Abstract
Background
At the time of the initial analysis of overall survival (OS) for the Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM) randomized, double-blind, phase III trial, approximately 50% of patients had died. A final analysis of OS was subsequently planned for when 75% of patients had died.
Methods
Patients were randomly assigned 1:1 to fulvestrant 500 mg administered as two 5-mL intramuscular injections on days 0, 14, and 28 and every 28 (±3) days thereafter or fulvestrant 250 mg administered as two 5-mL intramuscular injections (one fulvestrant and one placebo [identical in appearance to study drug]) on days 0, 14 (two placebo injections only), and 28 and every 28 (±3) days thereafter. OS was analyzed using an unadjusted log-rank test. No adjustments were made for multiplicity. Serious adverse events (SAEs) and best response to subsequent therapy were also reported. All statistical tests were two-sided.
Results
In total, 736 women (median age = 61.0 years) were randomly assigned to fulvestrant 500mg (n = 362) or 250mg (n = 374). At the final survival analysis, 554 of 736 (75.3%) patients had died. Median OS was 26.4 months for fulvestrant 500mg and 22.3 months for 250mg (hazard ratio = 0.81; 95% confidence interval = 0.69–0.96; nominal P = .02). There were no clinically important differences in SAE profiles between the treatment groups; no clustering of SAEs could be detected in either treatment group. Type of first subsequent therapy and objective responses to first subsequent therapy were well balanced between the two treatment groups.
Conclusions
In patients with locally advanced or metastatic estrogen receptor–positive breast cancer, fulvestrant 500mg is associated with a 19% reduction in risk of death and a 4.1-month difference in median OS compared with fulvestrant 250mg. Fulvestrant 500mg was well tolerated, and no new safety concerns were identified.
Fulvestrant is a pure estrogen receptor (ER) antagonist devoid of the agonistic properties displayed by tamoxifen in some tissues (1–4). After phase III studies, which demonstrated similar efficacy and an acceptable safety profile for fulvestrant 250mg compared with anastrozole (1,5), fulvestrant 250mg was approved as treatment in postmenopausal women with advanced hormone receptor–positive breast cancer that had progressed or recurred after prior antiestrogen therapy. However, previous preoperative studies showed that short-term exposure to fulvestrant was associated with a dose-dependent reduction in the levels of ER, progesterone receptor, and the cell proliferation–related antigen Ki67 (6,7) for fulvestrant doses up to 250mg. Other phase I and phase III studies also suggested a dose–response effect for fulvestrant (1,5,8).
The phase III Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM) trial compared the then-approved dose and dosing schedule of fulvestrant (250mg every 28 days) with a higher-dose regimen (500mg every 28 days plus an additional 500mg on day 14 of the first month only) in postmenopausal women with locally advanced or metastatic ER-positive breast cancer that had recurred or progressed after prior endocrine therapy. The initial results showed that fulvestrant 500mg was associated with a statistically significant increase in progression-free survival (PFS) without increased toxicity, therefore corresponding to a clinically meaningful improvement in benefit vs risk compared with fulvestrant 250mg (9). Based on these data, the 500-mg dose of fulvestrant is now the approved dose in the European Union (approved in March 2010), United States (approved in September 2010), Japan (approved in November 2011), and other countries worldwide.
In the CONFIRM study, the assessment of the therapeutic efficacy of both doses of fulvestrant was evaluated by several secondary outcome measures, including overall survival (OS). At the time of the initial analysis, approximately 50% of patients had died. After the reporting of the 50% survival data, which showed a trend in favor of 500mg over 250mg, it was agreed to perform a final survival analysis after 75% of patients had died. Here we report the results of this final OS analysis.
Methods
Study Design and Patients
The CONFIRM study design, including eligibility criteria, exclusion criteria, and the calculation of sample size, has been described in detail elsewhere (9). Briefly, CONFIRM was a randomized, phase III, double-blind trial that evaluated two different doses of fulvestrant (500mg vs 250mg) in postmenopausal patients who had either locally advanced or metastatic ER-positive breast cancer (ClinicalTrials.gov identifier: NCT00099437; http://www.clinicaltrials.gov/ct2/show/NCT00099437). The primary study endpoint was PFS (the time elapsing between the date of randomization and the date of earliest evidence of objective disease progression or death from any cause). Secondary endpoints included objective response rate, clinical benefit rate, duration of response, duration of clinical benefit, OS, tolerability, and quality of life (9).
After initial analysis, all patients, regardless of whether they were still receiving randomized treatment, entered a survival follow-up phase. Patients remaining on randomized treatment during this follow-up phase continued on blinded randomized treatment until progression and were assessed for serious adverse events (SAEs) and survival status. Patients who had discontinued randomized treatment were assessed for their survival status and best response to their first subsequent systemic breast cancer therapy received after treatment discontinuation.
Ethics
The study was performed in accordance with the Declaration of Helsinki, consistent with International Conference on Harmonisation/Good Clinical Practice requirements. All patients gave written informed consent before study entry, and the study protocol was approved by the institutional review board of each participating institution.
Randomization and Masking
Patients were randomly assigned to treatment in balanced blocks using a computer-generated randomization schedule; all study personnel were blinded to randomized treatment. Eligible patients were randomly assigned 1:1 to either fulvestrant 500mg administered as two 5-mL intramuscular injections on days 0, 14, and 28 and every 28 (± 3) days thereafter or fulvestrant 250mg administered as two 5-mL intramuscular injections (one fulvestrant and one placebo [identical in appearance to study drug]) on days 0, 14 (two placebo injections only), and 28, and every 28 (± 3) days thereafter (9).
Fulvestrant was supplied in the form of a single dose in a prefilled syringe. Each active prefilled syringe contained 250mg of fulvestrant at a concentration of 50mg/mL in a volume of 5mL, designated fulvestrant 5% weight/volume injection. The placebo prefilled syringe was identical to the active prefilled syringe and also had a volume of 5mL.
Survival analysis
OS was defined as the number of days from randomization to death from any cause. Patients who died after the data cutoff or who were known to be alive after the data cutoff were right-censored at the date of the data cutoff. Patients who were last known to be alive before the data cutoff or who were lost to follow-up before the data cutoff were right-censored at the date they were last known to be alive.
After the initial analysis, patients on fulvestrant 250mg were permitted to switch to 500mg before entering the survival follow-up phase. Irrespective of whether they were still receiving randomized treatment, all patients in the follow-up phase continued to have their survival status monitored every 12±2 weeks until cutoff for the final 75% OS analysis (October 31, 2011).
Best Response to First Subsequent Therapy
Details of the first subsequent systemic breast cancer therapy received after discontinuation of randomized treatment, and of the best response (complete response, partial response, stable disease, progressive disease, not evaluable) to this therapy were collected.
Tolerability
SAEs were reported to the Patient Safety Database and collated during the survival follow-up phase for those patients still receiving randomized treatment.
Statistical Analysis
OS was first analyzed in 2009, in parallel with the primary analysis of PFS, after the proportion of reported deaths exceeded 50% of the total number of patients randomized across the two treatment groups. The analysis was performed using an unadjusted log-rank test. An additional exploratory analysis, which used a Cox proportional hazards model adjusting for six predefined covariables (age at baseline, response to last endocrine therapy received before fulvestrant, receptor status at diagnosis, visceral involvement at baseline, last therapy before fulvestrant, and measurable disease at baseline) was also performed to assess the robustness of the unadjusted OS result.
An updated analysis is presented here of more mature survival data, performed after the proportion of reported deaths exceeded 75% of the total number of patients randomized across the two treatment groups. The data were analyzed using log-rank statistics, confirmed by Cox proportional hazards model, and summarized by the method of Kaplan–Meier. P values presented are nominal without adjustment for multiplicity, and no alpha was retained for this analysis (the 5% error was used at the initial OS analysis). All statistical tests were two-sided.
For SAEs, summaries and analyses were prepared according to the treatment actually received.
Results
Patients
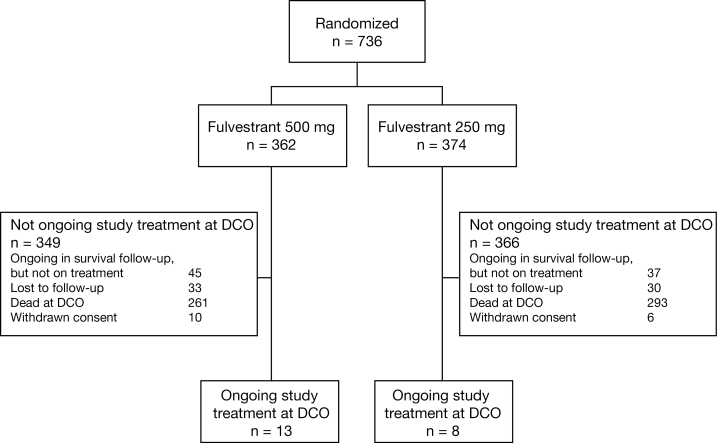
In total, 736 women (median age = 61.0 years) were randomly assigned between February 2005 and August 2007 from 128 centers in 17 countries (Belgium, Brazil, Chile, Colombia, Czech Republic, Hungary, India, Italy, Malta, Mexico, Poland, Russia, Slovakia, Spain, the United States, Ukraine, and Venezuela) (fulvestrant 500 mg: n = 362; fulvestrant 250 mg: n = 374) (Figure 1). Baseline patient and tumor characteristics, reported previously, were comparable between the treatment groups (9). At the time of the final analysis, 63 patients (8.6%) were lost to follow-up, 16 patients (2.2%) had withdrawn consent, 103 patients (14.0%) were still being followed up (n = 21 [2.9%] on treatment; n = 82 [11.1%] not on treatment), and 554 patients (75.3%) had died.
Figure 1.
CONSORT diagram. DCO = data cutoff.
For 34 of the 736 patients (4.6%), fulvestrant dose was unblinded after progression to the study drug.
Eight patients (2.1%) crossed over from fulvestrant 250mg to fulvestrant 500mg.
Survival Analysis
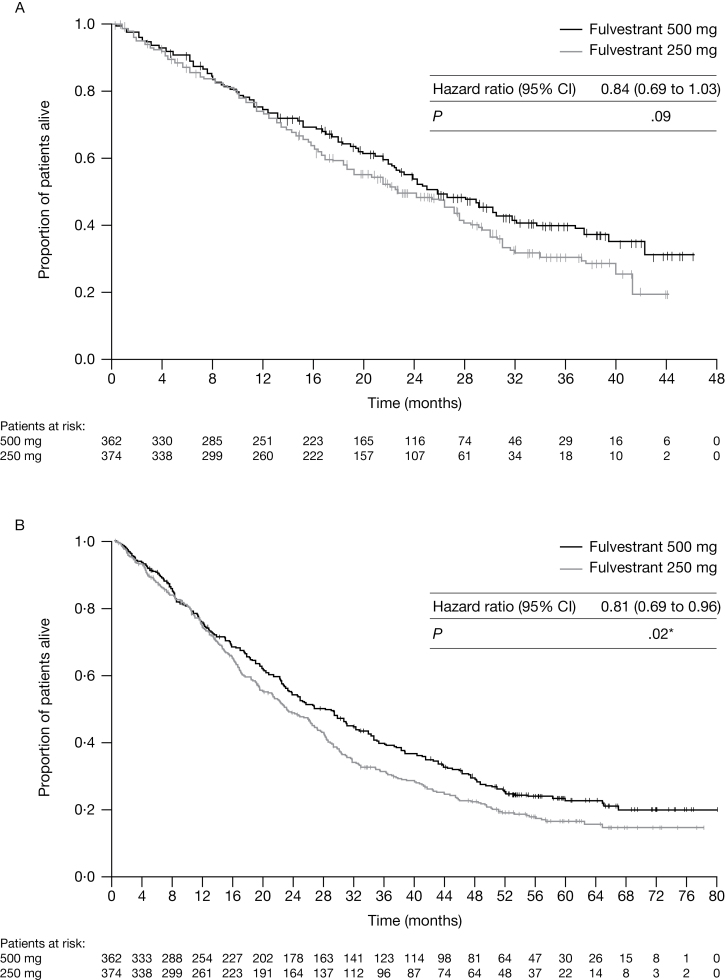
At the initial data cutoff, 378 of 736 patients (51.4%) had died (n = 175 [48.3%] in the fulvestrant 500mg group; n = 203 [54.3%] in the fulvestrant 250mg group) (Table 1). There was a trend for improved OS for patients in the fulvestrant 500mg group compared with those in the fulvestrant 250mg group (25.1 months vs 22.8 months, respectively; hazard ratio (HR) = 0.84, 95% confidence interval (CI) = 0.69 to 1.03, P = .09 for the unadjusted analysis; HR = 0.81, 95% CI = 0.66 to 1.00, P = .049 for the retrospective adjusted analysis) (Table 1; Figure 2A).
Table 1.
Summary of overall survival*
| Information on overall survival | Initial analysis (50% survival analysis) | Update (75% survival analysis) | ||
|---|---|---|---|---|
| Fulvestrant 500mg (n = 362) | Fulvestrant 250mg (n = 374) | Fulvestrant 500mg (n = 362) | Fulvestrant 250mg (n = 374) | |
| No. died (%) | 175 (48.3) | 203 (54.3) | 261 (72.1) | 293 (78.3) |
| Median time to death, mo | 25.1 | 22.8 | 26.4 | 22.3 |
| Median time to death, d | 764 | 693 | 805 | 679 |
| Time to death, mo: 25% percentile | 12.2 | 11.5 | 11.7 | 11.5 |
| Time to death, mo: 75% percentile | NC | 41.7 | 51.1 | 41.7 |
* NC = not calculable.
Figure 2.
Overall survival from date of randomization. A) Overall survival for when 50% of patients had died. B) Overall survival for when 75% of patients had died. Analysis by log-rank test. P values are two-sided. *No adjustments for multiplicity were made. Tick marks indicate censored observations. CI = confidence interval. © 2010 American Society of Clinical Oncology. All rights reserved (9).
At the final survival update, 554 of 736 patients (75.3%) had died (n = 261 [72.1%] in the fulvestrant 500mg group; n = 293 [78.3%] in the fulvestrant 250mg group) (Table 1). There was continued separation of the survival curves for fulvestrant 500mg compared with fulvestrant 250mg. The median time to death for patients in the fulvestrant 500mg group vs the fulvestrant 250mg group was 26.4 months vs 22.3 months, respectively (HR = 0.81, 95% CI = 0.69 to 0.96, nominal P = .02 for the unadjusted analysis; HR = 0.79, 95% CI = 0.67 to 0.94, nominal P = .007 for the adjusted analysis) (Table 1; Figure 2B).
No statistically significant interaction was observed between the six predefined variables indicated in the Method section and fulvestrant activity (global interaction test P = .62), indicating that the overall treatment effect was consistent across the predefined covariables.
Best Response to First Subsequent Therapy
Information on first subsequent therapies was available for 230 (63.5%) and 239 (63.9%) patients treated with fulvestrant 500mg or 250mg, respectively. Best response to subsequent therapy is detailed in Table 2. For those randomized patients who had subsequent therapy, response to subsequent therapies was similar between treatment groups: 8.3% vs 8.4% of patients had either complete response or partial response in the fulvestrant 500mg vs 250mg groups, respectively; 24.8% and 32.2% of patients had stable disease in the fulvestrant 500mg vs 250mg groups, respectively; and 33.5% and 28.5% of patients had progressive disease in the fulvestrant 500mg vs 250mg groups, respectively.
Table 2.
Best response to subsequent therapy*
| Available information on first subsequent therapy | Fulvestrant 500mg (n = 362) | Fulvestrant 250mg (n = 374) |
|---|---|---|
| 230 | 239 | |
| Category of subsequent therapy, No. | ||
| Radiotherapy | 8 | 8 |
| Endocrine therapy | 80 | 74 |
| Chemotherapy | 135 | 142 |
| HER2 directed | 0 | 1 |
| Unknown/other | 3 | 5 |
| Fulvestrant† | 4 | 9 |
| Best response to subsequent therapy, No. (%) | ||
| Complete response | 2 (0.9) | 0 |
| Partial response | 17 (7.4) | 20 (8.4) |
| Stable disease | 57 (24.8) | 77 (32.2) |
| Progressive disease | 77 (33.5) | 68 (28.5) |
| Not evaluable | 77 (33.5) | 74 (31.0) |
* Subsequent endocrine therapy included: anastrozole, exemestane, letrozole, medroxy progesterone, megestrol acetate, and tamoxifen. HER2 = human epidermal growth factor receptor 2.
† Fulvestrant was either given at a dose of 250mg or the dose was not specified.
Tolerability
A summary of patients with an SAE during the entire treatment period (main trial plus follow-up phase) is shown in Table 3. During the entire treatment period, a total of 35 (9.7%) and 27 (7.2%) patients had at least one SAE in the fulvestrant 500mg and fulvestrant 250mg groups, respectively. SAEs that were causally related to study treatment were reported for eight (2.2%) and four (1.1%) patients, and SAEs with an outcome of death were reported for five (1.4%) and seven (1.9%) patients in the fulvestrant 500mg and fulvestrant 250mg groups, respectively, during the entire treatment period. Overall, there were no clinically important differences in the profiles of SAEs between the treatment groups, and no clustering of SAEs could be detected in either treatment group.
Table 3.
Summary of patients experiencing SAEs during the treatment period*
| Available information on SAEs | No. of patients (%) | |
|---|---|---|
| Fulvestrant 500mg (n = 361) | Fulvestrant 250mg (n = 374) | |
| Patients with at least 1 SAE during the whole trial | ||
| Any SAE | 35 (9.7) | 27 (7.2) |
| Any SAE with outcome other than death† | 32 (8.9) | 22 (5.9) |
| Any causally related SAE | 8 (2.2) | 4 (1.1) |
| SAEs occurring in >1 patient | ||
| Acute myocardial infarction | 0 (0) | 2 (0.5) |
| Anemia | 3 (0.8) | 1 (0.3) |
| Bronchitis | 2 (0.6) | 0 (0) |
| Dyspnea | 2 (0.6) | 1 (0.3) |
| Femur fracture | 1 (0.3) | 2 (0.5) |
| Hyperglycemia | 2 (0.6) | 0 (0) |
| Pneumonia | 2 (0.6) | 0 (0) |
| Vomiting | 2 (0.6) | 1 (0.3) |
| SAEs with outcome of death, preferred term | ||
| Acute myocardial infarction | 0 (0) | 2 (0.5) |
| Acute renal failure | 0 (0) | 1 (0.3) |
| Aspiration | 0 (0) | 1 (0.3) |
| Cardiopulmonary failure | 1 (0.3) | 0 (0) |
| Suicide | 0 (0) | 1 (0.3) |
| Death, cause unknown | 1 (0.3) | 0 (0) |
| Dyspnea | 2 (0.6) | 0 (0) |
| Hypertension | 0 (0) | 1 (0.3) |
| Intestinal adenocarcinoma | 1 (0.3) | 0 (0) |
| Meningitis | 0 (0) | 1 (0.3) |
* SAEs = serious adverse events.
† All patients experiencing an SAE with nonfatal outcome (regardless of whether they later had a fatal SAE).
Discussion
Preclinical and preliminary clinical data prompted the activation of the CONFIRM trial comparing fulvestrant 500mg with fulvestrant 250mg in postmenopausal patients with ER-positive advanced breast cancer (1,5,6,10). The PFS analysis (primary study endpoint of the CONFIRM trial) demonstrated the superiority of 500mg over 250mg (9). At the time of the PFS analysis, a first OS analysis was also performed, and approximately 50% of events had been reported. The OS analysis suggested a numerical trend in favor of 500mg over 250mg despite the lack of a statistically significant difference (9). This observed numerical trend favoring fulvestrant 500mg led to a decision by the study Steering Committee to plan for a second OS analysis at 75% maturity.
This article reports the results of the final 75% OS analysis and suggests that fulvestrant 500mg is superior to fulvestrant 250mg, with a 19% relative reduction in the risk of death and a 4.1-month increase in median OS. However, a limitation of this study is that the 75% OS analysis is considered exploratory because it was planned after the results of the PFS and 50% OS events analyses were available; accordingly, no alpha was retained for this analysis and no adjustment for multiplicity was possible. Nevertheless, the reported results are consistent with the previously reported PFS and 50% OS events results (9).
In the attempt to rule out the hypothesis that the observed difference in OS in favor of fulvestrant 500mg was mainly the consequence of an imbalance in subsequent therapies delivered after progression on the study drug, an investigation of first subsequent therapies after progression on fulvestrant was carried out. Information on first subsequent therapies was available for approximately two-thirds (64%) of the study population, with 153 and 165 patients treated with fulvestrant 500mg and 250mg, respectively, evaluable for best response. The findings show that there was no imbalance in type of first subsequent therapies given after progression on fulvestrant. Last but not least, the analysis shows that the objective response rate and stable disease rate for first subsequent therapies are very similar between the two treatment groups. In summary, the analysis on first subsequent therapies suggests that the observed improvement in OS in favor of the 500mg dose was not due to an imbalance in subsequent treatments delivered after progression on fulvestrant.
An additional investigation carried out in this study focused on the cross-over rate for patients initially treated with 250mg. The study design did not initially allow for a cross-over after progression on fulvestrant 250mg. However, when the PFS results were available, the study protocol was amended, and patients on treatment with 250mg were offered the option to cross over to 500mg. Most patients had already progressed on fulvestrant by the time the PFS results were available and the study protocol had been amended. Accordingly, fulvestrant dose was unblinded after progression to the study drug for only 34 of the 736 patients. Twenty-four patients were eligible for crossover (per protocol amendment), but the actual cross-over rate was low, with only eight of 374 patients (2.1%) receiving fulvestrant 500mg after prior treatment with 250mg. Considering that there is no clinical evidence on the activity of fulvestrant 500mg in patients pretreated with 250mg, it seems unlikely that the suggested OS benefit of 500mg over 250mg is due to the low cross-over rate in this trial.
With regard to the safety profile, the reported results do not support any clinically relevant difference either in the rate or causality of related SAEs between the two treatment groups. Furthermore, the number of SAEs with an outcome of death was very similar between the two groups (five events for the 500mg group vs seven events for the 250mg group). In addition, the 500-mg safety profile reported in this article is comparable with the safety profile of the same dose observed at the time of the PFS analysis.
The results of this study raise a number of questions that need to be addressed in future trials. Is fulvestrant given at the 500mg dose a better option than aromatase inhibitors as a first-line therapy for postmenopausal patients with ER-positive advanced breast cancer? Results from a phase II randomized trial appear to suggest that this might be the case (11,12). A phase III trial (the FALCON trial) is currently ongoing (ClinicalTrials.gov identifier: NCT01602380) in an attempt to address this question.
A trial recently reported by the Southwest Oncology Group (SWOG) has suggested that a poly-endocrine therapy approach, consisting of the combination of fulvestrant 250mg with an aromatase inhibitor, is superior to single-agent treatment with the same aromatase inhibitor (13). No clinical data on the comparison between the poly-endocrine therapy and fulvestrant 500mg are available. Ideally, it would be important to have markers driving our treatment decisions when a first-line endocrine therapy approach has to be started. Unfortunately, no markers are currently available to support our treatment strategies. Interestingly, a subgroup analysis run in the context of the SWOG trial seems to suggest that most of the benefit from poly-endocrine therapy is observed in patients with no prior exposure to endocrine therapy, either in the adjuvant or in the advanced setting (13). This subgroup analysis might explain the contradictory results reported in another poly-endocrine therapy trial (the Fulvestrant and Anastrozole Combination Therapy [FACT] trial) whose design completely overlaps with the SWOG trial but where a significantly higher proportion of patients were previously exposed to endocrine therapies (14,15).
Last but not least, our results provide a rationale that if fulvestrant is to be combined with other nonendocrine agents targeting molecular pathways involved in the induction of primary or secondary endocrine resistance, then the dose should be 500mg. Results of the Breast Cancer Trials of Oral Everolimus 2 (BOLERO-2) show that the combination of an endocrine treatment with a compound targeting the PI3K pathway can improve the antitumor activity of single-agent endocrine therapy (16). Fulvestrant might be an ideal partner for future combination studies considering that its unique mechanism of action leads to ER downregulation, thus preventing not only the ER-mediated transcription of several genes but also the cross-talks between cytoplasmic ER and several downstream effectors of molecular pathways involved in resistance to endocrine therapies (17,18).
In summary, based on the final results of the CONFIRM trial, which suggest an OS benefit for fulvestrant 500mg over 250mg, and taking into account that the previously reported PFS results were statistically significantly in favor of fulvestrant 500mg, we believe that whenever treatment with fulvestrant should be initiated, this should be at the dose of 500mg, according to the same schedule of this trial.
Funding
This study was designed and funded by AstraZeneca Pharmaceuticals, Macclesfield, UK, which was involved in the reviewing and interpretation of data, the writing of the manuscript, and the decision to submit for publication.
A. Di Leo, S. Garnett, and M. Martin were responsible for conception and design of the study. A. Di Leo, G. Jerusalem, L. Petruzelka, R. Torres, I. N. Bondarenko, R. Khasanov, D. Verhoeven, J. L. Pedrini, I. Smirnova, M. R. Lichinitser, K. Pendergrass, and M. Martin were responsible for provision of study materials or patients. S. Garnett and Y. Rukazenkov were responsible for collection and assembly of data. A. Di Leo, G. Jerusalem, L. Petruzelka, R. Torres, I. N. Bondarenko, R. Khasanov, D. Verhoeven, J. L. Pedrini, I. Smirnova, M. R. Lichinitser, K. Pendergrass, L. Malorni, S. Garnett, Y. Rukazenkov, and M. Martin were responsible for data analysis and interpretation, manuscript writing, and final approval of the manuscript.
S. Garnett and Y. Rukazenkov are employed or have leadership positions at AstraZeneca and have stock ownership in AstraZeneca. A. Di Leo has a consultant or advisory role at and has received honoraria and research funding from AstraZeneca, Pfizer, and Novartis. M. Martin has a consultant or advisory role and has receive honoraria from AstraZeneca. I. Smirnova and M. R. Lichinitser have a consultant or advisory role at AstraZeneca. G. Jerusalem has received honoraria and research funding from AstraZeneca.
Medical writing assistance was provided by Dr Varinia Munoz from Complete Medical Communications Ltd, funded by AstraZeneca.
Data were presented previously at the 35th Annual San Antonio Breast Cancer Symposium, San Antonio, Texas, December 4–8, 2012.
References
- 1. Howell A, Robertson JFR, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–3403 [DOI] [PubMed] [Google Scholar]
- 2. Cano A, Matallin P, Legua V, Tortajada M, Bonilla-Musoles F. Tamoxifen and the uterus and endometrium. Lancet. 1989;1(8634):376 [PubMed] [Google Scholar]
- 3. Ewer MS, Glück S. A woman’s heart: the impact of adjuvant endocrine therapy on cardiovascular health. Cancer. 2009;115(9):1813–1826 [DOI] [PubMed] [Google Scholar]
- 4. Santen RJ. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96(2):308–319 [DOI] [PubMed] [Google Scholar]
- 5. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–3395 [DOI] [PubMed] [Google Scholar]
- 6. DeFriend DJ, Howell A, Nicholson RI, et al. Investigation of a new pure antiestrogen (ICI 182780) in women with primary breast cancer. Cancer Res. 1994;54(2):408–414 [PubMed] [Google Scholar]
- 7. Robertson JF, Nicholson RI, Bundred NJ, et al. Comparison of the short-term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5, (10)-triene-3,17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 2001;61(18):6739–6746 [PubMed] [Google Scholar]
- 8. Addo S, Yates RA, Laight A. A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers. Br J Cancer. 2002;87(12):1354–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250mg with fulvestrant 500mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–4600 [DOI] [PubMed] [Google Scholar]
- 10. Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003;98(2):229–238 [DOI] [PubMed] [Google Scholar]
- 11. Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500mg versus anastrozole 1mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–4535 [DOI] [PubMed] [Google Scholar]
- 12. Robertson JF, Lindemann J, Llombart-Cussac A, et al. Fulvestrant 500mg versus anastrozole 1mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503–511 [DOI] [PubMed] [Google Scholar]
- 13. Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367(5):435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergh J, Jönsson PE, Lidbrink EK, et al. FACT: an open-label randomized Phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30(16):1919–1925 [DOI] [PubMed] [Google Scholar]
- 15. Di Leo A, Malorni L. Polyendocrine treatment in estrogen receptor-positive breast cancer: a “FACT” yet to be proven. J Clin Oncol. 2012;30(16):1897–1900 [DOI] [PubMed] [Google Scholar]
- 16. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res. 2007;13(7):1950–1954 [DOI] [PubMed] [Google Scholar]
- 18. Robertson JFR. Fulvestrant (Faslodex)—how to make a good drug better. Oncologist. 2007;12(7):774–784 [DOI] [PubMed] [Google Scholar]