Abstract
Background and Aims
The timing of flowering has a direct impact on successful seed production in plants. Flowering of soybean (Glycine max) is controlled by several E loci, and previous studies identified the genes responsible for the flowering loci E1, E2, E3 and E4. However, natural variation in these genes has not been fully elucidated. The aims of this study were the identification of new alleles, establishment of allele diagnoses, examination of allelic combinations for adaptability, and analysis of the integrated effect of these loci on flowering.
Methods
The sequences of these genes and their flanking regions were determined for 39 accessions by primer walking. Systematic discrimination among alleles was performed using DNA markers. Genotypes at the E1–E4 loci were determined for 63 accessions covering several ecological types using DNA markers and sequencing, and flowering times of these accessions at three sowing times were recorded.
Key Results
A new allele with an insertion of a long interspersed nuclear element (LINE) at the promoter of the E1 locus (e1-re) was identified. Insertion and deletion of 36 bases in the eighth intron (E2-in and E2-dl) were observed at the E2 locus. Systematic discrimination among the alleles at the E1–E3 loci was achieved using PCR-based markers. Allelic combinations at the E1–E4 loci were found to be associated with ecological types, and about 62–66 % of variation of flowering time could be attributed to these loci.
Conclusions
The study advances understanding of the combined roles of the E1–E4 loci in flowering and geographic adaptation, and suggests the existence of unidentified genes for flowering in soybean,
Keywords: Glycine max, soybean, E locus, flowering time, single nucleotide polymorphism, SNP, haplotype, ecological type, marker-assisted selection
INTRODUCTION
Timing of flowering is critical for successful seed production of plants. Flowering time and maturity of crops are important factors that determine geographic adaptation, seed quality and yield. Soybean is a typical short-day (SD) plant (Garner and Allard, 1920). Several maturity loci designated as E loci have been characterized in soybean, including E1 and E2 (Bernard, 1971), E3 (Buzzell, 1971), E4 (Buzzel and Voldeng, 1980), E5 (McBlain and Bernard, 1987), E6 (Bonato and Vello, 1999), E7 (Cober and Voldeng, 2001) and E8 (Cober et al., 2010). Except for E6, the dominant alleles condition late flowering. Three flowering time quantitative trait loci (QTLs), FT1, FT2 and FT3, were identified using an F2 population (Yamanaka et al., 2001) and recombinant inbred lines (Watanabe et al., 2004) derived from a cross between ‘Misuzudaizu’ and ‘Moshidou Gong 503’. The FT1, FT2 and FT3 loci were found to correspond to the E1, E2 and E3 loci, respectively, based on their map positions or allelism tests. To date, the genes responsible for the E1, E2, E3 and E4 loci have been identified (Liu et al., 2008; Watanabe et al., 2009, 2011; Xia et al., 2012).
The E1 locus was limited to a 17·4 kb region containing an intron-free gene and identified by positional cloning (Xia et al., 2012). This gene (E1 gene) encodes a protein that contains a putative bipartite nuclear localization signal and a region distantly related to the B3 domain, suggesting that this protein is a novel transcription factor. The E1 gene is considered to be a suppressor of the expression of GmFT2a and GmFT5a, which are both orthologues of arabidopsis FLOWERING LOCUS T (FT) (Kong et al., 2010), under the regulation of the E3 and E4 loci (Xia et al., 2012). Allelic variation in the E1 gene includes a single nucleotide polymorphism (SNP) at nucleotide 44 leading to a missense mutation (arginine to threonine) (e1-as), a single base (adenine) deletion at nucleotide 49 leading to a premature stop codon at nucleotide 124 (e1-fs), and a null allele (e1-nl) in which the entire E1 gene was deleted (Xia et al., 2012).
GmGIa (Glyma10g36600), an orthologue of the arabidopsis GIGANTEA (GI) gene, was found by positional cloning to be the gene responsible for the E2 locus (Watanabe et al., 2011). GI is a circadian clock-controlled gene that functions upstream of CONSTANS (CO) and FT in arabidopsis (Huq et al., 2000). A nonsense mutation was found in the Misuzudaizu allele (e2-ns) where an SNP (A to T) at nucleotide 1561 introduces a premature stop codon (Watanabe et al., 2011). An allele-specific derived cleaved amplified polymorphic sequence (dCAPS) marker for e2-ns has been developed.
The gene responsible for the E3 locus was found by positional cloning to be a phytochrome A gene, GmPhyA3, (Watanabe et al., 2009). The corresponding Misuzudaizu allele, GmPhyA3-Mi (E3-Mi), displayed normal features of the phytochrome A gene, while the Moshidou Gong 503 allele, GmPhyA3-Mo (e3-Mo), showed a large insertion in the fourth intron and a single non-synonymous amino acid substitution (glycine to arginine) in the third exon. The inserted sequence is 2·5 kb in length, a part of which was found to be highly similar to the non-long-terminal retrotransposon reverse transcriptase element (Watanabe et al., 2009). The Harosoy allele, GmPhyA3-E3 (E3-Ha), harboured a large retrotransposon-like insertion sequence similar to that in e3-Mo, but the amino acid sequences encoded by E3-Mi and E3-Ha were identical (Watanabe et al., 2009). A large deletion of 13·33 kb at a position after the third exon was detected in the Harosoy-e3 allele, GmPhyA3-e3 (designated as e3-tr hereafter) (Watanabe et al., 2009).
The gene responsible for the E4 locus was identified to be another phytochrome A gene, GmPhyA2, by a candidate gene approach (Liu et al., 2008). A Ty1/copia-like retrotransposon was inserted in exon 1 of a recessive allele (e4-SORE-1) at the E4 locus (Liu et al., 2008). Alelle-specific primer pairs for E4 and e4-SORE-1 were reported by Liu et al. (2008).
The distribution of the e4-SORE-1 allele was analysed based on specific DNA markers using many cultivated soybean and wild soybean (Glycine soja) accessions, and this allele was detected only in cultivated soybean accessions grown in northern regions of Japan (Kanazawa et al., 2009). Recently, new dysfunctional recessive alleles, two at the E3 locus and four at the E4 locus, have been detected by sequencing in soybean accessions from high latitudes (Tsubokura et al., 2012; Xu et al., 2013). However, a comprehensive survey of natural variation at the E1–E4 loci has not been performed.
In Japan, soybeans are cultivated over a wide range of latitudes, from 24 °N to 45 °N. Soybean varieties with a wide variation of characteristics in flowering and maturity were geographically differentiated depending on the daylength, frost-free period, crop rotation system and escape from pest damage. Fukui and Arai (1951) classified Japanese soybean varieties into nine ecological types, namely Ia, Ib, IIa, IIb, IIc, IIIb, IIIc, IVc and Vc, by a combination of days from germination to flowering (I–V) and days from flowering to maturity (a–c). The groups, I, II, III, IV and V displayed extremely early, early, intermediate, late and extremely late flowering times, respectively. The ecological types have been associated with geographic differentiation and adaptability. However, genotypes at the E1–E4 loci in varieties covering several ecological types have not been identified because classical test crosses are too laborious to identify genotypes at these loci for many varieties.
In the present study, the sequences of the genes responsible for the E1, E2, E3 and E4 loci and their flanking regions were determined or partly analysed for 39 accessions by primer walking to detect new alleles for flowering genes. Systematic discrimination among alleles, including newly identified ones, was achieved based on DNA markers. The alleles at the E1–E4 loci in 63 accessions covering a wide range of flowering time were identified by DNA markers and sequencing. Frequencies and geographic distribution of the alleles, and association between allelic combinations at the E1–E4 loci and ecological types in Japanese soybean cultivars were examined. Furthermore, the effect of each locus and the combined effect of these loci on flowering time were analysed. Our research advanced understanding of the role of the E1–E4 loci in flowering and geographic adaptation of soybean.
MATERIALS AND METHODS
Plant materials and investigation of flowering time
Sixty-three accessions representing landraces and cross-breeding varieties of soybean (Glycine max), and experimental lines including two wild soybeans were selected for identification of alleles at the E1–E4 loci. The 63 accessions were grown in the greenhouse at three different sowing dates in 2010, i.e. 14 May, 3 June and 23 June, designated as ‘early’, ‘standard’ and ‘late’, respectively. Two weeks after sowing, three seedlings of each accession were transplanted in the experimental field of the National Institute of Agrobiological Sciences (NIAS) (36 °3'N, 140 °04'E). All the plants were grown under natural daylength conditions. The number of days from sowing to the initiation of flowering was recorded and the average value of two or three plants for each accession was used for the statistical analyses.
DNA isolation
Genomic DNA was extracted from fresh trifoliate leaves, based on the standard cetyltrimethyl ammonium bromide (CTAB) method (Murray and Thompson, 1980).
Sequencing by primer walking
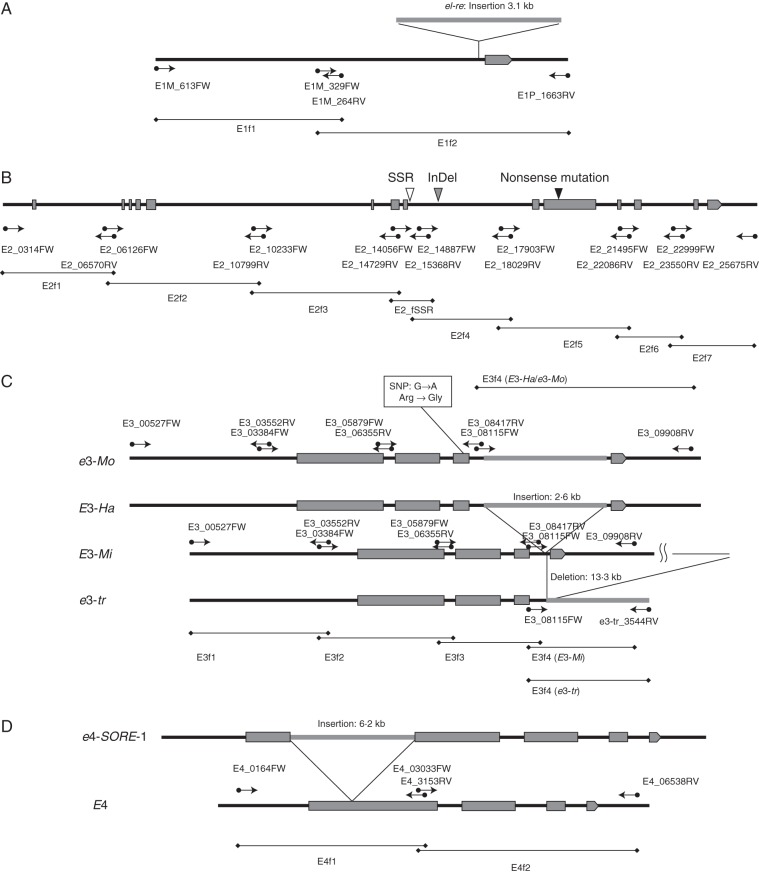
The E1 regions were amplified with two primer pairs, and two PCR products were designated as E1f1 and E1f2. The positions of these primers and PCR fragments are indicated in Fig. 1A. The E2 regions were amplified with eight primer pairs, resulting in eight PCR products designated as E2f1–E2f7 and E2_fSSR, respectively. The positions of these primers and PCR products are shown in Fig. 1B. The E3 regions were amplified with four specific primer pairs, and the four PCR products were designated as E3f1–E3f4. As the e3-tr allele lacked a portion of the third intron and the downstream region, the reverse primer for E3f4 was different from that for the other alleles. The positions of these primers and PCR fragments are shown in Fig. 1C. The E4 regions were amplified by two primer sets, resulting in two PCR products designated as E4f1 and E4f2 (Fig. 1D). The sequences of these primers are listed in Supplementary Data Table S1.
Fig. 1.
Variation of E1, GmGIa, GmPhyA3 and GmPhyA2 gene structures with the positions and directions of primers for PCR walking. Shaded boxes, solid lines and shaded lines indicate exons, introns and flanking regions, and insertions or deletions, respectively. Lines at the bottom of each figure with lozenges at both ends indicate PCR products for genome walking. (A) E1 regions were amplified with two primer pairs. (B) GmGIa (E2) regions were amplified with eight primer pairs. (C) GmPhyA3 (E3) regions were amplified with four primer pairs. As the e3-tr allele lacked a portion of the third intron and downstream region, the reverse primer for E3f4 was different from that for other alleles. (D) GmPhyA2 (E4) regions were amplified by two primer sets.
Target regions were amplified using total DNA of 39 accessions with Ex-Taq HS (Takara Bio Inc., Japan) in 30 cycles at 94 °C for 20 s, 58 °C for 20 s and 72 °C for 4 min. The PCR products were purified with shrimp alkaline phosphatase (GE Life Sciences, USA) and exonuclease I (GE Life Sciences). Sequencing primers were constructed at intervals of approx. 500 bases on the fragments. All the primers for sequencing are also presented in Supplementary Data Table S1. The PCR products were sequenced using a Big Dye Terminator v3·1 Cycle Sequencing Kit (Applied Biosystems, USA) and analysed with the capillary sequencer ABI3730Xl (Applied Biosystems). Alignment of the DNA sequences was carried out using ATGC version 4.2.1 (GENETYX Corporation, Japan). The haplotype of each region was displayed at the positions where at least two accessions have different bases.
Discrimination of alleles by DNA markers
Three primers were designed to discriminate between E1 and e1-re; E1M0535FW and E1P0305_RV for E1, and E1M0535FW and e1re_0188RV for e1-re, respectively (marker e1-re_STS). E1/e1-fs and e1-as were discriminated by the TaqαI digestion of PCR products generated with specific primers G33snpTaqcutF and G33snpTaqcutR1 (marker E1_TaqαI/HinfI) as described by Xia et al. (2012). The e1-fs allele was distinguished from E1/e1-as by the HinfI digestion of PCR products using the same primers as those for e1-as. The e1-nl allele was characterized by faint bands with different molecular sizes derived from the paralogues of the E1 gene using this CAPS marker and by the absence of bands using the DNA marker, e1-re_STS. The positions of these primers are indicated in Fig. 2A.
Fig. 2.
Variation of E1, E2 and E3 gene structures with the positions and directions of PCR primers. Shaded boxes, solid lines and shaded lines indicate exons, introns and flanking regions, and insertions, respectively. (A) The E1 gene and its natural mutations. (B) The E2 gene and its natural mutations. (C) The E3 gene and its natural mutations.
E2-in and E2-dl were discriminated based on the molecular size of PCR products using specific primers, E2_15345FW and E2_15856RV (marker E2_InDel). The e2-ns allele was distinguished from E2-in/E2-dl by the DraI digestion of PCR products (marker E2_DraI) as described by Watanabe et al. (2011). The positions of these primers are indicated in Fig. 2B.
Three primer pairs were also designed to discriminate E3-Mi, E3-Ha/e3-Mo and e3-tr from each other. These primer combinations consisted of E3_08557FW and E3_099080RV for E3-Mi, E3_08557FW and E3Ha_1000RV for E3-Ha/e3-Mo, and E3_08557FW and e3tr_0716RV for e3-tr (marker E3_Mix). The e3-Mo allele was distinguished from E3-Mi/E3-Ha/e3-T by the MseI digestion of the PCR products using specific primers, E3_08094FW and E3_08417RV (marker E3_MseI). The positions of these primers are indicated in Fig. 2C. The e4-SORE-1 allele was distinguished from E4 by allele-specific primer pairs as described by Liu et al. (2008). E3-Mi, E3-Ha/e3-Mo, e3-tr, E4 and e4- SORE-1 were each discriminated using mixed primers that consisted of E3_8420FW, E3_099080RV, E3Ha_1000RV, e3tr_0716RV, PhyA2-For, PhyA2-Rev/E4 and PhyA2-Rev/e4 (marker E3E4_Mix).
The sequences of these primers are shown in Table 1. Polymerase chain reaction was performed using Ex-Taq HS (Takara Bio Inc.) with 30 cycles at 94 °C for 20 s, 58 °C for 30 s and 72 °C for 1 min. The PCR products or digested fragments were separated by 10 % polyacrylamide gel electrophoresis. For combined discrimination of alleles at the E3 and E4 loci, the extension time was changed to 68 °C and the PCR products were separated on a 1·5 % agarose gel.
Table 1.
Sequences of DNA markers for identification of alleles at the E1, E2, E3 and E4 loci
| Marker | Primer | Sequence (5′–3′) |
|---|---|---|
| E1_TaqαI/HinfI | G33snpTaqcutF | TCAGATGAAAGGGAGCAGTGTCAAAAGAAGT |
| G33snpTaqcutR1 | TCCGATCTCATCACCTTTCC | |
| e1-re_STS | E1M0535_FW | CCGTTTGATTGGTTTTTGGT |
| E1P0305_RV | CCCTTCAGTTTCTGCAGCTC | |
| e1re_0188RV | GAGAAGACAAACAATTCGAG | |
| E2_DraI | SoyGI_dCAPaMs19300FW | GAAGCCCATCAGAGGCATGTCTTATT |
| SoyGI_dCAPa19440RV | GAGGCAGAGCCAAAGCCTAT | |
| E2_InDel | E2_15345FW | TGTTGATATTACATGCACATGCAT |
| E2_15856RV | GGCAGTTTCACCTTCTTAGC | |
| E3_Mix | E3_08557FW | TGGAGGGTATTGGATGATGC |
| E3_09908RV | CTAAGTCCGCCTCTGGTTTCAG | |
| E3Ha_1000RV | CGGTCAAGAGCCAACATGAG | |
| e3tr_0716RV | GTCCTATACAATTCTTTACGACG | |
| E3E4_Mix | E3_08420FW | TGGGTCTTCAGTTCAGTTGG |
| E3_09908RV | CTAAGTCCGCCTCTGGTTTCAG | |
| E3Ha_1000RV | CGGTCAAGAGCCAACATGAG | |
| e3T_0716RV | GTCCTATACAATTCTTTACGACG | |
| PhyA2-for | AGACGTAGTGCTAGGGCTAT | |
| PhyA2-Rev/E4 | GCATCTCGCATCACCAGATCA | |
| PhyA2-Rev/e4 | GCTCATCCCTTCGAATTCAG | |
| E3_MseI | E3_08094FW | TTGCATGAAGTTTTGGTTGC |
| E3_08417RV | CAACTGAACTGAAGACCCACAA |
Identification of genotypes at the E1–E4 loci in accessions with multiple flowering times
Genotypes at the E1–E4 loci in 63 accessions were identified by DNA markers and sequencing. Thirty accessions including Japanese varieties from several ecological types were selected for the determination of structural variation of E1, E2 (GmGIa), E3 (GmPhyA3) and E4 (GmPhyA2) genes and their flanking regions by sequencing. These accessions are marked with an asterisk in Table 2. E1 genes of ‘Gokuwase-Kamishunbetsu’, ‘Yakuwadaizu’ and ‘Harosoy_E1’; E2, E3 and E4 genes of ‘Toyomusume’; the E2 gene of ‘Harosoy_E2’; the E3 gene of ‘Harosoy_e3’; and E4 genes of ‘QT2’, ‘T-106’ and ‘Harosoy_e4’ were similarly analysed.
Table 2.
Days from sowing to flowering initiation and the alleles identified at the E1, E2, E3 and E4 loci
| Accessions | Accession no. | Origin | Maturity | E1 | E2 | E3 | E4 | Early | Sowing standard | Late |
|---|---|---|---|---|---|---|---|---|---|---|
| QT2 | Wild soybean | Japan | E1 | E2-dl | E3-Mi | E4† | NA | NA | (74·5) | |
| Qinghuangdou* | JP 28296 | China | E1 | E2-in | E3-Ha | E4 | (106) | (86) | (66) | |
| Akisengoku* | JP 29559 | Japan | Vc | E1 | E2-dl | E3-Mi | E4 | 103·0 | 84·7 | 64·0 |
| Hyuga | JP 29640 | Japan | IVc | E1 | E2-dl | E3-Mi | E4 | 93·0 | 79·0 | 61·7 |
| Tamanishiki* | JP 27959 | Japan | Vc | E1 | E2-dl | E3-Mi | E4 | 90·0 | 74·0 | 59·3 |
| Oushokuakidaizu* | JP 28222 | Japan | Vc | E1 | E2-dl | E3-Mi | E4 | 77·0 | 63·0 | 55·0 |
| Fukuyutaka* | JP 29668 | Japan | IVc | E1 | E2-dl | E3-Mi | E4 | 75·7 | 63·0 | 55·3 |
| Hyokeikuro3* | Japan | IVc | E1 | E2-dl | E3-Mi | E4 | 76·7 | 62·7 | 55·3 | |
| Nattoukotsubu | JP 29161 | Japan | IIIc | E1 | e2-ns | E3-Mi | E4 | 67·5 | 59·3 | 43·5 |
| Bay* | PI 553043 | USA | E1 | E2-dl | E3-Ha | E4 | 63·7 | 53·3 | 42·3 | |
| Zihua4 | JP 28134 | China | E1 | e2-ns | E3-Mi | E4 | NA | NA | NA | |
| Akishirome* | JP 29669 | Japan | IIIc | E1 | e2-ns | E3-Mi | E4 | 66·3 | 54·3 | 42·7 |
| Sachiyutaka | Japan | IIIc | E1 | E2-dl | e3-tr | E4 | 62·0 | 48·0 | 41·0 | |
| Gedenshirazu* | Japan | IIIc | E1 | e2-ns | E3-Mi | E4 | 60·7 | 49·0 | 39·0 | |
| Misuzudaizu* | JP 28856 | Japan | IIIc | E1 | e2-ns | E3-Mi | E4 | 64·3 | 50·3 | 39·7 |
| Peking* | JP 28432 | China | E1 | E2-in | E3-Ha | E4 | 61·7 | 51·0 | 39·3 | |
| Kosuzu | JP 68389 | Japan | E1 | e2-ns | E3-Mi | E4 | 58·7 | 47·0 | 37·7 | |
| Akasaya* | JP 28750 | Japan | IIIc | E1 | e2-ns | E3-Mi | E4 | NA | NA | NA |
| Tanishidaizu | JP 29743 | Korea | E1 | e2-ns | e3-tr | E4 | 58·7 | 46·3 | 36·7 | |
| Ani* | JP 27814 | Japan | IIc | E1 | e2-ns | e3-tr | E4 | 59·0 | 48·0 | 37·7 |
| Suzuyutaka | JP 68385 | Japan | IIc | E1 | e2-ns | E3-Ha | E4 | 58·3 | 48·7 | 37·3 |
| Gindaizu | JP 27579 | Japan | IIb | E1 | e2-ns | E3-Mi | E4 | 56·3 | 45·0 | 36·3 |
| T-106 | Wild soybean | USSR | E1 | E2-dl | E3-Mi | E4† | 53·0 | 43·0 | 33·7 | |
| Enrei* | JP 28862 | Japan | IIc | E1 | e2-ns | e3-tr | E4 | 54·7 | 44·0 | 35·7 |
| Tamatsukuri* | JP 27857 | Japan | IIIc | E1 | e2-ns | e3-tr | E4 | 56·7 | 45·0 | 36·0 |
| Tachiyutaka | JP 68387 | Japan | IIc | E1 | e2-ns | E3-Mi | E4 | 54·0 | 44·0 | 37·0 |
| Norin2* | Japan | IIb | E1 | e2-ns | e3-tr | E4 | 53·3 | 44·7 | 35·0 | |
| Okushirome | JP 28030 | Japan | IIc | E1 | e2-ns | e3-tr | E4 | 50·3 | 42·0 | 33·3 |
| Harosoy_E1 | PI 591430 | Canada | E1† | e2-ns | E3-Ha | E4 | 54·0 | 44·7 | 35·3 | |
| Ryuho | Japan | E1 | e2-ns | E3-Mi | E4 | 50·7 | 42·0 | (36·0) | ||
| Tachinagaha | JP 67666 | Japan | IIc | E1 | e2-ns | e3-tr | E4 | 54·0 | 44·7 | (33·0) |
| Aohigu* | JP 80038 | Japan | IIa | E1 | e2-ns | e3-tr | E4 | 50·0 | 41·7 | 33·5 |
| Matsuura* | JP 29470 | Japan | IIa | E1 | e2-ns | E3-Mi | E4 | 52·7 | 43·0 | 33·3 |
| Kin* | JP 29490 | Japan | IIa | E1 | e2-ns | e3-tr | E4 | 52·0 | 42·7 | 33·5 |
| Ohsuzu | Japan | E1 | e2-ns | E3-Mi | E4 | 52·7 | 42·0 | 34·0 | ||
| Koganedaizu | JP 29504 | Japan | IIa | E1 | e2-ns | e3-tr | E4 | 51·7 | 42·3 | 34·0 |
| Wasekin* | JP 29490 | Japan | IIa | E1 | e2-ns | e3-tr | E4 | 52·0 | 40·7 | (32·0) |
| Clark | PI 548533 | USA | e1-as | E2-in | E3-Ha | E4 | 47·3 | 39·7 | 32·3 | |
| Williams82* | PI 518671 | USA | e1-as | E2-in | E3-Ha | E4 | 48·3 | 38·7 | 33·0 | |
| Harosoy_E5 | Canada | e1-as | E2-dl | E3-Ha | E4 | NA | NA | NA | ||
| Tokachinagaha* | JP 27439 | Japan | E1 | e2-ns | e3-tr | E4 | 45·0 | 35·7 | 29·0 | |
| Toyosuzu | JP 27540 | Japan | Ib | e1-nl | e2-ns | E3-Mi | E4 | 40·7 | 34·0 | 29·7 |
| Moshidou Gong 503* | JP 27603 | China | e1-as | E2-in | e3-Mo | E4 | 40·3 | 36·0 | 29·0 | |
| Jinyuan1* | JP 28265 | China | E1 | e2-ns | E3-Ha | E4 | 43·3 | 38·0 | 29·0 | |
| NIL-13-E1* | Japan | E1 | e2-ns | e3-tr | E4 | NA | NA | NA | ||
| Suzumaru | JP 67771 | Japan | e1-as | e2-ns | E3-Mi | E4 | 43·0 | 35·3 | 29·0 | |
| Wasesuzunari | JP 68390 | Japan | Ib | E1 | e2-ns | e3-tr | NA | 41·7 | 34·7 | 28·0 |
| Oyachi2 | JP 53264 | Japan | Ib | E1 | e2-ns | e3-tr | e4-SORE-1 | 41·0 | 34·0 | 29·0 |
| Harosoy_E2 | PI 547768 | Canada | e1-as | E2-in† | E3-Ha | E4 | NA | 39·7 | 31·7 | |
| Kariyutaka* | Japan | Ib | E1 | E2-in | e3-tr | e4-SORE-1 | 41·0 | 34·0 | 28·7 | |
| Suzuhime | JP 200461 | Japan | Ib | e1-as | E2-in | e3-tr | E4 | 40·7 | (33·0) | 28·0 |
| Toyomusume | JP 27541 | Japan | Ib | e1-nl | e2-ns† | E3-Mi† | E4† | 40·3 | 34·0 | 30·0 |
| Hayahikari | Japan | Ib | E1 | e2-ns | e3-tr | e4-SORE-1 | 40·0 | 35·3 | 29·3 | |
| Harosoy* | PI 548573 | Canada | e1-as | e2-ns | E3-Ha | E4 | 40·3 | 32·0 | 28·3 | |
| Yukihomare | Japan | Ib | e1-nl | e2-ns | E3-Mi | e4-SORE-1 | 39·7 | 32·7 | 28·0 | |
| NIL-11-e4* | Japan | E1 | e2-ns | e3-tr | e4-SORE-1 | NA | NA | NA | ||
| Toshisdai7910 | JP 27451 | Japan | e1-nl | e2-ns | e3-tr | e4-SORE-1 | 39·7 | 31·0 | 27·0 | |
| NIL-13-e2* | Japan | E1 | e2-ns | e3-tr | e4-SORE-1 | NA | NA | NA | ||
| Harosoy_e4 | PI 591435 | Canada | e1-as | e2-ns | E3-Ha | e4-SORE-1† | 40·3 | 32·3 | 27·3 | |
| Tokei758 | JP 200291 | Japan | e1-as | e2-ns | E3-Ha | E4 | NA | NA | NA | |
| Harosoy_e3 | PI 591433 | Canada | e1-as | e2-ns | e3-tr† | E4 | 38·3 | 31·3 | 28·0 | |
| Fiskeby V | JP 30465 | Sweden | e1-nl | e2-ns | e3-tr | e4-SORE-1 | 35·7 | 29·0 | 25·3 | |
| Sakamotowase* | JP 27450 | Japan | Ia | e1-fs | e2-ns | e3-tr | E4 | 35·0 | 30·0 | 25·5 |
JP, accession number of NIAS Genebank; PI, accession number from the USDA-ARS National Plant Germplasm System.
Maturity refers to ecological types based on days from germination to flowering (I– V) and days from flowering to maturity (a–c) (Fukui and Arai, 1951).
Data obtained from a single plant are shown in parenthesis.
*The E1–E4 regions were sequenced.
†These loci were sequenced.
Statistical analyses
Analysis of variance (ANOVA) was performed using the statistical software R (Version 2. 10. 1) based on five factors consisting of genotypes at the E1–E4 loci and sowing time. The genotypes at each locus were classified into dominant and recessive alleles. Multiple regression analysis was based on a model in which the flowering phenotype is designated as a dependent variable, and alleles at the E1–E4 loci and sowing time as explanatory variables. Statistical tests for regression coefficients and estimation of regression coefficients were performed. Pearson product–moment correlation coefficients between different sowing times were also calculated.
Data deposition
The DNA sequence data described in this study have been deposited at the DNA Data Bank of Japan (DDBJ) under the accession numbers AB795360–AB795392, for E1, AB797147–AB797178 for GmGIa and AB797179–AB797210 for GmPhyA3.
RESULTS
Haplotypes for E1, E2 (GmGIa) and E3 (GmPhyA3) regions
The E1, GmGIa, GmPhyA3 and GmPhyA2 regions in 30 accessions and a total of 11 of these regions in nine other accessions were sequenced (Table 2, Fig. 3). The haplotypes for the E1, GmGIa and GmPhyA3 regions are shown in Fig. 3. A new allele with an insertion of a long interspersed nuclear element (LINE) at the position 148 bases upstream from the start codon of the E1 gene was identified in ‘Gokuwase-Kamishunbetsu’. This accession showed an earlier flowering time by 14 d compared with ‘Oyachi2’ (Tokachi Agricultural Experiment Station, 1988) that harboured the same alleles at the E2, E3 and E4 loci (Xu et al., 2013) and almost the same genetic background (Kaga et al., 2012; Tsubokura et al., 2012). We designated this allele as e1-re because ‘Oyachi2’ has an E1 allele. A cultivar ‘Peking’ showed a unique haplotype with the same sequence for the coding region as that in the E1 allele and a different sequence for the 5′ upstream region, compared with the other accessions that harboured E1 alleles (Fig. 3A). Because ‘Peking’ had a recessive allele at the E1 locus in the F2 generation derived from the cross with ‘Enrei’ (data not shown), we designated this allele as e1-p.
Fig. 3.
Haplotypes of the E1, E2, E3 and E4 gene regions. Filled and open squares indicate insertions and deletions, respectively. (A) The E1 gene and flanking regions including about 6 kb in the 5′ upstream region and about 1 kb in the 3′ downstream region were sequenced. (B) The E2 gene and about 1 kb flanking regions on both sides were sequenced. (C) The E3 gene and about 4 kb upstream and 1 kb downstream regions were sequenced. (D) The E4 gene and about 1 kb flanking regions on both sides were sequenced.
The dominant alleles at the E2 locus were classified into two alleles by insertion and deletion of 36 bases in the eighth intron (E2-in and E2-dl) with each specific sequence in the 5′ upstream, coding region, intron and 3′ downstream region (Fig. 3B). An AT simple sequence repeat (SSR) with 11–36 replications was detected in the eighth intron (Fig. 3B).
No new allele was identified at the E3 locus. The sequences of the 5′ upstream region, coding region and intron of E3-Mi, E3-Ha and e3-tr alleles were conserved, respectively, among accessions (Fig. 3C). An AG SSR with 16–18 replications was observed at the promoter region (Fig. 3C).
For the E4 locus, no new allele was identified among the 34 soybean accessions (Table 2).
Identification of alleles at the E1–E4 loci by DNA markers
The gel electrophoretic patterns for the identification of alleles at the E1–E4 loci are shown in Fig. 4. Examples of discrimination between E1/e1-fs, e1-as and e1-nl, and E1/e1-as/e1-fs, e1-re and e1-nl are shown in Fig. 4A. Clear distinctions between E2-in/E2-dl and e2-ns, and between E2-in and E2-dl/e2-ns were observed (Fig. 4B). An example of electrophoresis of PCR products by mixed primers is shown in Fig. 4C, with clear discrimination of the E3-Mi, e3-tr and E3-Ha/e3-Mo alleles. Two bands with smaller sizes of e3-Mo, compared with the single band of E3-Mi/E3-Ha/e3-tr, were produced by MseI digestion of the PCR product (Fig. 4C). The e4-SORE-1 allele was also clearly distinguished from the E4 allele by mixed primers, as reported previously (Liu et al., 2008). Furthermore, three E3 alleles, E3-Mi, E3-Ha/e3-Mo and e3-tr, and two E4 alleles, E4 and e4-SORE-1, were successfully discriminated from each other by using a modified primer and changing the PCR conditions and agarose content for electrophoresis (Fig. 4D). Thus allele-specific DNA markers were developed for all the alleles identified, except for e1-p.
Fig. 4.
Identification of alleles at the E1, E2, E3 and E4 loci. (A) Discrimination between E1, e1-as, e1-fs and e1-nl by combination of the dCAPS and CAPS markers (upper panel) and distinction of e1-re from E1, e1-as and e1-fs with specific primer pairs (lower panel). (B) Distinction of e2-ns from E2-in/E2-dl and discrimination between E2-in and E2-dl. (C) Discrimination of E3-Mi, e3-tr and E3-Ha/e3-Mo alleles and distinction of e3-Mo from other E3 alleles. (D) Discrimination between E3-Mi, E3-Ha/e3-Mo, e3-tr, E4 and e4-SORE-1 with mixed primers.
Genotypes at the E1–E4 loci of 63 accessions
The number of days from sowing to flowering initiation and the alleles at the E1–E4 loci identified by DNA markers and sequencing analyses in 63 accessions are listed in Table 2. At the E1 locus, E1 was the most abundant, followed by e1-as. On the other hand, e1-fs, e1-nl and e1-re were found to be rare. At the E2 locus, e2-ns was abundant, followed by E2-in and E2-dl. For the E3 locus, E3-Mi and e3-tr alleles were abundant, followed by the E3-Ha allele, while the e3-Mo allele was seldom found. The E3-Ha allele was detected in the accessions from China and North America. A Japanese cultivar ‘Suzuyutaka’ also harboured the E3-Ha allele. It was developed from the crossing of ‘Nemashirazu’ × ‘Harosoy’, followed by another crossing with ‘Okushirome’. Because the alleles at the E3 locus of ‘Nemashirazu’, ‘Harosoy’ and ‘Okushirome’ were E3-Mi, E3-Ha and e3-tr types, respectively, it was assumed that the E3-Ha allele of ‘Suzuyutaka’ was derived from ‘Harosoy’. The E4 allele was predominant at the E4 locus, and e4-SORE-1 was detected with a relatively high frequency in early-flowering accessions from Hokkaido (the north island of Japan). The autumn season type soybeans from the western region of Japan, which are classified into the IVc and Vc ecological types, flowered >62 d after sowing when sown at the standard sowing time. These accessions harboured dominant alleles at the E1, E2, E3 and E4 loci. Many accessions in ecological type IIIc possessed recessive alleles at the E2 locus and dominant alleles at the E3 locus. Many accessions in the ecological types IIb and IIc showed recessive alleles at the E2 locus and recessive or dominant alleles at the E3 locus. Most early-flowering accessions in IIa from the southern area had recessive alleles at both the E2 and E3 loci. Interestingly, all of the Japanese accessions except for those from the Hokkaido area harboured the dominant allele at the E1 locus. The early-flowering accessions from Hokkaido of the ecological types Ia and 1b harboured two or three recessive alleles at the E1–E4 loci. In particular, the e1-as allele in Hokkaido may have been derived mainly from the crossing with accessions from north-east China. Our data suggest that various combinations of alleles at the E1, E3 and E4 loci contributed to early flowering of soybeans in Hokkaido, which may have eventually led to adaptation of soybean to high-latitude environments.
Association between flowering times and genotypes at the E1–E4 loci
The effects of the E1–E4 loci on flowering time were analysed based on the data obtained at different sowing times using ANOVA and a multiple regression model. Among the 63 accessions, ten accessions were excluded because of incomplete or missing phenotype data due to abnormal growth or lodging, and unavailable genotype data at the E4 locus in one accession (‘Wasesuzunari’). As a result, only the data collected from 52 accessions were used for statistical analysis. High Pearson product–moment correlations of flowering phenotypes among three sowing conditions were observed: 0·99 for ‘early’ and ‘standard’; 0·98 for ‘early’ and ‘late’; and 0·98 for ‘standard’ and ‘late’. Although the range of flowering times decreased to 38·7 d in the ‘late’ condition from 68·0 d in the ‘early’ condition, the order of flowering times was highly conserved among these accessions (Table 2). These correlations indicate that the flowering times were determined by the stable effects of flowering genes. The effects of the E1–E4 loci on flowering time were highly significant based on ANOVA. There was no significant interaction between the effects of the loci and sowing conditions (data not shown). Multiple regression coefficients for the E1–E4 loci were estimated at 13·1, 9·0, 5·9 and 4·9, respectively (Table 3). When the regression coefficients were estimated separately, the values for the E1–E4 loci were relatively higher (16·4, 10·4, 7·2 and 6·4), intermediate (13·2, 9·0, 5·7 and 5·2) and lower (9·8, 7·7, 4·9 and 3·0) for ‘early’, ‘standard’, and ‘late’ sowing conditions, respectively (Table 3). However, the order of magnitude of the regression coefficients was conserved among the sowing conditions. Though interactions between loci could not be evaluated and differences in promoting effects on flowering among recessive alleles at each locus could not be included in the analyses, the results of ANOVA and estimation of regression coefficients confirmed the effects of the loci on flowering. The integrated contribution to flowering of these loci which was evaluated by the ratio of variance attributed to these loci to the total variance was estimated at about 62–66 % (Table 3), suggesting that other unknown genes may also exert significant effects on flowering.
Table 3.
Analysis of variance and estimation of regression coefficients of flowering genes
| Data used | Factor | d.f. | Mean square | F-value | Regression coefficient |
|---|---|---|---|---|---|
| Combined | Sowing condition | 2 | 3889·6 | 84·1*** | |
| E1 | 1 | 5766·7 | 124·7*** | 13·1*** | |
| E2 | 1 | 3604·5 | 78·0*** | 9·0*** | |
| E3 | 1 | 1594·8 | 34·5*** | 5·9*** | |
| E4 | 1 | 370·7 | 8·0** | 4·9** | |
| Residuals | 144 | 46·2 | |||
| Contribution of E1, E2, E3 and E4 | 44·0 % | ||||
| Early | E1 | 1 | 3099·3 | 49·5*** | 16·4*** |
| E2 | 1 | 1566·4 | 25·0*** | 10·4*** | |
| E3 | 1 | 796·3 | 12·7*** | 7·2** | |
| E4 | 1 | 212·0 | 3·4 | 6·4 | |
| Residuals | 46 | 62·7 | |||
| Contribution of E1, E2, E3 and E4 | 66·3 % | ||||
| Standard | E1 | 1 | 1916·1 | 44·3*** | 13·2*** |
| E2 | 1 | 1236·4 | 28·6*** | 9·0*** | |
| E3 | 1 | 507·2 | 11·7** | 5·7** | |
| E4 | 1 | 139·6 | 3·2 | 5·2 | |
| Residuals | 46 | 43·3 | |||
| Contribution of E1, E2, E3 and E4 | 65·6 % | ||||
| Late | E1 | 1 | 1038·8 | 33·0*** | 9·8*** |
| E2 | 1 | 843 | 26·8*** | 7·7*** | |
| E3 | 1 | 334·1 | 10·6** | 4·9** | |
| E4 | 1 | 47·3 | 1·5 | 3·0 | |
| Residuals | 44 | 31·5 | |||
| Contribution of E1, E2, E3 and E4 | 62·0 % | ||||
Statistical analyses were performed using data with three sowing times or separately at each sowing time. Complete data sets were used by omitting the missing and incomplete data. The test for regression coefficient was based on t-statistics.
The integrated contribution of the E1–E4 loci to flowering was evaluated by the ratio of variance attributed to these loci to the total variance.
**, ***Significant at P < 0·01 and P < 0·001, respectively.
DISCUSSION
Identification of novel alleles at the E1 and E2 loci by sequencing
A new allele with an insertion of a LINE at the promoter of the E1 gene was identified in ‘Gokuwase-Kamishunbetsu’ and designated as e1-re. The effect of this insertion on the expression of the E1 gene and GmFT genes should be confirmed. For the e1-p allele, it is necessary to identify the sequence in the 5′ upstream region that may regulate the expression of the E1 gene. An insertion and deletion of 36 bases in the eightth intron of the E2 gene were designated as E2-in and E2-dl, respectively, although the influence of this variation on flowering is not clear. Further research is necessary to reveal the effect of this variation on the expression of the E2 and GmFT genes. Many more accessions covering a wide range of latitudinal distribution may be necessary to detect novel alleles.
Allele discrimination with DNA markers is useful for diagnosis and marker-assisted selection of flowering genes
The allele-specific markers for the wild and e2-as alleles at the E2 locus and the multiplex PCR method with a mixture of one forward and two reverse primers to discriminate E3-Mi, E3-Ha and e3-tr alleles at the E3 locus were reported (Shin and Lee, 2012). The methods developed in our study can be used to discriminate systematically all the alleles that were identified in the present study at the E1–E3 loci, except for the e1-p allele.
Allele discrimination with DNA markers developed in the present study may become a useful method for the diagnosis of parental lines and for selection in breeding programmes in soybean. Genotypes at the E1–E4 loci identified by DNA markers and sequencing in multiple accessions covering many ecological types may be helpful to evaluate parental lines. To expand the adaptability of ‘Enrei’, two near-isogenic lines of ‘Enrei’ were developed with later flowering by introducing E2 and E3 alleles, based on backcrossing with maker-assisted selection (Yamada et al., 2012). An accurate selection of flowering genotypes and minute modification of flowering time could be obtained by the use of DNA markers developed for the alleles at the E1, E2 and E3 loci.
Allelic combinations at the E1–E4 loci are associated with ecological types
The relationship between ecological types and genotypes at the E1–E4 loci was revealed for the first time: E1/E2/E3/E4 was only detected in IVc and Vc; E1/e2/E3/E4 was a major genotype in IIIc; E1/e2/E3/E4 and E1/e2/e3/E4 were the genotypes in IIa, IIb and IIc; and E1/E2/e3/e4, E1/e2/e3/e4, e1/E2/e3/E4, e1/e2/E3/E4 and e1/e2/E3/e4 were the genotypes in Ib. Fukui and Matsumoto (1961) compared the days from cotyledon expansion to flower bud initiation, the days from bud initiation to blooming and the days from blooming to ripening among soybean varieties from each ecological type. The rates of shortening of the number of these days under SD conditions showed remarkable differences among ecological types in increasing order I, II, IV and V. The results could be interpreted primarily based on the number of dominant alleles at the E1, E3 and E4 loci which are involved in photoperiod sensitivity. The information obtained in the present study could be used to design allelic constitutions for breeding soybean varieties adapted to desired areas or cropping systems. McBlain et al. (1987) reported that the E1 allele delayed flowering but did not lengthen the reproductive period, while the E2 and E3 alleles lengthened both the pre-flowering and post-flowering periods. Saindon et al. (1989) found that the E4 allele delayed flowering and the beginning of maturity. For a precise understanding of ecological type, it is necessary to identify other genes related to flower initiation and the duration from flowering to maturity.
The integrated effect of the E1–E4 loci on flowering suggests other significant genes for flowering
The magnitude of effects of the dominant allele at these loci estimated by regression analysis decreased in the order E1, E2, E3 and E4, and was comparable with those reported in previous studies (Bernard, 1971; McBlain et al., 1987; Saidon et al., 1989; Yamanaka et al., 2001; Watanabe et al., 2004; Yamada et al., 2012). Analysis of association performed in the present study did not enable the evaluation of the interactions among these loci, presumably because genotypes expected in biparental populations were lacking in the data. In general, QTL analysis performed with a biparental population provides more accurate estimation of the effects of related loci, interactions among the loci and genotype × environmental interaction. Because flowering time varies depending on the genetic backgrounds, cultivated areas and sowing times, the differences in promoting effects on flowering among recessive alleles could not be included in the statistical analyses.
The integrated contribution to flowering of the E1–E4 loci was estimated at only 62–66 %, suggesting that the flowering time is controlled not only by the E1–E4 genes but also by other genes which are still unknown at present. Further studies should be carried out to identify other genes that may be involved in flowering time and to analyse their interactions. A combined approach including QTL analysis and use of candidate gene information from model plants may be useful for identification of flowering genes in soybean (Jung et al., 2012; Kim et al., 2012; Watanabe et al., 2012). Kaga et al. (2012) reported the analysis of the soybean collection conserved at the NIAS and their mini core collections. The association analysis using a large number of soybean accessions with genome-wide DNA markers may provide some useful insights into the identification of novel flowering time genes (Le Gouis et al., 2012). Furthermore, re-sequencing analysis of known flowering time genes in more accessions, including wild soybeans, would be necessary to elucidate the soybean domestication process and the roles of flowering time genes in soybean, particularly in the adaptation to higher latitudinal areas.
In conclusion, the present study advances the understanding of the combined roles of the E1–E4 loci in flowering and geographic adaptation and strongly suggested the need to identify other genes for flowering in soybean.
SUPPLEMENTARY DATA
ACKNOWLEGEMENTS
This work was supported by the Ministry of Agriculture, Forestry and Fisheries (Genomics for Agricultural Innovation Grants DD-2040 and Development of Mitigation and Adaptation Techniques to Global Warming in the Sectors of Agriculture Forestry and Fisheries 3002).
LITERATURE CITED
- Bernard RL. Two major genes for time of flowering and maturity in soybeans. Crop Science. 1971;11:242–247. [Google Scholar]
- Bonato ER, Vello NA. E6 a dominant gene conditioning early flowering and maturity in soybeans. Genetics and Molecular Biology. 1999;22:229–232. [Google Scholar]
- Buzzell RI. Inheritance of a soybean flowering response to fluorescent-daylength conditions. Canadian Journal of Genetics and Cytology. 1971;13:703–707. [Google Scholar]
- Buzzel RI, Voldeng HD. Inheritance of insensitivity to long daylength. Soybean Genetics Newsletter. 1980;7:26–29. [Google Scholar]
- Cober ER, Voldeng HD. A new soybean maturity and photoperiod-sensitivity locus linked to E1 and T. Crop Science. 2001;41:698–701. [Google Scholar]
- Cober ER, Molnar SJ, Charette M, Voldeng HD. A new locus for early maturity in soybean. Crop Science. 2010;50:524–527. [Google Scholar]
- Fukui J, Arai M. Ecological studies on Japanese soy-bean varieties. I Classification of soy-bean varieties on the basis of the days from germination to blooming and from blooming to ripening with special reference to their geographical differentiation. Japanese Journal of Breeding. 1951;1:27–39. [Google Scholar]
- Fukui J, Matsumoto S. On the varietal difference of the effect of short day-length upon flower initiation, its development and the seed ripening period of soy-bean. Japanese Journal of Breeding. 1961;11:1–6. [Google Scholar]
- Garner WW, Allard HA. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Journal of Agricultural Research. 1920;18:553–606. [Google Scholar]
- Huq E, Tepperman JM, Quail PH. GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2000;97:9789–9794. doi: 10.1073/pnas.170283997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C-H, Wong CE, Singh MB, Bhalla PL. Comparative genomic analysis of soybean flowering genes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038250. e38250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga A, Shimizu T, Watanabe S, et al. Evaluation of soybean germplasm conserved in NIAS genebank and development of mini core collections. Breeding Science. 2012;61:566–592. doi: 10.1270/jsbbs.61.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa A, Liu B, Kong F, Arase S, Abe J. Adaptive evolution involving gene duplication and insertion of a novel Ty1/copia-like retrotransposon in soybean. Journal of Molecular Evolution. 2009;29:164–175. doi: 10.1007/s00239-009-9262-1. [DOI] [PubMed] [Google Scholar]
- Kim MY, Shin JH, Kang YJ, Shim SR, Lee S-H. Divergence of flowering genes in soybean. Journal of Biosciences. 2012;37:857–870. doi: 10.1007/s12038-012-9252-0. [DOI] [PubMed] [Google Scholar]
- Kong FJ, Liu B, Xia Z, et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiology. 2010;154:1220–1231. doi: 10.1104/pp.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gouis J, Bordes J, Ravel C, et al. Genome-wide association analysis to identify chromosomal regions determining components of earliness in wheat. Theoretical and Applied Genetics. 2012;124:597–611. doi: 10.1007/s00122-011-1732-3. [DOI] [PubMed] [Google Scholar]
- Liu B, Kanazawa A, Matsumura H, Takahashi R, Harada K, Abe J. Genetic redundancy in soybean photoresponses associated with duplication of phytochrome A gene. Genetics. 2008;180:995–1007. doi: 10.1534/genetics.108.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBlain BA, Bernard RL. A new gene affecting the time of flowering maturity in soybeans. Journal of Heredity. 1987;178:68–70. [Google Scholar]
- McBlain BA, Hesketh JD, Bernard RL. Genetic effects on reproductive phenology in soybean isolines differing in maturity genes. Canadian Journal of Plant Science. 1987;67:105–116. [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Research. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saindon G, Beversdorf WD, Voldeng HD. Adjustment of the soybean phenology using the E4 locus. Crop Science. 1989;29:1361–1365. [Google Scholar]
- Shin JH, Lee S.-H. Molecular markers for the E2 and E3 genes controlling flowering and maturity in soybean. Molecular Breeding. 2012;30:1793–1798. [Google Scholar]
- Tokachi Agricultural Experiment Station. Characteristics and history of soybean variety preservations in Tokachi Agricultural Experiment Station. Miscellaneous Publication of Hokkaido Prefectural Tokachi Agricultural Experiment Station No. 1988;11:120–126. [Google Scholar]
- Tsubokura Y, Matsumura H, Xu M, et al. Genetic variation in the maturity locus E4 is involved in adaptation to high latitudes in soybean. Agronomy. 2012;3:117–134. [Google Scholar]
- Watanabe S, Tajuddin T, Yamanaka N, Hayashi M, Harada K. Analysis of QTLs for reproductive development and seed quality traits in soybean using recombinant inbred lines. Breeding Science. 2004;54:399–407. [Google Scholar]
- Watanabe S, Hideshima R, Xia Z, et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics. 2009;182:1251–1262. doi: 10.1534/genetics.108.098772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Xia Z, Hideshima R, et al. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics. 2011;188:395–407. doi: 10.1534/genetics.110.125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Harada K, Abe J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breeding Science. 2012;61:531–543. doi: 10.1270/jsbbs.61.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Watanabe S, Yamada T, et al. Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering. Proceedings of the National Academy Sciences, USA. 2012;109:E2155–E2164. doi: 10.1073/pnas.1117982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Xu Z, Liu B, et al. Genetic variation in four maturity genes affects photoperiod insensitivity and PHYA-regulated post-flowering responses of soybean. BMC Plant Biology. 2013;13:91–104. doi: 10.1186/1471-2229-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Hajika M, Yamada N, et al. Effects on flowering and seed yield of dominant alleles at maturity loci E2 and E3 in Japanese cultivar, Enrei. Breeding Science. 2012;61:653–660. doi: 10.1270/jsbbs.61.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Ninomiya S, Hoshi M, et al. An informative linkage map of soybean reveals QTLs for flowering time, leaflet morphology and region of segregation distortion. DNA Research. 2001;8:61–72. doi: 10.1093/dnares/8.2.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.