Abstract
Background and Aims
Environmental change is increasingly impacting ecosystems worldwide. However, our knowledge about the interacting effects of various drivers of global change on sexual reproduction of plants, one of their key mechanisms to cope with change, is limited. This study examines populations of poorly regenerating and threatened common juniper (Juniperus communis) to determine the influence of four drivers of global change (rising temperatures, nitrogen deposition, potentially acidifying deposition and altering precipitation patterns) on two key developmental phases during sexual reproduction, gametogenesis and fertilization (seed phase two, SP2) and embryo development (seed phase three, SP3), and on the ripening time of seeds.
Methods
In 42 populations throughout the distribution range of common juniper in Europe, 11 943 seeds of two developmental phases were sampled. Seed viability was determined using seed dissection and related to accumulated temperature (expressed as growing degree-days), nitrogen and potentially acidifying deposition (nitrogen plus sulfur), and precipitation data.
Key Results
Precipitation had no influence on the viability of the seeds or on the ripening time. Increasing temperatures had a negative impact on the viability of SP2 and SP3 seeds and decreased the ripening time. Potentially acidifying depositions negatively influenced SP3 seed viability, while enhanced nitrogen deposition led to lower ripening times.
Conclusions
Higher temperatures and atmospheric deposition affected SP3 seeds more than SP2 seeds. However, this is possibly a delayed effect as juniper seeds develop practically independently, due to the absence of vascular communication with the parent plant from shortly after fertilization. It is proposed that the failure of natural regeneration in many European juniper populations might be attributed to climate warming as well as enhanced atmospheric deposition of nitrogen and sulfur.
Keywords: Juniperus communis, juniper, seed viability, ripening time, climate change, increasing temperature, nitrogen deposition, acidifying deposition
INTRODUCTION
The early life history stages of plants, including seed production, are among the most important processes that drive plant community structure (HilleRisLambers et al., 2009; Linkies et al., 2010). Sexual reproduction aids plants to adapt to changing environments and to colonize previously unoccupied habitats (Fenner and Thompson, 2005). Worldwide, environmental conditions and ecosystems are undergoing rapid change (Millennium Ecosystem Assessment, 2005), and the effects of the drivers of this global change (e.g. climate change, nitrogen deposition, habitat fragmentation and invasive species) will increase in the coming decades (Rands et al., 2010). Hence, the role of sexual reproduction in the adaptation of organisms to changing environments might become more important. However, sexual reproduction itself is also affected by global change. Several studies have investigated the influence of different drivers of global change on sexual reproduction of plants, including warming (Peñuelas et al., 2004; De Frenne et al., 2011; Koivuranta et al., 2012), elevated CO2 concentrations (Thurig et al., 2003), nitrogen deposition (Callahan et al., 2008) and drought (Demirtas et al., 2010). Fewer studies have investigated the integrated effect of different drivers on sexual reproduction (Hovenden et al., 2008; HilleRisLambers et al., 2009; Verheyen et al., 2009; Li et al., 2011). Even less is known about the different processes acting during subsequent phases of the sexual reproductive cycle of plants (but see Owens et al., 2001; Hedhly, 2011). For example, Hedhly et al. (2009) underlined the importance of studying the sensitive stages (e.g. fertilization and embryogenesis) independently in order to obtain a better understanding of the effect of temperature on sexual reproduction. Here we follow such an approach in the coniferous shrub common juniper (Juniperus communis L.) to assess the effects of various drivers of global change across its distribution range. Juniperus communis has a relatively long sexual reproduction cycle (2–3 years) with clearly distinguishable phases (Gruwez et al., 2012) and it is widely distributed across much of the northern hemisphere (Adams, 2008). Populations of common juniper are declining throughout several European regions, including the north-western European lowlands [e.g. Belgium (Frankard, 2004; Adriaenssens et al., 2006), The Netherlands (Oostermeijer and De Knegt, 2004), northern and western Germany (Hüppe, 1995), and England (Clifton et al., 1997)], and the Mediterranean mountain regions (García et al., 1999). In contrast, the species is still abundant and exhibits good regeneration in other regions [e.g. in the Alps, Scandinavia and Poland(Falinski, 1980; Rosén, 1995; Rosén and Bakker, 2005)]. Nevertheless, due to their threatened status in several regions in Europe, J. communis communities are listed in Annex I of the EU Habitat Directive (code 5130).
Verheyen et al. (2005) identified habitat destruction, habitat degradation and limited sexual reproduction as the most important causes for this decline. In addition, a triangular relationship between the fraction of recently recruited individuals and the percentage of viable seeds in a population has been identified (Verheyen et al., 2009). Hence, a low percentage of viable seeds results in negligible recruitment, while in the case of a high percentage of viable seeds other factors such as herbivory, summer drought and the absence of suitable microsites for germination can explain the differences in recruitment between populations (Ward, 1973, 1982; Fitter and Jennings, 1975; Gilbert, 1980; García, 2001). Seed viability showed large inter-regional as well as intraregional variation across Europe (Verheyen et al., 2009). In addition, Verheyen et al. (2009) found a negative relationship between seed viability on the one hand and increasing temperature (expressed as mean annual growing degree-days above 0 °C) and enhanced nitrogen deposition on the other hand. In combination with climate warming, changing precipitation patterns are often put forward as important drivers of sexual reproduction in plants (e.g. Owens, 1995; Walck et al., 2011). Although Verheyen et al. (2009) found no relationship between seed viability of ripe common juniper seeds and precipitation, there can still be an influence during other phases of sexual reproduction. To date, landscape fragmentation has not led to genetic impoverishment of common juniper in north-western Europe (Vanden Broeck et al., 2011); thus, loss of genetic diversity is unlikely to be a cause of low seed viability.
The most critical phase of pre-dispersal seed development in common juniper occurs during embryo development (seed phase three; see Gruwez et al., 2012). However, it remains unclear whether the reasons for the failure of embryo development occur in this phase or in the previous phase of the growth of the pollen tube, gametogenesis, fertilization and early embryo development (seed phase two; see Gruwez et al., 2012), as different processes during seed development can regulate each other (Fig. 1). Two processes that need to be successful to allow fertilization are pollen tube growth and female gametophyte development (Fig. 1D, E). A prerequisite for pollen tube growth is that pollination supplies healthy pollen (Fig. 1A). There also seems to be an interaction between the pollen tube and the female gametophyte (Fig. 1B, C). In some species, a normal development of the ovule is promoted by the pollen or pollen tube that triggers the production of hormones such as auxins, gibberellins and cytokinins (Fernando et al., 2005; Owens et al., 2005). However, many gymnosperms show normal development of the female gametophyte until shortly after the period of fertilization, even without pollen (Owens and Blake, 1986; Owens, 1995; Owens and Morris, 1998). Growth of the pollen tube, on the other hand, often requires the presence of a healthy female gametophyte (e.g. Takaso and Owens, 1996; Fernando et al., 1997; Drews and Koltunow, 2011), probably because it provides specific secretions (Fernando et al., 2005) (Fig. 1B). During the pre-fertilization stage, the female gametophyte not only forms the archegonia and egg cells but also prepares, for instance, for seed reserve storage (Owens et al., 2008). Thus, the female gametophyte in gymnosperms accumulates nutrients before fertilization (Vuosku et al., 2009) and, at the moment of fertilization, the megagametophytes have almost reached their full development and there is practically no vascular communication between the seeds and the ovuliferous scales. Therefore, in general, the seeds are autonomous after fertilization (Owens and Blake, 1986; Owens, 1991) and the megagametophyte nourishes the developing embryo (Durzan and Chalupa, 1968; Vuosku et al., 2009). Thus, the development of the female gametophyte not only directly influences the fertilization (Fig. 1E) and indirectly influences the growth of the pollen tube (Fig. 1B), but anomalies during this phase can also lead to nutritional problems during embryo development (Fig. 1G).
Fig. 1.
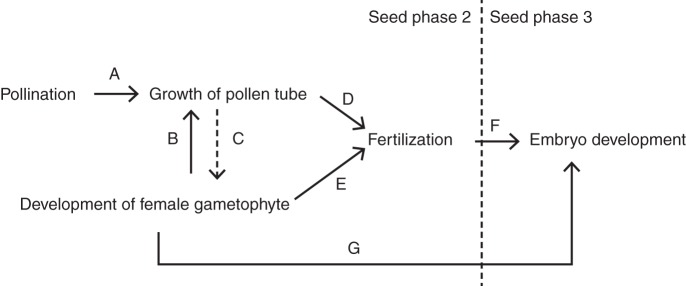
Schematic of the relationships between the key processes during the seed development of Juniperus communis (solid arrows for important relationships, dotted arrows for less important relationships). See text for a detailed explanation.
In common juniper, an additional complexity is that seeds can ripen in 2 years or in 3 years. In the latter case, fertilization is postponed for 1 year. A lag between pollination and fertilization is found in different conifers (e.g. different Pinus species; Singh, 1978), but the reasons remain unclear. It appears that the pollen tube enters dormancy while the female gametophyte is slowly developing. Shortly before fertilization, the pollen tube revives due to unknown cues (Williams, 2009). Willson and Burley (1983) suggested that delayed fertilization increases the time for selection of male gametophytes and female archegonia, but they also mentioned the possibility that short reproductive seasons force plants to spread pollination and fertilization over 1 year (Willson and Burley, 1983). However, there is still no consensus. In common juniper, the pattern of seed ripening can be dichotomized, with some seeds requiring a time interval of a few months and others a full year. These two strategies may appear within the same shrub (Gruwez et al., 2012). To our knowledge, this dual strategy is absent in other conifers. In addition, a complex relationship between the ripening time of the seeds of common juniper and seed viability appears to exist. Most seeds ripening in 3 years already have low viability shortly after fertilization, whereas the viability of seeds ripening in 2 years decreased mostly during embryo development (Gruwez et al., 2012).
In the present study, we sampled the hitherto spatially widest spread of populations (to our knowledge) of common juniper and collected seeds of two different development phases (shortly after fertilization and at the end of embryo ripening). By sampling in 42 populations throughout Europe (from Sweden to Spain and from Ireland to Poland), we are able to take advantage of the wide climatic and deposition gradients (De Frenne et al., 2013) in this area. We examined the influence of four drivers of global change (increasing temperature, nitrogen deposition, potentially acidifying depositions and altering precipitation patterns) on the seed viability of common juniper after both seed development phases and on the ripening time. The aim of the present study is therefore to test the following hypotheses: (1) that the influence of drivers of global change on seed viability is more pronounced after seed phase two, which indicates that this phase is more vulnerable and (2) that these drivers are determinants of which ripening time strategy occurs in seed.
MATERIALS AND METHODS
Species studied
Juniperus communis is a gymnosperm and one of the most widespread plant species, with a geographic distribution covering most of the northern hemisphere (Adams, 2008). It is a dioecious, wind-pollinated coniferous shrub or tree. The females annually produce fleshy, spherical, berry-like cones of approx. 6·5 mm in diameter that take 2 or 3 years to ripen (Ottley, 1909; García et al., 2000; Thomas et al., 2007; Ward, 2010). Sexual reproduction starts with the cone initiation in autumn or early winter (Singh, 1978), with the female strobili usually containing three ovules (Thomas et al., 2007). As common juniper has a dual seed ripening strategy, both a 2 year and a 3 year cycle can occur (Fig. 1; Gruwez et al., 2012). In a 2 year cycle, pollination takes place in the following spring and fertilization follows in the summer of the same year. After fertilization, embryo development starts and, by the end of the summer of the second year, the seeds are ready for dispersal. In a 3 year cycle, fertilization is postponed by 1 year and only takes place in the summer of the second year, so seeds are ripe for dispersal by the end of the summer of the third year (Ottley, 1909; García et al., 2000; Thomas et al., 2007; Ward, 2010; Gruwez et al., 2012). A detailed description of the seed and cone development is available in Gruwez et al. (2012). We here refer to seeds from the developmental phase shortly after fertilization as seed phase two seeds (SP2 seeds), while seeds that have a ripe embryo are referred to as seed phase three seeds (SP3 seeds; Gruwez et al., 2012).
Population, shrub and seed characteristics and sampling
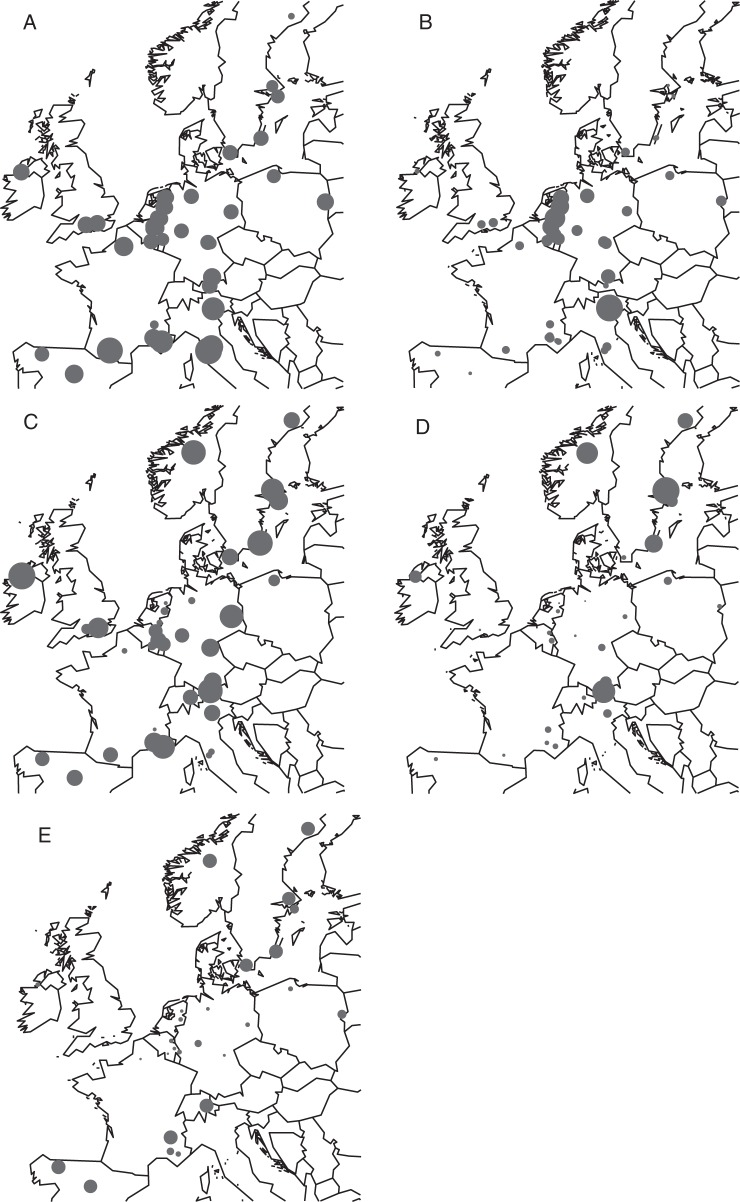
Seeds of 42 populations across the species' distribution range in Europe (Fig. 2A; Supplementary Data Table S1, Fig. S1) were sampled in autumn of 2008 and 2010 (eight and 34 populations, respectively). Populations consisted of at least 30 individual shrubs growing in unshaded conditions (i.e. not below other tree species). In each population, 3–8 (with a median of five) cone-bearing shrubs were randomly selected. Per shrub, three branches were randomly selected, of which on average 28·7 (±9·4 s.d.) SP2 seeds and 23·2 (±9·9 s.d.) SP3 seeds were sampled.
Fig. 2.
The locations and characteristics of the 42 sampled European common juniper (Juniperus communis) populations. For each map, the size of the dots is a relative measure for the GDD>0°C during embryo development (A), the amount of potentially acidifying deposition (N + S; keq ha−1 year−1) (B), the percentage of viable SP2 (C) and the percentage of viable SP3 seeds (D) per population; and the percentage seeds that ripen in 3 years instead of 2 years (E).
Different characteristics were measured and estimated on three different levels: population, shrub and seed. The age of the seeds was deduced from the age of the woody branches on which the cones (containing the seeds) were growing (the cones are always 1 year younger than the wood). The age of the wood can be determined by counting the growing shoot internodes that are separated by the annual bud scars. By taking the seed phase and the age of the seed into account, we then calculated the ripening time of the seeds (an SP2 seed of 1 year old or an SP3 seed of 2 years old has a ripening time of 2 years, an SP2 seed of 2 years old or an SP3 seed of 3 years old has a ripening time of 3 years). For every seed, the number of growing degree-days above 0 °C base temperature (GDD>0°C; cf. Hall et al., 2002) was calculated for three important processes during seed development: pollination, fertilization and embryo development. Depending on the collection date, seed phase and the ripening time, data of different years and seasons were used (Supplementary Data Table S2). Daily minimum and maximum temperatures of each population were obtained from the nearest weather stations (see Supplementary Data Table S1) and used to calculate the GDD>0°C. When the population and the weather station had different altitudes, a mean adiabatic lapse rate of 5·5 K km−1 (Körner, 2007) was applied. The GDD>0°C ranged between 257·6 and 1633·8, with an average of 979·5 (±245·6 s.d.) for the spring of pollination, between 775·7 and 2164·1 with an average of 1554·8 (±243·8 s.d.) for the summer of fertilization and between 1217·9 and 5074·7 with an average of 3344·8 (±786·7 s.d.) for the year of embryo development (Fig. 2A).
Nitrogen and sulfur deposition data were obtained from the European Monitoring and valuation Programme database (EMEP; http://www.emep.int). The EMEP is the ‘Co-operative Programme for Monitoring and Evaluation of the Long-range Transmission of Air pollutants in Europe’ and provides scientific information on the emission, transport and deposition of air pollutants. Here, data for 2008 were used: total (wet + dry) inorganic nitrogen (NHx + NOy) depositions expressed as kg ha−1 year−1 and potentially acidifying (NHx + NOy + SOx) depositions expressed as keq ha−1 year−1 in 50 × 50 km grid cells covering Europe. Nitrogen depositions ranged from 1·84 to 36·05 kg ha−1 year−1 with an average of 12·21 kg ha−1 year−1 (±6·9 s.d.). Potentially acidifying depositions ranged from 0·20 to 3·03 keq ha−1 year−1 with an average of 1·17 keq ha−1 year−1 (±0·60 s.d.) (Fig. 2B).
Next, the annual amount of precipitation in the year preceding the time of sampling was calculated per population using the monthly precipitation data from Climatic Research Unit (CRU) time-series data sets (Harris et al., 2013). Yearly precipitation ranged from 467·9 to 2280·8 mm year−1 with an average of 858·9 mm year−1 (±301·1 s.d.).
Correlations between temperature variables, atmospheric depositions and annual precipitation were low (Supplementary Data Table S3).
Finally, for each population, we estimated two soil characteristics in different classes: texture of the first 50 cm (sandy, sandy loam, loamy or clayey) and bedrock type (calcareous vs. non-calcareous).
At the shrub level, three characteristics, namely proportion of needle loss, cone density and plant height, were estimated. Different classes of needle loss (<20, <40 and ≥40 %) were used as an indicator for the health of each shrub. Cone density was sub-divided into three classes: low (the cones appear scattered and it is difficult to find any), normal (the cones appear scattered, but they are rather abundant) or dense (large clusters of cones are abundant). Finally, the height of each shrub was measured and classified into five different height classes (<0·5, <1, <2, <3 and ≥3 m).
Seed analyses
The viability of all sampled seeds was assessed by means of stereoscopic observations of dissected seeds (6609 seeds for SP2 and 5333 seeds for SP3). Seeds that had no visible signs of anomalies were considered to have the potential to develop to the next phase and are further referred to as ‘viable seeds’. Although this method generates an overestimation of the real seed viability, Adriaenssens (2006) stated that there is a clear correlation (R = 0·681 and P < 0·01) with the results of more precise methods such as a tetrazolium test (Miller, 2004). Viable SP2 seeds presented a megagametophyte and nucellus consisting of green-white and moist tissue, not completely filling the space within the seed coat (Gruwez et al., 2012). Viable SP3 seeds consisted of an embryo and megagametophyte with a smooth, white and moist surface. In this phase, almost all space within the seed coat is filled (Gruwez et al., 2012). In both SP2 and SP3, seeds were occasionally damaged by mites [e.g. Trisetacus quadrisetus (Acarina, Eriophyiidae)] or by the seed predator chalcid Megastigmus bipunctatus [Hymenoptera, Torymidae; see Roques and Skrzypczynska (2003) for a review of the seed-infesting chalcids of the genus Juniperus]. The content of seeds attacked by mites is completely distorted and, mostly, the mites are still present. Damage by M. bipunctatus could be recognized by the granular content, an exit hole or the presence of larvae. For both seed phases, seeds were scored on the basis of viability (non-viable or viable), presence of mites and M. bipunctatus (absent or present) and the ripening time (2 or 3 years). Mean infection rates with mites and M. bipunctatus were calculated for each shrub and population.
Data analysis
To study the relationships between the seed viability of SP2 seeds (viable or not), SP3 seeds (viable or not) and the ripening time (2 or 3 years; seed-level data throughout) on the one hand, and the climatic, environmental, soil, shrub and seed (ripening time) variables (fixed-effect terms) on the other hand, generalized linear mixed modelling with binomial distributions was applied, using the glmmML function of the glmmML library and the lmer function of the lme4 library in R 2.15.1 (R Development Core Team, 2012). Ripening time was only included in the models concerning the viability of SP2 and SP3 seeds. Populations were treated as clusters within the glmmML function and as random effects within the lmer function. In a first step, all variables were entered in the model on a one-by-one basis. Per dependent variable (viability of SP2 and SP3 seeds and ripening time), variables with a significance level of 0·1 were selected for multivariate modelling. Autocorrelation between the selected variables was checked by calculating the variance inflation factor (Quinn and Keough, 2002). In the case of autocorrelation (notably between GDD>0°C during pollination, GDD>0°C during fertilization and GDD>0°C during embryo development, and between nitrogen deposition and potentially acidifying depositions), only the most significant variables were selected.
Subsequently, all possible models for the three dependent variables (i.e. built by each combination of the selected fixed-effects terms, giving 384 models in total) were compared using the Akaike's information criterion, adjusted for sample size (AICc) (Hurvich and Tsai, 1989). The ΔAICc of a model was then calculated as the difference between the AICc of the model with the best fit and the AICc of that model. Models with ΔAICc ≤4 were considered equivalent (Bolker, 2008). To determine the relative importance of the explanatory variables, the sum of Akaike weights of the set of all top models (ΔAICc ≤4) in which the variable appeared (Burnham and Anderson, 2002) was used. The Aikake weight reflects the weight of evidence in support of a particular model relative to the entire model set, and varies from 0 (no support) to 1 (complete support). For each explanatory variable, the relative importance was calculated by summing the Aikake weights of the models containing the variable. Finally, the averaged parameters of the top models were calculated using the model averaging function based on the AICc of the MuMin package in R.
Finally, to visualize the effects of temperature, nitrogen deposition and potentially acidifying depositions on seed viability and ripening time, we calculated the proportion of viable SP2 and S3 seeds and the proportion of seeds that ripened in 3 years per population. A similar procedure was followed for shrub height, cone density and needle loss, where the proportion per class was calculated.
RESULTS
As expected, seed viability declined between seed phase two and three (Fig. 2). The average percentage of viable SP2 seeds per population was 38·2 % (±18·6 s.d.) with a minimum of 2·3 % and a maximum of 73·8 %. For SP3 seeds, the average was 10·9 % (±14·6 % s.d.) with a minimum of 0 % and a maximum of 58·2 %. The seed viability exhibits a large variability for both SP2 seeds and SP3 seeds. For example, 12 populations (in Belgium, France, Germany, Italy, The Netherlands, Spain and the UK) had extremely low percentages (<1 %) of viable SP3 seeds. Conversely, populations with higher percentages of viable SP2 seeds (>40 %) were situated in Scandinavia, on the axis of eastern Germany towards north-eastern Italy, on the axis of north-central Spain towards south-eastern France, in Ireland and in the south-west of the UK (Fig. 2C). After SP3, most of the Scandinavian populations still had relatively high percentages of viable seeds (>20 %), together with three populations in southern and central Germany and Austria and the Irish population (Fig. 2D).
Infection rates with mites and M. bipunctatus were relatively low for populations (mean 8·3 and 3·1 %, respectively), although infection rates of individual shrubs can be significant (e.g. 93·3 and 76·9 %, respectively). In only one population, more than half of the seeds were infected with mites (53·4 %), while M. bipunctatus infection rates always stayed below 20 %.
Most of the seeds ripened in 2 years (64·5 %). Fourteen populations had >90 % of their seeds ripening in 2 years and for nine populations this percentage dropped below 10 %. Seeds with a 3 year ripening time were mainly found in Scandinavia, but populations with high percentages were also present in Spain and south-eastern France (Fig. 2E).
Selection of the variables for multivariate modelling led to different results for the viability of SP2 and SP3 seeds and ripening time. For the viability of SP2 seeds, the factors precipitation, mite infection rate, type of bedrock, soil texture, needle loss and ripening time did not have a significance level lower than 0·1. These variables were therefore considered as not important and were excluded from the model selection procedure. In a similar way, precipitation, mite and M. bipunctatus infection rate, type of bedrock, soil texture and needle loss were excluded for SP3 seeds, and precipitation, mite infection rate and soil texture for ripening time. In addition, autocorrelation occurred between the temperature variables, and between the atmospheric deposition variables, resulting in a selection (on the basis of the factor with the highest significance level) of GDD>0°C during pollination and potentially acidifying deposition for SP2 seeds; of GDD>0°C during embryo development and potentially acidifying deposition for SP3 seeds; and of GDD>0°C in the year before sampling and nitrogen deposition for ripening time.
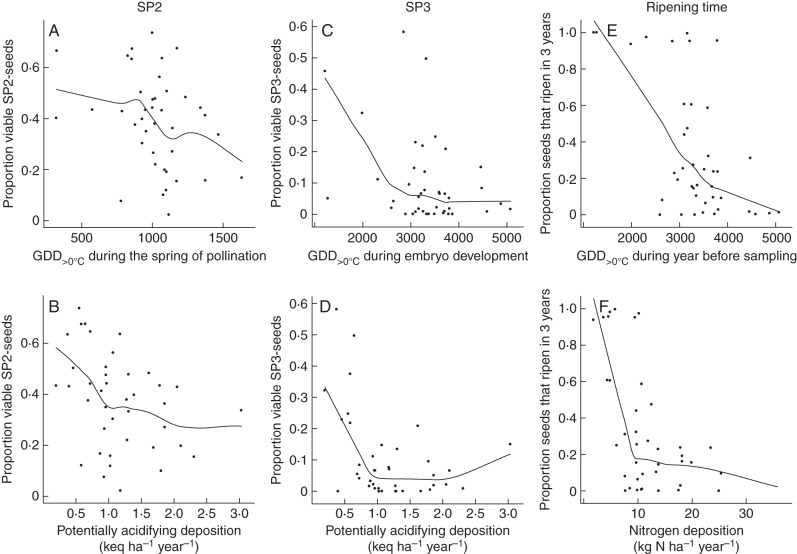
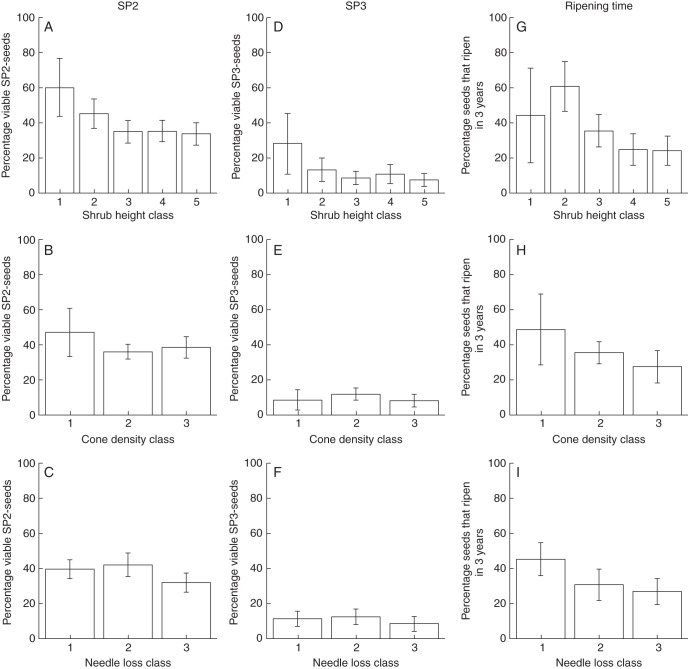
The most important variables affecting the viability of SP2 seeds were temperature (GDD>0°C during pollination), infection rate with M. bipunctatus on a population level and shrub height, which all had a negative influence (Table 1, Figs 3A, 4A). Potentially acidifying deposition had only a marginally negative influence (Table 1, Fig. 3B). The ripening time strategy for a given seed had no influence on the viability of SP2 seeds.
Table 1.
Model selection statistics for the analysis of the effects of GDD>0°C during the spring of pollination, potentially acidifying deposition, their interaction, infection rate with M. bipunctatus, cone density and shrub height on the viability of SP2 seed
| Intercept | Growing degree-days (GDD) pollination | Potentially acidifying deposition (PAD) | GDD:PAD | Infection rate with M. bipunctatus | Cone density | Shrub height | d.f. | ΔAICc | Weight |
|---|---|---|---|---|---|---|---|---|---|
| 3·50 | –3·09 × 10−3 | –11·9 | + | 8 | 0 | 0·48 | |||
| 3·55 | –3·02 × 10−3 | –0·0979 | –12·1 | + | 9 | 1·85 | 0·19 | ||
| 3·40 | –3·12 × 10−3 | –11·7 | + | + | 10 | 1·92 | 0·18 | ||
| 3·96 | –3·41 × 10−3 | –0·447 | 3·27 × 10−4 | –12·4 | + | 10 | 3·72 | 0·08 | |
| 3·45 | –3·06 × 10−3 | –0·0951 | –11·9 | + | + | 11 | 3·78 | 0·07 | |
| Importance | 1·00 | 0·34 | 0·07 | 1·00 | 0·26 | 1·00 | – | – | – |
+, a factorial variable is included in the model.
ΔAICc, difference in values of the corrected Akaike Information Criterion between a model and the best model; weight, Akaike weight indicating the relative support for the model; importance, the relative importance of the explanatory variables based on the sum of the Akaike weights of the models in which the variables appear.
Fig. 3.
Relationships between accumulated temperature (GDD>0°C during the spring of pollination for seed phase two, GDD>0°C during embryo development for seed phase three and GDD>0°C during the year before sampling for the ripening time) (A, C, E), potentially acidifying deposition (keq ha−1 year−1) for seed phase two and three, and nitrogen deposition (kg N ha−1 year−1) for ripening time (B, D, F) on the one hand, and viability of SP2 seeds (left column), viability of SP3 seeds (middle column) and the seed ripening time (right column). A smoothing spline is fitted to the continuous data.
The patterns were slightly different for SP3 seeds (Table 2). Here both potentially acidifying depositions and temperature (GDD>0°C during embryo development) had a strong negative influence on seed viability (Figs 3C, D). In addition, their interaction was also important and indicated that the negative influence of temperature was more pronounced in populations with a lower potentially acidifying deposition. In addition, shrub height and cone density had an important negative and positive influence, respectively (Fig. 4D, E). Seeds that ripened in 3 years had a slightly greater chance of being viable than seeds that ripened in 2 years, but this effect was less pronounced (Table 2).
Table 2.
Model selection statistics for the analysis of the effects of GDD>0°C during embryo development, potentially acidifying deposition, their interaction, cone density, shrub height and ripening time on the viability of SP3 seeds
| Intercept | Growing degree-days (GDD) embryo development | Potentially acidifying deposition (PAD) | GDD:PAD | Cone density | Shrub height | Ripening time | d.f. | ΔAICc | Weight |
|---|---|---|---|---|---|---|---|---|---|
| 4·70 | –2·07 × 10−3 | –6·95 | 1·72 × 10−3 | + | + | + | 12 | 0 | 0·65 |
| 5·15 | –2·16 × 10−3 | –7·18 | 1·77 × 10−3 | + | + | 11 | 1·20 | 0·36 | |
| Importance | 1 | 1 | 1 | 1 | 1 | 0·65 | – | – | – |
+, a factorial variable is included in the model.
ΔAICc, difference in values of the corrected Akaike Information Criterion between a model and the best model; weight, Akaike weight indicating the relative support for the model; importance, the relative importance of the explanatory variables based on the sum of the Akaike weights of the models in which the variables appear.
Fig. 4.
Relationships between shrub height (A, D, G), cone density (B, E, H) and needle loss (C, F, I) on the one hand, and viability of SP2 seeds (left column), viability of SP3 seeds (middle column) and the seed ripening time (right column). Shrub height classes: 1, <0·5 m; 2, <1 m; 3, <2 m; 4, <3 m; 5, ≥ 3 m; needle loss classes: 1, <20 %; 2, <40 %; 3, ≥40 %; cone density classes: 1, low; 2, normal; 3, dense. Values are means and error bars represent the 95 % confidence interval.
Ripening time was mostly determined by nitrogen deposition, the bedrock type, cone density, shrub height and needle loss (Table 3, Figs 3F, 4G–I). Seeds originating from populations with a higher nitrogen deposition or growing on soil with a calcareous bedrock more often had a ripening time of 2 years. Shrubs with a greater cone density had more seeds that ripened in 2 years, and vice versa for taller shrubs or those with a larger needle loss. Higher temperatures (GDD>0°C during embryo development) led to shorter ripening times, but this effect was less pronounced (Fig. 3E). The interaction between temperature and nitrogen deposition was of minor importance.
Table 3.
Model selection statistics for the analysis of the effects of GDD>0°C during the year before sampling, nitrogen deposition, their interaction the, bedrock type, cone density, shrub height and needle loss on the ripening time of the seeds
| Intercept | Growing degree-days (GDD) year before sampling | Nitrogen deposition | GDD:ND | Bedrock type | Infection rate with M. bipunctatus | Cone density | Shrub height | Needle loss | d.f. | ΔAICc | Weight |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6·18 | –2·29 × 10−3 | –0·553 | 1·06 × 10−4 | + | 15·8 | + | + | + | 15 | 0 | 0·22 |
| 2·14 | –1·20 × 10−3 | –0·156 | + | 17·5 | + | + | + | 14 | 0·11 | 0·21 | |
| 3·17 | –1·47 × 10−3 | –0·132 | 23·0 | + | + | + | 13 | 1·08 | 0·13 | ||
| 8·24 | –2·69 × 10−3 | –0·714 | 1·43 × 10−4 | + | + | + | + | 14 | 1·15 | 0·13 | |
| 7·27 | –2·56 × 10−3 | –0·518 | 1·03 × 10−4 | 20·0 | + | + | + | 14 | 1·22 | 0·12 | |
| –1·91 | –0·188 | + | 19·6 | + | + | + | 13 | 1·64 | 0·10 | ||
| 2·84 | –1·21 × 10−3 | –0·184 | + | + | + | + | 13 | 2·67 | 0·06 | ||
| –1·15 | –0·218 | + | + | + | + | 12 | 3·99 | 0·03 | |||
| Importance | 0·87 | 1 | 0·47 | 1 | 0·78 | 1 | 1 | 1 | – | – | – |
+, a factorial variable is included in the model.
ΔAICc, difference in values of the corrected Akaike Information Criterion between a model and the best model; weight, Akaike weight indicating the relative support for the model; importance, the relative importance of the explanatory variables based on the sum of the Akaike weights of the models in which the variables appear.
DISCUSSION
The aim of this study was to achieve a better understanding of the influence of four drivers of global change (increasing temperatures, enhanced nitrogen, potentially acidification deposition and altering precipitation patterns) on the viability and ripening time of common juniper seeds (Juniperus communis). We focused on two key seed developmental phases, i.e. growth of the pollen tube, megagametogenesis, fertilization and early embryo development (SP2) and ripening of the embryo (SP3), that were identified as crucial for the sexual reproductive cycle of this species (Gruwez et al., 2012). Both needle loss and shrub height (as a rough proxy for age, Breek, 1978; Forbes and Proctor, 1986) can be seen as a measure of senescence. Cone density, on the other hand, relates to the vitality of the shrub. This can explain the relationships between cone density, shrub height and seed viability. Precipitation was shown to have no influence. In the following sections, we focus on the important effects of temperature and enhanced atmospheric depositions on seed viability of common juniper.
Temperature effects
We showed that increasing temperatures have a negative influence on seed viability of both SP2 and SP3 seeds of common juniper and promote a ripening time of 2 years. Different patterns can be seen between the two studied developmental phases. For the viability of the SP2 seeds, the GDD>0°C during springtime were most important, while, for the SP3 seeds, only the GDD>0°C during embryo development had a significantly negative influence.
Our results differ from what is found in other studies on conifers where a positive relationship between higher temperatures and seed viability was revealed (e.g. Despland and Houle, 1997; Noland et al., 2006; Meunier et al., 2007). However, the latter studies were often performed at the northern distribution range limits, whereas our study included common juniper populations from a wider geographical area. Low temperatures and late frosts during pollination are often mentioned as reasons for failing pollination and ovule abortion during further seed development (e.g. Owens, 1995; Thomas et al., 2007). Therefore, a lack of pollination cannot explain the negative relationship between seed viability and the temperature during the spring.
During SP2, there are two processes (the development of the female gametophyte and growth of the pollen tube) that can help to explain the negative effects of temperature on seed viability (Fig. 1).
First, higher temperatures can induce abnormalities in the female gametophyte (Franz and Jolliff, 1989; Kozai et al., 2004) or other female structures (Saini et al., 1983; Hedhly et al., 2003, 2004, 2005), which can lead to abortion of the seed. Secondly, little is known about the direct effects of higher temperatures on the viability of the germinating pollen (but see Young et al., 2004; Steinacher and Wagner, 2012). However, several studies hypothesized that the ovule and female gametophyte might be important regulators of pollen tube growth (e.g. Gifford and Foster, 1989; Takaso and Owens, 1996; Fernando et al., 2005; Drews and Koltunow, 2011). Thus, through influencing the female gametophyte, increased temperatures can have an indirect effect.
In addition, due to their separate influences on the pollen tube and female gametophyte, high temperatures also have detrimental effects on the male–female synchrony in the pre-ferilization phases (Zinn et al., 2010; Hedhly, 2011).
Hence, both mechanisms, i.e. the negative influence of higher temperatures on the viability of the female gametophyte and the different effects on the growth speed of both female gametophyte and pollen tube, may lead to unviable SP2 seeds.
Higher temperatures during the spring of the pollination and the summer of the fertilization seem to have no influence on viability of SP3 seeds. Perhaps cones with aborted seeds have been shed by the third year, which can mask these effects. On the other hand, GDD>0°C during embryo development had a negative influence on seed viability. Possibly, higher temperatures may disrupt the meristimatic activity of the female gametophyte with accumulation of resources such as lipids, starch and proteins (Singh, 1978; Owens et al., 2008) and the nutrition and growth of the embryo. Owens et al. (2001) and Cross et al. (2003), for instance, found a higher rate of abortion in Picea abies and Linum usitatissimum, respectively, under higher temperatures during embryo development. An indirect negative effect of temperature on the viability of SP3 seeds due to malfunctions during the pre-fertilization development of the female gametophyte could not be detected, as GDD>0°C during that period had no significant effects.
Warmer temperatures during the last year before sampling (for SP2 and SP3 seeds) led to shorter ripening times. The influence of temperature on ripening time can be explained by an altered development of the male and female gametophytes and their interactions. As common juniper can be considered a cold-adapted species (cf. its relatively northerly distribution range and the assumption of its survival in Central Europe during the last glacial maximum; Michalczyk et al., 2010), there is a probability that the mechanism of delayed fertilization to overcome shorter reproduction seasons has developed in this species (Willson and Burley, 1983). Longer reproduction seasons due to higher temperatures can reduce the need for such mechanisms, leading to a higher chance of shorter ripening times.
To further our understanding of the role of higher temperature in the described processes, research on a biochemical level is needed.
Effects of nitrogen and potentially acidifying deposition
Acidifying depositions appear to have negative impacts on seed viability after SP3. We can assume that nitrogen deposition has a similar effect as both variables were highly correlated. This effect was almost absent after seed phase two. In addition, the effects of temperature and potentially acidifying depositions had a significant interaction after seed phase three: in populations with low potentially acidifying depositions, the negative effect of temperature was pronounced, while the effect was slightly reversed in populations with high depositions. Seeds sampled from individuals growing in areas with high nitrogen depositions also ripened more in 2 years instead of 3 years.
Our results agree with those of different studies demonstrating that nitrogen (e.g. Vergeer et al., 2003; Li et al., 2011) or potentially acidifying depositions (Wertheim and Craker, 1987; Feret et al., 1990; Munzuroglu et al., 2003; Vergeer et al., 2003) negatively affect plant performance in terms of seed quality. However, other studies have shown that nitrogen depositions can also increase seed quality (e.g. Drenovsky and Richards, 2005).
Nitrogen and potentially acidifying depositions can influence seed viability in a direct way by creating nutrient imbalances and causing decreased uptake and leaching of cations including K+, Ca2+ and Mg2+ in the plant (Bobbink et al., 1992; Krupa, 2003), leading to nutritional deficiencies (e.g. Pearson and Stewart, 1993). As for the influence of temperature, the biochemical mechanisms behind the effects on seed viability need more research. For example, Ca2+ plays a role in the control of conifer pollen tube growth (Fernando et al., 2005), and detoxification products such as arginine, needed in case of a higher uptake of NH4+ and NH3 through canopy exchange (Krupa, 2003), can have disturbing effects (Durzan and Chalupa, 1968; Durzan, 2002).
Nitrogen and potentially acidifying depositions can also indirectly influence plant performance as they cause a reduction of mycorrhizae associations in several species (Malcová et al., 1999; Krupa, 2003). For common juniper, symbiosis with mycorrhiza [especially with arbuscular mycorrhiza (Thomas et al. (2007)) can be important; Bakker (1988) suggested a possible relationship between the presence of mycorrhiza and the viability of J. communis shrubs.
Our results show an effect of potentially acidifying depositions only after seed phase three. One would expect a larger effect after seed phase two since, as in most conifers, seeds are largely autonomous shortly after fertilization. However, it is possible that anomalies during seed phase two (e.g. a badly developed megagametophyte due to nutrient deficits) only lead to seed abortion during seed phase three, when the megagametophyte nourishes the developing embryo. Similarly, nutrient imbalances and signalling disturbance can also alter the ripening time of the seed.
The effect of ripening time on seed viability was absent after seed phase two and of much less importance than the effect of potentially acidifying depositions and temperature after seed phase three. Thus, there is no clear evidence that the negative effects of increased temperatures, nitrogen depositions and potentially acidifying depositions on seed viability are acting through changed ripening duration of the seeds.
The negative influences of temperature, nitrogen deposition and potentially acidifying deposition on the viability of both SP2 and SP3 seeds of common juniper indicate that these drivers of global change affect different key processes of the sexual reproductive cycle of J. communis including pollen tube growth, megagametogenesis and embryo development. Thus, the failure of natural regeneration in many European juniper populations might be attributed to climate warming as well as high atmospheric deposition of nitrogen and sulfur.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to Natalia Filipowicz, Bernard Prévosto, Fiona Cooper, Cristina Blandino, Anna Shevtsova, Gérald Berger, Lena Ward, Daniel García, Eje Rosén, Massimo Nepi, Stefan Mayr, Aurélien Jamoneau, Guilaume Decocq, Inga Michalczyk, Dieter Michalczyk, Jake Alexander, Annette Kolb, Dirk, Lutz Eckstein, Tobias Donath, Sara Cousins, Marc Deconchat, Bente Graae, Jörg Brunet, Chris Melhuish, Chris Ford, Wolfgang Petrick, Thilo Heinken, Ana Isabel García-Cervigón Morales, José Miguel Olano Mendoza, Adrián Escudero, Georges Kunstler and Gnesotto Massimiliano for their help with the seed sampling, and to Luc Willems for help with the processing of the seeds. This paper was written while R.G. was funded by the Special Research Fund of Ghent University (BOF). P.D.F. and A.D.S. held a post-doctoral fellowship from the Research Foundation-Flanders (FWO). O.L. was funded by IRC. P.V. held a scholarship from the Flemish Institute for Technological Research (VITO).
LITERATURE CITED
- Adams RP. Junipers of the world: the genus Juniperus. 2nd edn. Vancouver: Trafford Publishing; 2008. [Google Scholar]
- Adriaenssens S. 2006. Vergelijkend onderzoek naar de productie en kiemkracht van jeneverbeszaden in Vlaanderen en omliggende regio's. MSc Thesis, Ghent University, Belgium.
- Adriaenssens S, Baeten L, Crabbe S, Verheyen K. Evolutie (1985–2006) en toekomst van de jeneverbes (Juniperus communis L.) in de provincie Limburg. Ghent: Ghent University & Likona; 2006. [Google Scholar]
- Bakker A. 1988. Gymnosporangium bij Juniperus communis L. en het verband tussen taksterfte in de toppen van Juniperus communis en het VAM-percentage. PhD Thesis, Wageningen Univerisity, The Netherlands.
- Bobbink R, Jeil GW, Raessen MBAG. Atmospheric deposition and canopy exchange processes in heathland ecosystems. Environmental Pollution. 1992;75:29–37. doi: 10.1016/0269-7491(92)90053-d. [DOI] [PubMed] [Google Scholar]
- Bolker BM. Ecological models and data. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- Breek J. 1978. De kiemingsecologie van Juniperus communis L. MSc Thesis, Utrecht University, The Netherlands.
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- Callahan HS, Del Fierro K, Patterson AE, Zafar H. Impacts of elevated nitrogen inputs on oak reproductive and seed ecology. Global Change Biology. 2008;14:285–293. [Google Scholar]
- Clifton SJ, Ward LK, Ranner DS. The status of juniper Juniperus communis L. in North-East England. Biological Conservation. 1997;79:67–77. [Google Scholar]
- Cross RH, McKay SAB, McHughen AG, Bonham-Smith PC. Heat-stress effects on reproduction and seed set in Linum usitatissimum L. (flax) Plant, Cell and Environment. 2003;26:1013–1020. [Google Scholar]
- De Frenne P, Brunet J, Shevtsova A, et al. Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Global Change Biology. 2011;17:3240–3253. [Google Scholar]
- De Frenne P, Graae BJ, Rodríguez-Sánchez F, et al. Latitudinal gradients as natural laboratories to infer species' responses to temperature. Journal of Ecology. 2013;101:784–795. [Google Scholar]
- Demirtas C, Yazgan S, Candogan BN, Sincik M, Buyukcangaz H, Goksoy AT. Quality and yield response of soybean (Glycine max L. Merrill) to drought stress in sub-humid environment. African Journal of Biotechnology. 2010;9:6873–6881. [Google Scholar]
- Despland E, Houle G. Climate influences on growth and reproduction of Pinus banksiana (Pinaceae) at the limit of the species distribution in Eastern North America. American Journal of Botany. 1997;84:928–937. [PubMed] [Google Scholar]
- Drenovsky RE, Richards JH. Nitrogen addition increases fecundity in the desert shrub Sarcobatus vermiculatus. Oecologia. 2005;143:349–356. doi: 10.1007/s00442-004-1821-y. [DOI] [PubMed] [Google Scholar]
- Drews GN, Koltunow AMG. The female gametophyte. The Arabidopsis Book. 2011;9:e0155. doi: 10.1199/tab.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durzan DJ, Chalupa V. Free sugars, amino acids, and soluble proteins in the embryo and female gametophyte of jack pine as related to climate and seed source. Canadian Journal of Botany. 1968;46:417–428. [Google Scholar]
- Durzan DJ. Stress-induced nitric oxide and adaptive plasticity in conifers. Journal of Forest Science. 2002;48:281–291. [Google Scholar]
- Falinski JB. Vegetation dynamics and sex structure of the populations of pioneer dioecious woody plants. Vegetatio. 1980;43:23–38. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Feret PP, Diebel KE, Sharik TL. Effect of simulated acid rain on reproductive attributes of red spruce (Picea rubens Sarg.) Environmental and Experimental Botany. 1990;30:309–312. [Google Scholar]
- Fernando DD, Owens JN, von Anderkas P, Takaso T. In vitro pollen tube growth and penetration of female gametophyte in Douglas fir (Pseudotsuga menziesii) Sexual Plant Reproduction. 1997;10:209–216. [Google Scholar]
- Fernando DD, Lazzaro MD, Owens JN. Growth and development of conifer pollen tubes. Sexual Plant Reproduction. 2005;18:149–162. [Google Scholar]
- Fitter AH, Jennings RD. The effects of sheep grazing on the growth and survival of seedling junipers (Juniperus communis L.) Journal of Applied Ecology. 1975;12:637–642. [Google Scholar]
- Forbes ARD, Proctor J. The Glen Artney juniper wood. Transactions of the Botanical Society of Edinburgh. 1986;45:63–72. [Google Scholar]
- Frankard P. Evolution de la population de Juniperus communis L. dans la réserve naturelle domaniale de la genévrière de Cour pendant ces vingt dernières années et impact des mesures de gestion appliquées. Parcs et Réserves. 2004;59:32–37. [Google Scholar]
- Franz RE, Jolliff GD. Temperature effects on megagametophytic development in meadowfoam. Crop Science. 1989;29:133–141. [Google Scholar]
- García D, Zamora R, Gómez JM, Hódar JA, Jordano P. Age structure of Juniperus communis L. in the Iberian peninsula: conservation of remnant populations in Mediterranean Mountains. Biological Conservation. 1999;87:215–220. [Google Scholar]
- García D. Effects of seed dispersal on Juniperus communis on a Mediterranean mountain. Journal of Vegetation Science. 2001;12:839–848. [Google Scholar]
- García D, Zamora R, Gómez JM, Jordano P, Hódar JA. Geographical variation in seed production, predation and abortion in Juniperus communis throughout its range in Europe. Journal of Ecology. 2000;88:436–446. [Google Scholar]
- Gifford E, Foster A. Morphology and evolution of vascular plants. 3rd edn. New York: W. H. Freeman; 1989. [Google Scholar]
- Gilbert OL. Juniper in Upper Teesdale. Journal of Ecology. 1980;68:1013–1024. [Google Scholar]
- Gruwez R, Leroux O, De Frenne P, Tack W, Viane R, Verheyen K. Critical phases in the seed development of common juniper (Juniperus communis) Plant Biology. 2012;15:210–219. doi: 10.1111/j.1438-8677.2012.00628.x. [DOI] [PubMed] [Google Scholar]
- Hall B, Motzkin G, Foster DR, Syfert M, Burk J. Three hundred years of forest and land-use change in Massachusetts, USA. Journal of Biogeography. 2002;29:1319–1335. [Google Scholar]
- Harris I, Jones PD, Osborn TJ, Lister DH. Updated high-resolution grids of monthly climatic observations – the CRU TS3·10 Dataset. International Journal of Climatology. 2013. (in press)
- Hedhly A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environmental and Experimental Botany. 2011;74:9–16. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. The effect of temperature on stigmatic receptivity in sweet cherry (Prunus avium L.) Plant, Cell and Environment. 2003;26:1673–1680. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rocaceae) American Journal of Botany. 2004;91:558–564. doi: 10.3732/ajb.91.4.558. [DOI] [PubMed] [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biology. 2005;7:476–483. doi: 10.1055/s-2005-865850. [DOI] [PubMed] [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends in Plant Science. 2009;14:30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- HilleRisLambers J, Harpole WS, Schnitzer S, Tilman D, Reich PB. CO2, nitrogen, and diversity differentially affect seed production of prairie plants. Ecology. 2009;90:1810–1820. doi: 10.1890/07-1351.1. [DOI] [PubMed] [Google Scholar]
- Hovenden MJ, Wills KE, Chaplin RE, et al. Warming and elevated CO2 affect the relationship between seed mass, germinability and seedling growth in Austrodanthonia caespitosa, a dominant Australian grass. Global Change Biology. 2008;14:1–9. [Google Scholar]
- Hüppe J. Zur Problematik der Verjüngung des Wacholders (Juniperus communis) unter dem Einfluβ von Wildkaninchen in Hudegebieten pleistozäner Sandlandschaften. Zeitschrift für Ökologie und Naturschutz. 1995;4:1–8. [Google Scholar]
- Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76:297–307. [Google Scholar]
- Koivuranta L, Latva-Karnanmaa T, Pulkkinen P. The effect of temperature on seed quality and quantity in crosses between European (Populus tremula) and hybrid aspens (P. tremula×P. tremuloides) Sylva Fennica. 2012;46:17–26. [Google Scholar]
- Körner The use of ‘altitude’ in ecological research. Trends in Ecology and Evolution. 2007;22:569–574. doi: 10.1016/j.tree.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Kozai N, Beppu K, Mochioka R, Boonprakob U, Subhadradandhu S, Katoaka I. Adverse effects of high temperature on the development of reproductive organs in ‘Hakuho’ peach trees. Journal of Horticultural Science and Biotechnology. 2004;79:533–537. [Google Scholar]
- Krupa SV. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environmental Pollution. 2003;124:179–221. doi: 10.1016/s0269-7491(02)00434-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang H, Xia J, Zhang W, Wan S, Li L. Effects of increased nitrogen deposition and precipitation on seed and seedling production of Potentilla tanacetifolia in a temperate steppe ecosystem. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028601. pe28601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkies A, Graeber K, Knight C, Leubner-Metzger G. The evolution of seeds. New Phytologist. 2010;186:817–831. doi: 10.1111/j.1469-8137.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- Malcová R, Vosátka M, Albrechtová J. Influence of arbuscular mycorrhizal fungi and simulated acid rain on the growth and coexistence of the grasses Calamagrostis villosa and Deschampsia flexuosa. Plant and Soil. 1999;207:45–57. [Google Scholar]
- Meunier C, Sirois L, Bégin Y. Climate and Picea mariana seed maturation relationships: a multiple-scale perspective. Ecological Monographs. 2007;77:361–376. [Google Scholar]
- Michalczyk IM, Opgenoorth L, Luecke Y, Huck S, Ziegenhagen B. Genetic support for perglacial survival of Juniperus communis L. in Central Europe. Holocene. 2010;20:887–894. [Google Scholar]
- Millennium Ecosystem Assessment (MEA) Ecosystems and human well-being: scenarios. Washington, DC: Island Press; 2005. [Google Scholar]
- Miller AL. Tetrazolium testing for flower seeds. In: Mcdonald MB, Kwong FY, editors. Flower seeds: biology and technology. Wallingford, UK: CABI Publishing; 2004. pp. 229–310. [Google Scholar]
- Munzuroglu O, Obek E, Geckil H. Effects of simulated acid rain on the pollen germination and pollen tube growth of apple (Malus sylvestris Miller cv. Golden) Acta Biologica Hungarica. 2003;54:95–103. doi: 10.1556/ABiol.54.2003.1.10. [DOI] [PubMed] [Google Scholar]
- Noland TL, Parker WC, Morneault AE. Natural variation in seed characteristics of eastern white pine (Pinus stobus L.) New Forests. 2006;32:87–103. [Google Scholar]
- Oostermeijer JGB, De Knegt B. Genetic population structure of the wind-pollinated, dioecious shrub Juniperus communis in fragmented Dutch heathlands. Plant Species Biology. 2004;19:175–184. [Google Scholar]
- Ottley AM. The development of the gametophytes and fertilization in Juniperus communis and Juniperus virginia. Botanical Gazette. 1909;48:31–46. [Google Scholar]
- Owens JN, Blake MD. Production de Semences Forestières. Rapport d'information PI-X-53F du Service Canadien des Forêts. Canada: Institut forestier national de Petawawa; 1986. [Google Scholar]
- Owens JN. Physiology of trees. New York: John Wiley; 1991. Flowering and seed set In: Raghavendra AS; pp. 247–271. [Google Scholar]
- Owens JN. Constraints to seed production: temperate and tropical forest trees. Tree Physiology. 1995;15:447–484. doi: 10.1093/treephys/15.7-8.477. [DOI] [PubMed] [Google Scholar]
- Owens JN, Morris SJ. Factors affecting seed and cone development in Pacific silver fir (Abies amabilis) Canadian Journal of Forest Research. 1998;28:1146–1163. [Google Scholar]
- Owens JN, Johnsen Ø, Dæhlen OG, Skrøppa T. Potential effects of temperature on early reproductive development and progeny performance in Picea abies (L.) Karst. Scandinavian Journal of Forest Research. 2001;16:21–237. [Google Scholar]
- Owens JN, Bennet J, L'Hirondelle S. Pollination and cone morphology affect cone and seed production in lodgepole pine seed orchards. Canadian Journal of Forest Research. 2005;35:383–400. [Google Scholar]
- Owens JN, Kittirat T, Mahalovich MF. Whitebark pine (Pinus albicaulis Engelm.) seed production in natural stands. Forest Ecology and Management. 2008;255:803–809. [Google Scholar]
- Pearson J, Stewart GR. The deposition of atmospheric ammonia and its effects on plants. New Phytologist. 1993;125:283–305. doi: 10.1111/j.1469-8137.1993.tb03882.x. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Gordon C, Llorens L, et al. Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a north–south European gradient. Ecosystems. 2004;7:598–612. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, ctVienna; 2012. [Google Scholar]
- Rands MRW, Adams WM, Bennun L, et al. Biodiversity conservation: challenges beyond 2010. Science. 2010;329:1298–1303. doi: 10.1126/science.1189138. [DOI] [PubMed] [Google Scholar]
- Roques A, Skrzypczynska M. Seed-infesting chalcids of the genus Megastigmus Dalman, 1820 (Hymenoptera: Torymidae) native and introduced to the West Palearctic region: taxonomy, host specificity and distribution. Journal of Natural History. 2003;37:127–238. [Google Scholar]
- Rosén E. Periodic droughts and long-term dynamics of alvar grassland vegetation on Öland, Sweden. Folia Geobotanica Phytotaxonomica. 1995;30:131–140. [Google Scholar]
- Rosén E, Bakker JP. Effects of agri-environment schemes on scrub clearance, livestock grazing and plant diversity in a low-intensity farming system on Öland, Sweden. Basic and Applied Ecology. 2005;6:195–204. [Google Scholar]
- Saini HS, Sedgley M, Aspinall D. Effect of heat stress during floral development on pollen tube growth and ovary anatomy in wheat (Triticum aestivum L.) Australian Journal of Plant Physiology. 1983;10:137–144. [Google Scholar]
- Singh H. Embryology of gymnosperms. Encyclopedia of plant anatomy, vol 10, pt. 2. Berlin: Gerbruder Borntraeger; 1978. [Google Scholar]
- Steinacher G, Wagner J. Effects of temperature on the progamic phase in high-mountain plants. Plant Biology. 2012;14:295–305. doi: 10.1111/j.1438-8677.2011.00498.x. [DOI] [PubMed] [Google Scholar]
- Takaso T, Owens JN. Postpollination–prezygotic ovular secretions into the mycropylar canal in Pseudotsuga menziesii (Pinaceae) Journal of Plant Research. 1996;109:147–160. [Google Scholar]
- Thomas PA, El-Barghathi M, Polwart A. Biological Flora of the British Isles: Juniperus communis L. Journal of Ecology. 2007;95:1404–1440. [Google Scholar]
- Thurig B, Körner C, Stocklin J. Seed production and seed quality in a calcareous grassland in elevated CO2. Global Change Biology. 2003;9:873–884. [Google Scholar]
- Vanden Broeck A, Gruwez R, Cox K, Adriaesnssens S, Michalczyk IM, Verheyen K. Genetic structure and seed-mediated dispersal rates of an endangered shrub in a fragmented landscape: a case study for Juniperus communis in northwestern Europe. BMC Genetics. 2011;12:73. doi: 10.1186/1471-2156-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergeer P, Rengelink R, Ouborg NJ, Roelofs JGM. The effects of population size and genetic variation on the response of Succisa pratensis to eutrophication and acidification. Journal of Ecology. 2003;91:600–609. [Google Scholar]
- Verheyen K, Schreurs K, Vanholen B, Hermy M. Intensive management fails to promote recruitment in the last large population of Juniperus communis (L.) in Flanders (Belgium) Biological Conservation. 2005;124:113–121. [Google Scholar]
- Verheyen K, Adriaenssens S, Gruwez R, et al. Juniperus communis: victim of the combined action of climate change and nitrogen deposition? Plant Biology. 2009;11:49–59. doi: 10.1111/j.1438-8677.2009.00214.x. [DOI] [PubMed] [Google Scholar]
- Vuosku J, Sarjala T, Jokela A, et al. One tissue, two fates: different roles of megagametophyte cells during Scots pine embryogenesis. Journal of Experimental Botany. 2009;60:1375–1386. doi: 10.1093/jxb/erp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biology. 2011;17:2145–2161. [Google Scholar]
- Ward LK. The conservation of juniper. I. Present status of juniper in southern England. Journal of Applied Ecology. 1973;10:165–188. [Google Scholar]
- Ward LK. The conservation of juniper: longevity and old age. Journal of Applied Ecology. 1982;19:917–928. [Google Scholar]
- Ward LK. Variation in ripening years of seed cones of Juniperus communis. Watsonia. 2010;28:11–19. [Google Scholar]
- Wertheim FS, Craker LE. Acid rain and pollen germination in corn. Environmental Pollution. 1987;48:165–172. doi: 10.1016/0269-7491(87)90031-5. [DOI] [PubMed] [Google Scholar]
- Williams CG. Conifer reproductive biology. New York: Springer; 2009. [Google Scholar]
- Willson MF, Burley N. Mate choice in plants: tactics, mechanisms, and consequences. Princeton, NJ: Princeton University Press; 1983. [Google Scholar]
- Young LW, Wilen RW, Bonham-Smith PC. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. Journal of Experimental Botany. 2004;55:485–495. doi: 10.1093/jxb/erh038. [DOI] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany. 2010;61:1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.