Abstract
Humans excel in cooperative exchanges between unrelated individuals. Although this trait is fundamental to the success of our species, its evolution and mechanisms are poorly understood. Other social mammals also build long-term cooperative relationships between non-kin, and recent evidence shows that oxytocin, a hormone involved in parent–offspring bonding, is likely to facilitate non-kin as well as kin bonds. In a population of wild chimpanzees, we measured urinary oxytocin levels following a rare cooperative event—food sharing. Subjects showed higher urinary oxytocin levels after single food-sharing events compared with other types of social feeding, irrespective of previous social bond levels. Also, urinary oxytocin levels following food sharing were higher than following grooming, another cooperative behaviour. Therefore, food sharing in chimpanzees may play a key role in social bonding under the influence of oxytocin. We propose that food-sharing events co-opt neurobiological mechanisms evolved to support mother–infant bonding during lactation bouts, and may act as facilitators of bonding and cooperation between unrelated individuals via the oxytocinergic system across social mammals.
Keywords: cooperation, food-sharing, bonding mechanism, chimpanzee, oxytocin, non-invasive hormone sampling
1. Introduction
The ability to cooperate is seen as crucial to the exceptional biological success of humans as a species [1–3]. Sharing food and allo-maternal childcare, among other behaviours, are common forms of cooperation, which frequently occur among related and unrelated individuals in hunter–gatherer and industrialized societies [4,5]. Despite the significance of cooperation as a biological phenomenon, its evolution and mechanisms remain poorly understood [3,6]. This is especially true for cooperation occurring between unrelated individuals in non-reproductive contexts.
Recent studies show that, in addition to humans, other social mammals form cooperative relationships between unrelated adults, which can last over months or years (chimpanzees [7], baboons [8,9] and feral horses [10]). Crucially, there is evidence that individuals who maintain such cooperative relationships have more offspring than those who do not [8,10–12]. Long-lasting cooperative relationships have also been referred to as strong social bonds [8–13], which are characterized by high rates of cooperative behaviours, such as grooming and food sharing [8–13].
Recent evidence suggests that an endocrinological mechanism promoting non-kin cooperative relationships may involve the oxytocinergic system [14,15]. In humans and other social mammals, the hormone oxytocin plays a central role in facilitating bonding between mother and offspring, and between mating partners [16–18]. Physiologically, oxytocin acts in the brain by reducing anxiety and fear [19,20], enhancing social memory [16–18,21] and activating neural reward circuits [21]. If exogenously administered, oxytocin enhances cooperative behaviour [22,23] between related animals, and behaviours associated with trust [24–26] and generosity [27] between unrelated humans. When centrally administered, oxytocin enhances both affiliation with bonding partners and aversion to strangers in unrelated female voles [14].
Exactly how cooperative bonds are established under the influence of oxytocin, however, is not well understood [16–18]. In humans, the concentration of plasma oxytocin is related to the perceived closeness to [28] or support received by [29] a partner, suggesting a relation between higher oxytocin levels and stronger social bonding. Such a link is also evident in chimpanzees, where grooming among bonding partners is associated with higher urinary oxytocin concentration than grooming between non-bonding partners [15]. Additionally, in humans, exogenously administered oxytocin enhances the encoding of social memory, both of positive [30] and negative [31] events, suggesting that higher oxytocin levels improve memorization of social interactions. This is particularly relevant for the question of how cooperative relationships are maintained and formed between two partners, especially if the cooperation is based on reciprocation [32,33]. In sum, higher oxytocin concentrations may be related to better cooperation via stronger bonding and better social memory.
Methodologically important is the fact that oxytocin not only acts within the brain, but is also released into the peripheral circulation before being excreted in urine, although the exact nature of the relationship between central and peripheral releases have yet to be clarified [34–39]. Significantly, peripheral measures of oxytocin from plasma or urine correlate positively with psychological and behavioural patterns, such as aversion reduction in male mice [19], social contact in marmosets [40], lactation in rhesus macaques [41], rates of affiliative behaviours in pair-bonded tamarins [42] and grooming in closely bonded chimpanzees [15], indicating that peripheral oxytocin levels reflect central processing of oxytocin.
In this study, we examine the association of urinary oxytocin levels with food-sharing events between kin and non-kin. This cooperative behaviour is widespread across the animal kingdom, with well-documented cases in insects, birds and mammals [43–47]. Food sharing has been defined as the owner of a food resource allowing others to access it, despite the fact that it could be monopolized [47]. The benefits of getting access to food already in somebody else's possession are self-evident, but what do the donors gain? Plausible explanations are that donors benefit through mating advantages [43,44], maintenance of pair bonds [43], reduction of harassment [47], or future reciprocation and exchange [48].
In most species, food sharing occurs between kin, such as parents provisioning their offspring (birds [44], mammals [45,47,49]), or between mating partners (insects [44], birds [43], mammals [45,47,50], humans [51]). Food sharing between unrelated individuals outside the mating context is rare but has been reported for humans, vampire bats, chimpanzees and bonobos [13,46,48,49,52]. In chimpanzees and vampire bats, food sharing typically occurs between individuals that also engage in other cooperative behaviours at higher rates, such as grooming and providing coalitionary support [13,49,52–54]. In chimpanzees, it is often discussed in the context of meat sharing after hunting other primates [50,52–56]. Meat is a high-value, monopolizable resource, which frequently precipitates fighting and sharing [56]. There is evidence that males share meat with other males in exchange for receiving support [54], or with females to receive mating opportunities [50] (cf. [54]). Additional reasons proposed for meat sharing are to reinforce cooperative hunting by discriminating against cheaters [56], or to reduce harassment of begging in order to allow the donor time to eat [55]. Food sharing in chimpanzees, however, is not restricted to meat but has also been reported with non-meat foods, for example honey [52], or large fruits such as Carica papaya [57] or Treculia africana [52]. For some food, the access to food is monopolizable and can be shared, such as when access to stone hammers for cracking Panda oleosa nuts is limited [52] or when holes in dead trunks of Raphia farinifera trees are too small for more than one chimpanzee to simultaneously access the rotting wood inside [58].
To investigate whether food sharing in chimpanzees is linked to higher urinary oxytocin levels, we compared subjects' urinary oxytocin levels after single food-sharing events and after other types of social feeding without sharing in the Sonso community of Budongo Forest, Uganda [59]. If urinary oxytocin levels after food sharing were higher than after social feeding, this would indicate that food sharing and bonding are closely linked. Given the hypothesized importance of food sharing for kin and pair bonding [44,46,51], we examined the relation between urinary oxytocin concentrations, relatedness and bondedness. With regard to the proposed importance of meat sharing in male–male bonding [54] and in the sex-for-meat hypothesis [50], we further investigated the relationship between urinary oxytocin concentrations, meat sharing and the sex of sharers. Finally, to examine whether different cooperative acts might be associated with different urinary oxytocin levels, we investigated the magnitude of the difference in urinary oxytocin levels after food-sharing events compared with grooming events.
2. Material and methods
We analysed 79 urine samples from 26 chimpanzees (females: 10 adults, i.e. more than 15 years of age; three subadults, i.e. between 10 and 15 years of age; males: six adults, seven subadults; mean sample per chimpanzee ± s.d. = 3.0 ± 1.97) of the Sonso community in Budongo Forest, Uganda [59], between January 2009 and July 2010. We collected urine samples if subjects had engaged in no affiliative behaviours (e.g. grooming or copulation) other than the target behaviour in the hour prior to urination. This was determined through focal sampling [60] of subjects or all-occurrence sampling [60] of chimpanzee subgroups (or ‘parties’). The Composite Relationship Index (CRI) and dominance hierarchies were also determined from focal or all-occurrence sampling conducted between October 2009 and July 2010 by C.C., R.M.W. and seven experienced field assistants. Feeding time, food source and party composition were recorded in 15 min scan samples [60]. Faecal samples for genetic analysis of kinship were collected throughout the study period.
(a). Behavioural criteria for food sharing
Food sharing occurred when one individual was allowed access to food in possession of another, in the absence of aggression [47] (see electronic supplementary material, text S1). This could happen in one of two ways. Food was passively shared, such that the possessor allowed another to take the food, or to take over access to the food supply, in the absence of overt coercion in the form of aggression or screaming (see electronic supplementary material, video S1). Alternatively, food was actively shared, such that the possessor extended the food towards the receiver and released it in the absence of aggression (see electronic supplementary material, video S2). Food-sharing events could thus be single momentary events, multiple momentary events or protracted events (see electronic supplementary material, table S1). Food-sharing events could occur with begging behaviour. Begging definitions were taken from Gilby [55], with our additions shown in brackets. Begging was either (i) sitting and staring at the food item (or possessor), (ii) reaching towards but not touching the food item or possessor (with or without whimpering), (iii) touching the food item or possessor, or (iv) placing a hand directly over the possessor's mouth. Begging behaviours (iii) and (iv) were considered to be low and high harassment, respectively [47,55,61].
(b). Long-term cooperation level and composite relationship index
We assessed the quality of relationships by calculating all-occurrence rates of the following behaviours over the current and preceding annual quarters: coalitionary support, food sharing, grooming, staying in (less than 1 m) proximity and aggression [62,63]. For all behaviours, each occurrence was recorded as a single event. From the resulting rates, we calculated the CRI, a measure of social bond strength [15,64]. The CRI is calculated over a period of three months and gives socio-positive (given or received food sharing, coalitionary support, allo-grooming and resting in less than 1 m proximity) and socio-negative (aggression given or received) behaviours equal weight:
where SP1 = rate of grooming bouts plus rate of resting in 1 m proximity, SP2 = rate of food sharing plus rate of coalitionary support, NP = rate of aggression, i = individual and j = dyad partner. The index is positive when each individual within a dyad initiates on average more socio-positive than socio-negative interactions. ‘Bond partners’ were defined as dyads having a net socio-positive relationship lasting more than or equal to six months (at least two consecutive blocks of three months). This can occur through either a mutual socio-positive relationship (CRI > 0) during the annual quarter of the experiment and the preceding quarter, or a large mutual socio-positive relationship (CRI > 10) during one of the quarters and a socio-neutral or positive relationship (CRI ≥ 0) during the other quarter. According to this, 1.9% of kin dyads and 1.6% of non-kin dyads reached bond-partner status.
(c). Urine sampling and oxytocin extraction
Our target behaviours were single food-sharing events or 1 h of feeding in the presence of chimpanzees without sharing food. We collected urine samples as described by Crockford et al. [15]. Specifically, urine was collected 15–60 min after the target behaviour (time window of urinary clearance of oxytocin for primates [65]). Occasionally, subjects were sampled after engaging in more than one food-sharing event within the required time window. In both conditions, samples were not collected if grooming or copulation also occurred within 60 min prior to urination, as both of these behaviours are likely to independently increase urinary oxytocin levels [39]. A volume of 1.1 ml of the collected urine was pipetted into a cryovial containing 100 µl of 0.5 N phosphoric acid and stored on ice in a thermo flask. Solid-phase extraction was conducted later the same day [40]. All samples were then frozen until transported for assaying in the Assay Services Unit at the NPRC, Madison, WI, using an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI; catalogue no. 901-153). To compensate for variation in urine concentration, we measured creatinine (crea) levels in each sample [66] and expressed all oxytocin values as pg mg−1 crea. We validated the measurement of urinary chimpanzee oxytocin levels through parallelism and accuracy tests, as described in a previous paper [15].
(d). Dominance relationships
We collected pant-grunt vocalizations as a unidirectional indicator of dominance relationships, given by the subordinate to the dominant [53]. We calculated a linear dominance hierarchy for Sonso chimpanzees on the basis of the pant-grunts using Mat Man v. 1.1 (see electronic supplementary material, text S2). Donors and receivers of shared food were assigned a relative dominance relationship according to the hierarchy matrix.
(e). Kin relationships
We collected fresh faecal samples, stored and extracted DNA following protocol of [15]. Dyads were classified as kin (n = 11) or non-kin (n = 10) according to a combination of (i) parentage analyses based on autosomal microsatellites and (ii) mitochondrial DNA and Y-chromosome microsatellite haplotype sharing information [13,67]. We were able to show that that all kin partners were either mother–offspring (n = 10) or maternal siblings (n = 1), and none of the non-kin partners were such close maternal relatives (see electronic supplementary material, text S3, S4 and table S5).
(f). Statistics
Urinary oxytocin (OT) concentrations were log10-transformed to fit a normal distribution. Five generalized linear mixed models (GLMM) were run with maximum-likelihood estimates in SPSS v. 20, testing the effect of the predictor variables shown in table 1 on the response variable of log10-transformed urinary oxytocin levels. In model 1, we compared food-sharing events with social feeding without food sharing. We tested the effect of whether food is shared or not and its monopolizability on the urinary oxytocin concentration after controlling for subjects’ sex and age. Subjects’ identity was included as a random factor. Model 2 was divided into two separate tests due to small sample size. Model 2a investigated the variation within the food-sharing samples with regard to the sharers’ relationship. We tested the effect of close kinship and bond quality on the urinary oxytocin concentration after controlling for whether the subject received the food. Subjects’, partners’ (interaction partner) and dyads’ identity were included as random factors. Model 2b examined the variation within the food-sharing samples with regard to the possible function of meat sharing. We tested the effect of whether the shared food was meat, and the sex combination of the sharers, on the urinary oxytocin concentration. Subjects’, partners’ and dyads’ identity were included as random factors. Finally, in model 3, we compared urinary oxytocin levels after food sharing with those after another cooperative behaviour, grooming (taken from [15]). In model 3a, we tested the effect of food sharing compared with grooming on the urinary oxytocin concentrations after controlling for subjects’ sex. Subjects’ identity, partners’ identity and dyads’ identity were all included as random factors. In model 3b, we tested the effect of five different behavioural contexts (food sharing with bond partner, food sharing with non-bond partner, grooming with a bond partner, grooming with a non-bond partner and control situations) on the urinary oxytocin concentrations, while controlling for subjects’ sex. Subjects’ identity was included as a random factor.
Table 1.
Predictor variables tested in the GLMMs. Model 1 refers to the GLMM shown in table 3, which tests sharing and non-sharing samples together (n = 79). Model 2 refers to the GLMM presented in table 4, using only food-sharing samples (n = 33). Model 3 refers to GLMM presented in table 5, contrasting food-sharing and grooming samples (n = 182).
| model | name | score | definition | n |
|---|---|---|---|---|
| 1,3 | sex of subject | male | subject is male | 46, 102 |
| female | subject is female | 33, 80 | ||
| 1 | age of subject | adult | age >15 years | 51 |
| subadult | age ≤15 years | 28 | ||
| 1 | monopolizability of food | high | food clumped in one piece or cluster | 37 |
| low | many food clusters, but single cluster is defendable by one individual | 42 | ||
| 1 | food share | yes | partners share food | 33 |
| no | partners do not share food | 46 | ||
| 2a | bond type | bond | sharers are bond partners | 24 |
| non-bond | sharers are not bond partners | 9 | ||
| (2a) | CRIa | continuous | mean CRI over two consecutive quarters of the year | 33 |
| 2a | kin relation | kin | sharers are kin-related | 18 |
| non-kin | sharers are not kin-related | 15 | ||
| 2a | sharing direction | receiving | subject receives food | 20 |
| not receiving | subject does not receive food | 13 | ||
| 2b | sex combination | F–F | sharers are both female | 10 |
| M–F | one sharer is male, the other is female | 14 | ||
| M–M | sharers are both males | 9 | ||
| 2b | food category | meat | meat is shared | 13 |
| not meat | non-meat resource is shared | 20 | ||
| 3 | behavioural context | control | resting or social feeding without food sharing | 71 |
| groom non-bond | grooming with a non-bond partner | 34 | ||
| groom bond | grooming with a bond partner | 44 | ||
| food share non-bond | sharing food with a non-bond partner | 9 | ||
| food share bond | sharing food with a bond partner | 24 |
aIn a control run of model 2a the continuous variable Composite Relationship Index (CRI) replaced categorical variable ‘bond type’ in model 2a (see Material and methods).
Five outliers (more than 2 s.d.) were excluded from the food-sharing dataset (three non-sharing and two sharing samples) to be sure that they were not driving any main effects. Their distribution relative to the main dataset is shown in the electronic supplementary material (figure S1). We excluded 13 outliers (more than 2 s.d.) from the grooming dataset for the comparison between grooming and food sharing. Nonetheless, when we ran the GLMMs with the full dataset, including the outliers, the results remained remarkably similar to the GLMM results excluding the outliers (see electronic supplementary material, tables S2–S4). Variables did not exhibit problems of collinearity [68] (Kendall's τ and Spearman's r < 0.7 in all cases). As a check of the overall significance of all predictor variables, we ran likelihood ratio tests comparing the full model with the respective null model (comprising only the random effects). We only considered significant effects of the individual predictors if the full model explained the variance significantly better than the null model.
As models 2a and 2b investigated many predictor variables relative to the number of cases (n = 33, d.f. = 3 or d.f. = 5), reduced power may have led to false negatives (i.e. erroneously non-significant effects), as well as some risk of instability in the derived estimates. Hence, we ran an additional set of univariate GLMMs with each including only one of the predictor variables at a time (and the same random effects as in the full model). None of the predictor variables tested in the univariate models reached significance (bond type: F1,33 = 0.074, p = 0.787; CRI: F1,33 = 0.277, p = 0.602; kin relationship: F1,33 = 0.020, p = 0.888; sharing direction: F1,33 = 0.008, p = 0.931; food category: F1,33 = 1.289, p = 0.264; sex combination: F2,33 = 1.200, p = 0.314), which showed that the lack of significance in models 2a and 2b was unlikely to be due to power issues.
3. Results
(a). Characteristics of food-sharing events
In 2009, we observed 42 food-sharing events (without overt aggression occuring during the begging event) in the Sonso community. Controlling for the overall observation time per male or female, respectively, males showed an average sharing rate of 0.00187 h−1 and females showed an average sharing rate of 0.00176 h−1. All food-sharing events sampled included begging behaviour by the receiver before receiving food from the donor. While the lower level of harassment did occur often, the highest level of harassment, the receiver placing his hand over the mouth of the donor, was never observed. Urine samples were obtained after 33 food-sharing and 46 social feeding events. Food items shared were meat (46%), fruit (30%), rotten wood pith (18%) and honey (6%; electronic supplementary material, table S1). Two-thirds of all donors were dominant over the receivers (table 2). Among non-kin food-sharing partners, three quarters of all donors were dominant. In 25% of the cases, we sampled food sharing between unrelated males. Less than 10% of the events sampled involved sharing between non-kin males and females in a possible meat-for-sex exchange context (table 2).
Table 2.
Food-sharing events described with respect to relative dominance and sex combination of sharing dyads.
| kin (n) | non-kin (n) | total (n) | |
|---|---|---|---|
| dominant donor | 10 | 11 | 21 |
| mutual food sharing | 3 | 2 | 5 |
| subordinate donor | 5 | 2 | 7 |
| sex | |||
| ♂ → ♂ | 1 | 8 | 9 |
| ♂ → ♀ | 3 | 4 | 7 |
| ♀ → ♂ | 7 | 0 | 7 |
| ♀ → ♀ | 7 | 3 | 10 |
(b). Link between food sharing and urinary oxytocin levels
In model 1, we investigated whether urinary oxytocin levels were higher after food sharing compared with social feeding events without food sharing. The full model explained the variation better than the null model (likelihood ratio test: χ2 = 20.24, d.f. = 4, p < 0.002). Urinary oxytocin concentrations after social feeding events were significantly higher with food sharing than without (F1,79 = 9.623, p = 0.003, table 3 and figure 1; mean log10OTshare ± s.e. = 1.407 ± 0.102 pg mg−1 crea versus mean log10OTnon-share ± s.e. = 0.884 ± 0.066 pg mg−1 crea). Potentially confounding variables, such as the sex or age of the subjects, or the monopolizability of the food, did not explain a significant amount of the variance in the urinary oxytocin levels (table 3).
Table 3.
Variables influencing urinary oxytocin concentrations across both food-sharing and non-food-sharing samples (model 1). Predictor variables and parameters entered in the models: sex of subject (male, female), age of subject (adult, subadult), monopolizability of food (high, low), food share (yes, no). n = 79 samples collected either after sharing events (n = 33 urine samples) or after non-sharing events (n = 46 urine samples) from 26 subjects (n = 13 males, n = 13 females). Likelihood ratio test: χ2 = 20.24, d.f. = 4, p < 0.001. Response variable: log10 oxytocin (pg mg−1 crea). Random factor: subject ID. Bold: p < 0.05.
| predictor | d.f. | F | p | parameter | β | t | p |
|---|---|---|---|---|---|---|---|
| sex of subject | 1 | 0.127 | 0.725 | male | −0.043 | −0.357 | 0.725 |
| age of subject | 1 | <0.000 | 0.989 | adult | −0.002 | −0.014 | 0.989 |
| monopolizability of food | 1 | 1.522 | 0.221 | high | 0.171 | 1.234 | 0.221 |
| food share | 1 | 9.623 | 0.003 | share | 0.427 | 3.102 | 0.003 |
Figure 1.
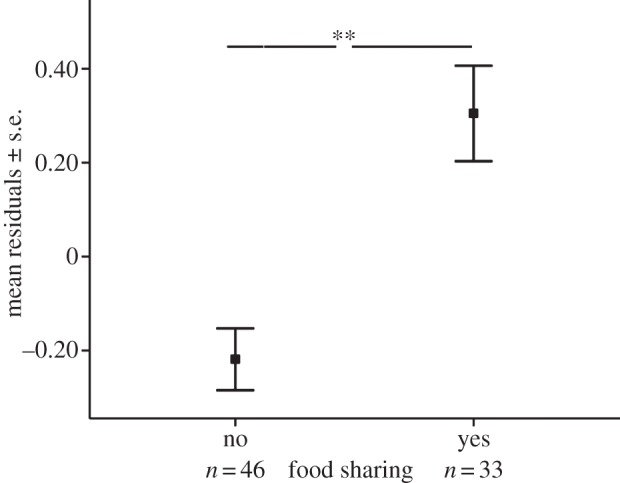
Effect of food sharing versus social feeding without sharing on the urinary oxytocin concentrations in wild chimpanzees. Residuals are shown ± standard error following model 1 (total of n = 79 samples; 26 subjects; **p < 0.01).
Model 2 included only the urine samples that followed food-sharing events to determine what factors were linked to variation in urinary oxytocin levels during sharing events. Neither models 2a or 2b explained the variation better than the null model (likelihood ratio test model 2a: χ2 = 0.08, d.f. = 3, n.s.; model 2b: χ2 = 6.24, d.f. = 5, n.s.). The results of model 2a were similar when exchanging the binary variable of bond quality with the continuous variable of the CRI (likelihood ratio test model: χ2 = 0.36, d.f. = 3, n.s.). Following sharing events, we found no significant differences in urinary oxytocin levels for any of the predictor variables (table 4a,b): kin or non-kin sharers (kin: mean log10OT ± s.e. = 1.42 ± 0.14 pg mg−1 crea; non-kin: mean log10OT ± s.e. = 1.39 ± 0.15 pg mg−1 crea), bond or non-bond partners (bond: mean log10OT ± s.e. = 1.42 ± 0.11 pg mg−1 crea; non-bond: mean log10OT ± s.e. = 1.36 ± 0.23 pg mg−1 crea), subject received food or not (receive: mean log10OT ± s.e. = 1.40 ± 0.16 pg mg−1 crea; not receive: mean log10OT ± s.e. = 1.41 ± 0.14 pg mg−1 crea), meat was shared compared with other foods (meat: mean log10OT ± s.e. = 1.55 ± 0.18 pg mg−1 crea; other foods: mean log10OT ± s.e. = 1.32 ± 0.12 pg mg−1 crea), and food was shared between females, males or between both sexes (F–F: mean log10OT ± s.e. = 1.36 ± 0.11 pg mg−1 crea; M–F: mean log10OT ± s.e. = 1.57 ± 0.17 pg mg−1 crea; M–M: mean log10OT ± s.e. = 1.21 ± 0.17 pg mg−1 crea). Finally, the interaction of meat and sex combination in sharing dyads was not significant (table 4b).
Table 4.
Variables influencing urinary oxytocin concentrations of food-sharing samples only. (a) Model 2a—predictor variables and parameters entered in the model: bond type (bond, non-bond), kin relationship (kin, non-kin), sex combination (female–female, male–female, male–male). Likelihood ratio test: χ2 = 0.08, d.f. = 3, n.s.. (b) Model 2b—predictor variables and parameters entered in the model: food category (meat, not meat), sharing direction (subject: receiving, not receiving) sex combination (female–female, male–female, male–male). Likelihood ratio test: χ2 = 6.24, d.f. = 5, n.s.; n = 33 samples, 18 subjects. (a,b) Response variable: log10 oxytocin (pg mg−1 crea); random factors: subject ID, partner ID, dyad ID.
| predictor | d.f. | F | p | parameter | β | t | p |
|---|---|---|---|---|---|---|---|
| (a) model 2a | |||||||
| bond type | 1 | 0.059 | 0.809 | bond | 0.074 | 0.244 | 0.809 |
| kin relationship | 1 | 0.005 | 0.944 | kin | −0.020 | −0.071 | 0.944 |
| sharing direction | 1 | 0.004 | 0.951 | receiving | −0.013 | −0.062 | 0.951 |
| (b) model 2b | |||||||
| food category | 1 | 1.940 | 0.173 | meat | 0.443 | 1.055 | 0.299 |
| sex combination | 2 | 2.371 | 0.109 | FF | 0.480 | 1.171 | 0.250 |
| MF | 0.522 | 1.274 | 0.212 | ||||
| food category × sex combination | 2 | 0.218 | 0.805 | FF–meat MF–meat MM–meat |
−0.350 0.065 0 |
−0.499 0.128 0 |
0.621 0.899 0 |
(c). Comparison of urinary oxytocin levels following either food-sharing or grooming events
Model 3a explained the variation better than the null model (likelihood ratio test model: χ2 = 19.11, d.f. = 2, p < 0,001). Urinary oxytocin levels following food-sharing events (mean log10OT ± s.e. = 1.41 ± 0.10 pg mg−1 crea) were significantly higher than following grooming events (mean log10OT ± s.e. = 0.99 ± 0.05 pg mg−1 crea; table 5a and figure 2a). This result was confirmed when analysing a subset of the data for a within-subject comparison of urinary oxytocin levels after sharing food (mean log10OT ± s.e. = 1.79 ± 0.32 pg mg−1 crea) and grooming (mean log10OT ± s.e. = 1.11 ± 0.22 pg mg−1 crea) using only cases when subjects had interacted with the same partner (Wilcoxon exact: n = 9, T+ = 40, p = 0.035). We also found that females had marginally significantly higher urinary oxytocin levels following food sharing than males (table 5a).
Table 5.
Comparison of variables influencing urinary oxytocin concentrations across different behavioural or social contexts. (a) Model 3a—contrasting grooming and food sharing only. Predictor variables and parameters entered in the model: behavioural context (grooming, food sharing), sex of subject (female, male). Random factors: subject ID, partner ID, dyad ID. Likelihood ratio test: χ2 = 19.11, d.f. = 2, p < 0.001. (b) Model 3b—contrasting behavioural context and dyads' bond quality. Predictor variables and parameters entered in the model: behavioural context (control (resting or feeding), grooming with non-bond partner, grooming with bond partner, food sharing with non-bond partner, food sharing with bond partner), sex of subject (female, male). Random factor: subject ID. Likelihood ratio test: χ2 = 36.47, d.f. = 5, p < 0.001. n = 182 samples, 34 subjects. (a,b) Parameter estimates: the context with 0 was compared with remaining contexts; parameter estimates of variables in italics were taken from a re-run of the same model with a different order of parameter entry. Bold: p < 0.05. Response variable: log10 oxytocin (pg mg−1 crea).
| predictor | d.f. | F | p | parameter | β | t | p |
|---|---|---|---|---|---|---|---|
| (a) model 3a | |||||||
| behavioural context | 1 | 16.496 | <0.001 | grooming | −0.42 | −4.062 | <0.001 |
| food sharing | 0 | 0 | 0 | ||||
| sex of subject | 1 | 4.193 | 0.044 | female | 0.20 | 2.048 | 0.044 |
| (b) model 3b | |||||||
| behavioural context | 4 | 9.821 | <0.001 | control | −0.51 | −4.667 | <0.001 |
| groom non-bond | −0.64 | −5.183 | <0.001 | ||||
| groom bond | −0.28 | −2.403 | 0.017 | ||||
| food-share non-bond | −0.03 | −0.145 | 0.885 | ||||
| food-share bond | 0 | 0 | 0 | ||||
| control | −0.48 | −2.959 | 0.004 | ||||
| groom non-bond | −0.61 | −3.538 | 0.001 | ||||
| groom bond | −0.25 | −1.522 | 0.130 | ||||
| food-share non-bond | 0 | 0 | 0 | ||||
| sex of subject | 1 | 0.245 | 0.624 | female | 0.04 | 0.494 | 0.624 |
Figure 2.
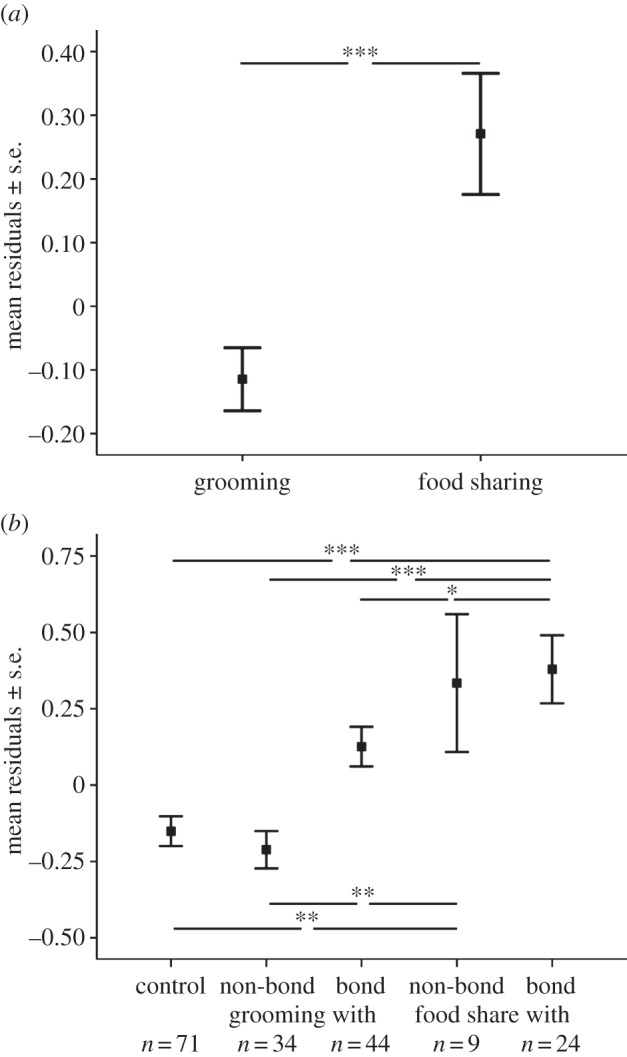
Effect of (a) food sharing compared with grooming and (b) five behavioural and social contexts on the urinary oxytocin concentration in wild chimpanzees. Residuals are shown ± standard error following model 3. Number of samples included in each context are shown beneath each plot (total n = 182 samples, 34 subjects; *p < 0.05, **p < 0.01, ***p < 0.001).
When examining urinary oxytocin levels in association with the type of cooperative behaviour and quality of the relationship (model 3b), urine collected after food sharing between bond partners showed the highest oxytocin concentrations (mean log10OT ± s.e. = 1.42 ± 0.11 pg mg−1 crea; F1,182 = 9.821, p < 0.001). This was not significantly higher than urinary oxytocin levels collected after food sharing with non-bond partners (mean log10OT ± s.e. = 1.36 ± 0.23 pg mg−1 crea) but was significantly higher than urinary oxytocin levels collected after grooming with bond partners (mean log10OT ± s.e. = 1.13 ± 0.07 pg mg−1 crea). By contrast, urine collected after grooming with non-bond partners (mean log10OT ± s.e. = 0.80 ± 0.07 pg mg−1 crea) and after control events (mean log10OT ± s.e. = 0.90 ± 0.05 pg mg−1 crea) both showed the lowest urinary oxytocin levels (table 5b and figure 2b).
4. Discussion
Our results provide empirical evidence that food-sharing events in chimpanzees are associated with significantly higher urinary oxytocin levels than non-sharing social feeding events. These effects were independent of the age of the subject and the monopolizability of the food. In one model, however, the sex of the subject showed a marginally significant effect such that female urinary oxytocin levels, in contrast to those of males, were higher following food sharing. High urinary oxytocin levels following food sharing were independent of whether subjects gave or received food, shared with kin or non-kin, shared with an established bond partner or not, or shared meat or other food types. Thus, our results suggest a direct link between food-sharing events and urinary oxytocin levels.
When comparing current results with results from our previous study on oxytocin and grooming [15], we found that urinary oxytocin levels associated with food-sharing events were significantly higher than those associated with grooming [15]. The high oxytocin concentrations could indicate that the relatively rare behaviour of food sharing had a stronger bonding effect compared with the more frequent grooming behaviour, although this remains to be tested. Furthermore, in contrast to grooming contexts, an existing social bond with the food-sharing partner was not associated with higher oxytocin levels, although it should be noted that there were relatively few food-sharing samples between non-bond partners. Sobolewski et al. [69] have shown that food sharing is linked to low urinary testosterone levels in wild male chimpanzees. As in some species, low testosterone is linked to nurturance (i.e. behaviours that involve gentle warm contact with others) [70] and high oxytocin is linked to social bonding [70], and together these results suggest that food sharing might provide the optimal conditions for social bonding (see [70]). We therefore hypothesize that food sharing may be a key behaviour for social bonding in chimpanzees.
Our results are inconsistent with the hypothesis that food sharing between non-kin is caused by manipulation, particularly through harassment via persistent begging [47,55], whereby only recipients gain benefits [6,71]. Given that in this study we found a positive association between urinary oxytocin levels and food sharing, it seems likely that food sharing is an act linked with social bonding. We do not exclude the possibility that harassment avoidance may be a motivation for some forms of food sharing, nor that bonding can only occur in the absence of harassment. Indeed, the possibility that food sharing may serve several functions in chimpanzees seems supported by several studies [61,72]. Also, it should be noted that in contrast to the Gombe field site (Tanzania) where the harassment avoidance hypothesis was examined [55], in Budongo we only observed low and not high harassment levels. One important finding in our study was that both receivers and donors of food had higher urinary oxytocin levels after food-sharing compared with non-sharing events, suggesting that both perceived the interaction positively [70].
(a). Food-sharing–lactation hypothesis
Food sharing is often observed in sexual interactions of insects and birds, with males offering food to females in return for matings [43,44], as well as in parent–offspring interactions (e.g. provisioning) [43,45]. In mammals, lactation has been described as the primary form of food sharing [73], and is well known to be connected to peripheral and central oxytocin release in mammals [34,74]. This positive feedback circuitry is considered a key mechanism for bond formation between mother and infant [34,75]. In some mammals, maternal care and provisioning of food, however, aids infant survival beyond the age of lactation [75]. It is thought that the interface between oxytocin and the dopaminergic reward system may contribute to the success of mother–infant and pair-bonding processes [18,75–77]. We therefore posit that food-sharing events between unrelated adults link into ancient mammalian neural ‘hardware’ that evolved in the context of lactation-related oxytocin release. Initially, this mechanism may have evolved to maintain bonds between mother and offspring beyond the age of weaning. It may then have evolved further, promoting bond formation and maintenance in non-kin cooperative relationships.
(b). Oxytocin circuitry co-opted for non-kin cooperation hypothesis
What makes food sharing in chimpanzees unusual in the animal kingdom is that, as well as occurring between kin [52] and in sexual contexts [50], food sharing also occurs between non-kin adults in non-sexual contexts [13,15,52,53]. Its occurrence is non-random, given that food sharing is more likely within dyads that engage in high rates of other cooperative behaviours [7,15,52,78]. Our results, however, suggest that urinary oxytocin levels are not based on prior bonding status of the sharing dyad, although given that we had relatively few samples following food sharing with non-bond partners, this is a tentative result. In addition, urinary oxytocin levels were high for both donors and receivers of food, suggesting that food sharing may have a bonding effect between sharers. This, in conjunction with recent evidence showing similar levels of urinary oxytocin in both groomers and groomees [15], leads us to posit the second hypothesis that the oxytocin-related mechanism associated with food-sharing and grooming events, although initially evolved to enable kin and sexual bonds, has, at least in some species, been co-opted to promote non-kin social bonds in non-sexual contexts. Therefore, such a hormonal mechanism would enable long-term cooperative relationships between non-kin to evolve.
As urinary oxytocin had similar levels in both donors and receivers, both may experience an immediate reward from sharing, as oxytocin is known to act on areas in the brain associated with reward and reinforcement [18]. Also, as oxytocin is thought to act on positive feedback circuits [16,17,21], sharers may experience a mutual increase in positive attitude [15,79,80], resulting in the promotion of stronger social bonds and longer-term benefits of more frequent cooperation [23,26,80]. Such links between food sharing and long-term benefits have been described on a behavioural level for chimpanzees reciprocally sharing meat [7,78], and exchanging meat for sex [50] or agonistic support [54]. Food sharing may, in effect, act as a trigger and predictor of cooperative relationships. This link between food sharing and oxytocin found in chimpanzees may also be relevant for humans [70], where pro-social behaviour has often been linked to food sharing and provisioning [46,81,82]. In the end, the word ‘companion’ (Lat.: com [=with], panis [=bread]) may be more literal than previously thought.
Acknowledgements
We thank Fred Babweteera and our field assistants Monday Gideon, Jackson Okuti, Sam Adue and Jacob Alio, Anja Weltring for organizing sample shipments, L. Vigilant, V. Reynolds and Z. Zommers for providing additional faecal samples, and Carolyn Rowney for genetic laboratory analyses. The Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and the President's office gave permission to conduct this study.
Funding statement
Financial support was provided by the British Academy, the Wenner-Gren Foundation for Anthropological Research, the Leverhulme Trust (Research Leadership Award), the Max Planck Institute for Evolutionary Anthropology and the Wisconsin National Primate Research Center (NIH NCRR000167 support of laboratory). The data of this study are available on request. We acknowledge the Royal Zoological Society of Scotland for providing core funding to the Budongo Conservation Field Station.
References
- 1.Bowles S. 2006. Group competition, reproductive levelling, and the evolution of human altruism. Science 314, 1569–1572 (doi:10.1126/science.1134829) [DOI] [PubMed] [Google Scholar]
- 2.Boyd R. 2006. The puzzle of human sociality. Science 315, 1555–1556 (doi:10.1126/science.1136841) [DOI] [PubMed] [Google Scholar]
- 3.Nowak MA. 2006. Five rules for the evolution of cooperation. Science 314, 1560–1563 (doi:10.1126/science.1133755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd R, Richerson PJ. 2009. Culture and the evolution of human cooperation. Phil. Trans. R. Soc. B 364, 3281–3288 (doi:10.1098/rstb.2009.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill KR, et al. 2011. Co-residence patterns in hunter–gatherer societies show unique human social structure. Science 331, 1286–1289 (doi:10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 6.Clutton-Brock TH. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 7.Mitani JC. 2009. Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640 (doi:10.1016/j.anbehav.2008.11.021) [Google Scholar]
- 8.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104 (doi:10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Female chacma baboons form strong, equitable, and enduring social bonds. Behav. Ecol. Sociobiol. 64, 1733–1747 (doi:10.1007/s00265-010-0986-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853 (doi:10.1073/pnas.0812917106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 (doi:10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 12.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210 (doi:10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 13.Langergraber KE, Mitani JC, Vigilant L. 2007. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790 (doi:10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beery AK, Zucker I. 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169, 665–673 (doi:10.1016/j.neuroscience.2010.05.023) [DOI] [PubMed] [Google Scholar]
- 15.Crockford C, Wittig RM, Langergraber KE, Ziegler TE, Zuberbühler K, Deschner T. 2013. Oxytocin and social bonding in unrelated chimpanzees. Proc. R. Soc. B 280, 20122765 (doi:10.1098/rspb.2012.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soares MC, Bshary R, Fusani L, Goymann W, Hau M, Hirschenhauser K, Oliveira RF. 2010. Hormonal mechanisms of cooperative behaviour. Phil. Trans. R. Soc. B 365, 2737–2750 (doi:10.1098/rstb.2010.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curley JB, Keverne EB. 2005. Genes, brains and mammalian social bonds. Trends Ecol. Evol. 20, 561–567 (doi:10.1016/j.tree.2005.05.018) [DOI] [PubMed] [Google Scholar]
- 18.Insel TR. 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 25, 768–779 (doi:10.1016/j.neuron.2010.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring RH, et al. 2006. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 185, 218–225 (doi:10.1007/s00213-005-0293-z) [DOI] [PubMed] [Google Scholar]
- 20.Churchland PS, Winkielman P. 2012. Modulating social behaviour with oxytocin: how does it work? What does it mean? Horm. Behav. 61, 392–399 (doi:10.1016/j.yhbeh.2011.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim MM, Young LJ. 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 50, 506–517 (doi:10.1016/j.yhbeh.2006.06.028) [DOI] [PubMed] [Google Scholar]
- 22.Madden JR, Clutton-Brock TH. 2011. Experimental peripheral administration of oxytocin elevates a suit of cooperative behaviours in a wild social mammal. Proc. R. Soc. B 278, 1189–1194 (doi:10.1098/rspb.2010.1675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Dreu CKW, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW. 2010. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411 (doi:10.1126/science.1189047) [DOI] [PubMed] [Google Scholar]
- 24.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature 435, 673–676 (doi:10.1038/nature03701) [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. 2008. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650 (doi:10.1016/j.neuron.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 26.de Dreu CKW. 2012. Oxytocin modulates the link between adult attachment through reduced betrayal aversion. Psychoneuroendocrinology 37, 871–880 (doi:10.1016/j.psyneuen.2011.10.003) [DOI] [PubMed] [Google Scholar]
- 27.Zak PJ, Syanton AA, Ahmadi S. 2007. Oxytocin increases generosity in humans. PLoS ONE 2, e1128 (doi:10.1371/journal.pone.0001128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grewen KM, Girdler SS, Amico J, Light KC. 2005. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom. Med. 67, 531–538 (doi:10.1097/01.psy.0000170341.88395.47) [DOI] [PubMed] [Google Scholar]
- 29.Light KC, Grewen KM, Amico JA. 2005. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol. Psychol. 69, 5–21 (doi:10.1016/j.biopsycho.2004.11.002) [DOI] [PubMed] [Google Scholar]
- 30.Guastella AJ, Mitchell PB, Mathews F. 2008. Oxytocin enahnces the encoding of positive social memories in humans. Biol. Psychaitry 64, 256–258 (doi:10.1016/j.biopsych.2008.02.008) [DOI] [PubMed] [Google Scholar]
- 31.Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. 2010. Effects of oxytocin on recollections of maternal care and closeness. Proc. Natl Acad. Sci. USA 107, 21 371–21 375 (doi:10.1073/pnas.0914051107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melis AP, Semmann D. 2010. How is human cooperation different? Phil. Trans. R. Soc. B 365, 2663–2674 (doi:10.1098/rstb.2010.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheney DL. 2011. Extent and limits of cooperation in animals. Proc. Natl Acad. Sci. USA 108, 10 902–10 909 (doi:10.1073/pnas.1100291108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross HE, Young LJ. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 30, 534–547 (doi:10.1016/j.yfrne.2009.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arletti R, Benelli A, Bertolini A. 1992. Oxytocin involvement in male and female sexual behaviour. Annu. NY Acad. Sci. 652, 180–193 (doi:10.1111/j.1749-6632.1992.tb34354.x) [DOI] [PubMed] [Google Scholar]
- 36.Caldwell JD, Walker CH, O'Rourke ST, Faggin BM, Morris M, Mason GA. 1996. Analogies between oxytocin systems of the uterus and brain. Horm. Metabol. Res. 28, 65–74 (doi:10.1055/s-2007-979131) [DOI] [PubMed] [Google Scholar]
- 37.Jin D, et al. 2007. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45 (doi:10.1038/nature05526) [DOI] [PubMed] [Google Scholar]
- 38.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. 2009. Characterization of the oxytocin system regulating affiliative behaviour in female prairie voles. Neuroscience 162, 892–903 (doi:10.1016/j.neuroscience.2009.05.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman R. 2012. Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391 (doi:10.1016/j.yhbeh.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 40.Seltzer LJ, Ziegler TE. 2007. Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Horm. Behav. 51, 436–442 (doi:10.1016/j.yhbeh.2006.12.012) [DOI] [PubMed] [Google Scholar]
- 41.Maestripieri D, Hoffman CL, Anderson GM, Carter S, Higley JD. 2009. Mother–infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol. Behav. 96, 613–619 (doi:10.1016/j.physbeh.2008.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. 2010. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm. Behav. 58, 614–618 (doi:10.1016/j.yhbeh.2010.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lack D. 1940. Courtship feeding in birds. Auk 57, 169–178 (doi:10.2307/4078744) [Google Scholar]
- 44.Vahed K. 1998. The function of nuptial feeding in insects: a review of empirical studies. Biol. Rev. 73, 43–78 (doi:10.1017/S0006323197005112) [Google Scholar]
- 45.Brown GR, Almond REA, van Bergen Y. 2004. Begging, stealing, offering: food transfer in non-human primates. Adv. Study Behav. 34, 265–295 (doi:10.1016/S0065-3454(04)34007-6) [Google Scholar]
- 46.Gurven M. 2004. To give and not to give: the behavioural ecology of human food transfers. Behav. Brain Sci. 27, 543–583 (doi:10.1017/S0140525X04000123) [Google Scholar]
- 47.Stevens JR, Gilby IC. 2004. A conceptual framework for non-kin food sharing: timing and currency benefits. Anim. Behav. 67, 603–614 (doi:10.1016/j.anbehav.2003.04.012) [Google Scholar]
- 48.Jaeggi AV, Stevens JMG, van Schaik CP. 2010. Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am. J. Phys. Anthropol. 143, 41–51 (doi:10.1002/ajpa.21288) [DOI] [PubMed] [Google Scholar]
- 49.Carter GG, Wilkinson GS. 2013. Food sharing in vampire bats: reciprical help predicts donations more than relatedness or harrassment. Proc. R. Soc. B 280, 20122573 (doi:10.1098/rspb.2012.2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomes CM, Boesch C. 2009. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE 4, e5116 (doi:10.1371/journal.pone.0005116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marlow FW. 2003. A critical provisioning by Hadza men: implications for pair bonding. Evol. Hum. Behav. 24, 217–229 (doi:10.1016/S1090-5138(03)00014-X) [Google Scholar]
- 52.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest. Oxford, UK: Oxford University Press [Google Scholar]
- 53.Wittig RM, Boesch C. 2003. Food competition and linear dominance hierarchy among female chimpanzees of the Taï National Park. Int. J. Primatol. 24, 847–867 (doi:10.1023/A:1024632923180) [Google Scholar]
- 54.Mitani JC, Watts DP. 2001. Why do chimpanzees hunt and share meat? Anim. Behav. 61, 915–924 (doi:10.1006/anbe.2000.1681) [Google Scholar]
- 55.Gilby IC. 2006. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 71, 953–963 (doi:10.1016/j.anbehav.2005.09.009) [Google Scholar]
- 56.Boesch C. 1994. Cooperative hunting in wild chimpanzees. Anim. Behav. 48, 653–667 (doi:10.1006/anbe.1994.1285) [Google Scholar]
- 57.Hockings KJ, Humle T, Anderson JR, Biro D, Sousa C, Ohashi G, Matsuzawa T. 2007. Chimpanzees share forbidden fruit. PLoS ONE 2, e886 (doi:10.1371/journal.pone.0000886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds V, Lloyd W, Babweteera F, English CJ. 2009. Decaying Raphia farinifera palm trees provide a source of sodium for wild chimpanzees in Budongo forest, Uganda. PLoS ONE 4, e6194 (doi:10.1371/journal.pone.0006194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds V. 2005. The chimpanzees of the Budongo forest. Oxford, UK: Oxford University Press [Google Scholar]
- 60.Altman J. 1974. Observational study of behavior: sampling methods. Behavior 49, 227–267 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 61.Silk JB, Brosnan SF, Henrich J, Lambeth SP, Shapiro S. 2013. Chimpanzees share food for many reasons: the role of kinship, reciprocity, social bonds and harrasment on food transfers. Anim. Behav. 85, 941–947 (doi:10.1016/j.anbehav.2013.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wittig RM, Boesch C. 2003. ‘Decision-making’ in conflicts of wild chimpanzees (Pan troglodytes): an extension to the relational model. Behav. Ecol. Sociobiol. 54, 491–504 (doi:10.1007/s00265-003-0654-8) [Google Scholar]
- 63.Fraser O, Shino G, Aureli F. 2008. Components of relationship quality in chimpanzees. Ethology 114, 834–843 (doi:10.1111/j.1439-0310.2008.01527.x) [Google Scholar]
- 64.Crockford C, Wittig RM, Mundry R, Zuberbühler K. 2012. Wild chimpanzees inform ignorant group members of danger. Curr. Biol. 22, 142–146 (doi:10.1016/j.cub.2011.11.053) [DOI] [PubMed] [Google Scholar]
- 65.Amico JA, Ulbrecht JS, Robinson AG. 1987. Clearance studies of OT in humans using radioimmunoassay measurements of the hormone in plasma and urine. J. Clin. Endocrinol. Metab. 64, 340–345 (doi:10.1210/jcem-64-2-340) [DOI] [PubMed] [Google Scholar]
- 66.Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M. 2000. Comparative aspects of the metabolism and extraction of cortisol in three individual nonhuman primates. Gen. Comp. Endocrinol. 117, 427–438 (doi:10.1006/gcen.1999.7431) [DOI] [PubMed] [Google Scholar]
- 67.Langergraber KE, Mitani JC, Vigilant L. 2009. Kinship and social bonds in female chimpanzees (Pan troglodytes). Am. J. Primatol. 71, 840–851 (doi:10.1002/ajp.20711) [DOI] [PubMed] [Google Scholar]
- 68.Tabachnick BG, Fidell LS. 2007. Using multivariate statistics, 5th edn Boston, MA: Allyn and Bacon [Google Scholar]
- 69.Sobolewski M, Brown J, Mitani J. 2012. Territoriality, tolerance and testosterone in wild chimpanzees. Anim. Behav. 84, 1469–1474 (doi:10.1016/j.anbehav.2012.09.018) [Google Scholar]
- 70.Van Anders SM, Goldey KL, Kuo PX. 2011. The steroid peptide theory of social bonds: Integrating testosterone and peptide responses for classifying social behavioural contexts. Psychoneuroendocrinology 36, 1265–1275 (doi:10.1016/j.psyneuen.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 71.Clutton-Brock TH, Parker GA. 1995. Punishment in animal societies. Nature 373, 209–216 (doi:10.1038/373209a0) [DOI] [PubMed] [Google Scholar]
- 72.Gomes C, Boesch C. 2011. Reciprocity and trades in wild West African chimpanzees. Behav. Ecol. Sociobiol. 65, 2183–2196 (doi:10.1007/s00265-011-1227-x) [Google Scholar]
- 73.Hruschka DJ. 2010. Friendship: development, ecology and evolution of a relationship. Berkeley, CA: University of California Press [Google Scholar]
- 74.Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. 1986. Cerebrospinal-fluid levels of acetylcholinesterase, monoamines and oxytocin during labor, parturition, vaginocervical stimulation, lamb separation and suckling in sheep. Neuroendocrinology 44, 149–156 (doi:10.1159/000124638) [DOI] [PubMed] [Google Scholar]
- 75.Broad KD, Curley JP, Keverne EB. 2006. Mother-infant bonding and the evolution of social relationships. Phil. Trans. R. Soc. B 361, 2199–2214 (doi:10.1098/rstb.2006.1940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young LJ, Wang Z. 2004. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054 (doi:10.1038/nn1327) [DOI] [PubMed] [Google Scholar]
- 77.Skuse DH, Gallagher L. 2009. Dopaminergic–neuropeptide interactions in the social brain. Trend Cog. Sci. 13, 27–35 (doi:10.1016/j.tics.2008.09.007) [DOI] [PubMed] [Google Scholar]
- 78.Wittig RM, Boesch C. 2010. Receiving post-conflict affiliation from the enemy's friend reconciles former opponents. PLoS ONE 5, e13995 (doi:10.1371/journal.pone.0013995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Waal FBM. 2000. Attitudinal reciprocity in food sharing among brown capuchin monkeys. Anim. Behav. 60, 253–261 (doi:10.1006/anbe.2000.1471) [DOI] [PubMed] [Google Scholar]
- 80.Schino G, Aureli F. 2009. Reciprocal altruism in primates: partner choice, cognition, and emotions. Adv. Stud. Behav. 39, 45–69 (doi:10.1016/S0065-3454(09)39002-6) [Google Scholar]
- 81.Lovejoy CO. 1981. The origin of man. Science 211, 341–350 (doi:10.1126/science.211.4480.341) [DOI] [PubMed] [Google Scholar]
- 82.Gurven M, Hill K. 2009. Hunting as subsistence and mating effort? A re-evaluation of ‘Man the hunter’, the sexual division of labor and the evolution of the nuclear family. Curr. Anthropol. 5, 51–74 (doi:10.1086/595620) [DOI] [PubMed] [Google Scholar]


