Abstract
Background and Aims
Biomass accumulation and allocation patterns are critical to quantifying ecosystem dynamics. However, these patterns differ among species, and they can change in response to nutrient availability even among genetically related individuals. In order to understand this complexity further, this study examined three ephemeral species (with very short vegetative growth periods) and three annual species (with significantly longer vegetative growth periods) in the Gurbantunggut Desert, north-western China, to determine their responses to different nitrogen (N) supplements under natural conditions.
Methods
Nitrogen was added to the soil at rates of 0, 0·5, 1·0, 3·0, 6·0 and 24·0 g N m−2 year−1. Plants were sampled at various intervals to measure relative growth rate and shoot and root dry mass.
Key Results
Compared with annuals, ephemerals grew more rapidly, increased shoot and root biomass with increasing N application rates and significantly decreased root/shoot ratios. Nevertheless, changes in the biomass allocation of some species (i.e. Erodium oxyrrhynchum) in response to the N treatment were largely a consequence of changes in overall plant size, which was inconsistent with an optimal partitioning model. An isometric log shoot vs. log root scaling relationship for the final biomass harvest was observed for each species and all annuals, while pooled data of three ephemerals showed an allometric scaling relationship.
Conclusions
These results indicate that ephemerals and annuals differ observably in their biomass allocation patterns in response to soil N supplements, although an isometric log shoot vs. log root scaling relationship was maintained across all species. These findings highlight that different life history strategies behave differently in response to N application even when interspecific scaling relationships remain nearly isometric.
Keywords: Biomass allocation, allometric relationship, nitrogen availability, optimal partitioning, ephemerals, annuals, Erodium oxyrrhynchum, Hyalea pulchella, Alyssum linifolium, Ceratocarpus arenarius, Salsola ruthenica, Horaninowia ulicina
INTRODUCTION
The rate at which plants accumulate biomass is important when estimating ecosystem productivity. However, this important ecological factor is known to differ among species and to change as a function of environmental conditions. Therefore, quantifying how growth rates vary among species and how they change in response to resource limitations is important, particularly in light of global change. Nevertheless, understanding plant biomass accumulation is a complex issue. For example, biomass accumulation is responsive to differences in nitrogen (N) availability but it is also affected by plant maturity, life form, soil moisture and many other environmental factors (Padgett and Allen, 1999; Bai et al., 2010; Su et al., 2013). Another related and equally complex issue in plant ecology is how plants allocate biomass among their different organs in response to variations in resource availability (Poorter and Nagel, 1999). This feature is also known to vary among species and to change as a function of environmental conditions (Thornley, 1972; Olff et al., 1990; Poorter et al., 1990).
The growth and allocation of desert plants have attracted a number of researchers owing to the often extreme environmental conditions under which plants grow (Wang et al., 2006; Allen et al., 2008; Su et al., 2013). Most studies report that the root/shoot ratio in desert plants is much greater than in plants of other ecosystems such as forests and grasslands (Barbour, 1973). The relatively higher biomass allocated to roots in comparison with above-ground parts would in theory provide plants with greater access to moisture and soil nutrients for growth and a smaller surface area for transpiration. Consequently, it should be possible to manipulate soil conditions to favour increases in above-ground biomass allocation if soil nutrient limitations (such as N) are reduced or eliminated. Indeed, in recent years, N deposition rates are increasing in desert ecosystems due to expanding metropolitan centres or large agricultural operations (Fenn et al., 2003). In desert ecosystems, biological activity is limited by water (Noy-Meir, 1973). In addition to water, this ecosystem is also limited by N, which has become the second most important factor influencing plant growth rates, community structure and soil microbial processes (Brooks, 2003; McCrackin et al., 2008; Zhou et al., 2011).
Plants with different life history strategies or life forms could show varying responses to N deposition in deserts as well as other ecosystems. For example, a 4-year field study found that perennial grasses benefited from N enrichment more than annuals and perennial forbs in a desert steppe, particularly during wet years (Su et al., 2013). Likewise, annuals contribute greatly to the yearly productivity of desert communities, e.g. desert annuals can provide over half of the total vegetation cover and are considered as a nutrient reservoir and provider (Chen et al., 2009). Although annuals are often used to test partitioning models (McConnaughay and Coleman, 1999; Bernacchi et al., 2000; Iwasa, 2000), the biomass partitioning patterns of desert annuals are rarely reported, particularly under field conditions.
Importantly, plants that live for only one growing season can be divided into two life history strategies, i.e. those with short and rapid periods of vegetative growth and those with longer and thus less rapid periods of vegetative growth (ephemerals and annuals sensu stricto, respectively). In the Gurbantunggut Desert, seed germination and seedling growth of the ephemeral plants are strongly dependent on temperature and water conditions in spring (Wang et al., 2006). Annuals often germinate later than the ephemerals, and more typically experience the relatively drier conditions of summer. Although Gurbantunggut ephemerals are more sensitive to soil moisture than Gurbantunggut annuals, with little or no differences in responses to N applications in pot experiments (Zhou et al., 2011), it is unknown if this holds under field conditions. In addition, the Gurbantunggut Desert is suffering from an increasing N deposition in recent years. Therefore, the Gurbantunggut Desert is an optimal area for understanding these ecologically important species. In addition, measurements of biomass accumulation and partitioning in field experimental plots would be helpful for the evaluation of biomass production in response to increased N deposition and for the study of the two contrasting biomass partitioning models described below.
In order to cope with this complexity in understanding biomass allocation in a changing environment, two general types of partitioning models have been proposed: optimal partitioning models and allometric models. The former postulates that plants respond to environmental changes by partitioning their biomass to different organs in a manner that permits the acquisition of the most limiting resource(s) such as N, light, water or carbon dioxide (Thornley, 1972; Bloom et al., 1985). For example, under light-limiting conditions, plants are predicted to allocate more biomass to the construction of stems and leaves rather than roots in order to maximize or at least increase light capture (Hunt and Burnett, 1973), whereas factors that limit the acquisition of below-ground resources are predicted to result in increased root growth as opposed to above-ground growth (Green et al., 1994; Gebauer et al., 1996). Allometric models offer an alternative (but not a mutually exclusive) framework in which to examine biomass accumulation and partitioning (McCarthy and Enquist, 2007). These models link metabolism to ecosystem dynamics based on size-dependent relationships that influence resource use, plant growth and architecture. Many but not all empirical studies support the existence of scaling exponents predicted by these models (e.g. Niklas and Enquist, 2002). For example, an isometric scaling relationship (α = 1) between log-transformed shoot and root biomass is predicted and observed for non-woody plants and the juveniles of woody species (Niklas, 2005); also, an isometric allocation pattern best describes the allometry of Chinese forested communities (Cheng and Niklas, 2007) and Tibetan grasslands (Yang et al., 2009). Nevertheless, other studies reveal ontogenetic changes in biomass allocation patterns as plants germinate, mature and reach reproductive status. These ‘ontogenetic shifts’ are real and sometimes optimal. Most allometric models fail to account for them. This can be particularly true in the case of rapidly growing annual species for which ontogenetic changes occur rapidly (McConnaughay and Coleman, 1999).
It is in the context of these two modelling frameworks that we examined the biomass allocation patterns of annuals and ephemerals in a desert ecosystem in response to differences in N and water availability. We hypothesized that the growth of ephemerals, which occurs in the spring under conditions of high soil moisture, would be more sensitive to N availability than that of annuals because N is the principal limiting resource to growth once drought stress is eliminated (McCrackin et al., 2008; Ladwig et al., 2012). We further hypothesized that more biomass would be allocated to above-ground body parts once N was provided in high concentrations, while an isometric relationship could be observed between the shoot and root biomass.
MATERIALS AND METHODS
Study site
The study site is situated in the centre of the Gurbantunggut Desert, north-western China (44·87 °N, 87·82 °E), which is the second largest desert in China. The annual mean temperature ranges from 6 to 10 °C. The mean annual precipitation is 70–150 mm, while the annual mean potential evaporation rate exceeds 2000 mm. Precipitation is unevenly distributed among seasons. Half of the annual precipitation falls between April and July (47·6 %). In winter, about 20 cm of snow covers the surface of the desert. In spring, melting snow and increased rainfall result in abundant soil moisture, which fosters the emergence and growth of many plant species. In May, the average ground coverage of annual and ephemeral plants can reach as much as 40 %. The major soil type is an aeolian sandy soil. Surface materials are predominantly medium-sized sand (0·5–0·25 mm) and finer materials (<0·25 mm), which together account for 78·74–94·56 % of the composition of the topsoil material (Chen et al., 2007). The soil water-holding capacity is about 16 % gravimetrically. The Gurbantunggut Desert is surrounded by and partially embedded within farmlands that inadvertently release large quantities of N into the soil, e.g. N deposition in the city near the desert had increased from 0·5 g N m−2 year−1 in 1991 to 2·9 g N m−2 year−1 in 2010 (Yuan and Wang, 1997; Zhang et al., 2011).
Field methods
In October 2008, sixty 8 × 8 m plots were established in interdune areas of the desert. The plots had the same plant composition, plant density and soil physicochemical properties before the N fertilizer treatment. Five different rates of N application plus one control without N addition were applied to each plot. The six applications with ten replicates were 0, 0·5, 1·0, 3·0, 6·0 and 24·0 g N m−2 year−1 (hereafter denoted as N0, N0·5, N1, N3, N6 and N24, respectively), the replicates were randomly selected. N0–N3 fell in the range of the natural N deposition around the desert. N6 and N24 were added to test the plant responses to high N input. Each application mixture consisted of 2:1 NH4+:NO3− (NH4NO3 and NH4Cl), which approximated the composition of N deposition reported for the nearby city of Urumqi (Zhang et al., 2008). All N treatments were applied in equal amounts in March after snow thaw and in October before snowfall each year. We chose these times because agricultural activities in lands adjoining the desert peak in March and October when fields are cultivated and N fertilizer applied, which might result in increased N emission and deposition onto desert soil. The first treatment began in October 2008 and continued in the following years to see the long-term effects of N deposition. To disperse N evenly over the plots, N was applied as a spray in 30 L of water, which was not sufficient to alter the water status of the soil or the plants.
Plants were harvested at the beginning in April 2011. Six dominant species were selected for study: three ephemerals (Erodium oxyrrhynchum, Hyalea pulchella and Alyssum linifolium) and three annuals (Ceratocarpus arenarius, Salsola ruthenica and Horaninowia ulicina). Due to the low species abundance of A. linifolium, S. ruthenica and H. ulicina in the plots from the beginning of the N treatments, these plants were sampled only once when plants began to flower. Alyssum linifolium was sampled on 29 April 2011; S. ruthenica and H. ulicina were sampled on 10 August 2011. The biomass of the other species was determined more frequently throughout the period of vegetative growth, i.e. at roughly 10-d intervals for E. oxyrrhynchum and H. pulchella, and at 20-d intervals for C. arenarius. The distance between each harvesting site was sufficient not to influence neighbouring sites as the season progressed. Soil gravimetrical moisture (%) in plots of each treatment was measured at 0–5 cm depth and 5–10 cm depth after each harvest. In addition, data for daily rainfall and daily mean air temperatures in 2011 were gathered from a nearby meteorological station. Soil volumetric moisture (%) and soil temperature (°C) during the growing season near the plots were also determined by a four-parameter soil monitoring system (Channel Corp., Beijing, China).
For each plot, 10–20 seedlings or 5–10 mature individuals of each species were harvested. A patch containing the whole or the majority of each root system was excavated using a spade (Wang et al., 2010). Patch size and shape were determined based on a previous study on the root morphology of each species (Wang et al., 2010). However, typically, a 20–30 cm diameter patch was excavated to a depth of 30–50 cm. For each sample, roots were carefully separated from the substrate and dead roots were removed under running water. The shoots and roots of each plant were separated in the laboratory and oven-dried at 65 °C to a constant mass. When some seedlings with very small biomass were harvested, multiple samples from one plot were pooled, weighed and averaged. In the case of A. linifolium and H. ulicina, the number of available plants was small for the N24 treatment and no data were obtained.
In May 2011, four replicate soil samples were randomly collected from 24 of the 60 plots. For each plot, three cores (diameter = 5 cm) from 0 to 5 cm were collected along a diagonal line and then thoroughly mixed to form one composite sample to measure soil physiochemical properties. The soil samples were transported to the laboratory on ice where plant roots were removed by sieving soil through a 2 mm mesh. All samples were air dried and used for measurements of soil physicochemical properties, including soil pH, electrical conductivity, organic carbon, total N, phosphorus (P) and potassium (K), and available N, P and K. Soil pH and electrical conductivity were determined in a 1:5 mixture of soil and deionized water using a PHS-3C digital pH meter and a DDS-307A conductivity meter (Precision and Scientific Corp., Shanghai, China), respectively. Soil organic carbon content was determined by the K2Cr2O7 method (Walkley-Black); total N by the CuSO4–Se powder diffusion method; total P by the NaOH fusion–Mo Te Sc colorimetry method; total K by the NaOH melting–flaming luminosity method; available N by the alkali hydrolyzation–diffusion method; available P by the 0·5 mol L−1 NaHCO3 leaching–Mo Te Sc colorimetry method; and available K by the 1 mol L−1 NH4OAc leaching–flaming luminosity method (Chen et al., 2007).
Data analysis
The shoot and root dry biomass of individual plants from each species was measured separately and then averaged for each N application. The relative growth rate (RGR) was calculated based on the increase in biomass per unit time: RGR = (log10 Mf – log10 Mi)/(tf – ti), where Mi and Mf are individual biomass harvested at the beginning (ti) and at the final harvest time (tf) of each growth interval. Repeated measures of a general linear model were performed with sampling date as the within-subject factor and N treatments as the between-subject factor. One-way analysis of variance (ANOVA) was used to assess differences in the shoot and root among the six different N treatments after the final harvest of the six species. Duncan's tests were also performed to evaluate whether differences in biomass and root/shoot ratios were statistically significant.
For the three frequently harvested species, second-order polynomial regression equations (Y = A + BX + CX2) were used for comparisons of log (root/shoot) ratio vs. log individual biomass among N treatments to study the effects of plant size (McConnaughay and Coleman, 1999). The fitted curves were statistically compared using methods described by Potvin et al. (1990). The relationship between log shoot and log root was determined with both ordinary least squares (OLS) and reduced major axis (RMA) regression. Thus, scaling exponents and y-intercepts were determined for log10-transformed shoot and root data (denoted as αRMA and log10 βRMA, respectively) using standardized major axis (SMA) regression SMART software package protocols. The software package was also used to provide the model type II equivalent of OLS standard analyses. The numerical values of αRMA were compared to determine if they were consistent with an isometric relationship (i.e. αRMA = 1·0), which was taken as the null hypothesis.
RESULTS
Climate and soil physicochemical characteristics
Mean air temperature increased from winter (January and February) to summer (June–August) and peaked in July and August in 2011 (Fig. 1A). More frequent rainfall events occurred in May and June than in any other months. Soil temperatures at 10, 20 and 30 cm depths during April and August increased (Fig. 1B), with some minor fluctuations following rainfall events. In contrast, soil volumetric moisture at the same three depths declined from April to August. A rainfall in early July increased soil moisture, particularly at 10 and 20 cm. Soil gravimetric moisture measured at each harvesting date in the plots also indicated an extreme dry period between 30 May and 10 August, with most soil moisture below 1 %. Soil moisture in plots with N24 treatment was higher than others during most of the vegetative growth period (P < 0·05) (Fig. 1C).
Fig. 1.
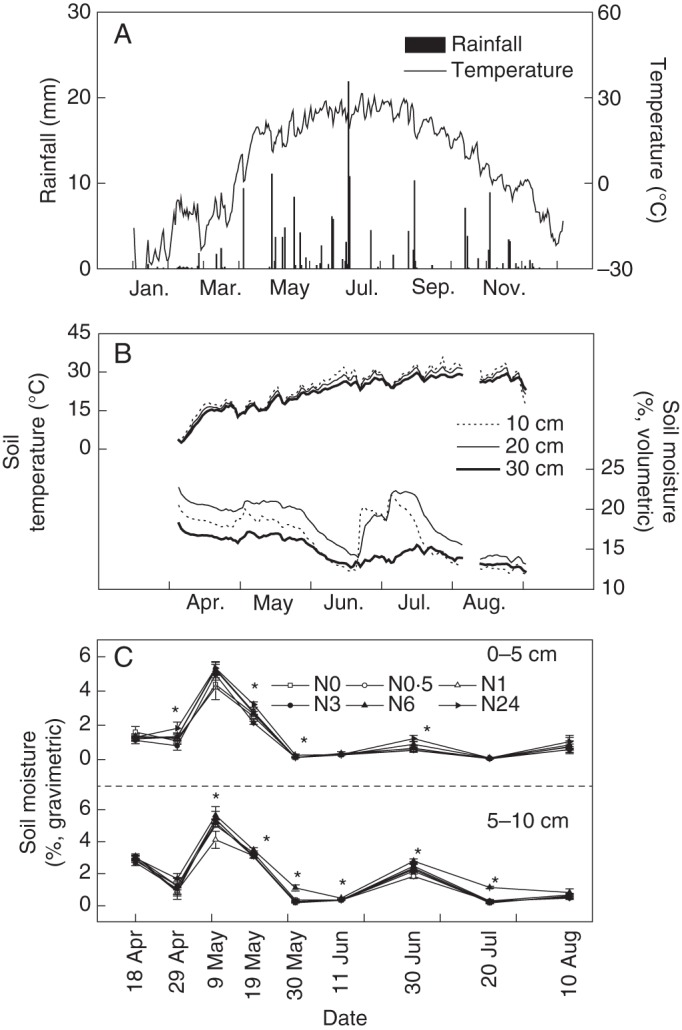
Data for physical variables of interest. (A) Variation in rainfall (mm) and mean daily air temperature (°C) in 2011. (B) Soil temperature and soil volumetric moisture at depths of 10, 20 and 30 cm near the plots. (C) Changes in soil gravimetric moisture (%) at depths of 0–5 cm and 5–10 cm (upper and lower panels, respectively) with different N application rates during the 2011 harvesting dates. * indicates a significant difference among treatments (n = 10, P < 0·05).
Soil pH, total N and total P did not vary significantly among the different N treatments (P > 0·05; Table 1). Nitrogen supplements increased soil electrical conductivity and the available N with increasingly higher rates of N application (P < 0·05). Total soil K in the N6 treatment and available K in the N24 treatment were significantly lower than in the N0 treatment (P < 0·05). The highest organic C content and available P were measured for the N1 treatment (P < 0·05).
Table 1.
Soil physicochemical characteristics (in May 2011)
| Treatments | pH | Electrical conductivity (μS cm−1) | Organic carbon (mg kg−1) | Total N (mg kg−1) | Total P (mg kg−1) | Total K (mg kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| N0 | 8·4 ± 0·1a | 93 ± 7c | 905 ± 14c | 113 ± 7a | 336 ± 7a | 12 050 ± 83a | 6·7 ± 1·7b | 4·6 ± 0·2d | 134·0 ± 1·7a |
| N0·5 | 8·2 ± 0·3a | 96 ± 2c | 956 ± 28c | 110 ± 14a | 346 ± 14a | 12 110 ± 52a | 6·0 ± 0·4b | 5·2 ± 0·2cd | 137·3 ± 3·8a |
| N1 | 8·1 ± 0·2a | 105 ± 7bc | 1199 ± 19a | 123 ± 13a | 335 ± 3a | 12 125 ± 35a | 6·4 ± 1·8b | 7·2 ± 0·2a | 158·3 ± 9·6a |
| N3 | 7·9 ± 0·2a | 103 ± 12bc | 962 ± 34c | 94 ± 11a | 346 ± 2a | 11 977 ± 51ab | 8·1 ± 1·3b | 5·5 ± 0·1bc | 149·7 ± 9·4a |
| N6 | 8·2 ± 0·4a | 119 ± 4b | 1099 ± 45b | 125 ± 20a | 338 ± 16a | 11 554 ± 302b | 17·5 ± 4·5b | 6·1 ± 0·2b | 139·3 ± 13·3a |
| N24 | 8·1 ± 0·4a | 164 ± 16a | 811 ± 13d | 102 ± 10a | 331 ± 21a | 11 772 ± 22ab | 56·3 ± 10·2a | 5·1 ± 0·1cd | 101·7 ± 12·0b |
Values are given as mean ± s.e. with ANOVA results (n = 4).
Different letters indicate significant difference levels between different nitrogen addition treatments (P < 0·05; Duncan's test).
Plant biomass accumulation and RGR
Shoot, root and individual biomass were significantly affected by different sampling dates and N treatments (Table 2). The biomass of the ephemerals E. oxyrrhynchum and H. pulchella increased during the late vegetative growth period and reached its maximum in a short time (Fig. 2A–C). In contrast, the annual C. arenarius grew relatively more slowly during the vegetative growth period. Nitrogen supplements significantly affected plant growth for each sampling date (P < 0·05). N3–N24 treatments increased the growth rates of ephemerals during the early vegetative growth period, e.g. growth rates were highest for E. oxyrrhynchum on day 20 and for H. pulchella on day 10 (Fig. 3A, B). However, no general RGR pattern among treatments was observed for C. arenarius (Fig. 3C).
Table 2.
F-values from repeated ANOVA on shoot, root, individual biomass and root/shoot ratios differing in N treatments, date (D) and interaction of N treatment × date (N × D)
| Life history | Species | Source of variation | d.f. | Shoot | Root | Individual | Root/shoot ratio |
|---|---|---|---|---|---|---|---|
| Ephemerals | E. oxyrrhynchum | N | 5 | 39·27 | 36·74 | 39·56 | 8·32 |
| D | 4 | 257·78 | 264·65 | 262·26 | 213·49 | ||
| N × D | 20 | 15·91 | 20·50 | 15·58 | 0·75 | ||
| H. pulchella | N | 5 | 16·73 | 13·95 | 16·75 | 1·72 | |
| D | 4 | 244·98 | 193·01 | 245·01 | 50·75 | ||
| N × D | 20 | 8·17 | 3·96 | 7·99 | 1·04 | ||
| Annuals | C. arenarius | N | 5 | 11·60 | 15·40 | 11·88 | 5·18 |
| D | 5 | 93·04 | 119·72 | 95·35 | 50·35 | ||
| N × D | 25 | 2·48 | 4·51 | 2·56 | 1·69 |
All N and D and N × D were significant at P < 0·01, except N × D for the root/shoot ratio in E. oxyrrhynchum and H. pulchella (P < 0·01).
Fig. 2.
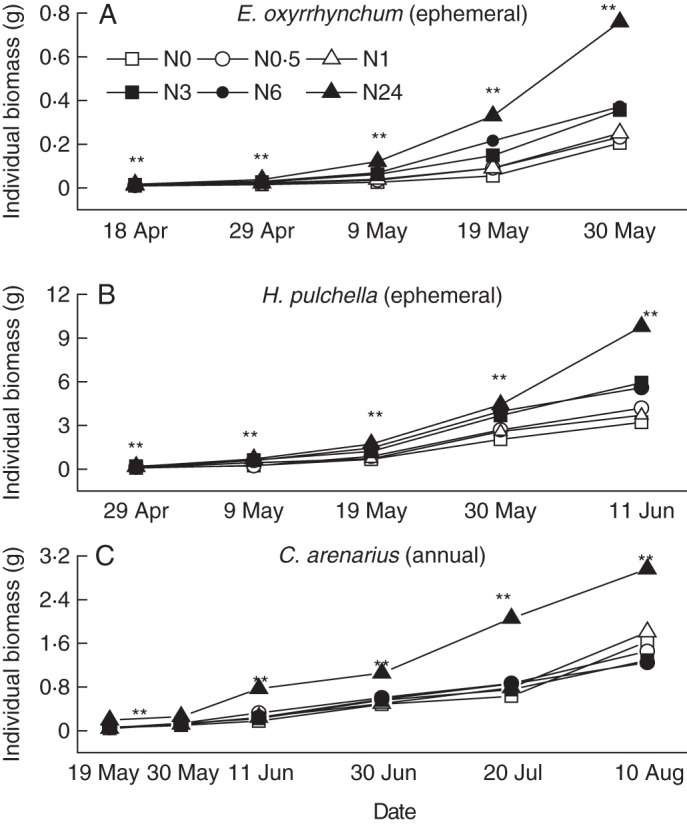
Changes in individual biomass (g) of E. oxyrrhynchum (A), H. pulchella (B) and C. arenarius (C) across sampling dates; ** indicates significant differences among treatments (P < 0·01).
Fig. 3.
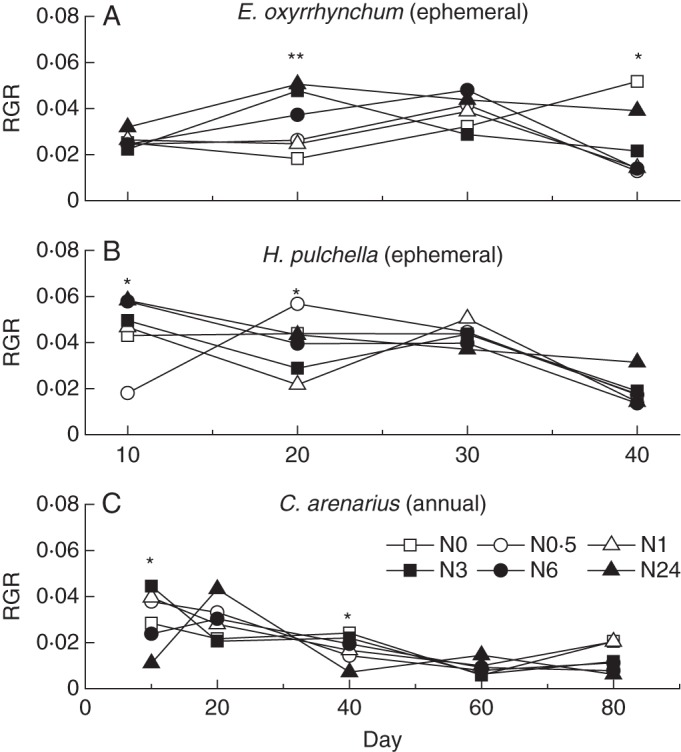
Changes in relative growth rate (RGR) of E. oxyrrhynchum (A), H. pulchella (B) and C. arenarius (C) during each sampling date, * indicates a significant difference at α = 0·05 and ** indicates significant differences at the α = 0·01 level (n = 10).
Shoot and root biomass responses to N applications
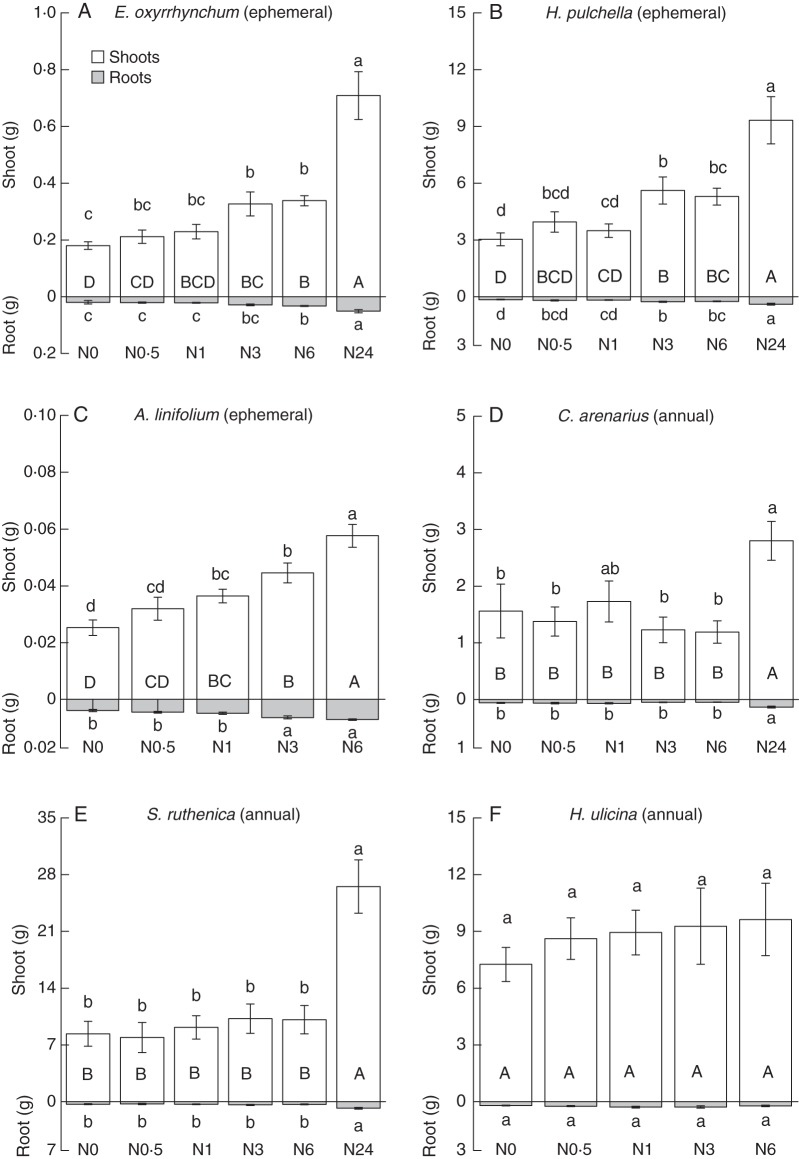
Final harvest shoot biomass increased with increasing N application rates for the three ephemeral plants, with the highest shoot biomass found for N24 treatments (Fig. 4A–C). However, N0·5–N6 treatments did not increase shoot biomass significantly for the three annual plants (Fig. 4D–F; P > 0·05). Root biomass also positively responded to N addition. No significant differences in root biomass were found for N0–N6 treatments for annuals. The responses of total individual biomass (shoot biomass + root biomass) to increasing N application rates were similar to the responses of shoot biomass for all six species.
Fig. 4.
Changes in shoot, root and total individual biomass (shoot + root) of ephemerals [E. oxyrrhynchum (A), H. pulchella (B) and A. linifolium (C)] and annuals [C. arenarius (D), S. ruthenica (E) and H. ulicina (F)] for six N application rates at the final harvest time. Different lower case letters represent significant differences in shoot or root; different upper case letters indicate significant differences in individual biomass among different N addition doses (n = 10, P < 0·05).
Root and shoot biomass ratios
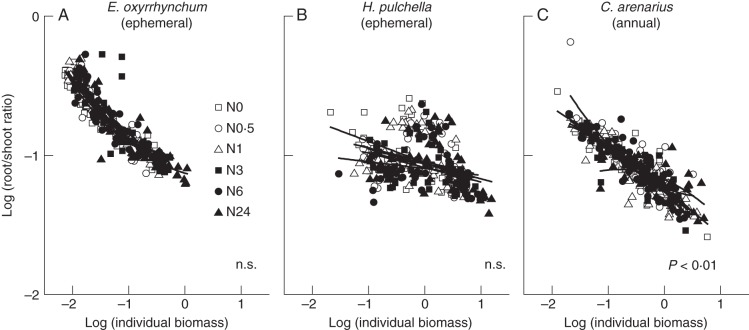
Root and shoot ratios were significantly affected by sampling dates, N treatments and their interactions, except for ephemerals E. oxyrrhynchum and H. pulchella (Table 2), but shared similar intraspecific trends across the different N treatments for the three growth periods (Fig. 5A–C). Significant effects of N addition were found between 29 April and 30 May for three ephemeral plants (P < 0·05). The N addition did not change the root/shoot ratios on 30 June and 10 August in the case of the annual C. arenarius. Comparisons of log (root/shoot) as a function of total plant size showed increased allocation to shoots for all three species during their vegetative growth periods (Fig. 6A–C). Root/shoot allocation did not change significantly with N addition for ephemerals E. oxyrrhynchum and H. pulchella (P > 0·05).
Fig. 5.
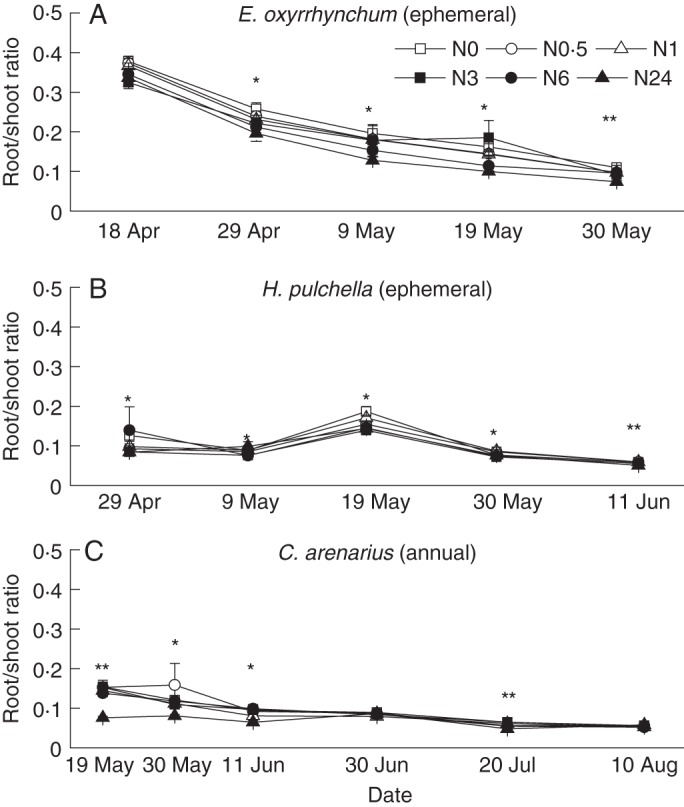
Changes in root and shoot ratios at different sampling dates for E. oxyrrhynchum (A), H. pulchella (B) and C. arenarius (C). * indicates a significant difference at α = 0·05 and ** indicates significant differences at the α = 0·01 level (n = 10).
Fig. 6.
Bivariate log–log plots of root/shoot vs. individual biomass for E. oxyrrhynchum (A), H. pulchella (B) and C. arenarius (C) individuals, over their vegetative period in response to six N treatments (all sampling dates were pooled). Curves represent best-fit second-order polynomial regressions. The probability values reported correspond to tests of the hypothesis that the curves for the different N treatments do not differ. ‘n.s.’ indicates that no significant differences were found.
Based on root/shoot ratios measured at the time of final harvesting, increased N applications resulted in increased allocation to shoot biomass for the ephemerals (Table 3). In contrast, the biomass partitioning patterns of annuals were insensitive to N addition. The root/shoot ratios of ephemerals were significantly decreased by N addition treatments (P < 0·05). However, no significant differences in root/shoot ratios were observed for the three annual plants (P > 0·05).
Table 3.
Comparisons of root/shoot biomass ratios for E. oxyrrhynchum, H. pulchella, A. linifolium, C. arenarius, S. ruthenica and H. ulicina in response to different N application rates
| Treatments | Ephemerals |
Annuals |
||||
|---|---|---|---|---|---|---|
| E. oxyrrhynchum | H. pulchella | A. linifolium | C. arenarius | S. ruthenica | H. ulicina | |
| N0 | 0·110 ± 0·006a | 0·058 ± 0·002a | 0·188 ± 0·013a | 0·056 ± 0·007a | 0·046 ± 0·003a | 0·033 ± 0·003a |
| N0·5 | 0·097 ± 0·004ab | 0·059 ± 0·002a | 0·176 ± 0·010ab | 0·055 ± 0·005a | 0·042 ± 0·003a | 0·033 ± 0·003a |
| N1 | 0·098 ± 0·006ab | 0·060 ± 0·001a | 0·159 ± 0·006bc | 0·052 ± 0·005a | 0·043 ± 0·004a | 0·037 ± 0·003a |
| N3 | 0·092 ± 0·005b | 0·056 ± 0·002a | 0·168 ± 0·006abc | 0·054 ± 0·007a | 0·046 ± 0·005a | 0·035 ± 0·004a |
| N6 | 0·096 ± 0·002ab | 0·055 ± 0·002ab | 0·149 ± 0·005c | 0·052 ± 0·005a | 0·042 ± 0·005a | 0·031 ± 0·004a |
| N24 | 0·074 ± 0·003c | 0·050 ± 0·002b | NA | 0·058 ± 0·003a | 0·035 ± 0·002a | NA |
Values are given mean ± s.e.; P < 0·05; n = 10.
Different letters for each species denote significant differences between treatments.
NA indicates that no data were available.
Allometric relationship between shoot and root biomass
When the data gathered across the different sampling dates and the six treatments were pooled, a non-isometric (allometric) relationship between shoot and root was observed and did not vary significantly for ephemerals (Fig. 7A, B). Nutrient availability only affected the allometric coefficient (i.e. the y-intercept) of the root vs. shoot scaling relationship, but had no effect on the scaling exponent (i.e. the slope) of the two species. Significantly different slopes were found for the data gathered for N0 − N6 treatments and for N24 treatments in the case of the annual C. arenarius (Fig. 7C, P < 0·01).
Fig. 7.
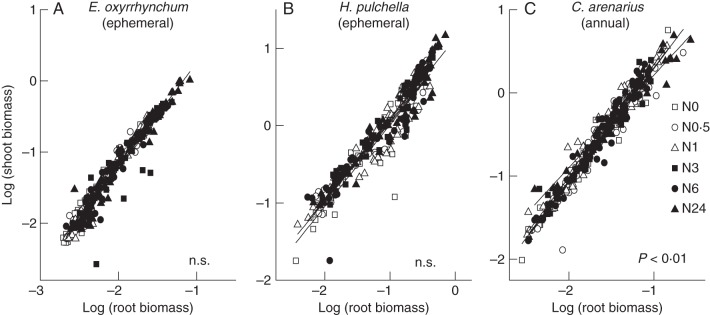
Bivariate log–log plots of shoot vs. root growth for E. oxyrrhynchum (A), H. pulchella (B) and C. arenarius (C) over the vegetative growth period in response to six different N treatments (all sampling dates were pooled). Linear curves take the form log shoot = αRMA log10 root + log10 βRMA. The probability values reported correspond to tests of the hypothesis that the curves do not differ. ‘n.s.’ indicates that no significant differences were found.
The OLS analyses for the data gathered at the final harvesting of the six species revealed a statistically significant positive correlation between shoot and root biomass (r2 > 0·72). The αOLS of the regression was approximately unity. The RMA analyses also showed no statistically significant numerical differences in the slopes or in the y-intercepts (αRMA and log10 βRMA, respectively) among the six species, nor did the numerical values of αRMA differ significantly from the null hypothesis, i.e. αRMA = 1·0 (Table 4). However, the pooled data for ephemerals showed an allometric relationship between shoot and root biomass, which is in contrast to those of annuals and all species (isometric). Consequently, the log shoot vs. log root scaling relationships between ephemerals and annuals were statistically distinguishable (Fig. 8).
Table 4.
Summary statistics of ordinary least squares (OLS) and reduced major axis (RMA) regression analysis of the relationship between log10-transformed data of final shoot (Y) and root biomass (X) for six species
| OLS analyses |
RMA analyses |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αOLS | r2 | OLS model error | Mean log Y | s.d. log Y | Mean log X | s.d. log X | n | α s.e. | β s.e. | αRMA | 95 % CI | log10 βRMA | 95 % CI | |||
| E. oxyrrhynchum | 1·056 | 0·801 | 0·715 | –0·552 | 0·247 | –1·576 | 0·209 | 60 | 0·472 | 0·836 | 1·179 | 0·708 | 1·651 | 1·307 | –0·332 | 2·945 |
| H. pulchella | 1·102 | 0·966 | 0·121 | 0·644 | 0·244 | –0·607 | 0·217 | 60 | 0·190 | 0·134 | 1·121 | 0·931 | 1·311 | 1·324 | 1·062 | 1·587 |
| A. linifolium | 1·153 | 0·867 | 0·213 | –1·441 | 0·181 | –2·222 | 0·146 | 50 | 0·338 | 1·005 | 1·238 | 0·900 | 1·577 | 1·310 | –0·660 | 3·280 |
| C. arenarius | 1·047 | 0·791 | 1·011 | 0·132 | 0·287 | –1·152 | 0·243 | 60 | 0·520 | 0·634 | 1·177 | 0·657 | 1·697 | 1·488 | 0·246 | 2·730 |
| S. ruthenica | 0·990 | 0·858 | 0·213 | 0·970 | 0·323 | –0·421 | 0·303 | 60 | 0·214 | 0·103 | 1·068 | 0·854 | 1·282 | 1·419 | 1·218 | 1·620 |
| H. ulicinia | 0·836 | 0·728 | 0·789 | 0·879 | 0·243 | –0·610 | 0·248 | 50 | 0·499 | 0·336 | 0·980 | 0·481 | 1·480 | 1·476 | 0·818 | 2·135 |
| All ephemerals | 1·270 | 0·993 | 0·924 | –0·391 | 0·876 | –1·427 | 0·687 | 170 | 0·175 | 0·170 | 1·275 | 1·100 | 1·449 | 1·428 | 1·094 | 1·762 |
| All annuals | 1·099 | 0·907 | 3·620 | 0·647 | 0·479 | –0·734 | 0·415 | 170 | 0·445 | 0·297 | 1·154 | 0·709 | 1·600 | 1·495 | 0·912 | 2·077 |
| All species | 1·300 | 0·971 | 7·420 | 0·128 | 0·876 | –1·081 | 0·664 | 340 | 0·356 | 0·282 | 1·319 | 0·963 | 1·675 | 1·553 | 1·000 | 2·107 |
Fig. 8.
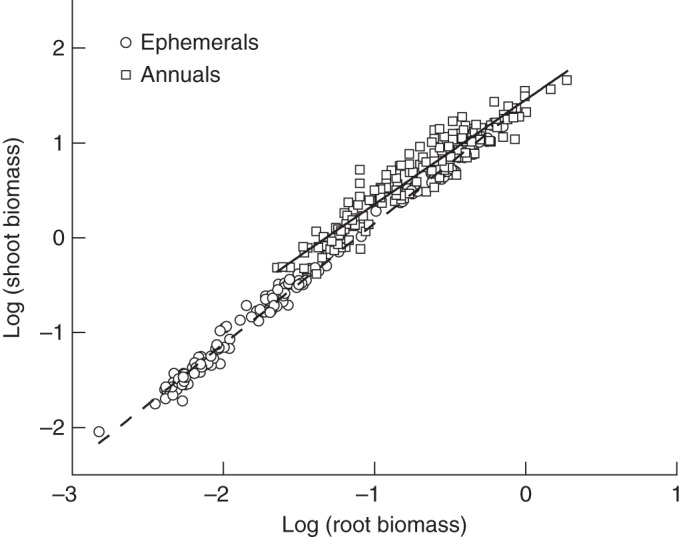
Log–log bivariate plot of shoot and root biomass for combined data from ephemeral and annual species at the final harvesting. Solid and dashed lines indicate the RMA regression curve for ephemerals and annuals, respectively.
DISCUSSION
Differences in growth patterns in response to N applications
As initially hypothesized, the growth of ephemerals was more sensitive to N applications compared with that of annuals in the field. The biomass of ephemerals increased significantly and rapidly with N soil enhancement, which only occurred for annuals with the highest N application rate (i.e. N24 = 24·0 g N m−2 year−1). Nitrogen application resulted in similar differences in the root vs. shoot biomass partitioning patterns for the two life history strategies.
Our study adds to a growing body of data showing that it is necessary to consider differences in functional traits when formulating mechanistic explanations for experimental results and specifically to our understanding of how plants differing in life history strategies or life forms respond to natural or artificial changes in N soil concentration. The contrasting responses of plants representing different life forms or functional groups to environmental changes have been well documented. For example, N seasonality affects the biomass and relative growth rates of both C3 and C4 grass species, while seasonal water regimes significantly affect biomass only in C3 grass species (Niu et al., 2008), whereas desert steppe perennial grasses respond more than annual or perennial forbs to the addition of N during wet as opposed to dry years (Su et al., 2013). Also, increasing N deposition is reported to elevate N assimilation and above-ground production in grasses but has the reverse effects on temperate steppe forbs (Song et al., 2011).
In the context of our study, we can propose two inter-related explanations for the differences observed between the growth rates and biomass partitioning patterns of the ephemeral and annual species. First, by virtue of their more extended vegetative growth periods, annuals have a longer time to adjust to environmental changes, and, secondly, they tend to grow during relatively drier soil conditions than ephemerals, where low soil moisture limits the stimulatory effects of N increase. On the one hand, ephemerals are triggered to germinate by snow thaw and rainfall, and usually grow only for a very short period of time. Consequently, they experience comparatively little water stress. We therefore surmise that the desert ephemerals examined in our study experience less intense selection pressure to develop structures or physiological mechanisms that vegetatively respond to long-term environmental changes compared with annuals, which grow for longer periods of time throughout a potentially hot summer. Ephemerals are considered to be opportunistic species. In a study of 27 herbaceous species, Müller et al. (2000) found that the more ‘opportunistic’ species responded more vigorously to nutrient availability than less opportunistic species. On the other hand, it is possible that the differences between the two life history strategies in response to N addition might be due to differences in soil moisture during the periods of active vegetative growth. Under desert conditions, plant growth and microbial activity are primarily limited by the availability of water and secondarily by soil nutrients such as N (McCrackin et al., 2008; Ladwig et al., 2012). The effect of N enrichment on herbaceous plant communities in desert steppes is also reported to be strongly dependent on natural precipitation patterns. For example, during wet years in arid Chinese regions, perennial grasses benefit more from N enrichment than do annuals, whereas, during dry years, all species groups appear to be equally responsive to precipitation (Su et al., 2013). Ladwig et al. (2012) found that N only becomes limiting once drought stress is alleviated under desert conditions. The significant effects of N addition on the root/shoot ratio of C. arenarius on 20 July might be due to the rainfall events in early July, which resulted in abundant soil moisture in early and mid July.
Although it is true that the application of high N rates can reduce plant growth (Wu et al., 2008), no negative effects of N application were observed during our study even when applications were increased to 24 g N m−2 year−1. The obvious stimulating effects of high N addition on individual plant growth might also be a result of less intra- and interspecific competition in high N plots (i.e. N24 treatments) than in plots where N is a limiting resource. In this study, plant cover and density declined in high N plots compared with low N plots (data not shown).
At a mechanistic level, changes in soil N in N-limited ecosystems might lead to differences in tissue N and then differences in shoot vs. root partitioning patterns (and thus differences in assimilation and growth) among species. Some workers argue that total N and even the soluble protein in plants might account for root/shoot ratio variations (Hilbert, 1990; Gleeson, 1993; Andrews et al., 2006). The cost of increasing tissue N concentration is primarily related to increases in biomass allocation to roots (Hilbert, 1990).
Optimal allocation vs. allometric models
The root/shoot ratios of ephemerals measured at the time of final harvesting decreased with increasing N application. This was not the case for annual species. Thus, the data for ephemerals are consistent with standard optimal allocation models, which predict that biomass partitioning patterns will be adjusted in response to changes in resource availabilities, e.g. increases in the levels of root resources are predicted to result in increases in biomass allocation to shoot parts. However, the results for the annual species examined in our study did not follow this trend. Increased N application had no observable effect on the root/shoot ratios of annuals. One explanation for these divergent responses is the effects of an ‘ontogenetic drift’ and changes in overall plant size. That is, developmental adjustments in biomass allocation can occur simply as a consequence of normal plant growth and development (Coleman et al., 1994; Niklas, 2004). In the case of the ephemerals E. oxyrrhynchum and H. pulchella, the influence of nutrient availability on biomass allocation patterns can be significant during the entire vegetative growth period. However, the effects of this influence can diminish or even disappear once plants reach their mature size. Thus, some species such as E. oxyrrhynchum and H. pulchella appear to alter their shoot vs. root biomass allocation patterns only slightly in response to broadly varying N soil conditions. Additional research is required to examine this explanation in greater detail since our current data set is insufficient to resolve the proximal causalities for the allocation patterns reported here. Under any circumstances, we are not indifferent to the effects of species composition on attempts to quantify biomass allocation patterns. What can be said based on our data is that (1) individual species differ in the allocation patterns and that (2) the two species groups examined here (ephemerals and annuals) differ in their allocation patterns, in their responses to N applications. More specifically, we can say that changes in the biomass allocation of some species (i.e. E. oxyrrhynchum) in response to N treatments are largely a consequence of changes in overall plant size, which is (taken at face value) inconsistent with an optimal partitioning model.
The claim that species-specific differences can confound attempts to generalize about the efficacy of optimal allocation models is supported by the allometric analyses reported here, which reveal that the allometry of E. oxyrrhynchum and H. pulchella shares the same slope but differs from that of C. arenarius in response to different N treatments. Specifically, only minor effects of N treatments on biomass allocation patterns for E. oxyrrhynchum and H. pulchella were observed, whereas C. arenarius showed a significant decrease in the log root vs. log shoot scaling exponent (αRMA) for N24 treatments. Comparisons between competing and non-competing populations of the three annual species demonstrate that plant allometry is altered by competition (Weiner and Thomas, 1992). In this regard, it is noteworthy that N24 treatments negatively affected plant cover and density and thus probably had an indirect effect on competition, which might account for the shoot and root allocation responses observed for C. arenarius.
Previous studies have shown that across all treatments and species, an isometric log root vs. log shoot scaling relationship on average holds true for non-woody species (see Niklas, 2004). This phenomenology has also been reported by others at the level of individual plants, conspecifics differing in size within individual populations and even across a broad spectrum of forested communities (Niklas, 2005; Cheng and Niklas, 2007; Allen et al., 2008; Yang et al., 2009; Yang and Luo, 2011). It is also reported to be independent of changes in soil moisture or N content (Yang et al., 2009). However, other studies do not support the expectation that shoot biomass will scale one-to-one with root biomass, e.g. the scaling relationship between shoot and root biomass across Chinese grassland herbaceous species is anisometric (i.e. allometric) (Wang et al., 2010). There are a number of possible explanations to account for why different studies draw different conclusions regarding the scaling of shoot with respect to root biomass. Among the most obvious explanations is the fact that many scaling exponents have fairly broad 95 % confidence intervals (CIs) that permit different workers to propose different scaling phenomenologies. For example, inspection of Table 4 shows that the 95 % CIs of the scaling exponent for the relationship between shoot and root biomass across all six species includes one, i.e. the null hypothesis (i.e. αRMA = 1·0) cannot be categorically rejected. However, the 95 % CIs for this scaling relationship span numerical values that include 5/4, 7/5 and 3/2, each of which cannot be rejected as a working hypothesis. Even though these three scaling values are not based on any theory or hypothesis known to us, each is a potential competing value used here to illustrate a simple but important fact – it is extremely difficult to pin-point the actual numerical value of a scaling exponent with comparatively broad 95 % CIs. Notice, however, that the actual numerical value of the exponent reported here is 1·3, which is allometric and consistent with the hypothesis that αRMA = 5/4. Nevertheless, it would be disingenuous not to point out that this range of values is sufficiently broad to permit alternative hypotheses to remain viable until additional data are brought to bear. Accordingly, our data do not permit us to reject the null hypothesis emerging from allometric theory. However, they do not permit us to exclude alternative hypotheses should they emerge from an alternative allometric theory.
Conclusions
In contrast to annuals (C. arenarius), ephemerals (E. oxyrrhynchum and H. pulchella) had rapid growth during the late period of their vegetative growth, and had higher growth rates under modest to high applications of N during early sampling dates. The root/shoot ratios of three ephemerals at the final harvesting time were also found to be more sensitive to N addition compared with that of the three annuals, with significant effects of N addition found in ephemerals. Root and shoot biomass ratios of ephemerals did not vary with overall plant size among the different N treatments, indicating that root vs. shoot allocation patterns are adaptively responsive in ways that are largely not consistent with optimal allocation models. Allometric analyses showed that the scaling relationship between shoot and root biomass is indistinguishable from an isometric relationship across all six species and all annuals. Nevertheless, all ephemerals revealed allometric relationships. These results indicate that (1) the selection of life history strategies can profoundly alter statistical results and thus the conclusions that can be drawn from experimental manipulation and (2) although an isometric model for the scaling relationship between shoot and root biomass could not be rejected, the typically broad 95 % CIs spanning most scaling exponents caution against drawing summary judgements about canonical scaling relationships.
ACKNOWLEDGEMENTS
We thank Ye Tao, Lin Wu, Guodong Li, Zhibin Zhou and Yaobin Liu for their help with the plant sampling and analysis. Two anonymous reviewers provided helpful suggestions to improve the manuscript. This work is supported by the National Natural Science Foundation of China (41001181, U1203301) and the College of Agriculture and Life Sciences, Cornell University.
LITERATURE CITED
- Allen AP, Pockman WT, Restrepo C, Milne BT. Allometry, growth and population regulation of the desert shrub Larrea tridentata. Functional Ecology. 2008;22:197–204. [Google Scholar]
- Andrews M, Raven JA, Lea PJ, Sprent JI. A role for shoot protein in shoot–root dry matter allocation in higher plants. Annals of Botany. 2006;97:3–10. doi: 10.1093/aob/mcj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YF, Wu JG, Clark CM, et al. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Global Change Biology. 2010;16:358–372. [Google Scholar]
- Barbour MG. Desert dogma reexamined: root–shoot productivity and plant spacing. American Midland Naturalist. 1973;89:41–57. [Google Scholar]
- Bernacchi CJ, Coleman JS, Bazzaz FA, McConnaughay KDM. Biomass allocation in old-field annual species grown in elevated CO2 environments: no evidence for optimal partitioning. Global Change Biology. 2000;6:855–863. [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA. Resource limitations in plants – an economic analogy. Annual Review of Ecology and Systematics. 1985;16:363–392. [Google Scholar]
- Brooks ML. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. Journal of Applied Ecology. 2003;40:344–353. [Google Scholar]
- Chen BM, Wang GX, Peng SL. Role of desert annuals in nutrient flow in arid area of Northwestern China: a nutrient reservoir and provider. Plant Ecology. 2009;201:401–409. [Google Scholar]
- Chen YN, Wang Q, LI WH, Ruan X. Microbiotic crusts and their interrelations with environmental factors in the Gurbantonggut desert, western China. Environmental Geology. 2007;52:691–700. [Google Scholar]
- Cheng DL, Niklas KJ. Above- and below-ground biomass relationships across 1534 forested communities. Annals of Botany. 2007;99:95–102. doi: 10.1093/aob/mcl206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JS, Mcconnaughay KDM, Ackerly DD. Interpreting phenotypic variation in plants. Trends in Ecology and Evolution. 1994;9:187–191. doi: 10.1016/0169-5347(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Fenn ME, Baron JS, Allen EB, et al. Ecological effects of nitrogen deposition in the western United States. Bioscience. 2003;53:404–420. [Google Scholar]
- Gebauer RLE, Reynolds JF, Strain BR. Allometric relations and growth in Pinus taeda: the effect of elevated CO2 and changing N availability. New Phytologist. 1996;134:85–93. [Google Scholar]
- Gleeson SK. Optimization of tissue nitrogen and root shoot allocation. Annals of Botany. 1993;71:23–31. [Google Scholar]
- Green TH, Mitchell RJ, Gjerstad DH. Effects of nitrogen on the response of loblolly-pine to drought. 2 Biomass allocation and C–N balance. New Phytologist. 1994;128:145–152. doi: 10.1111/j.1469-8137.1994.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Hilbert DW. Optimization of plant root:shoot ratios and internal nitrogen concentration. Annals of Botany. 1990;66:91–99. [Google Scholar]
- Hunt R, Burnett JA. The effects of light intensity and external potassium level on root/shoot ratio and rates of potassium uptake in perennial ryegrass (Lolium perenne L.) Annals of Botany. 1973;37:519–537. [Google Scholar]
- Iwasa Y. Dynamic optimization of plant growth. Evolutionary Ecology Research. 2000;2:437–455. [Google Scholar]
- Ladwig LM, Collins SL, Swann AL, Xia Y, Allen MF, Allen EB. Above- and belowground responses to nitrogen addition in a Chihuahuan Desert grassland. Oecologia. 2012;169:177–185. doi: 10.1007/s00442-011-2173-z. [DOI] [PubMed] [Google Scholar]
- McCarthy MC, Enquist BJ. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Functional Ecology. 2007;21:713–720. [Google Scholar]
- McConnaughay KDM, Coleman JS. Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology. 1999;80:2581–2593. [Google Scholar]
- McCrackin ML, Harms TK, Grimm NB, Hall SJ, Kaye JP. Responses of soil microorganisms to resource availability in urban, desert soils. Biogeochemistry. 2008;87:143–155. [Google Scholar]
- Müller I, Schmid B, Weiner J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:115–127. [Google Scholar]
- Niklas KJ. Plant allometry: is there a grand unifying theory? Biological Reviews. 2004;79:871–889. doi: 10.1017/s1464793104006499. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. Modelling below- and above-ground biomass for non-woody and woody plants. Annals of Botany. 2005;95:315–321. doi: 10.1093/aob/mci028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ, Enquist BJ. Canonical rules for plant organ biomass partitioning and annual allocation. American Journal of Botany. 2002;89:812–819. doi: 10.3732/ajb.89.5.812. [DOI] [PubMed] [Google Scholar]
- Niu SL, Liu WX, Wan SQ. Different growth responses of C(3) and C(4) grasses to seasonal water and nitrogen regimes and competition in a pot experiment. Journal of Experimental Botany. 2008;59:1431–1439. doi: 10.1093/jxb/ern051. [DOI] [PubMed] [Google Scholar]
- Noy-Meir I. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics. 1973;4:25–51. [Google Scholar]
- Olff H, Vanandel J, Bakker JP. Biomass and shoot root allocation of 5 species from a grassland succession series at different combinations of light and nutrient supply. Functional Ecology. 1990;4:193–200. [Google Scholar]
- Padgett PE, Allen EB. Differential responses to nitrogen fertilization in native shrubs and exotic annuals common to Mediterranean coastal sage scrub of California. Plant Ecology. 1999;144:93–101. [Google Scholar]
- Poorter H, Nagel O. International Conference on Assimilate Transport and Partitioning (ICATP 99). Australia: Newcastle; 1999. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. [Google Scholar]
- Poorter H, Remkes C, Lambers H. Carbon and nitrogen economy of 24 wild-species differing in relative growth-rate. Plant Physiology. 1990;94:621–627. doi: 10.1104/pp.94.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin C, Lechowicz MJ, Tardif S. The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology. 1990;71:1389–1400. [Google Scholar]
- Song L, Bao X, Liu X, et al. Nitrogen enrichment enhances the dominance of grasses over forbs in a temperate steppe ecosystem. Biogeosciences. 2011;8:2341–2350. [Google Scholar]
- Su JQ, Li XR, Li XJ, Feng L. Effects of additional N on herbaceous species of desertified steppe in arid regions of China: a four-year field study. Ecological Research. 2013;28:21–28. [Google Scholar]
- Thornley JHM. A balanced quantitative model for root:shoot ratios in vegetative plants. Annals of Botany. 1972;36:431–441. [Google Scholar]
- Wang LA, Niu KC, Yang YH, Zhou P. Patterns of above- and belowground biomass allocation in China's grasslands: evidence from individual-level observations. Science China-Life Sciences. 2010;53:851–857. doi: 10.1007/s11427-010-4027-z. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Jiang J, Wang YC, Luo WL, Song CW, Chen JJ. Responses of ephemeral plant germination and growth to water and heat conditions in the southern part of Gurbantunggut Desert. Chinese Science Bulletin. 2006;51:110–116. [Google Scholar]
- Weiner J, Thomas SC. Competition and allometry in 3 species of annual plants. Ecology. 1992;73:648–656. [Google Scholar]
- Wu FZ, Bao WK, Li FL, Wu N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environmental and Experimental Botany. 2008;63:248–255. [Google Scholar]
- Yang YH, Luo YQ. Isometric biomass partitioning pattern in forest ecosystems: evidence from temporal observations during stand development. Journal of Ecology. 2011;99:431–437. [Google Scholar]
- Yang YH, Fang JY, Ji CJ, Han WX. Above- and belowground biomass allocation in Tibetan grasslands. Journal of Vegetation Science. 2009;20:177–184. [Google Scholar]
- Yuan XM, Wang ZQ. Studies on the NO3− -N in the environment and soil. Arid Zone Research. 1997;14:52–55. (in Chinese with English abstract) [Google Scholar]
- Zhang W, Liu X, Hu Y, Li K, Shen J, Luo X, Song W. Analysis on input of atmospheric nitrogen dry deposition in Urumqi. Arid Zone Research. 2011;28:771–716. (in Chinese with English abstract) [Google Scholar]
- Zhang Y, Zheng LX, Liu XJ, et al. Evidence for organic N deposition and its anthropogenic sources in China. Atmospheric Environment. 2008;42:1035–1041. [Google Scholar]
- Zhou XB, Zhang YM, Ji XH, Downing A, Serpe M. Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China. Environmental and Experimental Botany. 2011;74:1–8. [Google Scholar]