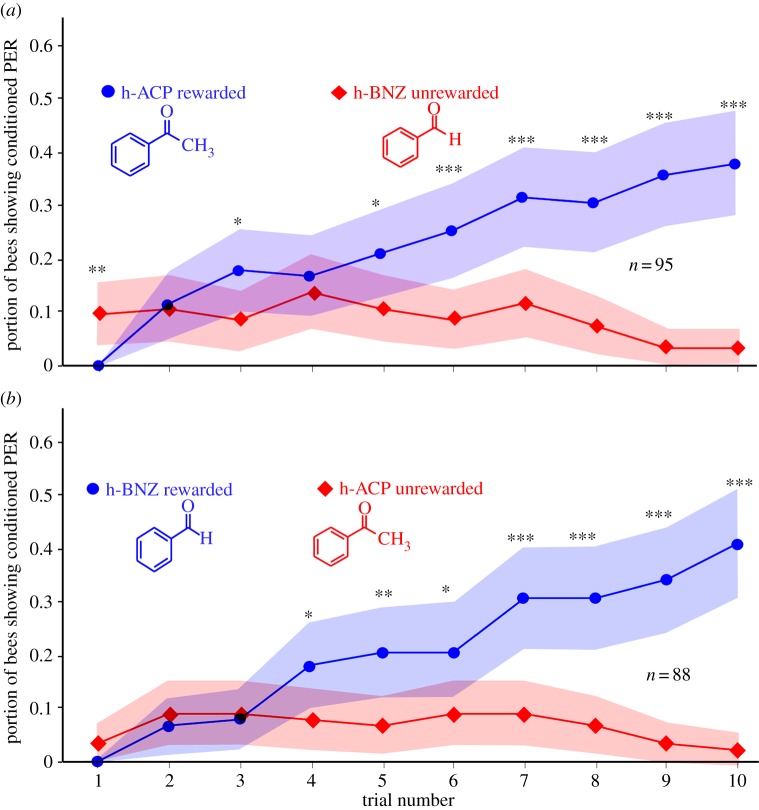
For differential conditioning experiments (figures 3–5), each bee was presented with two odorants (e.g. ‘h-ACP’ and ‘d8-ACP’), only one of which was rewarded with sucrose solution while the other odour was not sugar-rewarded (figure 1c). Rewarded (R) and unrewarded trials (U) were administered in a pseudorandom order pattern as follows: (R U R U U R U R R U U R R U R U U R U R) to avoid potential sequence learning by the bees. The bees had to learn to discriminate the two odours and to extend their proboscides only in response to the ‘correct’ odour, but not to the unrewarded one. During the unrewarded trials, the bees’ antennae were touched with a dry toothpick, as a control tactile stimulus, without sucrose to make the rewarded and unrewarded trials as similar as possible.
Figure 3.
Learning curves (differential conditioning; portion of bees showing conditioned proboscis extension response (PER)) for common (non-deuterated) compounds. (a) ‘h-ACP’ (rewarded) versus ‘h-BNZ’ (not rewarded); (b) ‘h-BNZ’ (rewarded) versus ‘h-ACP’ (not rewarded). Shaded areas show 95% CIs; differences between curves statistically significant (ANOVA; (a): p < 10−17; F = 78; n = 95 bees; (b): p < 10−18; F = 84; n = 88 bees); significance levels for comparison of individual trials (t-tests) are *p < 0.01; **p < 0.001; ***p < 0.0001. Note initial odour generalization during the first two (a) or three (b) trials. (Online version in colour.)
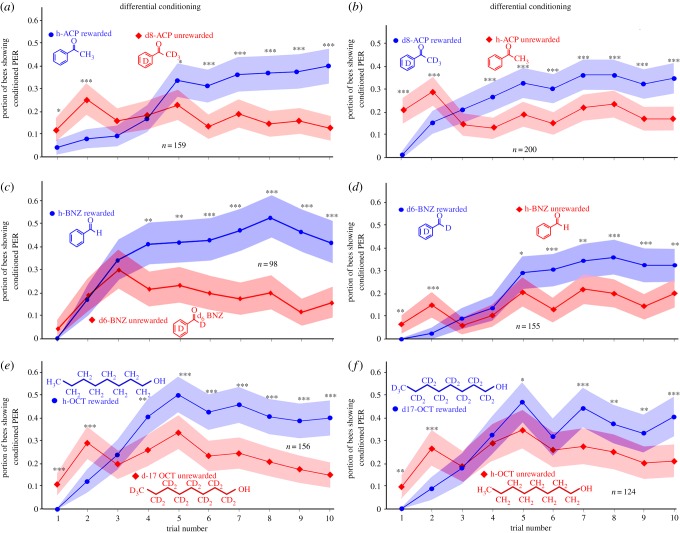
Figure 4.
Learning curves (differential conditioning; portion of bees showing conditioned proboscis extension response (PER)) for the three pairs of deuterated versus hydrogen-based compounds. (a) ‘h-ACP’ (rewarded) versus ‘d8-ACP’ (unrewarded); (b) the inverse (‘d8-ACP’ (rewarded) versus ‘h-ACP’ (unrewarded)); (c) ‘h-BNZ’ (rewarded) versus ‘d6-BNZ’ (unrewarded); (d) the inverse (‘d6-BNZ’ (rewarded) versus ‘h-BNZ’ (unrewarded)); (e) ‘h-OCT’ (rewarded) versus ‘d17-OCT’ (unrewarded); (f) the inverse (‘d17-OCT (rewarded) versus ‘h-OCT’ (unrewarded)). Shaded areas show 95% CIs; differences between curves statistically significant (ANOVA; (a): p < 10−8; F = 34.6; n = 159 bees; (b): p < 10−8; F = 33; n = 200 bees); (c): p < 10−14; F = 65.2; n = 98 bees; (d): p < 10−8; F = 33; n = 155 bees); (e): p < 10−13; F = 55; n = 156 bees); (f): p < 10−3; F = 9.9; n = 124 bees; significance levels for comparison of individual trials (t-tests) are *p < 0.01; **p < 0.001; ***p < 0.0001. Note initial odour generalization during the first three or four trials. (Online version in colour.)
Figure 5.
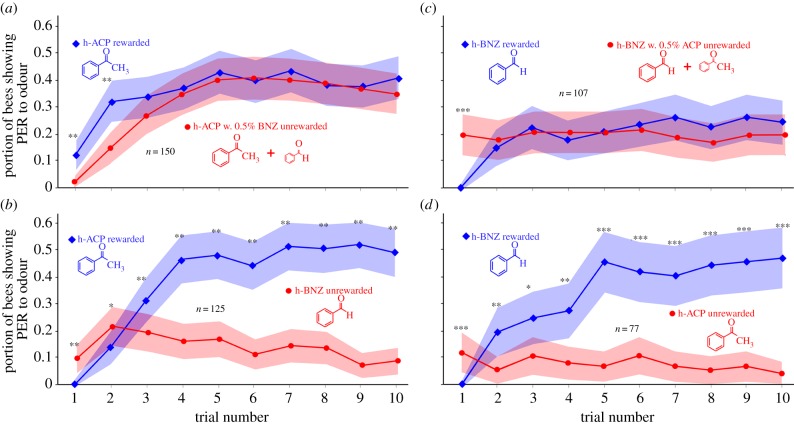
Learning curves (differential conditioning; portion of bees showing conditioned proboscis extension response (PER)) showing the effect of impurities in the test compounds. (a) Pure ‘h-ACP’ (rewarded) versus ‘h-ACP’ with 0.5% of ‘h-BNZ’ (unrewarded); (b) pure ‘h-BNZ’ (rewarded) versus ‘h-BNZ’ with 0.5% of ‘h-ACP’ (unrewarded); (c,d) control tests of the respective pure substance pairs: (c) pure ‘h-ACP’ (rewarded) versus pure ‘h-BNZ’ (unrewarded); (d) pure ‘h-BNZ’ (rewarded) versus ‘h-ACP’ (unrewarded). Shaded areas show 95% CIs; learning curves after second trial not statistically significant in (a) (ANOVA; p = 0.22; F = 1.49; n = 150 bees) and (c) (p = 0.87; F = 0.03; n = 107 bees); however, curves in (b,d), respectively, are significantly different (ANOVA; (b): p < 10−50; F = 223; n = 125 bees; (d): p < 10−40; F = 192; n = 77 bees); significance levels for comparison of individual trials (t-tests) are *p < 0.01; **p < 0.001; ***p < 0.0001. (Online version in colour.)
Control experiments (figure 5) were performed to examine whether the potentially different impurities in the ‘common’ and the deuterated compounds, which were derived from different suppliers, might cause response differences apart from the effects of deuteration. To do so, one compound (e.g. h-ACP) was tested against the same compound but to which 0.5% of another compound (e.g. h-BNZ) was added to mimic an impurity.
(e). Data analysis
At each trial, the proportion of individuals responding to the CS was computed, and the means of these data were then graphed together with their 95% CIs, and the resulting learning curves were compared with the respective alternative curves (e.g. ‘h-ACP rewarded’ versus ‘d8-ACP unrewarded’). F-tests were used to compare absolute conditioning trials (figure 2), and the proportions of responding individuals were used in ANOVA (as explained in [40]). For absolute conditioning (figure 2), odour was the independent factor, and the trial number was the repeated measure. Two-way ANOVA was used to test for significant differences between treatments (differential conditioning experiments; odour (rewarded versus unrewarded) and trial number were treated as repeated measures). Likewise, when comparing different differential conditioning subgroups, these were treated as independent factors. Individual data points were compared using a Student's t-test corrected for multiple comparisons. Excel 2010 (Microsoft, Inc.) was used to calculate statistical values.
3. Results
(a). Absolute conditioning
The worker honeybees were able to learn the four aromatic odorants. After five to seven trials, on average, more than half of the bees learned to associate the sucrose reward with the respective odour stimulus (figure 2). Slightly fewer bees appeared to learn the two forms of ACP (46–47%; figure 2a,b; triangles and squares in figure 2e) compared with the BNZ isotopes (56–60%, figure 2c,d; circles and diamonds in figure 2e), but F-tests did not show significant differences between the four learning curves (F = 0.87–1.25; p = 0.18–0.49; d.f. = 60–84) and individual t-tests comparing the responses for the four different odorants (‘h-ACP’, ‘d8-ACP’, ‘h-BNZ’ and ‘d6-BNZ’) at each learning trial did not show statistically significant differences. At trial no. 3, the conditioned responses to h-BNZ appeared to be smaller than those to h-ACP or d8-ACP (indicated by asterisk in figure 2e), but this difference turned out not to be significant after Bonferroni correction for multiple comparisons. This suggested that, when rewarded, the four odorants were learned equally well by the bees. Importantly, no statistically significant differences were found between the learning curves for ‘h-ACP’ and ‘d8-ACP’ and, respectively, ‘h-BNZ’ and ‘d6-BNZ’, when both odorants were rewarded (individual t-tests; p = 0.10–0.69), suggesting that replacing hydrogen for deuterium in the odorant molecules did not significantly affect the bees’ ability to perceive and learn the odorants.
(b). Differential conditioning
In an initial experiment, we tested the common aromatic odorants ‘h-ACP’ against ‘h-BNZ’, rewarding h-ACP and not rewarding h-BNZ (figure 3a) and, respectively, the inverse (figure 3b), effectively ‘asking the bees’ to discriminate these two chemically related odorant compounds (differing just in the presence or absence of one methyl group; see inset in figure 3). As shown in figure 3, the bees readily discriminated the two odorants after the second or third conditioning trial: the respective curves for the rewarded and unrewarded treatments are statistically significantly different (ANOVA; (i): p < 10−17; F = 78; n = 95 bees; (ii): p < 10−18; F = 84; n = 88 bees). However, we found considerable variation in the bees’ learning ability over time (on certain days, bees would learn faster and experiments would reach higher learning scores overall), and we also found much variation across bees, with some bees being able to discriminate the respective test odours after one or two trials, and a few bees taking up to nine trials. Some bees that had originally shown ‘correct’ learned responses would also stop responding to the odours after about six to seven trials, and all of these individual differences contributed to the fact that overall the learning curves are relatively flat and do not reach the high percentage of learned responses published in several earlier studies (e.g. [34]). This was true throughout, including the following experiments (figures 4 and 5).
The critical tests regarding the discrimination of deuterated compounds from their common, hydrogen-based counterparts, and which are at the core of this study, are shown in figure 4. When conditioning ‘h-ACP’ (rewarded) against ‘d8-ACP’ (unrewarded; figure 4a), the two learning curves are significantly different (ANOVA; p < 10−8; F = 34.6; n = 159 bees). The bees started discriminating the common (rewarded) odorant from its unrewarded deuterated counterpart after the fourth trial (p < 0.01; t-test). During the first two trials, the bees responded significantly more often to the unrewarded odour, as is common in differential learning paradigms and indicating initial generalization. This same initial overlap and crossing of the learning curves can be seen in the other differential stimulus combinations (figure 4b–f) and also, although less pronounced, in figure 3.
When the reward schedule was reversed for the ACP odour pair (‘d8-ACP’ rewarded, ‘h-ACP’ unrewarded; figure 4b), the resulting learning curves are similar to those in figure 3a: the rewarded and unrewarded curves are significantly different (ANOVA; p < 10−8; F = 33; n = 200 bees), both curves intersect initially (odour generalization during the first two trials; see above) and the two odours were reliably discriminated after the third trial. Despite minor differences in the trajectory of the learning curves, it did not matter for the bees’ learning performance whether ‘h-ACP’ or ‘d8-ACP’ was the rewarded odour. Under both experimental conditions, the common odour was distinguished from the deuterated one after only a few brief learning trials.
Honeybees showed similar learning results when conditioned to discriminate between ‘h-BNZ’ and ‘d6-BNZ’ (figure 4c,d). When ‘h-BNZ’ was rewarded (figure 4c), the learning curve for the rewarded common odorant was significantly different from the curve for the unrewarded ‘d6-BNZ’ compound (figure 4c; ANOVA; p < 10−14; F = 65.2; n = 98 bees), and the same was true for the inverse case, where ‘d6-BNZ’ was rewarded and ‘h-BNZ’ was unrewarded (figure 4d; ANOVA; p < 10−8; F = 33; n = 155 bees). While there are some differences in the trajectories of the graphs in figure 4c,d, in both cases, the bees initially generalized the two odours and then were able to discriminate the common from the deuterated BNZ after the third (figure 4c) or fourth (figure 4d) conditioning trial (t-tests).
This was also the case when the bees were differentially conditioned to discriminate hydrogen-based from deuterated octanol (figure 4e,f). After an initial response generalization, the common octanol is discriminated from the deuterated one after three (figure 4e) or four trials (figure 4f), and the curves for the rewarded odorant are significantly different from the ones for the unrewarded odorant (ANOVA; p < 10−13; F = 55; n = 156 bees (‘h-OCT’ rewarded; figure 4e); p < 10−3; F = 9.9; n = 124 bees (‘d17-OCT’ rewarded; figure 4f)). Based on tests using acetophenone, benzaldehyde and octanol, it therefore appears that honeybees are able to discriminate hydrogen-based organic volatile compounds from their respective fully deuterated counterparts (figure 4). It should be noted, however, that it appears that the deuterated compounds are confused to a higher degree: when bees are trained to discriminate two different common aromatic compounds (‘h-ACP’ and ‘h-BNZ’ in figure 3), fewer bees respond to the respective unrewarded odorants (compare unrewarded responses in figure 3, which are generally at or below 10%, with those in figure 4, which are generally above 15%).
(i). Controls
To confirm that the differential odour responses were the result of the hydrogen- or deuterium-based nature of the odorants rather than being caused by potential impurities of the compounds, we ran a set of experiments in which bees were differentially trained to discriminate pure compounds (‘h-ACP’ and ‘h-BNZ’) from the same respective compounds containing 0.5% of a different compound. Bees were not able to discriminate these ‘impure’ mixtures from their respective pure counterparts and showed the same response to both, even after 10 training cycles (figure 5a,c). Initial response differences represent odour generalization, not discrimination. By contrast, simultaneous experiments showed that control bees were discriminating two different pure odours after only one or two training cycles (figure 5b; ANOVA: p < 10−50; F = 223; n = 125 bees; figure 5d; ANOVA: p < 10−40; F = 192; n = 77 bees).
4. Discussion
Our results demonstrate that honeybees can learn deuterated odorants as fast as their common, hydrogen-based isotopic counterparts. More importantly, the bees learned to discriminate the common odorants from the deuterated ones, suggesting that they may rely on cues other than strictly structural ones and which may include intra-molecular vibrational spectra.
(a). Odour discrimination and generalization
Odours can be more reliably distinguished the more dissimilar odorant molecules are from one another. In insects and vertebrates, discrimination depends on differences in carbon chain lengths, molecular shape, differences in functional groups and other molecular parameters [11,41–44]. This explains why our two common aromatic test odours, ‘h-ACP’ and ‘h-BNZ’, are readily discriminated (figure 2b). However, for very similar odorants, discrimination is difficult even at high odour concentrations [44]; hence, we think that absolute odorant concentrations do not significantly affect our results, even though we have not measured them.
The trajectories of our differential learning curves (figures 3 and 4) indicate that the bees are sometimes confusing (generalizing) the two odours for several trials before they later discriminate them reliably. Our learning curves, however, differ from many published results where honeybees reach a high learning score (70–80%) after only two training trials. Our differential learning curves (figures 3 and 4) are more comparable with those shown by Paldie et al. [45] for similar compounds, where it takes the bees three (instead of one or two) trials to discriminate the odours. We therefore suggest that the respective common and deuterated compounds appear relatively similar to the bees, but different enough to be discriminated by them. For reasons that we do not know, the overall learning performance of the bees seemed lower than expected. We have used bees of the same strain and from the same source in previous learning experiments in which the bees generally reached higher learning scores [46]. The difference in treatment was that in the present study the bees were housed in a cage for 1–4 days, which may have affected their learning disposition. We also found much variation in the learning performance of individual bees, which was not further evaluated but is represented by the 95% CIs in figures 2–5. Similar inter-individual differences have been described in field studies and differences in learning performances may be involved in different foraging strategies of individual honeybee workers [47].
(b). Potential differences between odorants
When trained with a single odour (absolute conditioning), honeybees generalize (confuse) learned odours to a certain degree if they are chemically closely related (e.g. 1-octanol and 1-heptanol, or 1-octanol and 2-octanol) and the degree of generalization allows us to predict some kind of behavioural odour space, which seems to match physiological responses of the bee's primary olfactory centre in the brain [31]. In such an odour space, BNZ and ACP would be situated close together, differing in only one methyl group, and the respective hydrogen-based compounds should be indistinguishably close to their deuterated counterparts. However, using differential conditioning, bees could be trained to discriminate these odorants, suggesting that the latter occupy different locations in the odour space. Besides the effects that deuteration has on the vibrational properties of the molecules, the only other differences that might potentially affect the bees’ odour perception are the purity of the test compounds and potential differences in vapour pressures of the respective compounds. The gas chromatographs for the deuterated compounds supplied by the manufacturer (see the electronic supplementary material, figure S1) show only single, sharp peaks demonstrating that the compounds were of the highest purity. Likewise, the common odorants (‘h-ACP’, ‘h-BNZ’ and h-OCT) were of analytical standard purity, making it unlikely that impurities could have affected our results and the honeybee learning behaviours. This was confirmed by controls with deliberately introduced ‘impurities’, which the bees were not able to discriminate from the original compounds (figure 5).
Deuterated compounds may have a slightly lower volatility compared with their hydrogen-based counterparts based on their molecular mass. To the best of our knowledge, no data on the volatility of the deuterated compounds are available, differences should be small (about 3% difference in diffusivity for h-ACP versus d8-ACP [48] and insignificant as bees can only discriminate three- to fivefold (walking bees [49]), 10-fold (flying bees [50]) or 100-fold (proboscis extension reflex conditioning [51,52]) concentration differences in odorant mixtures. It is therefore unlikely that in our experiments the bees’ discrimination of deuterated and non-deuterated compounds was based on potentially present slight concentration differences. Rather, we suggest that the bees’ ability to discriminate common compounds from their respective deuterated counterparts is based on their difference in molecular-level vibrations.
(c). Molecular vibrations can explain the bees’ ability to discriminate isotopes
A molecule containing only non-exchangeable hydrogens, i.e. hydrogens that do not take part in bond breaking or formation will, from the shape point of view, be nearly indistinguishable from its hydrogen isotopomer. The electronic structure of the molecule remains unchanged. By contrast, deuteration causes large-scale changes in molecular vibration frequencies: it doubles the mass of hydrogen, and therefore lowers the vibrational frequencies of the carbon–hydrogen modes by 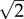 and of more complex modes by smaller amounts. It is therefore plausible that honeybees, like fruit flies [28], are responding to vibrational frequencies. This study does not prove this assumption, and it is likely that olfactory systems use any available cue for discriminating different odorants. Such cues might involve molecular vibrations as well as any of the other physico-chemical descriptors suggested to underlie olfactory discrimination [11]. More complex experiments can, in principle, resolve this issue. For example, fruit flies mistake an odorant containing a nitrile group for a deuterated one [28]. C–D and C ≡ N have little in common chemically but do share a vibrational stretch frequency.
and of more complex modes by smaller amounts. It is therefore plausible that honeybees, like fruit flies [28], are responding to vibrational frequencies. This study does not prove this assumption, and it is likely that olfactory systems use any available cue for discriminating different odorants. Such cues might involve molecular vibrations as well as any of the other physico-chemical descriptors suggested to underlie olfactory discrimination [11]. More complex experiments can, in principle, resolve this issue. For example, fruit flies mistake an odorant containing a nitrile group for a deuterated one [28]. C–D and C ≡ N have little in common chemically but do share a vibrational stretch frequency.
One important point to consider when discussing the potential of intra-molecular vibrations to contribute to, or underlie, OR interactions is the fact that insect OR proteins are different from those in vertebrates. While the latter are second messenger-coupled G proteins [1,3], the majority of insect ORs are most likely ligand-gated ion channels composed of one (or more) variable and probably odorant-specific subunit and one subunit (referred to as ORCO [53] present in all receptors [53–56]). It is therefore possible that intra-molecular vibrations affect or underlie the function of insect ORs in different ways than they do in vertebrate ORs. If that were the case, it might explain why compounds such as deuterated acetophenone can be discriminated from their hydrogen-based counterparts by flies [28] and bees (figure 4a,b), but apparently not by humans [22,27]. Insects, in general, have smaller numbers of OR proteins compared with vertebrates [8] and it is possible that vibrationally assisted olfaction [57] may provide an additional layer of odorant–OR interaction that could increase the insects’ ability to discriminate odorants.
Acknowledgements
We thank Dr Gloria DeGrandi-Hoffman of the USDA-ARS Carl Hayden Bee Research Center in Tucson, AZ, USA for permission to collect honeybees, Dr Luca Turin for advice and comments on the manuscript, and Jessica Burkey, Betsy Lukins, Marina Kinninger, Amar Raikhelkar, Audrey Roberts, Samantha Sahawneh, Veronica So, Swetha Uppalapati and Nicholas Woolstenhulme for help with the experiments.
References
- 1.Buck L, Axel R. 1991. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 (doi:10.1016/0092-8674(91)90418-X)90178-3) [DOI] [PubMed] [Google Scholar]
- 2.Firestein S, Darrow B, Shepherd GM. 1991. Activation of the sensory current in salamander olfactory receptor neurons depends on a G protein-mediated cAMP second messenger system. Neuron 6, 825–835 (doi:10.1016/0896-6273(91)90178-3) [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts P. 1999. Seven-transmembrane proteins as odorant and chemosensory receptors. Science 286, 707–711 (doi:10.1126/science.286.5440.707) [DOI] [PubMed] [Google Scholar]
- 4.Schild D, Restrepo D. 1998. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol. Rev. 78, 429–466 [DOI] [PubMed] [Google Scholar]
- 5.Bargmann CI. 2006. Comparative chemosensation from receptors to ecology. Nature 444, 295–301 (doi:10.1038/nature05402) [DOI] [PubMed] [Google Scholar]
- 6.Hildebrand JG, Shepherd GM. 1997. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595–631 (doi:10.1146/annurev.neuro.20.1.595) [DOI] [PubMed] [Google Scholar]
- 7.Strausfeld NJ, Hildebrand JG. 1999. Olfactory systems, common design, uncommon origins? Curr. Opin. Neurobiol. 9, 634–639 (doi:10.1016/S0959-4388(99)00019-7) [DOI] [PubMed] [Google Scholar]
- 8.Kaupp BU. 2010. Olfactory signaling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11, 188–200 (doi:10.1038/nrn2789) [DOI] [PubMed] [Google Scholar]
- 9.Araneda RC, Kini AD, Firestein S. 2000. The molecular receptive range of an odorant receptor. Nat. Neurosci. 3, 1248–1255 (doi:10.1038/81774) [DOI] [PubMed] [Google Scholar]
- 10.Hallem EA, Carlson JR. 2006. Coding of odors by a receptor repertoire. Cell 125, 143–160 (doi:10.1016/j.cell.2006.01.050) [DOI] [PubMed] [Google Scholar]
- 11.Haddad R, Khan R, Takahashi YK, Mori K, Harel D, Sobel N. 2008. A metric for odorant comparison. Nat. Methods 5, 425–429 (doi:10.1038/nmeth.1197) [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Firestein S. 2002. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 5, 124–133 [DOI] [PubMed] [Google Scholar]
- 13.Touhara K, Sengoku S, Inaki K, Tsuboi A, Hirono J, Sato T, Sakano H, Haga T. 1999. Functional identification and reconstitution of an odorant receptor in single olfactory neurons. Proc. Natl Acad. Sci. USA 96, 4040–4045 (doi:10.1073/pnas.96.7.4040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum DM, Rasmussen SGF, Kobilka BK. 2009. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (doi:10.1038/nature08144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobilka B. 2008. G-protein-coupled receptors. In Encyclopedia of molecular pharmacology: springer reference (http://www.springerreference.com) (eds Offermanns S, Rosenthal W.). Berlin, Germany: Springer; (doi:10.1007/SpringerReference_1378552011-01-3123:00:00UTC) [Google Scholar]
- 16.Hall SE, Floriano WB, Vaidehi N, Goddard WA. 2004. Predicted 3-D structures for mouse I7 and rat I7 olfactory receptors and comparison of predicted odor recognition profiles with experiment. Chem. Senses 29, 595–616 (doi:10.1093/chemse/bjh063) [DOI] [PubMed] [Google Scholar]
- 17.Zarzo M. 2007. The sense of smell: molecular basis of odorant recognition. Biol. Rev. Camb. Phil. Soc. 82, 455–479 (doi:10.1111/j.1469-185X.2007.00019.x) [DOI] [PubMed] [Google Scholar]
- 18.Moncrieff RW. 1949. What is odor? A new theory . Am. Perfumer 54, 453 [Google Scholar]
- 19.Amoore E. 1963. Stereochemical theory of olfaction. Nature 198, 271–272 (doi:10.1038/198271a0) [DOI] [PubMed] [Google Scholar]
- 20.Kato A, Touhara K. 2009. Mammalian olfactory receptors: pharmacology, G protein coupling and desensitization. Cell. Mol. Life Sci. 66, 3743–3753 (doi:10.1007/s00018-009-0111-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sell C. 2006. On the unpredictability of odor. Angew. Chem. 45, 6254–6261 (doi:10.1002/anie.200600782) [DOI] [PubMed] [Google Scholar]
- 22.Keller A, Vosshall LB. 2004. A psychophysical test of the vibration theory of olfaction. Nat. Neurosci. 7, 337–338 (doi:10.1038/nn1215) [DOI] [PubMed] [Google Scholar]
- 23.Dyson GM. 1938. The scientific basis of odor. Chem. Ind. 57, 647–651 (doi:10.1002/jctb.5000572802) [Google Scholar]
- 24.Turin L. 1996. A spectroscopic mechanism for primary olfactory reception. Chem. Senses 21, 773–791 (doi:10.1093/chemse/21.6.773) [DOI] [PubMed] [Google Scholar]
- 25.Turin L, Yoshii F. 2003. Structure–odor relations: a modern perspective. In Handbook of olfaction and gustation, (ed. Doty R.), pp. 275–294, 2nd edn. New York, NY: Marcel Dekker [Google Scholar]
- 26.Haffenden LJW, Yaylayan VA, Fortin J. 2001. Investigation of vibrational theory of olfaction with variously labelled benzaldehydes. Food Chem. 73, 67–72 (doi:10.1016/S0308-8146(00)00287-9) [Google Scholar]
- 27.Gane S, Georganakis D, Maniati K, Vamvakias M, Ragoussis N, Skoulakis EMC, Turin L. 2013. Molecular vibration-sensing component in human olfaction. PLoS ONE 8, e55780 1–8 (doi:10.1371/journal.pone.0055780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco MI, Turin L, Mershin A, Skoulakis EMC. 2011. Molecular vibration-sensing component in Drosophila melanogaster olfaction. Proc. Natl Acad. Sci. USA 108, 3979–3802 (doi:10.1073/pnas.1012293108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frisch Kv. 1914. Der Farbensinn und Formensinn der Biene. Zool. J. Physiol. 37, 1–238 [Google Scholar]
- 30.Menzel R, Erber J. 1978. Learning and memory in bees. Sci. Am. 239, 102–110 (doi:10.1038/scientificamerican0778-102)684402 [Google Scholar]
- 31.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. 2005. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 3, 718–732 (doi:10.1371/journal.pbio.0030060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giurfa M, Sandoz JC. 2012. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54–66 (doi:10.1101/lm.024711.111) [DOI] [PubMed] [Google Scholar]
- 33.Kuwabara M. 1957. Bildung des bedingten reflexes von Pavlovs Typus bei der Honigbiene, Apis mellifica. J. Fac. Hokkaido Univ. 13, 458–464 [Google Scholar]
- 34.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119 (doi:10.1037/0735-7036.97.2.107) [PubMed] [Google Scholar]
- 35.Ball P. 2011. The dawn of quantum biology. Nature 474, 272–274 (doi:10.1038/474272a) [DOI] [PubMed] [Google Scholar]
- 36.Page RE, Erber J, Fondrk MK. 1998. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J. Comp. Physiol. A 182, 489–500 (doi:10.1007/s003590050196) [DOI] [PubMed] [Google Scholar]
- 37.Scheiner R, Barnert M, Erber J. 2003. Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie 34, 67–72 (doi:10.1051/apido:2002050) [Google Scholar]
- 38.Pankiw T, Page RE. 2000. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav. Ecol. Sociobiol. 47, 265–267 (doi:10.1007/s002650050664) [Google Scholar]
- 39.Siegel AJ, Freedman C, Page RE. 2012. Ovarian control of nectar collection in the honey bee (Apis mellifera). PLoS ONE 7, e33465 (doi:10.1371/journal.pone.0033465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto Y, Menzel R, Sandoz JC, Giurfa M. 2012. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J. Neurosci. Methods 211, 159–167 (doi:10.1016/j.jneumeth.2012.08.018) [DOI] [PubMed] [Google Scholar]
- 41.Smith BH, Menzel R. 1989. The use of electromyogram recordings to quantify odorant discrimination in the honey bee, Apis mellifera. J. Insect Physiol. 35, 369–375 (doi:10.1016/0022-1910(89)90110-8) [Google Scholar]
- 42.Laska M, Galizia CG, Giurfa M, Menzel R. 1999. Olfactory discrimination ability and odor structure: activity relationships in honeybees. Chem. Senses 22, 457–465 (doi:10.1093/chemse/22.4.457) [DOI] [PubMed] [Google Scholar]
- 43.Sakura M, Okada R, Mizunami M. 2002. Olfactory discrimination of structurally similar alcohols by cockroaches. J. Comp. Physiol. A 188, 787–797 (doi:10.1007/s00359-002-0366-y) [DOI] [PubMed] [Google Scholar]
- 44.Wright GA, Smith BH. 2004. Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera). Chem. Senses 29, 127–135 (doi:10.1093/chemse/bjh016) [DOI] [PubMed] [Google Scholar]
- 45.Paldi N, Zilber S, Shafir S. 2003. Associative olfactory learning of honeybees to differential rewards in multiple contexts: effect of odor component and mixture similarity. J. Chem. Ecol. 29, 2515–2538 (doi:10.1023/A:1026362018796) [DOI] [PubMed] [Google Scholar]
- 46.Couvillon MJ, DeGrandi-Hoffman G, Gronenberg W. 2010. Africanized honeybees are slower learners than their European counterparts. Naturwis 97, 153–160 (doi:10.1007/s00114-009-0621-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomson JD, Chittka L. 2001. Pollinator individuality: when does it matter? In Cognitive ecology of pollination (eds Chittka L, Thomson JD.), pp. 191–213 Cambridge, UK: Cambridge University Press [Google Scholar]
- 48.Bittner ER, Madalan A, Czader A, Roman G. 2012. Quantum origins of molecular recognition and olfaction in Drosophila. J. Chem. Phys. 137, 1–7 (doi:10.1063/1.4767067) [DOI] [PubMed] [Google Scholar]
- 49.Kramer E. 1976. The orientation of walking honeybees in odour fields with small concentration gradients. Physiol. Entomol. 1, 27–37 (doi:10.1111/j.1365-3032.1976.tb00883.x) [Google Scholar]
- 50.Ditzen M, Evers J-F, Galizia CG. 2003. Odor similarity does not influence the time needed for odor processing. Chem. Senses 28, 781–789 (doi:10.1093/chemse/bjg070) [DOI] [PubMed] [Google Scholar]
- 51.Bhagavan S, Smith BH. 1997. Olfactory conditioning in the honey bee, Apis mellifera: effects of odor intensity. Physiol. Behav. 61, 107–117 (doi:10.1016/S0031-9384(96)00357-5) [DOI] [PubMed] [Google Scholar]
- 52.Pelz C, Gerber B, Menzel R. 1997. Odorant intensity as a determinant for olfactory conditioning in honeybees: roles in discrimination, overshadowing and memory consolidation. J. Exp. Biol. 200, 837–847 [DOI] [PubMed] [Google Scholar]
- 53.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (doi:10.1016/j.neuron.2004.08.019) [DOI] [PubMed] [Google Scholar]
- 54.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. 1999. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327–338 (doi:10.1016/S0896-6273(00)81093-4) [DOI] [PubMed] [Google Scholar]
- 55.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. 2008. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (doi:10.1038/nature06850) [DOI] [PubMed] [Google Scholar]
- 56.Wicher D, Schaefer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. 2008. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007–1011 (doi:10.1038/nature06861) [DOI] [PubMed] [Google Scholar]
- 57.Solov'yov IA, Po-Yao Chang P-Y, Schulten K. 2012. Vibrationally assisted electron transfer mechanism of olfaction: myth or reality? Phys. Chem. Chem. Phys. 14, 13 861–13 871 (doi:10.1039/c2cp41436h) [DOI] [PMC free article] [PubMed] [Google Scholar]