Abstract
Background and Aims
Epicotyl dormancy break in seeds that have deep simple epicotyl morphophysiological dormancy (MPD) requires radicle emergence and even a certain root length in some species. However, the mechanisms by which root length affects epicotyl dormancy break are not clear at present. This study aims to explore the relationship between root length and epicotyl dormancy release in radicle-emerged seeds of Tibetan peony, Paeonia ludlowii, with discussion of the possible mechanisms.
Methods
Radicle-emerged seeds (radicle length 1·5, 3·0, 4·5 and 6·0 cm) were incubated at 5, 10 and 15 °C. During the stratification, some seeds were transferred to 15 °C and monitored for epicotyl–plumule growth. Hormone content was determined by ELISA, and the role of hormones in epicotyl dormancy release was tested by exogenous hormone and embryo culture.
Key Results
Cold stratification did not break the epicotyl dormancy until the root length was ≥6 cm. The indole-3-actic acid (IAA) and GA3 contents of seeds having 6 cm roots were significantly higher than those of seeds with other root lengths, but the abscisic acid (ABA) content was lowest among radicle-emerged seeds. GA3 (400 mg L−1) could break epicotyl dormancy of all radicle-emerged seeds, while IAA (200 mg L−1) had little or no effect. When grown on MS medium, radicles of naked embryos grew and cotyledons turned green, but epicotyls did not elongate. Naked embryos developed into seedlings on a mixed medium of MS + 100 mg L−1 GA3.
Conclusions
A root length of ≥6·0 cm is necessary for epicotyl dormancy release by cold stratification. The underlying reason for root length affecting epicotyl dormancy release is a difference in the GA3/ABA ratio in the epicotyl within radicle-emerged seeds, which is mainly as a result of a difference in ABA accumulation before cold stratification.
Keywords: Seed, epicotyl dormancy release, hormone, root length, Paeonia ludlowii, Tibetan peony
INTRODUCTION
Paeonia ludlowii, the Tibetan peony, is a species endemic to, and distributed in, a small area of southern Tibet in western China. Growing at an altitude of 2900–3200 m a.s.l. in the Brahmaputra River valley on hillsides and along the forest edge, it is an important rare species with high economic, medicinal and ornamental value (Zhang et al., 1997). Moreover, the Tibetan peony is an important peony genetic resource due to its tall stature and pure yellow flowers. Paeonia ludlowii is an obligate breeder, and sexual reproduction resulting in seed is the only known viable option for Tibetan peony breeding and reproduction (Allen and Meyer, 1998). In natural conditions, the germination percentage of Tibetan peony seeds was determined to be 2·3 ‰ and it requires about 2–3 years to grow into seedlings (He, 2008; Su et al., 2010).
A low germination percentage and long germination periods are factors that have contributed to a sharp decrease in population and a narrower distribution range of Tibetan peony. This species is on the verge of extinction and is listed in the Endangered Species Red Book of China. Biologists wanting to save this species first need to address the most important issue of how to increase its seed germination percentage. He (2008) and Ma et al. (2012) successfully released the radicle dormancy by a variety of temperature stratification treatments and found that the optimal condition for radicle germination was warm stratification at 15 °C for 90 d. Furthermore, the cold stratification methods reported by Jing and Zheng (1999) did not promote epicotyl–plumule germination in Tibetan peony seeds. It is speculated that Tibetan peony seeds have deep epicotyl dormancy and that dormancy release requires strict treatment conditions.
In the nine different levels of morphophysiological dormancy (MPD), epicotyl dormancy has received considerable research attention from seed researchers, ecologists, physiologists and other scientists working in similar fields (Baskin and Baskin, 2004) for the following reasons. First, epicotyl dormancy is a common type of seed dormancy. Many species have this dormancy characteristic, such as Piperales, Liliales, Ranunculales, Saxifragales, Dipsacales, Boraginaceae, Chionanthus retusus, Psychotria sp. and Quercus spp. (Allen and Farmer, 1977; Farmer, 1977; Baskin and Baskin, 1998; Chien et al., 2004). Secondly, specific conditions are required for dormancy break. More interesting was the fact that radicle germination was a prerequisite for releasing epicotyl dormancy, in addition to stratification time and temperature (Jing et al., 1995; Zheng et al., 1995; Baskin and Baskin, 1998; Chien et al., 2011). For some species, such as Paeonia rockii, P. szechuanica and P. spontanea, the radicle must grow to a certain length before the epicotyl dormancy can be released by cold stratification. However, how root length affects epicotyl dormancy release is not very clear.
Plant hormones are one of the most important seed dormancy and germination regulators, of which GA3 and abscisic acid (ABA) are considered to be fundamental promoting and inhibiting hormones. Seed dormancy studies in species such as Fagus sylvatica, Arabidopsis sp., potato and Nicotiana plumbaginifolia have provided evidence that the ABA and GA3 balance regulates the onset, maintenance and termination of dormancy (Nicolás et al., 1996; Alvarado et al., 2000; Debeaujon and Koornneef, 2000; Grappin et al., 2000; Lorenzo et al., 2002). The effect of ABA in antagonizing gibberellin (GA) during germination of arabidopsis (Karssen et al., 1983; Kucea et al., 2005) might be via repression of the growth potential of the embryo, which is indispensable for overcoming the restraint conferred by the envelope structures. It has been suggested that GA has two functions during seed germination (Linkies and Leubner, 2012). First, GA enhances the growth potential of the embryo and promotes germination. Secondly, GA is essential to overcome the restraint imposed by the seed-covering layers through weakening of the tissues around the radicle. The regulatory effect of ABA and GA on seed dormancy release is therefore relatively clear, but their roles in determining how root length affects the epicotyl dormancy break require further studies.
Our study aims to investigate the condition of epicotyl dormancy break in Tibetan peony seeds in order to discuss: (1) how root length affects epicotyl dormancy release; and (2) the ecological significance of epicotyl dormancy.
MATERIALS AND METHODS
Plant materials
About 20 kg of mature seeds of Tibetan peony (Paeonia ludlowii) were collected from a population growing in the Brahmaputra River valley on hillsides and forest edge in Linzi (altitude 3100 m, latitude 94 °25'N, longitude 29 °59'E) Tibet in October 2006. Seeds were extracted from fruits by removing the pericarp by hand and were sealed inside transparent polyethylene ziplock bags (0·12 mm in thickness). Laboratory experiments were started on 15 October 2006 at the Beijing Botanical Garden Institute of Botany, Chinese Academy of Sciences. Before the experiment, the shrivelled seeds were removed from their bags and suspended in water. Seeds were soaked in distilled water at constant 40 °C for 48 h, mixed with disinfected moss which had been moistened with distilled water, and then incubated at 15 °C for 90 d (He, 2008). Seeds having different root lengths (root length 1·5, 3·0, 4·5 and 6·0 cm) were selected for the experiments.
Effect of temperature on epicotyl dormancy of radicle-emerged seeds
Seeds with radicles 1·5, 3·0, 4·5 and 6·0 cm long were selected and mixed with disinfected sphagnum moss moistened with distilled water in 30 × 50 cm nylon seed bags, and then stratified at constant temperatures of 5, 10 and 15 °C. During stratification, 20 seeds of each radicle length were taken out and monitored for epicotyl–plumule growth after 0, 20, 40, 60 and 80 d. The seeds with observed epicotyl–plumule growth were transferred into a light incubator and incubated at a constant temperature of 15 °C in nylon seed bags filled with disinfected moist sphagnum moss. At 2 d intervals, the contents of each bag were poured out onto a table to facilitate examination of seeds for germination; seeds and moss were then returned to the bags. Regular examination of the seeds for germination resulted in a re-shuffling of seeds with regard to their position in the sphagnum and thus the light and moisture they received. The photoirradiance of the light incubator was about 54 µmol m−2 s−1 (the photoperiod was 24 h) of cool white fluorescent light, 400–700 nm. At each temperature, three replications of 100 seeds were used.
Means and s.e. were calculated for each measurement, and the results of epicotyl–plumule growth were expressed as germination percentage and germination potential:
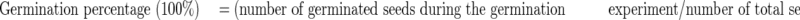 |
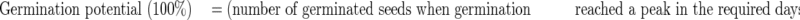 |
Effect of GA3 and IAA on epicotyl dormancy release of radicle-emerged seeds
The purpose of this experiment was to determine if GA3 would eliminate the impact of the radicle length on epicotyl dormancy release. Seeds having different radicle lengths (1·5, 3·0, 4·5 and 6·0 cm) were soaked in 400 mg L−1 GA3 (95 % purity, Sigma) and 200 mg L−1 IAA (95 % purity, Sigma) for 2 h at room temperature prior to being tested for epicotyl–plumule germination. The test operation and conditions were the same as described above. Each treatment consisted of three replications of 100 seeds.
Hormone determination
Radicle-emerged seeds (root lengths 1·5, 3·0, 4·5 and 6·0 cm) were mixed with disinfected sphagnum moss moistened with distilled water in 30 × 50 cm nylon seed bags, then bags were stratified at a constant temperature of 5 °C. Destructive samples were taken after 0, 20, 40 and 60 d stratification, respectively. Seeds with different root lengths were rinsed three times in sterile double-deionized water. Then the seed coat and endosperm were removed to excise the cotyledon, epicotyl, hypocotyl and root as material to be used for hormone determination. The fresh samples were frozen and stored in liquid nitrogen prior to measuring the hormone contents. The contents of GA3, ABA, indole-3-acetic acid (IAA) and zeatin riboside (ZR) were determined using an enzyme-linked immunosorbent assay (ELISA) method at the State Key Laboratory of Plant Physiology and Biochemistry of China Agricultural University. Every sample for hormone determination (root, cotyledon, epicotyl, and hypocotyl) was 0·5 g, and every experiment was performed in three replicates.
Embryo culture
Seeds were soaked in 70 % ethanol for 5 min, rinsed three times in sterile double-deionized water, surface sterilized in 0·1 % HgCl2 for 3 min and rinsed again three times in sterile double-deionized water prior to use. The seeds were cut in half with a razor blade and the seed coats and endosperm were removed to excise the embryo. The bare embryos were placed in a cheesecloth bag, sterilized in 0·1 % HgCl2 for 2 min, and rinsed three times in sterile double-deionized water. Embryo culture used revised Murashige and Skoog medium (MS) [KNO3 (1900 mg L−1), NH4NO3 (1650 mg L−1), KH2PO4 (170 mg L−1), MgSO4·7H2O (370 mg L−1), CaCl2·2H2O (440 mg L−1), KI (0·83 mg L−1), H3BO3 (6·2 mg L−1), MnSO4·4H2O (22·3 mg L−1), ZnSO4·7H2O (8·6 mg L−1), Na2MoO4·2H2O (0·25 mg L−1), CuSO4·5H2O (0·025 mg L−1), CoCl2·6H2O (0·025 mg L−1), Na2·EDTA (37·25 mg L−1), FeSO2·7H2O (27·85 mg L−1), inositol (100 mg L−1), VB1 (0·5 mg L−1), VB6 (0·5 mg L−1), nicotinic acid (0·5 mg L−1), glycine (2 mg L−1), sucrose (30 g L−1), agar (7 g L−1)]. There were two treatments of embryo cultures in this experiment: bare embryo + MS, and bare embryo + MS + 100 mg L−1 GA3. All the above operations were completed in a laminar flow cabinet (CJT-Z-1, China). Media were sterilized in an autoclave at 1·1 kg cm−2 and 121 °C for 30 min. Cultures were grown in a culture room at temperature 25 ± 2 °C, humidity 65–70 %, under lights (cool white fluorescent), 100–150 µmol−2 s−1 and a 18 h photoperiod.
Statistical analyses
All data were examined for normality distribution and homogeneity of variance before the analysis of variance (ANOVA) was carried out. The final percentage of plumule (epicotyl) emergence among the temperature and hormone treatments was analysed using one-way ANOVA followed by a Duncan multiple comparison tests using SPSS (version 12·0) at the level of 0·05 (P < 0·05). The means of the replicate experiments for categorical data variation are shown by s.e. bars in the figures. The graphics program Sigma-Plot (version 10·0) was used to create artwork.
RESULTS
Effect of stratification temperature and time on epicotyl dormancy release
The epicotyl–plumule germination percentage of seeds having roots 6·0 cm long was about 80 % after incubation at 5 °C for 80 d, while there was no epicotyl–plumule germination during stratification at 10 and 15 °C (data not shown). The epicotyl dormancy was not broken in seeds with root lengths <6 cm during 5, 10 and 15 °C stratification treatments.
The germination percentage and germination potential of the epicotyl–plumule for seeds with a root length of 6·0 cm was significantly enhanced or increased with cold stratification time (at 5 °C) (F = 102·88, P = 0·024) (Fig. 1A). The epicotyl–plumule germination of seeds with roots 6·0 cm long after 80 d stratification treatment reached 80 % in 8 d (Fig. 1B); this was not significantly different from the germination after 60 d stratification treatment. However, the seed germination potential and the time at which the highest germination percentage was reached in the 60 d stratification treatment were significantly lower, i.e. germination was slower, than that in the 80 d stratification treatment (Fig. 1B) (P < 0·05). Epicotyl–plumule germination percentage and potential after 20 and 40 d stratification were significantly lower than after 80 and 60 d stratification treatment.
Fig. 1.
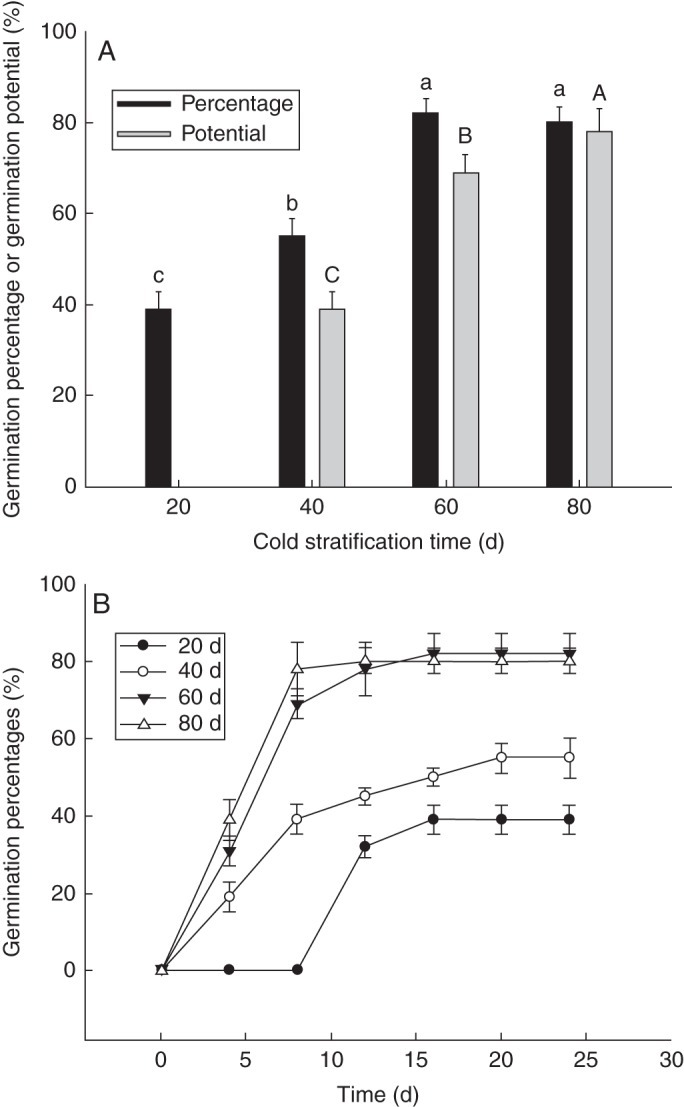
The effect of stratification time on epicotyl dormancy release of Tibetan peony seeds. (A) Epicotyl–plumule germination percentage and germination potential; (B) epicotyl–plumule germination process for seeds with different cold stratification times. Upper and lower case letters represent the effects of stratification time on epicotyl–plumule germination percentage and germination potential of Tibetan peony seeds, respectively, based on one-way ANOVA (P = 0·05). Mean ± s.e.
Effect of cold stratification on hormone content of seeds of different root lengths
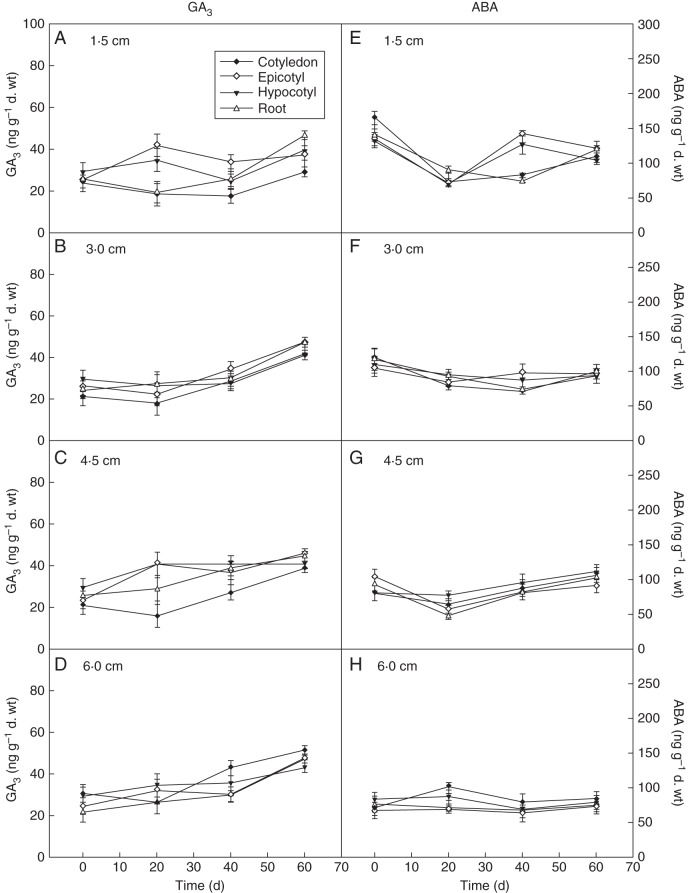
The changes in GA3 content in seeds having different root lengths (1·5, 3·0, 4·5 and 6·0 cm) were similar during 0–60 d cold stratification (Fig. 2A–D). The GA3 content in seeds with 6 cm roots was the highest (e.g. epicotyl, F = 8·42, P = 0·007), while the GA3 content was similar in the seeds with the other three root lengths after 60 d cold stratification. Comparison of GA3 accumulation in the different parts of seeds showed that the GA3 contents of the hypocotyl and root were higher than that of the epicotyl and cotyledons in seeds with 1·5 cm root length (Fig. 2A) (P < 0·05). The GA3 contents of cotyledons, epicotyl, hypocotyl and root were similar in seeds with 3·0 cm long roots (Fig. 2B), and, in seeds with 4·5 cm roots, the GA3 content of epicotyl and cotyledons was higher than that in hypocotyl and roots (Fig. 2C) (P < 0·05). The GA3 content of each part of the seeds with 6·0 cm long roots was significantly increased with cold stratification time (e.g. epicotyl, F = 13·50, P = 0·002), and the GA3 content order was cotyledon > epicotyl > root > hypocotyl (Fig. 2D).
Fig. 2.
Effect of cold stratification on GA3 (left) and ABA (right) content in seeds with different root lengths. Mean ± s.e. (A, E) Seeds with 1·5 cm root length; (B, F) seeds with 3·0 cm root length; (C, G) seeds with 4·5 cm root length; (D, H) seeds with 6·0 cm root length.
The ABA content in radicle-emerged seeds was higher than GA3 accumulation both before and after cold stratification (Fig. 2E–H). There was no significant change in ABA content in radicle-emerged seeds (e.g. epicotyl, F = 18·107, P = 0·089). The ABA accumulation in seeds of 1·5 cm root length significantly decreased during the process of cold stratification (P < 0·05), but the total content was higher (Fig. 2E) than in the other three groups of seeds (3·0, 4·5, 6·0 cm, Fig 2F–H) (e.g. epicotyl, F = 38·45, P = 0·006). The ABA accumulation in seeds of 6·0 cm root length was the lowest amongst all the seeds (Fig. 2H) (P < 0·05). Seeds with 4·5 and 3·0 cm long roots had similar ABA contents (Fig. 2G). The ABA accumulation in the epicotyl decreased with increase in root length (F = 181·23, P = 0·001), and the ABA accumulation in other parts also showed similar trends.
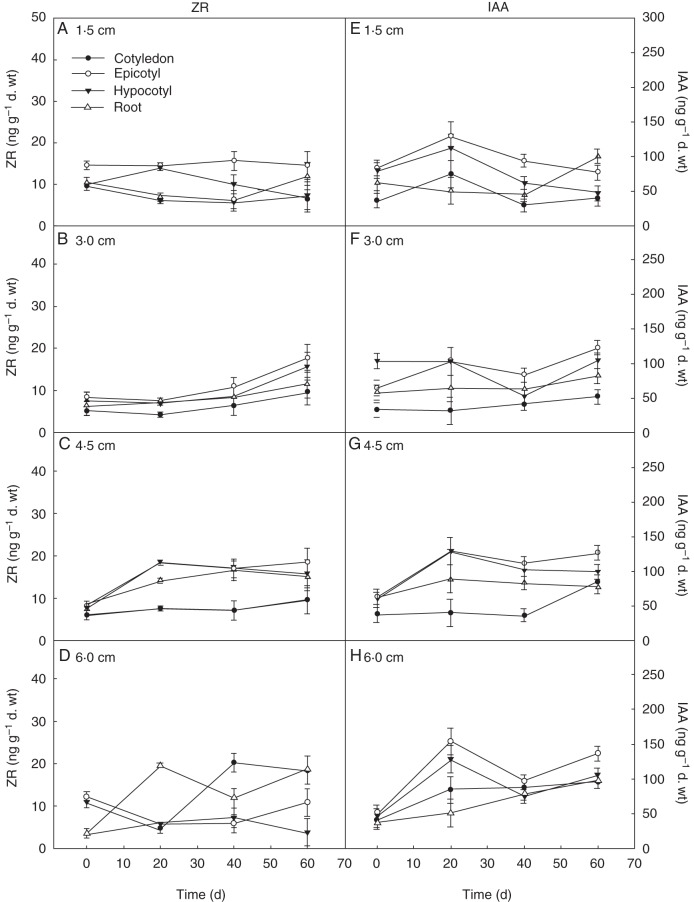
Changes in IAA content were more obvious than the changes in GA3 or ABA during the process of cold stratification (Fig. 3E–H) (P < 0·05). The IAA accumulation in seeds with 6·0 cm roots increased significantly with the cold stratification time (Fig. 3H) (e.g. epicotyl, F = 372·53, P = 0·017), while in seeds with other root lengths cold stratification had little effect on IAA accumulation. Comparison of the same parts of the seed revealed that the IAA content of roots and epicotyl changed significantly during the process of cold stratification, and the IAA accumulation of roots and epicotyl was also the highest among the different parts in seeds from all root length groups after cold stratification (Fig. 3E–H).
Fig. 3.
Effect of cold stratification on ZR (left) and IAA (right) content in seeds of different root lengths. Mean ± s.e. (A, E) Seeds with 1·5 cm root length; (B, F) seeds with 3·0 cm root length; (C, G) seeds with 4·5 cm root length; (D, H) seeds with 6·0 cm root length.
In comparison with the other three hormones, ZR accumulation was the lowest during the process of cold stratification (Fig. 3A–D) (P < 0·05). ZR accumulation increased in various parts of the seeds with 3·0 cm roots compared with that in seeds with 1·5 cm roots (Fig. 3A, B) (e.g. epicotyl, F = 174·37, P = 0·001). The whole ZR content was similar in seeds with 3·0, 4·5 and 6·0 cm long roots (Fig. 3B–D). ZR accumulation in roots and cotyledons was higher than in other parts of the seeds; this trend was similar among seeds with different root lengths.
Comparison of the hormone ratio of the epicotyl revealed that the IAA/ABA ratio was significantly higher than the GA3/ABA and ZR/ABA ratios in seeds with the same root lengths, and the ZR/ABA ratio was the lowest among all the hormone ratios (Fig. 4) (F = 713·95, P = 0·002). The GA3/ABA and IAA/ABA ratios in seeds with 6·0 cm long roots were significantly higher than those in seeds with root lengths of 1·5, 3·0 and 4·5 cm, and those with 1·5 cm long roots had the lowest ratios (Fig. 4) (P < 0·05). Seeds with 3·0 and 4·5 cm roots had similar GA3/ABA and IAA/ABA ratios. There was no significant difference in the ZR/ABA ratios for seeds of all root lengths.
Fig. 4.
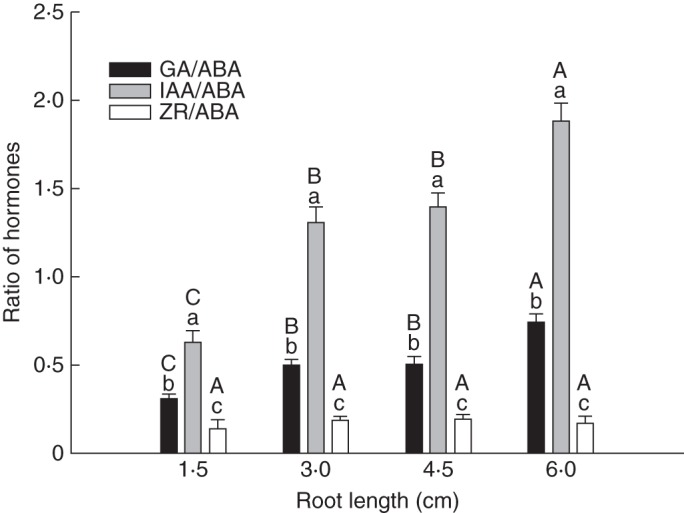
Effect of cold stratification on the hormone ratio of epicotyl in seeds of different root lengths. Upper and lower case letters represent the difference in the hormone ratio in seeds of different or the same root length, respectively, based on one-way ANOVA (P = 0·05). Mean ± s.e.
Effect of exogenous GA3 on epicotyl dormancy release
Cold stratification (5 °C, absence of GA3) only released the epicotyl dormancy of seeds with 6 cm long roots (Fig. 5A), while GA3 significantly increased the epicotyl–plumule germination percentage in seeds of all root lengths (Figs 5B, 6) (F = 5·207, P = 0·028). The epicotyl–plumule germination percentage of seeds with 1·5 cm long roots was significantly lower than that for seeds of all other root lengths (P < 0·05), but, even so, the germination percentage was >80 % (Fig. 5B). With increased root length, the rate of germination also increased (Fig. 5B). The epicotyl–plumule germination percentage reached a peak after 8 d in the seeds with 6·0 cm long roots, while it took 20 d to reach a peak in the seeds with 1·5 cm long roots (Fig. 5B). There were few lateral roots produced by seeds with 1·5 cm long roots (Fig. 6A). These results showed that the role of GA3 in the epicotyl–plumule was also influenced by the root length.
Fig. 5.
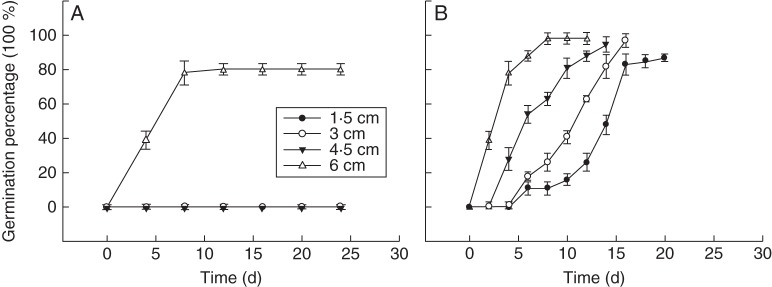
Effect of exogenous GA3 (A, absence; B, presence) on germination percentage and the germination process of epicotyl–plumule in seeds with different root lengths. Mean ± s.e. (A) Cold stratification (5 °C) treatment (absence of GA3); (B) GA3 treatment.
Fig. 6.
Effect of exogenous GA3 on epicotyl dormancy release in Tibetan peony seeds with different root lengths. (A) Seeds with 1·5 cm roots; (B) seeds with 3·0 cm roots; (C) seeds with 4·5 cms; (D) seeds with 6·0 cm roots.
Effect of exogenous IAA on epicotyl dormancy release
Exogernous IAA did not break the epicotyl dormancy in radicle-emerged seeds of Tibetan peony, as there was no germination following treatment of radicle-emerged seeds with 200, 400 and 600 mg L−1 IAA (data not shown).
Embryo culture experiment
Naked embryos were cultured in MS, and, 40 d later, the radicles had grown significantly and the cotyledons had turned green, but the epicotyl did not elongate (Fig. 7A). However, in the mixed medium of MS + 100 mg L−1 GA3, the radicle and epicotyl–plumule grew significantly after a 40 d culture (Fig. 7B), supporting the hypothesis that epicotyl–plumule dormancy is related to GA3.
Fig. 7.
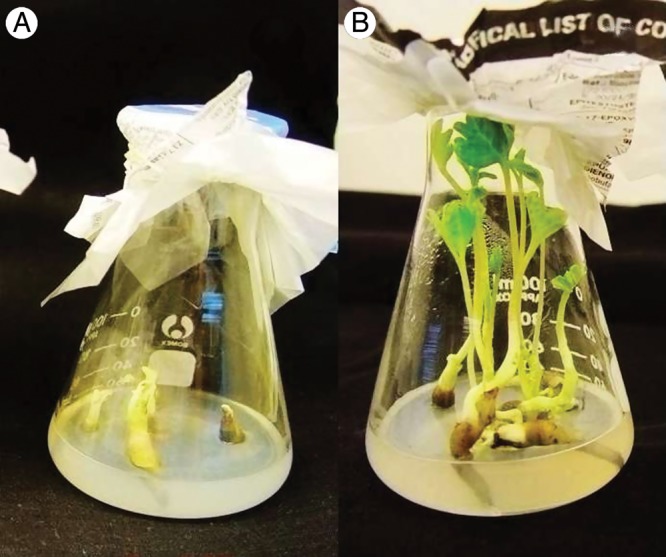
Effect of culture media on development of seedlings from embryo cultures. (A) MS medium; (B) MS + 100 mg L−1 GA3.
DISCUSSION
Our study showed that root length has a significant effect on epicotyl dormancy. Epicotyl dormancy of seeds with root lengths of 1·5, 3·0 and 4·5 cm cannot be broken by cold stratification (Fig. 5A), This differs from the findings of studies on epicotyl dormancy of Diploclisia, Glaucescens, Liliales, Ranunculales, Saxifragales, Dipsacales, Daphniphyllum, Hexastylis, Viburnum, Chionanthus retusus and Quercus spp. seeds, in which epicotyl dormancy break is not influenced by root length (Allen and Farmer, 1977; Farmer, 1977; Baskin and Baskin, 1998; Adams et al., 2003; Chien et al., 2004). Stratification at 10 and 15 °C for 80 d did not break the epicotyl dormancy in the seeds with 6·0 cm long roots in our experiment, as also seen in Viburnum betulifolium and Narcissus hispanicus where cold stratification was required for epicotyl dormancy release (Chien et al., 2011; Copete et al., 2011). The epicotyl–plumule germination percentage in seeds with 6·0 cm long roots reached 20 % when incubated at 5 °C for 20 d (Fig. 1), and the germination percentage increased with the stratification time, showing that stratification time has an effect on epicotyl dormancy release. Cold stratification for 20 d could significantly break epicotyl dormancy, in contrast to reports from preliminary studies where it was found that 60 d stratification was required to break epicotyl dormancy (Adams et al., 2003; Chien et al., 2004; Copete et al., 2011).
Treatment with GA3 could partly or completely offset the effect of root length, stratification temperature and time on epicotyl dormancy release. When all seeds of all root lengths were pre-treated with 400 mg L−1 GA3 and then incubated at 15 °C for 20 d, the germination percentages were all >80 %, and the highest germination percentage was 95 % (Fig. 5). Although the radicle of seeds with 1·5 cm long roots was weak, the plumule germinated when they were treated with GA3 (Fig. 6A). This suggests that the hormone content was the main factor affecting epicotyl dormancy. Embryo culture experiments further supported this speculation, since the epicotyl–plumule grew when the naked embryo was cultured in the presence of GA3 (Fig. 7B). GA3 treatment can therefore break the epicotyl dormancy of seeds of different root lengths and promote the naked embryo's growth into a seedling.
Exogenous GA3 can partly or completely substitute for cold stratification in epicotyl dormancy release (Hamilton and Carpenter, 1977; Bhatt et al., 2000; Chien et al., 2000). However, with double GA and ABA mutant studies, previous researchers found that the hormone balance, especially the ratio between GA3 and ABA, was the real factor that regulates seed dormancy and germination (Wareing and Saunders, 1971; Debeaujon and Koornneef, 2000; Finch-Savage and Leubner-Metzger, 2006). In our study, the hormone contents in different parts of the seeds were similar in seeds having different root lengths. For example, the GA3 content of the epicotyl was similar in seeds with roots 3·0, 4·5 and 6·0 cm long after cold stratification, but the cold stratification treatment only broke the epicotyl dormancy of seeds with 6·0 cm long roots (Fig. 2). Thus the total GA3 content of the epicotyl was not the main reason for the difference in epicotyl dormancy release in seeds of different root lengths.
The GA3/ABA and IAA/ABA ratios were significantly different in seeds of different root lengths (Fig. 4), with the highest proportion in seeds with roots 6·0 cm long and the lowest in seeds with roots 1·5 cm long. These results supported the evidence that cold stratification could only break the epicotyl dormancy in seeds with 6·0 cm long roots and the previous conclusion that hormone balance regulated dormancy and germination of seeds (Hamilton and Carpenter, 1977; Bhatt et al., 2000). Therefore, our results revealed that differences in the GA3/ABA and IAA/ABA ratios in the epicotyl could be the main reason for the effect of root length on epicotyl dormancy release in Tibetan peony seeds.
Changes in IAA/ABA and GA3/ABA ratios of epicotyls were similar in seeds with different root lengths after 5 °C cold stratification. The value for the IAA/ABA ratio of the epicotyl was significantly higher than that of GA3/ABA in seeds of all the root lengths (Fig. 4). In addition, the total IAA accumulation was higher than that of GA3 in the seeds of different root lengths (Figs 2, 3). Since IAA has no effect on epicotyl dormancy break, it can be concluded that the GA3/ABA ratio plays the key role in epicotyl dormancy break. The higher IAA accumulation in epicotyls may be because the whole seed was in an active state before germination. Bialek et al. (1992) and Birgit et al. (2005) also found that seeds accumulated high amounts of IAA during seed imbibition and seedling root growth and germination. In studies on pine seed dormancy and germination, Ljung et al. (2001) and Slavtcho et al. (2004) found that IAA accumulated before the germination of the seed. In our experiment, GA3 broke the epicotyl dormancy of either radicle-emerged seeds or naked embryos in Tibetan peony seeds (Figs 5, 6). These results showed that GA3 could partially regulate seed dormancy.
The absence of differences in ZR accumulation and the ZR/ABA ratio in seeds of different root lengths suggested that this ratio may not be the main regulator of epicotyl dormancy release (Figs 3, 4), as shown in a previous study (Brady and McCourt, 2003). The different germination percentage of the epicotyl–plumule in seeds of different root lengths may result from the GA3/ABA ratio in the epicotyl (Debeaujon and Koornneef, 2000; Finch-Savage and Leubner-Metzger, 2006). In our experiment, the total GA3 content of the epicotyl was similar in seeds with 3·0, 4·5 and 6·0 cm roots (Fig. 2); therefore, the difference in the GA3/ABA ratio in these seeds may result from the change in the amount of ABA (Ali-Rachedi et al., 2004). Thus, the effect of root length on epicotyl dormancy release may be due to (1) differences in ABA accumulation in the epicotyl in seeds of different root lengths, which caused the difference in the GA3/ABA ratio in the seeds of different root lengths; or (2) cold stratification which may have changed the sensitivity of seeds to GA3 (Derkx and Karssen, 1993a).
The trends in GA3 and ABA of seeds changed as the root length increased during the process of cold stratification. Comparing the hormone variations in all seeds, the most obvious change occurred between seeds of 1·5 and 3·0 cm root length (Figs. 2, 3). Therefore, we speculated that 3·0 cm may be a turning point for root length in the seeds' physiology. The experiment using exogenous GA3 supported this speculation (Fig. 6). Under GA3 treatment, the plumule was short and there were few lateral roots in seeds with 1·5 cm long roots. When root length was >3·0 cm, all the indicators improved (Fig. 6).
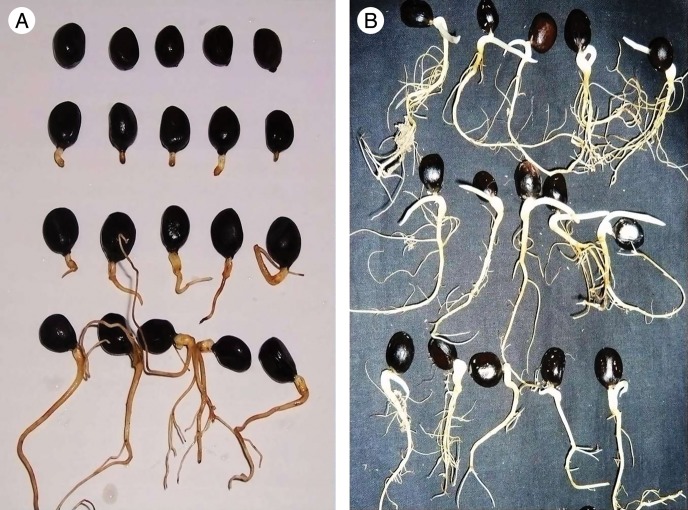
The climate in the Tibetan Plateau is characterized by low temperatures, low annual accumulated temperature, short growing seasons, and cold and dry long winter seasons. The unique climatic conditions required the seedlings to grow rapidly after seed germination in order to accumulate biomass and to prevent low temperature injury during the long autumn and winter. Seedlings must have a complete root system, so they can grow rapidly. When dormancy is released in the radicle of Tibet peony seeds, even those seeds with a 6·0 cm long root have only a primary root (Fig. 8A). The seeds then constructed complete root systems after cold stratification (Fig. 8B). Epicotyl dormancy plays an important role in the adaptation to the climatic conditions of the Tibetan Plateau and the reproduction of the Tibetan peony. The fact that the seedling has to attain a certain root length before it can be sensitive to cold stratification for epicotyl dormancy break may be an adaptation to the climate of the region.
Fig. 8.
The root growth of Tibetan peony seeds before (A) and after (B) epicotyl dormancy release. (A) Seeds were incubated at 15 °C for 90 d; (B) seeds with 6 cm roots were incubated at 5 °C for 60 d.
Conclusions
Root length had a significant effect on epicotyl dormancy release in Tibetan peony seeds. Root lengths ≥6 cm were essential for epicotyl dormancy release by cold stratification. Root length affected the GA3/ABA ratio of epicotyl in seeds, and thus the epicotyl became sensitive to cold stratification.
ACKNOWLEDGEMENTS
We express our thanks to Ms Jing Wang, and to Ethan Johnson at the Holden Arboretum, for their help with this article. This work was supported by the National Key Technology R&D Program (grant no. 2012BAC01B05), the Knowledge Innovation Engineering of the Chinese Academy of Sciences (grant no. KSCX2-EW-B-5) and the Strategic Technical Support System of Special Biological Resources Project (grant no. CZBZX-1).
LITERATURE CITED
- Adams CA, Baskin JM, Baskin CC. Epicotyl dormancy in the mesic woodland herb Hexastylis heterophylla (Aristolochiaceae) Journal of the Torrey Botanical Society. 2003;130:11–15. [Google Scholar]
- Ali-Rachedi S, Bouinot D, Wagner MH, et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. doi: 10.1007/s00425-004-1251-4. [DOI] [PubMed] [Google Scholar]
- Allen PS, Meyer S. Ecological aspects of seed dormancy loss. Seed Science Research. 1998;8:183–191. [Google Scholar]
- Allen R, Farmer RE., Jr Germination characteristics of bear oak. Southern Journal of Applied Forestry. 1977;1:19–20. [Google Scholar]
- Alvarado V, Hiroyaki H, Bradford KJ. Expression of endomannanase and SNF-related protein kinase genes in true potato seeds in relation to dormancy, gibberellin and abscisic acid. In: Viémont J-D, Crabbé J, editors. Dormancy in plants: from whole plant behavior to cellular control. Wallingford, UK: CABI Publishing; 2000. pp. 347–364. [Google Scholar]
- Basken CC, Baskin JM. Seeds: ecology,biogeography and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Bhatt ID, Rawal RS, Dhar U. Improvement in seed germination of Myrica esculenta Buch.-Ham. ex D. Don: a high value tree species of Kumaun Himalaya, India. Seed Science Technology. 2000;28:597–605. [Google Scholar]
- Bialek K, Michalczuk L, Cohen JD. Auxin biosynthesis during seed germination in Phaseolus vulgaris. Plant Physiology. 1992;100:509–517. doi: 10.1104/pp.100.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgit K, Marc AC, Gerhard LM. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- Brady SM, McCourt P. Hormone cross-talk in seed dormancy. Journal of Plant Growth Regulation. 2003;22:25–31. [Google Scholar]
- Chien CT, Chen YC, Chen SY, Hong KY. Dormancy-releasing strategies for Myrica seeds. Taiwan Journal of Forestry Science. 2000;15:473–481. [Google Scholar]
- Chien CT, Huang LLK, Shen YC, et al. Storage behavior of Chionanthus retusus seed and asynchronous development of the radicle and shoot apex during germination in relation to germination inhibitors, including abscisic acid and four phenolic glucosides. Plant and Cell Physiology. 2004;45:1158–1167. doi: 10.1093/pcp/pch129. [DOI] [PubMed] [Google Scholar]
- Chien CT, Chen SY, Tsai CC. Deep simple epicotyl morphophysiological dormancy in seeds of two Viburnum species, with special reference to shoot growth and development inside the seed. Annals of Botany. 2011;108:13–22. doi: 10.1093/aob/mcr096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copete E, Herranz JM, Ferrandis P, Baskin CC, Baskin JM. Physiology, morphology and phenology of seed dormancy break and germination in the endemic Iberian species Narcissus hispanicus (Amaryllidaceae) Annals of Botany. 2011;107:1003–1016. doi: 10.1093/aob/mcr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiology. 2000;122:415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkx MPM, Karssen CM. Effects of light and temperature on seed dormancy and gibberellin-stimulated germination in Arabidopsis thaliana: studies with gibberellin-deficient and -insensitive mutants. Physiologia Plantarum. 1993a;89:360–368. [Google Scholar]
- Farmer RE., Jr Epicotyl dormancy in white and chestnut oaks. Forest Science. 1977;23:329–332. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger GL. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M. Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta. 2000;210:279–285. doi: 10.1007/PL00008135. [DOI] [PubMed] [Google Scholar]
- Hamilton DF, Carpenter PL. Seed germination of Myrica pensylvanicum L. HortScience. 1977;12:565–566. [Google Scholar]
- He Z. 2008. Seed dormancy and germination characteristics of Paeonia Ludlowii, an endangered plant endemic to China. PhD Thesis, IBCAS, China.
- Jing XM, Zheng GH. The characteristics in seed germination and dormancy of four wild species of tree peonies and their bearing on endangerment. Acta Phytophysiologica Sinica. 1999;25:214–221. [Google Scholar]
- Jing XM, Zheng GH, Hong DY. Characteristic of germination and storage of seed in cultural Paeonia suffruticosa. Plant Physiology Communications. 1995;31:268–270. [Google Scholar]
- Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M. Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta. 1983;157:158–165. doi: 10.1007/BF00393650. [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- Linkies A, Leubner-Metzger G. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Reports. 2012;31:253–270. doi: 10.1007/s00299-011-1180-1. [DOI] [PubMed] [Google Scholar]
- Ljung K, Ostin A, Lioussanne L, Sandberg G. Developmental regulation of indole-3-acetic acid turnover in Scots pine seedlings. Plant Physiology. 2001;125:464–475. doi: 10.1104/pp.125.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Nicolás C, Nicolás G, Rodriquez D. GA3-induced expression of a new functional AAA-ATPase (FsA1) is correlated with the onset of germination in Fagus sylvatica L. seeds. Plant and Cell Physiology. 2002;43:27–34. doi: 10.1093/pcp/pcf009. [DOI] [PubMed] [Google Scholar]
- Ma H, Li ZH, Zhang YL, Wang Y, Liu XX, Wan YM. Release of seed dormancy of Paeonia ludlowii. Scientia Silvae Sinica. 2012;48:62–67. [Google Scholar]
- Nicolás C, Nicolás G, Rodriquez D. Antagonistic effects of abscisic acid and gibberellic acid on the breaking of dormancy of Fagus sylvatica seeds. Physiologia Plantarum. 1996;96:244–250. [Google Scholar]
- Slavtcho S, Henry VO, Rossitza B, Atanas A, Els P. IAA production during germination of Orobanche spp. seeds. Journal of Plant Physiology. 2004;161:847–853. doi: 10.1016/j.jplph.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Su JR, Liu WD, Lang XD, Zhang WY, Luo J, Wang SL. The relationships of Paeonia ludlowii and habitat community characteristics. Forest Research. 2010;23:487–492. [Google Scholar]
- Wareing PF, Saunders PF. Hormones and dormancy. Annual Review of Plant Physiology. 1971;22:261–288. [Google Scholar]
- Zhang XB, Ni AL, Zhang WM, Wang CC. Advances in researches on seed dormancy of officinal plants. Chinese Traditional and Herbal Drugs. 1997;6:376–378. [Google Scholar]
- Zheng XM, Zhou RB, Gu LP, Mao DJ, Zhou JH. The properties of dormancy and germination of Paeonia suffruticosa. Plant Physiology Communications. 1995;31:260–262. [Google Scholar]