Abstract
Background and Aims
Floral traits, such as floral volatiles, can contribute to pre-zygotic reproductive isolation by promoting species-specific pollinator foraging. When hybrid zones form, floral traits could also influence post-zygotic isolation. This study examined floral volatiles in parental species and natural hybrids in order to explore potential scent mediation of pre-zygotic and post-zygotic isolation.
Methods
Floral bouquets were analysed for the sister species Ipomopsis aggregata and I. tenuituba and their natural hybrids at two contact sites differing in both hybridization rate and temporal foraging pattern of hawkmoth pollinators. Floral volatiles were quantified in diurnal and nocturnal scent samples using gas chromatography–mass spectrometry.
Key Results
The bouquets of parental species and hybrids showed qualitative overlap. All flowers emitted similar sets of monoterpenoid, sesquiterpenoid, aliphatic and benzenoid compounds, but separated into groups defined by multivariate analysis of quantitative emissions. The parental species differed most strikingly in the nitrogenous compound indole, which was found almost exclusively in nocturnal bouquets of I. tenuituba. Natural hybrid bouquets were highly variable, and showed emission rates of several compounds that appeared transgressive. However, indole emission rates were intermediate in the hybrids compared with rates in the parents. Volatile bouquets at the contact site with lower hybridization did not show greater species specificity in overall scent emission, but I. tenuituba presented a stronger indole signal during peak hawkmoth activity at that site.
Conclusions
The two species of Ipomopsis differed in patterns of floral bouquets, with indole emitted in nocturnal I. tenuituba, but not in I. aggregata. Natural hybrid bouquets were not consistently intermediate between the parents, although hybrids were intermediate in indole emission. The indole signal could potentially serve as a hawkmoth attractant that mediates reproductive isolation both before and after hybrid formation.
Keywords: Floral volatiles, hawkmoth, hybrid zone, Ipomopsis, Polemoniaceae, reproductive isolation, scent
INTRODUCTION
The astounding diversity of floral features in the angiosperms is commonly attributed to biotic pollination (Grant, 1994; Fenster et al., 2004; Johnson, 2006), even though interactions with antagonists and abiotic stress factors can also play a role (Galen and Cuba, 2001; Frey, 2004; Strauss and Whittall, 2006; Dötterl et al., 2009). Pollinators can be involved not only in evolution of floral traits within a species, but also in speciation, as differences in floral traits can lead to reproductive isolation (Fulton and Hodges, 1999; Schemske and Bradshaw, 1999). In animal-pollinated plants, one form of pre-zygotic reproductive isolation is ethological isolation, which occurs when two types of pollinators each visit flowers of different species when foraging. Each pollinator could be attracted to a different suite of floral traits or may respond differently to traits perceived as repellent (Grant, 1994). If pollinators move between flowers of two species, pre-zygotic reproductive isolation is incomplete and hybridization occurs.
Many floral traits such as petal colour (Melendez-Ackerman and Campbell, 1998; Bradshaw and Schemske, 2003) and flower orientation (Fulton and Hodges, 1999) have been shown to influence ethological reproductive isolation. In contrast to these traits, the emission of floral volatile organic compounds (VOCs), or floral scent, has been largely ignored in studies of reproductive isolation until very recently (Raguso, 2008). Examples of species-specific scents that attract pollinators are known mostly from highly specialized sexually deceptive orchids and nursery pollination systems (Schiestl et al., 2003; reviewed by Hossaert-McKey et al., 2010; Ayasse et al., 2011; Xu et al., 2012). The potential of floral volatiles to mediate pre-zygotic ethological isolation between related plant species with generalized, food-rewarding pollination systems remains poorly understood (Waelti et al., 2008; Shuttleworth and Johnson, 2010).
Outside the realm of finely attuned sexually or food-deceptive systems, the role of floral scent as a facilitator or suppressor of hybridization between taxa has rarely been studied. One example of pronounced scent differences between closely related species is found in the interfertile white and red campions (Silene latifolia and S. dioica, respectively; Waelti et al., 2008). Experimental application of the dominant compound of the S. dioca bouquet to S. latifolia increased transfer of fluorescent dye (used as a pollen analogue) between those species. In this case, species-specific scents reduce the potential for gene flow between the two species, demonstrating the importance of odour for pre-zygotic reproductive isolation. A second case of floral volatiles acting as a species-specific cue occurs in hawkmoth-pollinated white Petunia axillaris and hummingbird-pollinated red P. exserta (Klahre et al., 2011). The bouquet of P. axillaris is dominated by hawkmoth-attracting benzenoid esters and alcohols, whereas the hummingbird-pollinated flowers of P. exserta are scentless. In wind tunnel experiments, captive Manduca sexta hawkmoths visited red flowers only when those flowers were laboratory crosses that also emitted scent. These few studies have focused mainly on the role of scent in pre-zygotic isolation. We know even less about the potential of floral volatiles to influence post-zygotic reproductive isolation, i.e. the role of floral scent in promoting or reducing visits to hybrids and thereby affecting hybrid fitness.
When closely related plant species lack pre-zygotic reproductive isolation, hybrids are produced, and the fate of those hybrids in principle might also depend on floral volatiles. Hybridization is relatively common in flowering plants (Whitney et al., 2010), and many natural hybrid zones contain hybrid plants that survive to flower. The scent emission profiles of these hybrid offspring could potentially influence whether hybrids are pollinated and thereby set seed, and whether advanced generation hybrids are formed. Hence, the degree of breakdown in post-zygotic reproductive isolation between the parental species could depend in part on the chemical composition of hybrid volatile bouquets. If hybrids are intermediate to the parents in scent emission, they could serve as a bridge promoting backcrossing to both species and introgression of traits between them. Such a hybrid bridge has been suggested for two species of Nicotiana, although the sensory cues involved are not fully understood for that case (Ippolito et al., 2004). If hybrids are more similar to one parent species, unidirectional backcrossing might instead be promoted. Natural hybrids might also emit unique combinations of scents that differ from those in the parental species in compound identity or quantitative compound ratios. These unique combinations could be the result of transgressive inheritance, in which hybrids have traits lying outside the range of either parental species (Rieseberg et al., 1999), or could result from selection favouring particular trait combinations within a hybrid zone, as has been seen for vegetative traits such as leaf succulence (e.g. Rieseberg et al., 2007). Unusual trait combinations might lead to reduced pollinator visitation of hybrids, ultimately maintaining reproductive isolation of the parental species or, alternatively, attraction of a novel pollinator type.
The few studies that have compared the floral volatiles of parental species and hybrids in their natural hybrid zones report contrasting findings, suggesting that we might expect different outcomes in different systems. A study by Cortis et al. (2009) on the floral scent of the sexually deceptive orchid species Ophrys iricolor and O. incubacea showed that their hybrids were intermediate in floral scent patterns. Similar patterns of intermediate scent composition were reported for hybrids of the Mediterranean food-deceptive orchids Orchis mascula and O. pauciflora (Salzmann et al., 2007). However, F1 hybrids of two different sexually deceptive Ophrys species, O. arachnitiformis and O. lupercalis, did not emit intermediate scent patterns, but produced a greater number of individual compounds than either of the progenitors. Furthermore, compounds not present in the scent of the parental species were detected in the hybrid bouquets (Vereecken et al., 2010). Outside of food-deceptive orchid systems, no previous study has compared the floral volatiles of advanced generations of natural hybrids with those of their parental species under field conditions to explore the potential for scent to influence post-zygotic isolation. Studying a natural hybrid zone allows determination of the scent phenotypes that actually persist in a hybrid zone and thus are perceived by pollinators in the wild.
When describing the ideal study system for the investigation of the biological and evolutionary significance of floral scent, Whitehead and Peakall (2009) called for a pair of plant species that are closely related, morphologically similar, animal pollinated and co-flowering. Ipomopsis aggregata subsp. aggregata and I. tenuituba ssp. tenuituba (Polemoniaceae) satisfy those stipulations (Campbell, 2004; Porter et al., 2010) and present a good model to study the importance of floral volatiles in reproductive isolation within a food-rewarding pollination system. The main pollinators of Ipomopsis flowers are hummingbirds and hawkmoths. The diurnal hummingbirds prefer to visit I. aggregata and are more efficient at transferring pollen from the wide corolla tubes that are characteristic of that species (Melendez-Ackerman et al., 1997; Campbell et al., 2002), whereas hawkmoths prefer the narrower corolla tubes typical of I. tenuituba (Campbell et al., 1997). Interestingly, the rate of hybridization between the two Ipomopsis species differs across contact sites in the western Rocky Mountains, from virtually no hybridization in some locations to the formation of extensive hybrid swarms in others (Aldridge, 2005), in part because of differences in the degree of hawkmoth specificity (Aldridge and Campbell, 2007). The hawkmoths forage nocturnally at a contact site with low hybridization, but during daylight at a contact site with high hybridization (Aldridge and Campbell, 2007). This geographical variation in hybridization offers the additional opportunity to investigate whether the degree of species specificity in scent cues is associated with reduced hybridization. In principle, floral scent is expected to play a role in hawkmoth flower choice (reviewed by Raguso and Willis, 2003), and hawkmoths can respond to individual compound combinations (Schlumpberger and Raguso, 2008).
A crucial first step in assessing the role of floral volatiles in pre-zygotic and post-zygotic reproductive isolation is to characterize the differences between the parental species and hybrids. We characterized both the qualitative (composition of chemical compounds) and quantitative (emission rates of compounds) differences between types of plants. We asked the following questions. (1) How do the two parental species I. aggregata and I. tenuituba differ in their floral volatile emissions during both day and night? (2) How do natural hybrids compare with the parental species in their floral bouquets? (3) How do the floral volatiles of the two parental species differ between two geographically separated contact sites with high vs. low hybrid frequency? We specifically asked whether a low hybridizing site has greater species specificity in volatiles overall, or a greater difference in key volatiles present at the actual time that key pollinator activity peaks.
MATERIALS AND METHODS
Study system
Ipomopsis aggregata subsp. aggregata (Pursh) V.E.Grant and I. tenuituba ssp. tenuituba (Rydb.) V.E.Grant are monocarpic perennials that commonly occur throughout the western USA and often form hybrid zones in sympatry (Grant and Wilken, 1988; Aldridge, 2005). Flowers of both species have tubular corollas that are coloured red in I. aggregata and pale pink to white in I. tenuituba. These flower colours and morphologies are consistent with hummingbird and hawkmoth pollination syndromes, respectively (Grant and Grant, 1965). Flowers in both species last for several days and remain open day and night.
Collection of floral volatiles was carried out in two contact sites: Poverty Gulch (PG) in Gunnison County, Colorado and Grizzly Ridge (GR) on the north rim of the Black Canyon of the Gunnison, in Montrose County, Colorado. These sites were chosen because they represent extremes in the level of natural hybridization between the two species of Ipomopsis (Aldridge and Campbell, 2007, 2009). Natural hybrids are virtually absent at the low-elevation site at GR (2375–2450 m a.s.l.), whereas an extensive hybrid swarm is present at the clinal high-elevation site at PG (2900–3250 m a.s.l.) (Aldridge, 2005). Plants at both contact sites show the patterns of species-specific floral morphology, with I. aggregata having shorter, wider corollas than I. tenuituba (Aldridge, 2005). Natural hybrids at PG are intermediate between the parental species in floral morphology (Campbell et al., 1997).
At GR, both hummingbirds (Selasphorus platycercus and S. rufus) and Hyles lineata, the only hawkmoth pollinators observed, are consistently abundant and visit I. aggregata and I. tenuituba, respectively, with seemingly complete species specificity and pronounced temporal separation, i.e. hummingbirds forage diurnally and H. lineata are strictly nocturnal (Aldridge and Campbell, 2007). At PG, H. lineata pollinators are absent in most years, potentially due to the cooler nights at this high-elevation site. When present, they are the only hawkmoth species observed and forage diurnally (Aldridge and Campbell, 2007). Hummingbirds, on the other hand, are abundant at PG and visit both species along with natural hybrids during the day, although they preferentially visit redder, wider flowers, exerting directional selection for corolla width in both Ipomopsis species (Campbell et al., 1997; Melendez-Ackerman et al., 1997).
Scent collection
We collected floral scent samples using dynamic headspace extraction methods for subsequent analysis with coupled gas chromatography and mass spectrometry (GC-MS). In total, 64 samples from the two parental species (six day samples and ten night samples per species, per contact site) and 26 samples from natural hybrids (11 day samples and 15 night samples at PG) were collected in the 2010 flowering season. In total, we sampled ten groups, including the eight combinations of species (I. aggregata or I. tenuituba) × sampling time (diurnal or nocturnal) × site (GR or PG), plus natural hybrids at the two sampling times at the PG site only.
At the GR site, I. aggregata plants were randomly sampled from populations G and H, and I. tenuituba samples were randomly collected from populations C, F and I (see topographic map in Aldridge and Campbell, 2009) between 9 and 21 June. At the PG site, scent samples were collected from random plants from the I. aggregata L population, the hybrid I population and the I. tenuituba B population between 13 July and 6 August (see population descriptions in Campbell et al., 1997). The hybrid I population is a well-established hybrid swarm that probably includes advanced generation hybrids, as both F1 and F2 hybrids have high fitness at that site (Campbell et al., 2008), although it is not presently possible to distinguish classes of hybrids based on molecular markers (Wu and Campbell, 2005). The samples were collected in the field between 1000 and 1200 h in sunny, calm conditions and at night between 2100 and 2300 h in dry weather. Both parental species and their hybrids experience heavy browsing by deer; however, only flowers of undamaged plants were sampled to prevent potential artefacts due to herbivory-induced volatile emissions. Although it was not possible for us to sample all types of plants at both sites on the same days, two lines of evidence indicate that differences between days did not confound results. First, daytime hybrids at PG were sampled on both 19 July and 23 July, and only one compound out of 32 present in these hybrids differed significantly between dates for emission rate [P < 0·05 following Bonferroni correction on analysis of variance (ANOVA) of log-transformed data]. Secondly, both parental species were sampled simultaneously during the daytime at GR, and we nevertheless saw large differences in scent profiles between these species that were consistent with comparisons between the species at PG (see the Results).
The samples were taken by enclosing single flowers in male phase (approximately the second day of anthesis) in nylon-6 bags (Reynold's oven bags, USA) with the dimensions 7 × 10 cm made with an impulse sealer (Easyway HS16, USA). A scent trap, consisting of a micro vial (see Gordin and Amirav, 2000) filled with 1 mg of Tenax TA® and 1 mg of Carbotrap® activated charcoal sealed with plugs of silanized quartz wool, was inserted within each bag. Headspace volatiles were left to equilibrate in the bag for 30 min. Air from the bag was then pumped through the trap with a micro air sampler (Supelco PAS-500) at a flow rate of 100 mL min−1 (Dötterl et al., 2005) for 15 min. Ambient controls were taken from an empty nylon-6 bag sampled for the same duration, and vegetative controls were obtained from undamaged leaves.
Scent analysis
We carried out GC-MS analysis of floral scent samples using a Varian CP-3800 GC (Varian, Palo Alto, CA, USA), with a 30 m × 0·25 mm internal diameter (film thickness 0·25 µm) Alltech EC-WAX column, coupled to a Varian 1200 quadrupole mass spectrometer in electron-impact ionization mode at 70 eV (Shuttleworth and Johnson, 2009). Scent traps were placed in a Varian 1079 injector equipped with a ‘Chromatoprobe’ thermal desorption device (Amirav and Dagan, 1997). The flow of helium carrier gas was 1 mL min−1. The injector was held at 40 °C for 2 min with a 20:1 split and then increased to 200 °C at 200 °C min−1 in splitless mode for thermal desorption. After a 3 min hold at 40 °C, the temperature of the GC oven was ramped up to 240 °C at 10 °C min−1 and held for 12 min.
Volatile compounds were tentatively identified using the Varian Workstation software with the NIST05 mass spectral library, and were verified with retention times of 12 authentic standards and published Kovats indices wherever possible. Compounds present at similar abundance in the ambient air and vegetative control samples were considered to be contaminants and were excluded from analysis.
When assessing scent differences, it is important to keep in mind that the volatile bouquets of flowers, including those of Ipomopsis, are usually highly complex. Therefore, analysis and interpretation of scent differences can be a challenge (Adler and Irwin, 2012). We performed two kinds of analyses. In qualitative analyses, we examined presence vs. absence of individual compounds. Exclusive emission of compounds that originate from a major biochemical pathway such as the shikimate pathway for nitrogenous compounds or the mevalonate pathway for sesquiterpenoids would indicate a fundamental qualitative difference between the chemical phenotype of any two groups (Levin et al., 2003). The presence or absence of individual compounds that have their chemical origin in the same biochemical pathway would represent a slightly less pronounced, yet still considerable difference. In quantitative analyses, we examined differences in the emission rates of compounds.
To compensate for the lack of an internal standard in our thermal desorption samples, quantification of the volatile emission rates per hour was carried out using injections of standardized volumes of a set of commercially available standards. One calibration compound per compound class was injected as a placeholder for all compounds in the class, under the assumption that chemically similar compounds will interact with the column material and the detector in similar ways. We selected a total of 12 synthetic standards that covered the majority of chemical structures in the Ipomopsis bouquets, e.g. α-pinene was injected as a standard for the cyclic monoterpenes, whereas (E)-β-ocimene acted as the placeholder for all acyclic monoterpenes (see Supplementary Data Table S1). We injected the compound that was the most abundant per compound class in the natural bouquets wherever a synthetic standard was commercially available. If a synthetic standard for the most abundant compound per compound class could not be obtained, we injected an appropriate compound satisfying three stipulations: abundance in the natural bouquet, commercial availability and similarity of the chemical structure (Majetic et al., 2010). Each compound was injected three times, following the same methods as for the biological samples. The peak areas in the chromatograms were averaged per injected standard, resulting in a conversion factor per compound class. All compounds in the compound class were subsequently standardized with this conversion factor. Finally, all samples were multiplied by the factor 1·3 to convert the emission rates in the sampling time of 45 min to the emission rate per hour. In the following, all emission rates are scaled to ng h−1.
Statistical analysis
For comparison of emissions among groups of plants, we used several methods of analysis, each with its own advantages and disadvantages. To compare the four groups of two parental species and two sampling times (Question 1), we first looked for compounds that were present in only some of those groups (qualitative analysis). To compare the four groups quantitatively, we began by log tranforming the data (after adding 1) to reduce the tendency for variance to increase with the mean, and the skew due to zero-inflated data. We then employed two multivariate methods [random forests (RF) and canonical discriminant analysis] along with univariate ANOVAs. Random forests is a machine learning algorithm designed to choose a minimum set of predictor variables for classifying pre-defined subgroups and is particularly useful for situations with a large number of variables compared with samples (Cutler et al., 2007), as is common with floral volatiles (Ranganathan and Borges, 2010). For each bootstrapped sub-set of data, RF fits a classification tree minimizing the prediction error for observations that were not in the bootstrap sample (out-of-bag). Based on all trees, a solution is chosen with the smallest set of predictor volatiles whose error rate meets a minimum requirement. We built 500 trees for each RF and iterated the process with 200 bootstrap iterations (following Parachnowitsch et al., 2012) using the package VarSelRF in the statistical software program R (R Development Core Team, 2012). Although RF selects a minimum set of volatiles needed to distinguish groups of plants, it does not provide significance tests.
To provide visualization of the data along dimensional axes, we employed canonical discriminant analysis, after removing scent variables with F ratios < 1 in univariate tests of mean emission rates (Huberty and Olejnik, 2006). Our sample size was relatively small for this method, but, with 33 scent variables remaining, permitted standard (rather than sparse) canonical discriminant analysis (Cao et al., 2011), as the total sample size was larger than the number of variables plus groups. We detected significant canonical discriminant functions based on similar variables to those detected by RF (see the Results). The first canonical discriminant function was the linear combination of the quantitative scent variables that explained the maximum variance among the group means. This analysis was implemented using Proc Candisc in SAS 9·2 (SAS Institute, 2008). Although principal components analysis and non-metric multidimensional scaling are sometimes employed with scent data, neither method is well suited to discriminating particular groups defined a priori, such as the two species.
To supplement the multivariate analyses, we ran two-way ANOVAs with the factors of species and time of day, focusing on indole and α-pinene. Indole was chosen because that compound represented the most important difference in the multivariate analyses. α-Pinene was chosen because it was the most abundant compound in the bouquet. Type III SS were employed, as appropriate for unequal sample sizes.
To compare the natural hybrids with the two parental species (Question 2) qualitatively, we used contingency analyses to compare presence vs. absence of indole across the three plant types at PG, separately for daytime and night-time samples. For quantitative analysis, we first plotted hybrid scent profiles in the multivariate odour space generated by canonical discriminant analysis to distinguish the two parental species at two sampling times. We determined the values of the first two canonical variates (i.e. linear combinations of scent variables) for each of the hybrid samples to examine how hybrids compared with the parents based on the same combinations of variables that separate the species. The above analysis allowed us to visualize variation in hybrids. To explore further how the hybrids compared with the parental species in mean values for specific volatiles, we examined the PG site alone, where a large natural hybrid zone as well as the parental species were present. Two-way ANOVA with the factors of plant type and time was used to analyse each specific compound. For indole, α-pinene and all compounds for which the overall type of plant was significant after adjusting P-values for multiple a posteriori comparisons with the sequential Bonferroni method, we then compared the means to see if hybrids were intermediate in volatile emission levels, similar to one parent or transgressive (beyond the range of both parental means). These analyses were performed on untransformed data, to simplify interpretation of whether hybrids were approximately half-way between the parental species (Campbell and Waser, 2007).
In addition to these analyses of specific compounds, we evaluated changes in classes of compounds (aliphatics, monoterpenes, benzenoids, nitrogenous compounds, sesquiterpenes and other) between day and night. Multivariate ANOVA (MANOVA) was used to compare the arcsin square-root-transformed proportions of pooled emissions in these classes (omitting ‘other’ from the analysis) among the three types of plants, time of day and their interactions. To test specifically for differences in emission by the five compound classes with either type of plant or time of day, we used profile analysis. This analysis employed a profile transformation in MANOVA (by specifying the appropriate matrix in the MANOVA statement of Proc GLM; SAS 9·2).
To analyse quantitatively the overall scent differences between the contact sites (Question 3), we employed RF to determine a minimum set of volatiles distinguishing the two species of Ipomopsis at each of the two sites (GR with few hybrids and PG with many hybrids). As before, we also examined the patterns in scent differences using canonical discriminant analysis after variable reduction. Finally, to compare species specificity of the two key compounds (α-pinene and indole) at GR during the night (when hawkmoths forage there) with species specificity at PG during daytime (when hawkmoths forage there), we used two-way ANOVAs to test whether the species difference in emission was higher for night-time GR samples than for daytime PG samples.
RESULTS
Question 1. Qualitative and quantitative differences between parental species bouquets
The floral bouquets of I. aggregata and I. tenuituba included a total of 53 volatiles detectable under our analytical conditions (see Supplementary Data Table S1). We combined together unidentified sesquiterpenes in Supplementary Data Table S1, which resulted in 50 compounds for statistical analyses. Regarding the composition of chemical compounds, terpenoids featured most prominently in all samples, accounting for approximately two-thirds of the identified compounds. Both monoterpenes and sesquiterpenes were present at nearly equal numbers of 18 and 17 individual compounds, respectively. α-Pinene dominated the bouquet as the most abundant compound in eight out of the ten groups [all except GR I. aggregata day samples, in which ylangene dominated, and PG hybrid night samples, in which (E,E)-α-farnesene was most abundant], accounting on average for 48 % of the bouquet. Other compounds in the floral headspace included a number of aliphatics, benzenoids and two lactones. Most of the terpenoids and aliphatics were also present in vegetation samples at low concentrations. The sole nitrogenous compound indole was detected consistently in I. tenuituba night samples (14 out of 20 samples) and in five out of 12 day samples of this species, whereas it was virtually absent from all I. aggregata bouquets (present in trace amount in one out of 32 samples and absent from the remainder). Apart from indole, few compounds appeared to be confined to a particular species or time of sampling. The sesquiterpenes (E,E)-farnesol and (E)-3,7-dimethyl-2,6-octadien-1-yl acetate were only emitted by I. tenuituba at night. Several other terpenoids were exclusively detected in I. tenuituba, but only in one sample. The only compound that was exclusive to I. aggregata was β-elemene emitted in one night sample. (E)- and (Z)-β-ocimene and β-farnesene were never found in day samples of any plant type.
The multivariate RF analysis of the quantitative emission rates selected three volatiles for distinguishing the four groups defined by the two Ipomopsis species sampled at day vs. night: indole, (E)-3-hexen-1-ol and camphor (Table 1A). The bootstrapped prediction error using these three variables was much lower than at random (0·39 vs. 0·69). The variable with the highest importance (indole) was also the variable with the highest correlation with the first canonical discriminant function, and the variable with second highest importance [(E)-3-hexen-1-ol] correlated highly with the second canonical discriminant function (Fig. 1). Thus the two multivariate methods gave largely concordant results. Canonical discriminant analysis was effective at separating the groups, as the first discriminant function was highly correlated with group membership (canonical correlation = 0·95, F96,87·7 = 3·14, P < 0·0001) and accounted for 67 % of the variance among groups. The second function accounted for an additional 22 % of the variance among groups and was also statistically significant (P = 0·0049). Together the two functions cleanly separated the night-time I. aggregata from night-time I. tenuituba (filled vs. open circles in Fig. 1), in large part because only I. tenuituba emitted consistently detectable amounts of indole (see Supplementary Data Table S1).
Table 1.
Predictor volatiles in random forest (RF) models and their correlations with canonical discriminant functions (CDFs)
| Groups in analysis | Predictor volatiles in RF | Model frequency | Correlation with CDF 1 | Correlation with CDF 2 | |
|---|---|---|---|---|---|
| A | I. aggregata day | Indole | 0·835 | –0·644 | –0·263 |
| I. aggregata night | (E)-3-Hexen-1-ol | 0·670 | 0·359 | –0·434 | |
| I. tenuituba day | Camphor | 0·535 | –0·099 | 0·167 | |
| I. tenuituba night | |||||
| B | I. aggregata GR | p-Anisaldehyde | 0·970 | 0·445 | 0·293 |
| I. aggregata PG | Caprolactone | 0·950 | 0·569 | 0·314 | |
| I. tenuituba GR | (E)-Sabinene hydrate | 0·875 | 0·657 | 0·131 | |
| I. tenuituba PG | α-Terpineol | 0·875 | 0·539 | 0·047 | |
| Indole | 0·615 | –0·031 | 0·471 | ||
| Benzyl acetate | 0·475 | 0·506 | 0·064 | ||
(A) Analysis of two species sampled at day vs. night. (B) Analysis of two species sampled at GR vs. PG site.
Fig. 1.
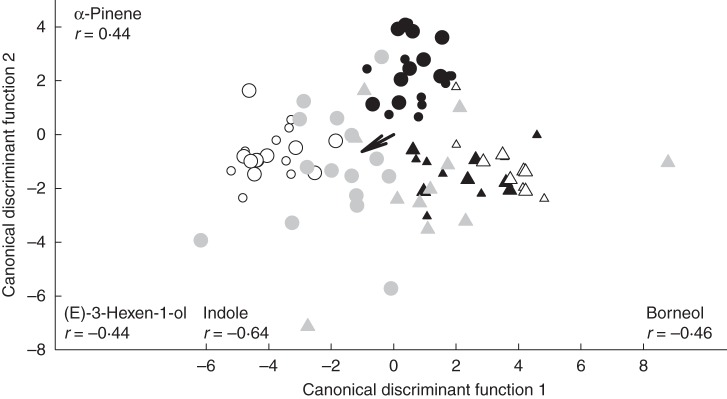
Differences in floral scent chemistry between the two Ipomopsis species during day (triangles) and night (circles) separated using canonical discriminant analysis. The first two canonical discriminant functions were defined based on the scent data set for the two parental species only, with I. aggregata in black and I. tenuituba in white. The natural hybrids (grey) were then mapped onto the resulting parental odour space. Small symbols show plants from Grizzly Ridge. Large symbols show plants from Poverty Gulch, where hybrids also were present. Compounds with the largest positive and negative correlations (r) with the discriminant functions are labelled along the axes. The arrow indicates the direction of the loadings on indole.
Two-way ANOVA detected effects of species (I. aggregata vs. I. tenuituba), sampling time and their interaction on log-transformed indole (P < 0·0001 for species, P < 0·01 for time and the interaction), indicating that indole emission is significantly higher in I. tenuituba at night. The compound α-pinene did not show significant differences between species, sampling time or their interaction in this two-way ANOVA (P > 0·05), although there was a significant increase between day and night for I. aggregata analysed separately (F1,30 = 11·46, P = 0·0020). Total scent emissions did not differ between the parental species (contrast F1,84 = 0·20, P = 0·6585), so species differences in terms of percentage of compounds should be similar to those expressed in terms of emission rates.
Question 2. Qualitative and quantitative differences between natural hybrid and parental bouquets
The qualitative comparison of natural Ipomopsis hybrids with the parental species showed that a number of hybrids emitted indole, as in I. tenuituba bouquets but not I. aggregata bouquets. In particular, four out of 15 natural hybrid samples taken at night contained indole. Contingency analyses on presence vs. absence of indole confirmed variation among the three plant types at PG for night samples (likelihood ratio χ2 = 6·215, P = 0·0447), but not for day samples (P = 0·2441). At a quantitative level, samples from natural hybrids were highly variable within the odour space of scent characteristics that separated the parental species. Most hybrids were not intermediate to their parents in terms of the two axes that best separated the species; instead, many fell outside of the parental ranges (Fig. 1). In an analysis restricted to the PG site only, there were differences in the amount of indole emitted among the two species and hybrids (plant type effect in two-way ANOVA also including time, F2,52 = 4·47, P = 0·0162), with the hybrids intermediate to the parents and statistically distinguishable from I. tenuituba, but not I. aggregata (Tukey a posteriori comparisons; Fig. 2). Absolute levels of α-pinene emission were lower in the natural hybrids than in either of the parental species (Fig. 2; Tukey a posteriori comparisons, P < 0·05).
Fig. 2.
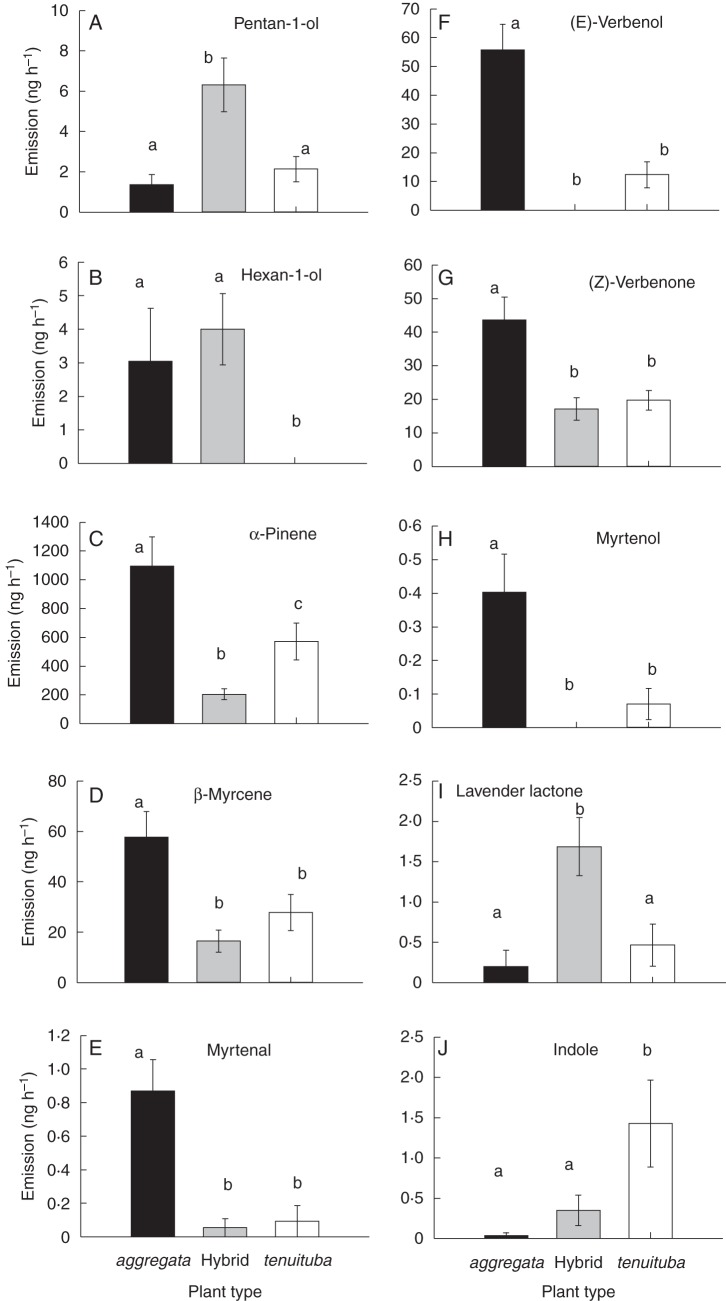
Comparisons of emission rates (mean ± SE) between Ipomopsis aggregata, I. tenuituba and natural hybrids at Poverty Gulch for compounds that differed significantly across plant type. For A–I, significance was based on sequential Bonferroni correction for tests of 47 compounds. Plant types that do not share the same letter differ significantly based on Tukey comparisons.
Other compounds besides indole and α-pinene that were evaluated a posteriori and found to differ among the three plant types (I. aggregata, I. tenuituba and hybrids) were two aliphatics, five monoterpenes and lavender lactone (Fig. 2A–I). For the ten compounds with differences among plant types (Fig. 2), only indole had a hybrid mean that fell intermediate between the parents (Fig. 2J). Three compounds showed transgressive patterns in which the hybrid mean emission rate was significantly larger (pentan-1-ol and lavender lactone) or smaller (α-pinene) than both of the parental means. For the other compounds, hybrids were similar to one of the parental species (Fig. 2). Hybrid flowers never emitted benzyl acetate, (E)-verbenol or a small number of unidentified mono- and sesquiterpenes; however, the emission rates of these compounds in the parental species were also relatively low. In sum, natural Ipomopsis hybrids did not cluster tightly within the odour space defined by the floral volatiles that best distinguish their parental species (Fig. 1). Hybrid flower bouquets differed from the parental species in some individual compounds, with hybrid emission rates not generally intermediate between the parental species (Fig. 2).
We also analysed the relative emission rates of classes of compounds, i.e. the ratios of the compound classes in the floral bouquets, for the two parental species and their hybrids during the day and night (Fig. 3). Relative emissions by the five compound classes differed among plant types (profile analysis, Wilks' Lambda = 0·77864, F8,162 = 2·70, P = 0·0082), because of the difference in the sole nitrogenous compound indole (univariate P = 0·0001). They also differed between day and night (Wilks' Lambda = 0·61077, F4,81 = 12·90, P < 0·0001); however, there was no interaction between time of emission and plant type (profile analysis for interaction, P = 0·3257). In general, at night, plants showed lower emission rates of aliphatic compounds (P < 0·0001 in univariate ANOVA) and higher emission of the sole nitrogenous compound indole (P = 0·0008; Fig. 3), with the other compound classes not showing significant differences (P > 0·05). The class of nitrogenous compounds was the only one for which a significant plant type × time interaction was detected (ANOVA, F2,84 = 5·15, P = 0·0078), reflecting the emission of indole at night only by I. tenuituba and hybrids.
Fig. 3.
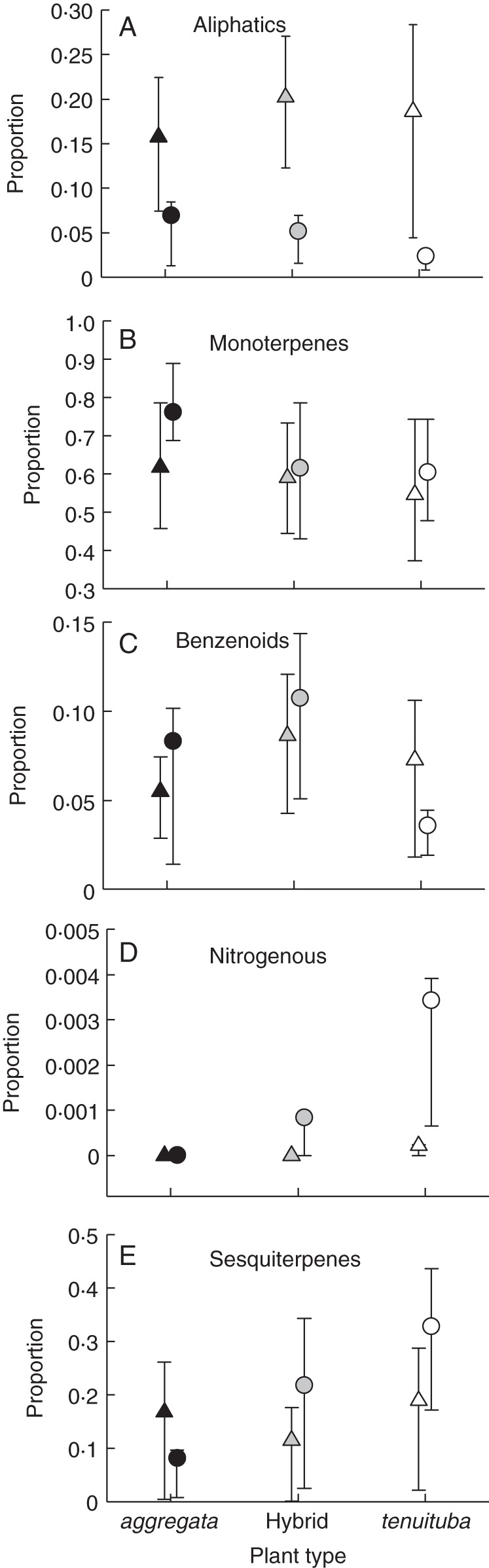
Proportion of floral volatile emissions representing (A) aliphatic compounds, (B) monoterpenes, (C) benzenoids, (D) nitrogenous compounds and (E) sesquiterpenes identified from the flowers of the two parental species Ipomopsis aggregata (black symbols) and I. tenuituba (white) and their natural hybrids (grey), collected during the day (triangles) and the night (circles). Values are means with 95 % confidence intervals, calculated by back-transforming from the arcsin square-root values.
Question 3. Differences in volatile emission between contact sites
When we analysed differences between contact sites and the two species in Supplementary Data Table S2, RF identified six volatiles needed to distinguish the groups (Table 1B). The bootstrap estimate of the prediction error was much smaller than at random (0·25 vs. 0·75). All six of these volatiles were also highly correlated (r > 0·44, n = 64, P < 0·01) with the first or second canonical discriminant function. The first canonical discriminant function was most highly correlated with (E)-sabinene hydrate, also among the most informative at separating groups in the RF analysis (Table 1B). The first two canonical correlations were highly significant at 0·98 (P < 0·0001) and 0·95 (P < 0·01), and together accounted for 90 % of the variance among the four groups. Samples from GR contained more (E)-sabinene hydrate, α-terpineol, α-ylangene, caprolactone, benzyl acetate, linalool and p-anisaldehyde (all uncorrected P < 0·001 for site effect in two-way ANOVA on log-transformed data) but no (E)-β-ocimene. Whereas I. tenuituba emitted (Z)-β-ocimene at both contact sites, I. aggregata bouquets contained that compound only at the PG site with high hybridization. Samples from the two sites were similar in indole (site effect in two-way ANOVA, F1,60 = 0·27, P = 0·6050). Plants from the site without hybrids, GR, did not show a greater difference between the species in indole (site × species interaction, F1,60 = 0·50, P = 0·4836) or α-pinene (P = 0·7828). Thus, samples from the two sites differed in scent profiles, but not in average emission of compounds that were important in defining the overall species difference.
The previously described analysis compared the two sites averaging over day and night. However, hawkmoths typically fly after dusk at GR and during the day at PG, making it of interest to compare species differences specifically between those two scenarios of GR night and PG day emissions. A foraging H. lineata at GR would be exposed to nocturnal emission rates of indole and α-pinene, whereas a day-flying hawkmoth at PG would experience daytime emission patterns. Therefore, the emission rate at the peak of hawkmoth activity needs to be compared. The most abundant component of the bouquet, α-pinene, did not show a significantly greater species difference for night-time GR than for daytime PG samples (interaction P = 0·4707). On the other hand, indole, the key compound separating the species, did show this pattern of interaction (interaction F1,28 = 6·00, P = 0·0208). Thus, hawkmoths would be exposed to a more species-specific indole signal at GR than at PG, given their respective flight patterns.
DISCUSSION
Our study discovered differences in emission of floral volatiles among two species of Ipomopsis and their natural hybrids. When interpreting these scent differences in the following, we employ a logical framework of three tiers to assess the biological relevance of each observed quantitative and qualitative difference in scent emission. A major difference is indicated if one or more compounds originating from a major biochemical pathway is exclusively present in any one group. The presence or absence of individual compounds originating in the same biochemical pathway represents a slightly less pronounced, but still substantial, difference. Quantitative differences in emission rates of shared compounds should be interpreted with caution. Emissions of VOCs are extremely sensitive to variation in the microhabitat (reviewed by Reinhard and Srinivasan, 2009). Therefore, differences in quantitative emission rates of the same compound should only be interpreted as meaningful separators of groups if the consistency of the result has been confirmed through intensive sampling with a rigorous sampling method. Our sampling technique, with Tenax TA® and Carbotrap® as trapping agents and subsequent thermal desorption, allowed for convenient, rapid screening in a remote location and can be considered the superior method for field sampling in difficult terrain. However, this method is semi-quantitative, and differences in emission rates should ideally be quantified using solvent extraction with an internal standard (e.g. Parachnowitsch et al., 2012) or closed-looped stripping (Tholl et al., 2006).
Question 1. Comparison of the parental species
The most striking difference between the volatile bouquets of the closely related I. aggregata and I. tenuituba was the virtually exclusive emission of the nitrogenous compound indole by I. tenuituba after nightfall. Indole is derived from the shikimate pathway and represents the only instance in our system where a major biochemical pathway is consistently active in one of the sister species but not the other. This species-specific pattern of floral scent suggests a potential role for volatiles in the pre-zygotic reproductive isolation between the species. The rhythmic release of scent is often correlated with the temporal activity pattern of the corresponding primary pollinator and may be controlled by a circadian clock or regulated by light (reviewed by Dudareva and Pichersky, 2006). Indole is a documented hawkmoth antennal stimulant, in particular for H. lineata (Raguso et al., 1996). It is often part of the bouquet of sphingid-pollinated flowers along with oxygenated terpenoids and benzenoid esters (Dobson, 2006), and there is phylogenetic evidence for the regular co-occurrence of those compound classes (Levin et al., 2003). The night-time indole signal of I. tenuituba may be part of a suite of traits including narrow corolla tubes (Campbell et al., 1997) favoured by H. lineata. It has been shown that foraging hawkmoths require odour to elicit the proboscis extension reflex (Raguso and Willis, 2002). We hypothesize that indole may be required for hawkmoth feeding, and the next step is to test behavioural responses of the hawkmoths to addition of various amounts of this scent compound to Ipomopsis flowers.
In addition to the difference in indole emission rates, I. aggregata and I. tenuituba exhibited qualitative differences in a small number of compounds that originated from the same biochemical pathway; however, the emission rates of the compounds in question were so low that they represented much less than 1 % of the total bouquet. Overall, monoterpenes were the most abundant class of compounds for both of the parental species, accounting for an average 59 % of the bouquet. Our results were consistent with those of Irwin and Dorsett's (2002) earlier qualitative examination of I. aggregata scent with a solid-phase microextraction technique, although our employment of dynamic headspace sampling with a sensitive trapping agent greatly extended the number of compounds detected in the bouquet and allowed quantification of emission rates.
Of the compounds shared between the two Ipomopsis species, the cyclic monoterpene α-pinene stood out as the most abundant compound (except for day samples from GR I. aggregata). Furthermore, α-pinene tripled in emission rate between day and night in I. aggregata. What purpose could this night-time increase serve in a day-blooming, largely hummingbird-pollinated species such as I. aggregata? The emission of terpenoids has traditionally been associated with chemical defence functions (Dudareva and Pichersky, 2006; Junker and Blüthgen, 2010). If α-pinene is acting as a deterrent in I. aggregata, there ought to be one or more potential night-time receivers of the signal. One such receiver might be foraging H. lineata, as α-pinene is a behaviourally active compound for moths (Cunningham et al., 2004). High α-pinene levels may act as a deterrent for foraging moths (Schiestl et al., 2011), since the addition of α-pinene can decrease insect receptor responses and behavioural attractiveness of certain compounds, at least for euglossine bees (Williams and Dodson, 1972; Schiestl and Roubik, 2003). Therefore, the high levels of α-pinene in the nocturnal floral bouquet of I. aggregata could potentially prevent flower visits from H. lineata, which might be advantageous if they are less effective pollinators than the diurnal hummingbirds. However, since α-pinene is also the major compound in bouquets of largely moth-pollinated I. tenuituba, it probably has another function besides acting as a deterrent to hawkmoth pollinators, such as influencing herbivores.
Emission of aliphatics, including a number of compounds also known as green-leaf volatiles, also changed between day and night. In both species of Ipomopsis emissions of aliphatics were higher during the day. Like terpenoids, green-leaf volatiles can be associated with herbivore deterrence (Pichersky and Gershenzon, 2002). The receiver of this potentially deterrent signal might be anthomyiid flies of the species complex Delia (=Hylemya) spp., herbivores with highly specialized oviposition behaviour on Ipomopsis and the related herb Polemonium foliosissimum (Polemoniaceae) (Brody, 1992). The emission of relatively high overall levels of α-pinene in combination with green-leaf volatiles may influence Delia oviposition, a hypothesis that would explain the diurnal increase in aliphatics emission during the day when Delia fly activity peaks.
Question 2. Comparison of volatiles between natural hybrids and the parental species
Floral volatiles of natural hybrids have the potential to contribute to post-zygotic reproductive isolation if they influence pollinator visitation. Our study provided some of the first measurements of floral volatiles of hybrids from a natural hybrid zone. Hybrids of I. aggregata and I. tenuituba at the contact site at PG generally did not show volatile patterns that were intermediate between the parents, except with regard to indole emission. This result is in contrast to the finding of intermediate hybrid bouquets reported from several deceptive orchids (Cozzolino and Widmer, 2005; Salzmann et al., 2007). However, there are several reasons why hybrids might not have intermediate phenotypes, including genetic composition of the hybrids and differences in environmental conditions among sites. Our scent samples probably included advanced generations as well as F1 hybrids. Hybrid fitness is high in this system (Campbell et al., 2008), allowing for complex segregation patterns. We did not find any unique compounds in the bouquets of hybrid flowers, and a small number of compounds were consistently absent compared with the parental bouquets. However, hybrids had mean quantitative emission rates for at least three compounds, including α-pinene, that could be interpreted as transgressive, ranging outside of the means of both parental species. For other compounds, hybrids appeared indistinguishable from one of the parental species.
An important point to consider when interpreting variation in scent emission is the influence of habitat and the conditions on the day of sampling (Adler and Irwin, 2012). Abiotic conditions vary among the sites, with the hybrid site being hotter and drier than either parental site at PG (Campbell et al., 2010). Our results indicate that hybrid floral volatiles depart significantly from those of their parents at our field site. We do not yet know whether the natural hybrids show putative transgressive floral emissions due to differences in environment, genetic effects such as complementary genes or epistasis (see Rieseberg et al., 1999), or selection for hybrids with particular genetic combinations. A follow-up study with common gardens and controlled crosses of F1 and F2 hybrids will be necessary to tease apart the influences of habitat and genetic background. In this study, however, it was our aim to quantify natural variation of the volatiles of the two parental species and their hybrids under field conditions in order to assess the chemical signals a foraging pollinator or seed predator would experience in the wild. Natural Ipomopsis hybrids could act as a conduit for introgression of the indole signal into the I. aggregata population, because backcrossing with the I. aggregata parent is probably common due to the behaviour of hummingbird pollinators (Campbell et al., 2002). How hawkmoths will respond specifically to the hybrid scent bouquets is ultimately difficult to predict, because indole emission was intermediate whereas some other scent compound emissions appeared transgressive. The presence of indole, if it is the primary hawkmoth attractant, could increase visitation to hybrids, increasing their potential as a hybrid bridge and reducing post-zygotic isolation.
Question 3. Comparison of contact sites with low and high hybridization
When comparing the parental species at two contact sites with low and high frequency in natural hybrids, we found significant differences in floral volatiles. However, the species-defining key compounds of the bouquet were similar across both sites, i.e. indole and α-pinene emission rates did not differ significantly. The scent bouquets of plants of the two parental Ipomopsis species growing at PG, the high hybrid frequency site, were not markedly more similar than at GR, the low hybrid frequency site, although a number of other floral traits show this pattern. Flowers from the two parental species at GR are more dissimilar with respect to corolla length and width compared with flowers from PG (Aldridge and Campbell, 2009), and the petal colour of I. tenuituba at GR often is almost pure white, whereas I. tenuituba flowers from PG frequently are pink and thus more similar to the red flowers of I. aggregata. The floral phenotypes appear more distinct at the low hybrid frequency site, consistent with the higher species specificity of flower visitors there (Aldridge and Campbell, 2007). Although we did not detect differences in the level of similarity of the floral bouquets between sites, it is interesting to observe that β-ocimene, a demonstrated hawkmoth antennal stimulant (Raguso et al., 1996), was present in nocturnal bouquets of I. tenuituba at both contact sites, whereas I. aggregata and hybrids emitted the compound at PG but never at GR. This could indicate introgression of a putative hawkmoth attractant into nocturnal I. aggregata bouquets at PG. In the case of β-ocimene, the bouquets of the parental species appear more similar at PG than at GR.
Finally, although the overall species specificity of scent bouquets was not higher at GR, the temporal shift in hawkmoth foraging behaviour from nocturnal activity at GR to diurnal foraging at PG might make the putative indole scent cue important to the difference in hybridization. Indole emission in I. tenuituba is a nocturnal cue, and we found greater species specificity in indole for GR night-time samples than for PG daytime samples. Thus, if indole attracts hawkmoths, it would have the potential to contribute to species-specific foraging much more so at GR than at PG.
In conclusion, the two closely related sister species I. aggregata and I. tenuituba differ in their floral volatiles both qualitatively, with only I. tenuituba producing the compound indole, and quantitatively. Natural hybrids were generally not intermediate in scent production between the parental species, except in emission of the key compound indole. The large qualitative overlap in the bouquets probably reflects a closely shared evolutionary history and therefore phylogenetic constraints (Steiner et al., 2011). The remaining differences between the species, particularly in indole emission, predict that pre-zygotic reproductive isolation in Ipomopsis may depend on floral scent in combination with the more extensively studied flower colour and shape (Melendez-Ackerman and Campbell, 1998; Campbell, 2004), a hypothesis now being tested experimentally. The scent differences between hybrids and the parental species illustrate the potential for floral scents to influence not only pre-zygotic but also post-zygotic reproductive isolation mediated by pollinators.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Kirsten Dales, Michelle Forster and Robert Schaeffer for assistance in the field, Sandy Steenhuisen for help with sample analysis, and Robert Raguso and two anonymous reviewers for helpful comments on a previous version of the manuscript. We also thank the US National Park Service for collection permits. This work was supported by the US National Science Foundation (grant no. DEB-0542876); a Rocky Mountain Biological Laboratory fellowship (to D.C.); the UC Irvine School of Biological Sciences; and the South African Research Foundation (grant no. NRF-70801).
LITERATURE CITED
- Adler LS, Irwin RE. What you smell is more important than what you see? Natural selection on floral scent. New Phytologist. 2012;195:510–511. doi: 10.1111/j.1469-8137.2012.04229.x. [DOI] [PubMed] [Google Scholar]
- Aldridge G. Variation in frequency of hybrids and spatial structure among Ipomopsis (Polemoniaceae) contact sites. New Phytologist. 2005;167:279–288. doi: 10.1111/j.1469-8137.2005.01413.x. [DOI] [PubMed] [Google Scholar]
- Aldridge G, Campbell DR. Variation in pollinator preference between two Ipomopsis contact sites that differ in hybridization rate. Evolution. 2007;61:99–110. doi: 10.1111/j.1558-5646.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- Aldridge G, Campbell DR. Genetic and morphological patterns show variation in frequency of hybrids between Ipomopsis (Polemoniaceae) zones of sympatry. Heredity. 2009;102:257–265. doi: 10.1038/hdy.2008.112. [DOI] [PubMed] [Google Scholar]
- Amirav A, Dagan S. A direct sample introduction device for mass spectrometry studies and gas chromatography mass spectrometry analyses. European Mass Spectrometry. 1997;3:105–111. [Google Scholar]
- Ayasse M, Stökl J, Francke W. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry. 2011;72:1667–1677. doi: 10.1016/j.phytochem.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Brody AK. Oviposition choices by a predispersal seed predator (Hylemya Sp). 1 Correspondence with hummingbird pollinators, and the role of plant size, density and floral morphology. Oecologia. 1992;91:56–62. doi: 10.1007/BF00317241. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Natural selection in Ipomopsis hybrid zones: implications for ecological speciation. New Phytologist. 2004;161:83–90. [Google Scholar]
- Campbell DR, Waser NM. Evolutionary dynamics of an Ipomopsis hybrid zone: confronting models with lifetime fitness data. American Naturalist. 2007;169:298–310. doi: 10.1086/510758. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Melendez-Ackerman EJ. Analyzing pollinator-mediated selection in a plant hybrid zone: hummingbird visitation patterns on three spatial scales. American Naturalist. 1997;149:295–315. [Google Scholar]
- Campbell DR, Waser NM, Pederson GT. Predicting patterns of mating and potential hybridization from pollinator behavior. American Naturalist. 2002;159:438–450. doi: 10.1086/339457. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Aldridge G, Wu CA. Lifetime fitness in two generations of Ipomopsis hybrids. Evolution. 2008;62:2616–2627. doi: 10.1111/j.1558-5646.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Wu CA, Travers SE. Photosynthetic and growth responses of reciprocal hybrids to variation in water and nitrogen availability. American Journal of Botany. 2010;97:925–933. doi: 10.3732/ajb.0900387. [DOI] [PubMed] [Google Scholar]
- Cao KL, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortis P, Vereecken NJ, Schiestl FP, Lumaga MRB, Scrugli A, Cozzolino S. Pollinator convergence and the nature of species' boundaries in sympatric Sardinian Ophrys (Orchidaceae) Annals of Botany. 2009;104:497–506. doi: 10.1093/aob/mcn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino S, Widmer A. The evolutionary basis of reproductive isolation in Mediterranean orchids. Taxon. 2005;54:977–985. [Google Scholar]
- Cunningham JP, Moore CJ, Zalucki MP, West SA. Learning, odour preference and flower foraging in moths. Journal of Experimental Biology. 2004;207:87–94. doi: 10.1242/jeb.00733. [DOI] [PubMed] [Google Scholar]
- Cutler DR, Edwards TC, Jr, Beard KH, et al. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- Dobson H. Relationship between floral fragrance composition and type of pollinator. In: Dudareva NA, Pichersky E, editors. Biology of floral scent. Boca Raton, FL: CRC/Taylor & Francis; 2006. pp. 147–198. [Google Scholar]
- Dötterl S, Wolfe LM, Jürgens A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry. 2005;66:203–213. doi: 10.1016/j.phytochem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jürgens A, Wolfe L, Biere A. Disease status and population origin effects on floral scent: potential consequences for oviposition and fruit predation in a complex interaction between a plant, fungus, and noctuid moth. Journal of Chemical Ecology. 2009;35:307–319. doi: 10.1007/s10886-009-9601-0. [DOI] [PubMed] [Google Scholar]
- Dudareva NA, Pichersky E. Floral scent metabolic pathways: their regulation and evolution. In: Dudareva NA, Pichersky E, editors. Biology of floral scent. Boca Raton, FL: CRC/Taylor & Francis; 2006. pp. 55–78. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Frey FM. Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae) Evolution. 2004;58:2426–2437. doi: 10.1111/j.0014-3820.2004.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society B: Biological Sciences. 1999;266:2247–2252. [Google Scholar]
- Galen C, Cuba J. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution. 2001;55:1963–1971. doi: 10.1111/j.0014-3820.2001.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Gordin A, Amirav A. SnifProbe: new method and device for vapor and gas sampling. Journal of Chromatography A. 2000;903:155–172. doi: 10.1016/s0021-9673(00)00877-3. [DOI] [PubMed] [Google Scholar]
- Grant V. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences, USA. 1994;91:3–10. doi: 10.1073/pnas.91.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V, Grant KA. Flower pollination in the Phlox family. New York: Columbia University Press; 1965. [Google Scholar]
- Grant V, Wilken DH. Natural hybridization between Ipomopsis aggregata and Ipomopsis tenuituba (Polemoniaceae) Botanical Gazette. 1988;149:213–221. [Google Scholar]
- Hossaert-McKey M, Soler C, Schatz B, Proffit M. Floral scents: their roles in nursery pollination mutualisms. Chemoecology. 2010;20:75–88. [Google Scholar]
- Huberty CJ, Olejnik S. Applied MANOVA and discriminant analysis. Hoboken, NJ: Wiley & Sons; 2006. [Google Scholar]
- Ippolito A, Fernandes GW, Holtsford TP. Pollinator preferences for Nicotiana alata, N. forgetiana, and their F-1 hybrids. Evolution. 2004;58:2634–2644. doi: 10.1111/j.0014-3820.2004.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Dorsett B. Volatile production by buds and corollas of two sympatric, confamilial plants, Ipomopsis aggregata and Polemonium foliosissimum. Journal of Chemical Ecology. 2002;28:565–578. doi: 10.1023/a:1014596129601. [DOI] [PubMed] [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 295–310. [Google Scholar]
- Junker RR, Blüthgen N. Floral scents repel facultative flower visitors, but attract obligate ones. Annals of Botany. 2010;105:777–782. doi: 10.1093/aob/mcq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U, Gurba A, Hermann K, et al. Pollinator choice in Petunia depends on two major genetic loci for floral scent production. Current Biology. 2011;21:730–739. doi: 10.1016/j.cub.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Levin RA, McDade LA, Raguso RA. The systematic utility of floral and vegetative fragrance in two genera of Nyctaginaceae. Systematic Biology. 2003;52:334–351. doi: 10.1080/10635150390196975. [DOI] [PubMed] [Google Scholar]
- Majetic CJ, Rausher MD, Raguso RA. The pigment–scent connection: do mutations in regulatory vs structural anthocyanin genes differentially alter floral scent production in Ipomoea purpurea? South African Journal of Botany. 2010;76:632–642. [Google Scholar]
- Melendez-Ackerman E, Campbell DR. Adaptive significance of flower color and inter-trait correlations in an Ipomopsis hybrid zone. Evolution. 1998;52:1293–1303. doi: 10.1111/j.1558-5646.1998.tb02011.x. [DOI] [PubMed] [Google Scholar]
- Melendez-Ackerman E, Campbell DR, Waser NM. Hummingbird behavior and mechanisms of selection on flower color in Ipomopsis. Ecology. 1997;78:2532–2541. [Google Scholar]
- Parachnowitsch AL, Raguso RA, Kessler A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytologist. 2012;195:667–675. doi: 10.1111/j.1469-8137.2012.04188.x. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Porter JM, Johnson LA, Wilken D. Phylogenetic systematics of Ipomopsis (Polemoniaceae): relationships and divergence times estimated from chloroplast and nuclear DNA sequences. Systematic Botany. 2010;35:181–200. [Google Scholar]
- Raguso RA. Start making scents: the challenge of integrating chemistry into pollination ecology. Entomologia Experimentalis et Applicata. 2008;128:196–207. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by naive hawkmoths, Manduca sexta. Animal Behaviour. 2002;64:685–695. [Google Scholar]
- Raguso RA, Willis MA. Hawkmoth pollination in Arizona's Sonoran Desert: behavioral responses to floral traits. In: Boggs CL, Watt WB, Ehrlich PR, editors. Butterflies: ecology and evolution taking flight. Chicago: University of Chicago Press; 2003. pp. 43–65. [Google Scholar]
- Raguso RA, Light DM, Pickersky E. Electroantennogram responses of Hyles lineata (Sphingidae: Lepidoptera) to volatile compounds from Clarkia breweri (Onagraceae) and other moth-pollinated flowers. Journal of Chemical Ecology. 1996;22:1735–1766. doi: 10.1007/BF02028502. [DOI] [PubMed] [Google Scholar]
- Ranganathan Y, Borges RM. Reducing the babel in plant volatile communication: using the forest to see the trees. Plant Biology. 2010;12:735–742. doi: 10.1111/j.1438-8677.2009.00278.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/ [Google Scholar]
- Reinhard J, Srinivasan S. The role of scents in honeybee foraging and recruitment. In: Hrincir M, Jarau S, editors. Food exploitation by social insects. New York, NY: CRC Press; 2009. pp. 165–182. [Google Scholar]
- Rieseberg LH, Archer MA, Wayne RK. Transgressive segregation, adaptation and speciation. Heredity. 1999;83:363–372. doi: 10.1038/sj.hdy.6886170. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Kim SC, Randell RA, et al. Hybridization and the colonization of novel habitats by annual sunflowers. Genetica. 2007;129:149–165. doi: 10.1007/s10709-006-9011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann CC, Cozzolino S, Schiestl FP. Floral scent in food-deceptive orchids: species specificity and sources of variability. Plant Biology. 2007;9:720–729. doi: 10.1055/s-2007-965614. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS version 9·2. Cary, NC, USA: SAS Institute; 2008. [Google Scholar]
- Schemske DW, Bradshaw HD. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences, USA. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Roubik DW. Odor compound detection in male euglossine bees. Journal of Chemical Ecology. 2003;29:253–257. doi: 10.1023/a:1021932131526. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. The chemistry of sexual deception in an orchid–wasp pollination system. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Huber FK, Gomez JM. Phenotypic selection on floral scent: trade-off between attraction and deterrence? Evolutionary Ecology. 2011;25:237–248. [Google Scholar]
- Schlumpberger BO, Raguso RA. Geographic variation in floral scent of Echinopsis ancistrophora (Cactaceae); evidence for constraints on hawkmoth attraction. Oikos. 2008;117:801–814. [Google Scholar]
- Shuttleworth A, Johnson SD. The importance of scent and nectar filters in a specialized wasp-pollination system. Functional Ecology. 2009;23:931–940. [Google Scholar]
- Shuttleworth A, Johnson SD. The missing stink: sulphur compounds can mediate a shift between fly and wasp pollination systems. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2811–2819. doi: 10.1098/rspb.2010.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner KE, Kaiser R, Dötterl S. Strong phylogenetic effects on floral scent variation of oil-secreting orchids in South Africa. American Journal of Botany. 2011;98:1663–1679. doi: 10.3732/ajb.1100141. [DOI] [PubMed] [Google Scholar]
- Strauss S, Whittall J. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 120–138. [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Röse USR, Schnitzler JP. Practical approaches to plant volatile analysis. The Plant Journal. 2006;45:540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- Vereecken NJ, Cozzolino S, Schiestl FP. Hybrid floral scent novelty drives pollinator shift in sexually deceptive orchids. BMC Evolutionary Biology. 2010;10:103. doi: 10.1186/1471-2148-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti MO, Muhlemann JK, Widmer A, Schiestl FP. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology. 2008;21:111–121. doi: 10.1111/j.1420-9101.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Whitehead MR, Peakall R. Integrating floral scent, pollination ecology and population genetics. Functional Ecology. 2009;23:863–874. [Google Scholar]
- Whitney KD, Ahern JR, Campbell LG, Albert LP, King MS. Patterns of hybridization in plants. Perspectives in Plant Ecology, Evolution and Systematics. 2010;12:175–182. [Google Scholar]
- Williams NH, Dodson CH. Selective attraction of male euglossine bees to orchid floral fragrances and its importance in long-distance pollen flow. Evolution. 1972;26:84–95. doi: 10.1111/j.1558-5646.1972.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Wu CA, Campbell DR. Cytoplasmic and nuclear markers reveal contrasting patterns of spatial genetic structure in a natural Ipomopsis hybrid zone. Molecular Ecology. 2005;14:781–792. doi: 10.1111/j.1365-294X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- Xu S, Schlüter PM, Schiestl FP. Pollinator-driven speciation in sexually deceptive orchids. International Journal of Ecology. 2012 Article ID 285081. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.