Abstract
Background
There is controversy on whether former smokers have increased risk for breast cancer recurrence or all-cause mortality, regardless of how much they smoked.
Methods
Data were from three US cohorts in the After Breast Cancer Pooling Project, with detailed information on smoking among 9975 breast cancer survivors. Smoking was assessed an average of 2 years after diagnosis. Delayed entry Cox proportional hazards models were used to examine the relationships of smoking status, cigarettes per day, years of smoking, and pack years with breast cancer prognosis. Endpoints included breast cancer recurrence (n = 1727), breast cancer mortality (n = 1059), and overall mortality (n = 1803).
Results
Compared with never smokers, former smokers with less than 20 pack-years of exposure had no increased risk of any outcome. However, former smokers with 20 to less than 34.9 pack-years of exposure had a 22% increased risk of breast cancer recurrence (hazard ratio [HR] = 1.22; 95% confidence interval [CI] = 1.01 to 1.48) and a 26% increased risk of all-cause mortality (HR = 1.26; 95% CI = 1.07 to 1.48). For former smokers with 35 or more pack-years of exposure, the probability of recurrence increased by 37% (HR = 1.37; 95% CI = 1.13 to 1.66), breast cancer mortality increased by 54% (HR = 1.54; 95% CI = 1.24 to 1.91), and all-cause mortality increased by 68% (HR = 1.68; 95% CI = 1.44 to 1.96). Current smoking increased the probability of recurrence by 41% (HR = 1.41; 95% CI = 1.16 to 1.71), increased breast cancer mortality by 60% (HR = 1.61; 95% CI = 1.28 to 2.03), and doubled the risk of all-cause mortality (HR = 2.17; 95% CI = 1.85 to 2.54).
Conclusions
Lifetime cigarette smoking was statistically significantly associated with a poor prognosis among women diagnosed with breast cancer, dose-dependent increased risks of recurrence, and breast cancer and all-cause mortality.
For years, experts have questioned whether cigarette smoking worsens the prognosis among women diagnosed with breast cancer. To date, most longitudinal studies have indicated that breast cancer survivors who smoke have increased risk of all-cause mortality but women who quit after diagnosis do not (1–8). However, it may be that the risk for former smokers is dependent upon the lifetime exposure to smoking, which is not captured by a simple question on current or former smoking. Therefore, the lack of association between former smoking and breast cancer prognosis could be an artifact of including former smokers with a wide range of lifetime exposure to cigarettes.
Cohort studies of breast cancer survivors have been limited by sample size (range = 166–5056) (2–7,9,10), breast cancer–specific death events (range = 111–628) (2–7,9,10), and follow-up times that average less than a decade. In contrast, Doll et al. studied more than 34000 British doctors with nearly 5300 cancer deaths over several decades to expose the link between smoking and lung cancer (11).
In addition to adequate study power, data on duration and intensity of smoking were needed to explain the lung cancer risk in major cohort studies (12,13). For example, the 50-year follow-up study of British doctors indicated that adults who smoke the equivalent of a pack a day appear to escape most health consequences if they quit smoking by age 35 (11). In 2010, more than 20% of US smokers quit successfully before age 30 (14); therefore a large proportion of former smokers are likely to be in this low-risk category. These data suggest that previous studies of smoking and breast cancer prognosis may have suffered from misclassification of the exposure or been underpowered to test this association.
The objective of this study is to investigate the association of lifetime smoking behavior with breast cancer outcomes. We used data from the 3 US cohorts in the After Breast Cancer Pooling Project (ABCPP), which provides data on duration and intensity of smoking among almost 10000 breast cancer survivors who experienced more than 1700 breast cancer events. This much larger population and number of events compared with previously published studies provide us with the power to detect even moderate effect sizes (eg, 20%) among subgroups. To our knowledge, this is the largest examination of lifetime smoking and breast cancer outcomes conducted. Results of this analysis can inform clinicians about the impact of smoking cessation on breast cancer prognosis.
Methods
Study Subjects
The details of the ABCPP have been described previously (15). For this analysis, the three US cohorts included in the ABCPP were the Women’s Healthy Eating and Living (WHEL) Study, the Life After Cancer Epidemiology (LACE) Study, and the Nurses’ Health Study (NHS). The fourth cohort (Shanghai) in this pooling project was excluded because they reported very low ever smoking rates. Dates of first breast cancer diagnosis ranged from 1976 to 2006 in the ABCPP. We excluded women who were diagnosed before 1991 (n = 3271) because this was before endocrine therapy became standard of care, those who had stage IV tumors at diagnosis (n = 120), or those who were missing data for all smoking variables (n = 81), leaving an analytical sample size of 9975. Data from the three cohorts were harmonized (15). Institutional review board approval was obtained for each cohort study, and all participants provided written informed consent.
Smoking status (current, past, or never), cigarettes per day, and years of smoking were asked on questionnaires an average of 2 years after breast cancer diagnosis. Lifetime pack years were computed. The timing of quitting compared with diagnosis for former smokers was not available for the LACE study and so could not be used in statistical models. Breast cancer events (recurrences or new primary breast cancers) were confirmed by medical records after being reported either on follow-up telephone calls (WHEL) or on mailed questionnaire responses (LACE, NHS). The NHS collected data on cancer recurrences but not on new breast primaries. Mortality was assessed by periodic reviews of the Social Security Death Index and the National Death Index for NHS and WHEL and additionally by Kaiser Permanente North California electronic data sources for LACE. Cause of death was extracted from National Death Index records, death certificates, or electronic medical records (Kaiser Permanente in LACE).
Statistical Analysis
Because the majority of deaths were due to breast cancer and insufficient other events were available for a competing risks analysis, we undertook a sensitivity analysis using a Lunn–McNeil model (16) to account for the different causes of death. Because entry time varied across studies (1 year after diagnosis for NHS; 2 years after diagnosis for WHEL and LACE), we used delayed entry Cox proportional hazards models (17) to estimate survival after diagnosis, with entry time being the time of the first postdiagnosis questionnaire. The proportionality assumption was tested by inclusion of product term of covariables and the log of time. We controlled for cancer stage and grade, age at diagnosis, race/ethnicity, education, and body mass index. Relationships were examined between smoking status, cigarettes per day, years of smoking, and pack-years with all-cause and breast cancer mortality and breast cancer recurrence by individual cohort and in aggregate models stratified by cohort. Following previous research (1), we used 20 pack-years as a primary cut point for classifying ever smokers with heavy exposure. Additionally, for some analyses, we split these heavily exposed ever smokers into two approximately equal groups using 35 pack-years as the cut point. Trends for outcome across smoking categories were assessed with Wald χ2 tests within each multivariable Cox model. There were no statistically significant differences between white and nonwhite subjects.
To further delineate risks associated with heavy former smoking (≥20 pack-years) vs current smokers, an adjusted Cox model was run subsetting these two risk groups. Nonproportionality of the Cox model fit was examined by including product terms for smoking and a binary time indicator (≥10 vs <10 years since breast cancer diagnosis). Using risk estimates for recurrence, breast cancer mortality, or all-cause mortality, random effects meta-analysis tested for heterogeneity between cohorts (Q statistic). Pooled hazard ratios stratified by study are presented whenever no heterogeneity by study was observed. Kaplan–Meier graphs are presented for four categories of smoking history for each of the two mortality endpoints and for breast cancer recurrence. Analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Our analytical sample included 9975 women aged 25 to 83 years at diagnosis (mean = 59.2 years) of early-stage invasive primary breast cancer between 1991 and 2006 (Table 1). Half of the sample had stage 1 disease, and a further 36.4% had stage 2 disease. More than half of the tumors were moderately or well differentiated, and 61.5% were hormone receptor positive. At the time of diagnosis, 23.9% or women were premenopausal, and 71.8% were postmenopausal. Half of the sample received chemotherapy, and 59.7% received radiation; 47.4% underwent mastectomy, and 49.9% had breast-conserving surgery. Median follow-up time was 11.1 years extending through at least 2010, and health endpoints included 1727 breast cancer recurrences, 1059 breast cancer deaths, and a total of 1803 deaths from any cause. Main causes of death in the pooled cohort were breast cancer (58.6%), other cancer deaths (14.8%), and cardiovascular diseases (10.1%).
Table 1.
Demographic and clinical characteristics among breast cancer survivors who provided smoking data (n = 9975)*
| Characteristics | WHEL | LACE | NHS | All |
|---|---|---|---|---|
| Age at diagnosis, y, mean (SD) | 51.3 (8.8) | 59.3 (11.0) | 64.8 (7.7) | 59.2 (10.6) |
| Age at smoking questionnaire, y, mean (SD) | 53.3 (9.0) | 60.2 (11.0) | 65.8 (7.8) | 60.7 (10.5) |
| Age at questionnaire, y, range | 27–74 | 27–82 | 44–84 | 27–84 |
| Cancer stage, No. (%) | ||||
| I | 1184 (38.7) | 1052 (46.6) | 2752 (59.1) | 4988 (50.0) |
| II | 1391 (45.5) | 952 (42.1) | 1287 (27.6) | 3630 (36.4) |
| III | 482 (15.8) | 254 (11.2) | 415 (8.9) | 1151 (11.5) |
| Unspecified | 0 | 1 (0.0) | 205 (4.4) | 206 (2.1) |
| Tumor grade, No. (%) | ||||
| Well differentiated | 481 (15.7) | 428 (19.0) | 811 (17.4) | 1720 (17.2) |
| Moderately differentiated | 1231 (40.3) | 941 (41.7) | 1572 (33.7) | 3744 (37.5) |
| Poorly differentiated | 1092 (35.7) | 676 (29.9) | 1122 (24.2) | 2895 (29.0) |
| Unspecified | 253 (8.3) | 214 (9.5) | 1149 (24.7) | 1616 (16.2) |
| Tumor hormone receptors, No. (%) | ||||
| ER+/PR+ | 1894 (62.0) | 1526 (67.6) | 2718 (58.3) | 6138 (61.5) |
| ER+/PR− | 366 (12.0) | 318 (14.1) | 669 (14.4) | 1353 (13.6) |
| ER−/PR+ | 126 (4.1) | 41 (1.8) | 120 (2.6) | 287 (2.9) |
| ER−/PR− | 606 (19.8) | 350 (15.5) | 648 (13.9) | 1604 (16.1) |
| Unspecified | 65 (2.1) | 24 (1.1) | 503 (10.8) | 592 (5.9) |
| Cancer treatment, No. (%) | ||||
| Radiation only | 588 (19.2) | 566 (25.1) | 1652 (35.5) | 2806 (28.1) |
| Chemotherapy only | 841 (27.5) | 435 (19.3) | 625 (13.4) | 1901 (19.1) |
| Radiation and chemotherapy | 1288 (42.1) | 856 (37.9) | 1011 (21.7) | 3155 (31.6) |
| Neither radiation nor chemotherapy | 334 (10.9) | 401 (17.8) | 1062 (22.8) | 1797 (18.0) |
| Unspecified | 2 (0.1) | 1 (0.0) | 307 (6.6) | 310 (3.1) |
| Surgery type, No. (%) | ||||
| Mastectomy | 1599 (52.3) | 1115 (49.4) | 2013 (43.2) | 4727 (47.4) |
| Breast conserving | 1457 (47.7) | 1144 (50.6) | 2376 (51.0) | 4977 (49.9) |
| Unspecified | 0 | 0 | 236 (5.1) | 236 (2.4) |
| Diagnosis menopause status, No. (%) | ||||
| Premenopausal | 1555 (50.9) | 513 (22.7) | 217 (4.7) | 2285 (23.9) |
| Postmenopausal | 1414 (46.3) | 1436 (6.6) | 4314 (92.6) | 7164 (71.8) |
| Equivocal | 88 (2.9) | 310 (13.7) | 128 (2.7) | |
* ER = estrogen receptor; LACE = Life After Cancer Study; NHS = Nurses Health Study; PR = progesterone receptor; WHEL = Women’s Healthy Eating and Living Study; SD = standard deviation.
Overall, only 7% of breast cancer survivors were current smokers, as determined by questionnaire on average 2 years after diagnosis, ranging from 4.5% to 8.6% across the three cohorts (Table 2). Almost half (45%) were former smokers (range = 39.4%–48.8%), and 48% were never smokers (range = 42.4%–53.8%). Among current smokers, women in the NHS were more likely to be heavier smokers (>15 cigarettes per day) (54% vs 32%–37% in WHEL and LACE; P < .0001). In addition, 95% of NHS current smokers had smoked for more than 30 years compared with 68% for LACE and only 40% for WHEL (P < .0001). Among former smokers, 19% had been light smokers (<5 cigarettes/day). Former smokers were divided approximately equally across decades of duration of smoking. For the two cohorts with data on timing of quitting, 87% of the former smokers had quit smoking at least 3 years before their cancer diagnosis. Tests for heterogeneity (Q statistic) across the three cohorts were non-statistically significant. Therefore we present pooled hazard ratios (HRs) for each model.
Table 2.
Smoking history among three cohorts of US breast cancer survivors (n = 9975)*
| Smoking history | WHEL No. (%) | LACE No. (%) | NHS No. (%) | All No. (%) |
|---|---|---|---|---|
| Smoking status | ||||
| Never | 1643 (53.8) | 1195 (52.9) | 1974 (42.4) | 4812 (48.2) |
| Current | 138 (4.5) | 173 (7.7) | 399 (8.6) | 710 (7.1) |
| Former | 1276 (41.7) | 891 (39.4) | 2272 (48.8) | 4439 (44.5) |
| Unspecified | 0 | 0 | 14 (0.3) | 14 (0.1) |
| Cigarettes/day | ||||
| Current smokers | ||||
| <5 | 32 (23.2) | 22 (12.7) | 38 (9.5) | 92 (13.0) |
| 5–14 | 62 (44.9) | 83 (48.0) | 125 (31.3) | 270 (38.0) |
| 15–24 | 32 (23.2) | 55 (31.8) | 155 (38.9) | 242 (34.1) |
| ≥25 | 12 (8.7) | 9 (5.2) | 60 (15.0) | 81 (11.4) |
| Unspecified | 0 | 4 (2.3) | 21 (5.3) | 25 (3.5) |
| Former smokers | ||||
| <5 | 350 (27.5) | 155 (17.4) | 342 (15.1) | 847 (19.1) |
| 5–14 | 388 (30.4) | 283 (31.8) | 736 (32.4) | 1407 (31.7) |
| 15–24 | 340 (26.7) | 293 (32.9) | 743 (32.7) | 1376 (31.0) |
| ≥25 | 189 (14.8) | 132 (14.8) | 376 (16.6) | 697 (15.7) |
| Unspecified | 9 (0.7) | 28 (3.1) | 75 (2.4) | 112 (2.5) |
| Years of smoking | ||||
| Current smokers | ||||
| <10 | 13 (9.4) | 4 (2.3) | 3 (0.8) | 20 (2.8) |
| 10–19 | 24 (17.4) | 11 (6.4) | 4 (1.0) | 39 (5.5) |
| 20–29 | 46 (33.3) | 38 (22.0) | 8 (2.0) | 92 (13.0) |
| ≥30 | 55 (39.9) | 118 (68.2) | 377 (94.5) | 550 (77.5) |
| Unspecified | 0 | 2 (1.2) | 7 (1.8) | 9 (1.3) |
| Former smokers | ||||
| <10 | 572 (44.8) | 208 (23.3) | 413 (18.2) | 1193 (26.9) |
| 10–19 | 335 (26.3) | 234 (26.3) | 525 (23.1) | 1094 (24.7) |
| 20–29 | 237 (18.6) | 207 (23.2) | 486 (21.4) | 930 (21.0) |
| ≥30 | 125 (9.8) | 234 (25.1) | 807 (35.5) | 1156 (26.0) |
| Unspecified | 7 (0.6) | 18 (2.0) | 41 (1.8) | 66 (1.5) |
| Pack-years | ||||
| 0 | 1644 (53.8) | 1197 (53.0) | 1974 (42.4) | 4815 (48.3) |
| 0.1–10 | 775 (25.4) | 467 (20.7) | 803 (17.2) | 2045 (20.5) |
| 10.1–19.9 | 245 (8.0) | 167 (7.4) | 457 (9.8) | 869 (8.7) |
| 20.0–34.9 | 199 (6.5) | 201 (8.9) | 545 (11.7) | 945 (9.5) |
| ≥35 | 179 (5.9) | 188 (8.3) | 796 (17.1) | 1163 (11.7) |
| Unspecified | 15 (0.5) | 39 (1.7) | 84 (1.8) | 138 (1.4) |
* LACE = Life After Cancer Study; NHS = Nurses Health Study; WHEL = Women’s Healthy Eating and Living Study;
Table 3 shows the results of the adjusted Cox models for associations of smoking with breast cancer recurrence, breast cancer mortality, and all-cause mortality. Compared with never smokers, former smokers with low lifetime smoking exposure (<20 pack-years) had no increased risk of any outcome. In former smokers with heavier exposures, there was a dose–response relationship between increasing lifetime smoking exposure and the risk for all-cause mortality (P trend < .0001) and breast cancer mortality (P trend < .0001). Specifically, compared with never smokers, former smokers with 20 to 34.9 pack-years of exposure had a 22% increased risk of breast cancer recurrence (HR = 1.22; 95% confidence interval [CI] = 1.01 to 1.48) and a 26% increased risk of all-cause mortality (HR = 1.26; 95% CI = 1.07 to 1.48). For former smokers with 35 or more pack-years of exposure, the probability of breast cancer recurrence increased by 37% (HR = 1.37; 95% CI = 1.13 to 1.66), breast cancer mortality increased by 54% (HR = 1.54; 95% CI = 1.24 to 1.91), and all-cause mortality increased by 68% (HR = 1.68; 95% CI = 1.44 to 1.96). Current smokers in this study had a mean exposure of 39 pack years. Compared with nonsmokers, the probability of a breast cancer recurrence in smokers was 41% higher (HR = 1.41; 95% CI = 1.16 to 1.71), breast cancer mortality was 60% higher (HR = 1.61; 95% CI = 1.28 to 2.03), and there was double the risk of all-cause mortality (HR = 2.17; 95% CI = 1.85 to 2.54). There appeared to be little difference in risk for any outcome between current smokers and former smokers with 35 or more pack-years of exposure.
Table 3.
Adjusted Cox models* examining the associations of smoking with breast cancer recurrence and mortality in a pooled cohort of US breast cancer survivors (n = 9755)
| Smoking status | No. | Breast cancer recurrence | Breast cancer mortality | All-cause mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event | HR (95% CI) | P† | Event | HR (95% CI) | P† | Event | HR (95% CI) | P† | ||
| Never smokers | 4812 | 824 | Referent | 499 | Referent | 780 | Referent | |||
| Former smokers | ||||||||||
| <20 pack-years | 2744 | 453 | 0.98 (0.87 to 1.11) | .78 | 259 | 0.99 (0.85 to 1.15) | .88 | 410 | 0.97 (0.86 to 1.09) | .58 |
| 20–34.9 pack-years | 808 | 156 | 1.22 (1.01 to 1.48) | .04 | 93 | 1.14 (0.91 to 1.43) | .26 | 177 | 1.26 (1.07 to 1.48) | .01 |
| ≥35 pack-years | 785 | 155 | 1.37 (1.13 to 1.66) | .001 | 111 | 1.54 (1.24 to 1.91) | <.001 | 227 | 1.68 (1.44 to 1.96) | <.001 |
| Current smokers‡ | 710 | 139 | 1.41 (1.16 to 1.71) | <.001 | 97 | 1.61 (1.28 to 2.03) | <.001 | 209 | 2.17 (1.85 to 2.54) | <.001 |
| P trend | <.001 | <.001 | <.001 | |||||||
* Hazard ratios (HRs) were from delayed-entry Cox regression models with study as a stratification variable and adjusted for age at diagnosis, cancer stage, tumor grade, race/ethnicity, education, and obesity. CI = confidence interval; SD = standard deviation.
† P values (two-sided) were from the Wald test within Cox proportional hazards regression.
‡ Current smokers had smoked for a mean of 39 (standard deviation = 25) pack-years.
Sensitivity analyses for outcomes in Table 3 were conducted, including diagnosis menopause status and treatment (eg, adjuvant hormonal therapy) as predictors in each model, and hazard ratios for former smokers were slightly stronger, but within 5% of those presented in Table 3. The Lunn–McNeil model hazard ratios for breast cancer mortality in former smokers, accounting for cardiovascular or other cancer deaths, differed by less than 10% from those in Table 3 (data not shown). The hazard ratio in the Lunn–McNeil model for current smokers was 2.06 (95% CI = 1.73 to 2.46), indicating double the risk of never smokers.
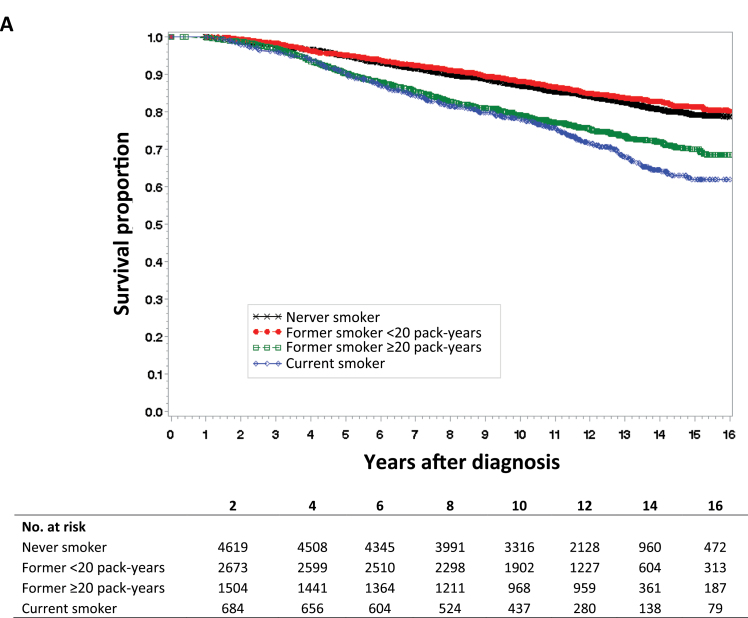
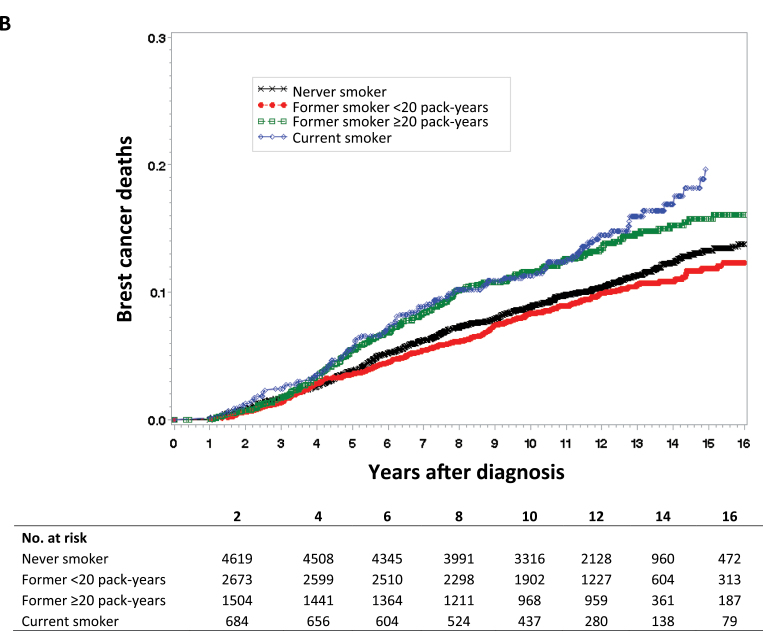
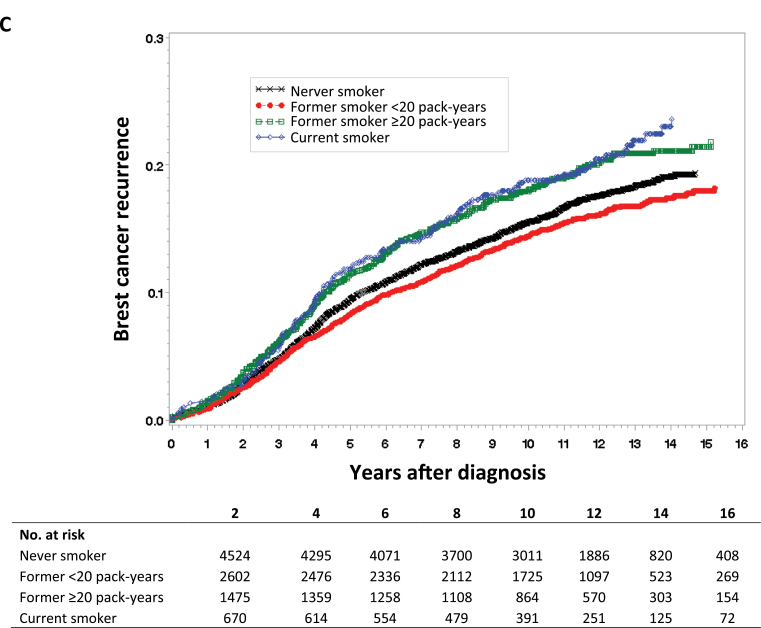
For Kaplan–Meier survival curves of all-cause mortality (Figure 1A), breast cancer mortality (Figure 1B), and breast cancer recurrence (Figure 1C), we categorized lifetime exposure among former smokers as less than or greater than 20 pack-years. As expected, there was no apparent difference between never smokers and former smokers without a heavy lifetime exposure (<20 pack-years) in the survival curves for any of the three study outcomes. Compared with never smokers, both current smoking and former smokers with a heavy lifetime exposure (>20 pack years) were at statistically significantly increased risk for all three study outcomes.
Figure 1.
Kaplan–Meier survival curves by smoking status. Data were analyzed for A) all-cause mortality, B) breast cancer mortality, and C) breast cancer recurrence. Tables of the number of patients at risk in each group at various time points are below each graph.
For all-cause mortality, the hazards for current smokers and former smokers with heavy exposure appeared to change over time. Therefore we tested an interaction with time at 10 years (χ2 = 4.06; P = .04). This interaction indicated that before the 10 year time point, current smokers had a 33% increased risk (HR = 1.33; 95% CI = 1.09 to 1.63) in comparison with former smokers. However, current smokers had a much greater risk after 10 years, after which they had a twofold greater risk than these former smokers (HR = 1.99; 95% CI = 1.42 to 2.80).
Discussion
To our knowledge, this is the first study to report a statistically significant, dose-dependent association of lifetime cigarette smoking with breast cancer recurrence. Study data indicate that cigarette smoking is associated with a poor prognosis among women diagnosed with breast cancer, statistically significantly increased risks of recurrence, and breast cancer and all-cause mortality. However, this risk is confined to heavy smokers, defined as former smokers with more than 20 pack-years of exposure and current smokers (who, in this study, had a mean of 39 pack-years of exposure). This analysis shows the risk associated with current smoking is higher, most likely because current smoking is a surrogate for high lifetime exposure to cigarettes. It is also notable that in this large pooled cohort approximately 60% of former smokers reported lifetime cigarette exposures of less than 20 pack-years. Therefore it is key to determine the extent of past smoking in breast cancer cohorts so that analyses can differentiate those with heavy lifetime exposures. Analyses that group all former-smokers into one category will dilute the ability to detect the true effect. This misclassification of exposure could potentially contribute to the conclusion from prior cohort studies that former smokers are not at greater risk than never smokers with regard to breast cancer outcomes (2–7,9).
Although numerous studies have noted that continued smoking after diagnosis increases the risk of all-cause mortality (2–10,18), only a few have identified an increased risk for breast cancer mortality (2,19). The inclusion of the LACE and NHS cohorts in this analysis confirms the recent WHEL report that lifetime exposure to cigarette smoking confers a dose-dependent increasing risk of mortality among breast cancer survivors (1). Importantly, this pooled cohort also had sufficient cases of breast cancer recurrence to examine this outcome in a rigorous manner. Finally, this large cohort allowed us to investigate a relatively uncommon exposure: extremely heavy smoking history (eg, ≥35 pack years) which occurred in only 12% of the population. Taken together, these data demonstrated a statistically significant dose–response relationship between lifetime smoking and all breast cancer outcomes, providing convincing evidence of causal relationships.
New in our study is that the survival curves for former smokers with statistically significant lifetime exposure were quite similar to current smokers for the first few years after diagnosis. This similarity may reflect the “smokers’ paradox” that has been reported for myocardial infarctions. Specifically, smokers with more severe disease may be more successful at quitting at the time of diagnosis (20). This so-called paradox could create a disease differential between current and former smokers that would minimize the difference in survival curves for the first few years after diagnosis. If this were the case, we would expect the survival curves to separate after a lag period, which was observed in this study.
The mechanisms by which smoking causes premature death are well documented and include major impacts on cardiovascular and respiratory health. These global health effects likely explain some of the impacts of smoking on mortality seen in most studies of breast cancer survivors. However, the finding of an association of smoking with breast cancer recurrence suggests cancer-specific mechanisms of risk. A Canadian Expert Panel on Smoking and Breast Cancer (21) concluded that there are persuasive biological reasons to suspect that exposure to the carcinogens in tobacco smoke may lead to breast cancer. For example, tobacco smoke contains more than a dozen fat-soluble compounds that are known to induce mammary tumors in rodents, and these carcinogens can be activated into electrophilic intermediates by enzymes active in the human breast epithelial cell. The findings regarding lifetime risk of smoking are also biologically plausible because there is considerable evidence that tobacco carcinogens accumulate with exposure over time (22). In the Women’s Health Initiative, the increased risk for incident invasive breast cancer persisted up to 20 years after smoking cessation (23). This persistence in risk may occur as a result of the complex disequilibrium of the genes controlling activation, detoxification, DNA repair, and cell cycle control (22).
This study is limited by the use of pack-years as the measure of lifetime cigarette exposure because there are problems with recall of intensity and duration of smoking. However, measurement of only current smoking status will miss important cancer relationships that could bias conclusions. Additionally, there has been a major consistent decline in the intensity of smoking over the past 40 years in the United States (24) that may not be captured in self-reported measures of average use.
A strength of this study is the large sample size (almost 10000 breast cancer survivors) and number of breast cancer–specific events (>1700) made possible by pooling the three cohort studies. By harmonizing the data across studies, we were able to use standardized exposure definitions and control for potential confounders in a unified analytic approach. Although there were differences in the sociodemographic variables and cancer characteristics across these studies, we were unable to find evidence of heterogeneity in the outcome results. There were differences in lifetime smoking patterns across studies that may relate to recruitment (one was a cohort recruited before diagnosis; another was a randomized trial of a health behavior intervention).
In summary, this pooling project of breast cancer survivors was large enough to identify the statistically significant and dose-dependent risks of poor prognosis associated with a lifetime history of cigarette smoking. From a research perspective, these results emphasize the importance of assessing lifetime cigarette exposure for both past and current smokers in studies of breast cancer. Clearly, including smoking as a key variable in clinical trials of breast cancer outcomes is very important (10,25). From a clinical perspective, these data suggest that there is an important opportunity to motivate women to quit smoking before they accumulate 20 pack-years of exposure, around which time our data suggest they will have acquired sustained changes to their risk of morbidity and mortality from breast cancer and other diseases. Finally, combined with recent robust analyses and reviews supporting an association of smoking with incident breast cancer (23,26), we believe that the data are sufficiently mature to issue guidelines indicating that cigarette smoking is a major risk factor for breast cancer outcomes and that smoking cessation counseling should be a standard part of breast cancer survivor care.
Funding
This Pooling Project was supported by the National Cancer Institute at the National Institutes of Health (3R01 CA118229-03S1 and 1RO1CA166293-01A1). Support for the individual cohorts came from Women’s Healthy Eating & Living Study (Susan G. Komen Foundation, KG100988); Life After Cancer Epidemiology Study (National Cancer Institute, R01 CA129059); and the Nurses’ Health Study (P01 CA87969).
The funding sources were not involved in the design of the study; the collections, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1. Saquib N, Stefanick ML, Natarajan L, Pierce JP. Mortlity risk in former smokers with breast cancer: pack-years vs. smoking status. Int J Cancer. 2013;133(10):2493–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manjer J, Andersson I, Berglund G, et al. Survival of women with breast cancer in relation to smoking. Eur J Surg. 2000;166(11):852–858 [DOI] [PubMed] [Google Scholar]
- 3. Fentiman IS, Allen DS, Hamed H. Smoking and prognosis in women with breast cancer. Int J Clin Pract. 2005;59(9):1051–1054 [DOI] [PubMed] [Google Scholar]
- 4. Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int J Cancer. 2007;120(12):2672–2677 [DOI] [PubMed] [Google Scholar]
- 5. Sagiv SK, Gaudet MM, Eng SM, et al. Active and passive cigarette smoke and breast cancer survival. Ann Epidemiol. 2007;17(5):385–393 [DOI] [PubMed] [Google Scholar]
- 6. Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123(9):2188–2194 [DOI] [PubMed] [Google Scholar]
- 7. Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19(5):366–373 [DOI] [PubMed] [Google Scholar]
- 8. Braithwaite D, Izano M, Moore DH, et al. Smoking and survival after breast cancer diagnosis: a prospective observational study and systematic review. Breast Cancer Res Treat. 2012;136(2):521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calle EE, Miracle-McMahill HL, Thun MJ, Heath CW., Jr Cigarette smoking and risk of fatal breast cancer. Am J Epidemiol. 1994;139(10):1001–1007 [DOI] [PubMed] [Google Scholar]
- 10. Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132(2):401–410 [DOI] [PubMed] [Google Scholar]
- 11. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Br Med J. 2004;328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flanders W, Lally C, Zhu B, Henley S, Thun M. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63(19):6556–6562 [PubMed] [Google Scholar]
- 14. Pierce JP, Cummins SE, White MM, Humphrey A, Messer K. Quitlines and nicotine replacement therapy for smoking cessation: do we need to change policy? Annu Rev Public Health. 2012;33:341–356 [DOI] [PubMed] [Google Scholar]
- 15. Nechuta SJ, Caan BJ, Chen WY, et al. The After Breast Cancer Pooling Project: rationale, methodology, and breast cancer survivor characteristics. Cancer Causes Control. 2011;22(9):1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleinbaum DG, Klein M, Gail M, et al., eds. Survival Analysis, A Self-Learning Text. 3rd ed New York: Springer; 2012 [Google Scholar]
- 17. Therneau TM, Grambsch PM, Dietz K, et al., eds. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000 [Google Scholar]
- 18. Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26(20):3310–3316 [DOI] [PubMed] [Google Scholar]
- 19. Tominaga K, Andow J, Koyama Y, et al. Family environment, hobbies and habits as psychosocial predictors of survival for surgically treated patients with breast cancer. Jpn J Clin Oncol. 1998;28(1):36–41 [DOI] [PubMed] [Google Scholar]
- 20. Kunz F, Pechlaner C, Hortnagl H, Pfister R. The smoker’s paradox and the real risk of smoking. Eur J Epidemiol. 2005;20(2):161–167 [DOI] [PubMed] [Google Scholar]
- 21. Collishaw NE, Boyd NF, Cantor KP, et al. Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk. Ontario Tobacco Research Unit Special Report Series. Toronto, Ontario: Tobacco Research Unit; 2009. [Google Scholar]
- 22. US Department of Health and Human Services How Tobacco Smoke Causes Disease: The Biological and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services; 2010 [Google Scholar]
- 23. Luo J, Margolis KL, Wactawski-Wende J, et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ. 2011;342:d1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965–2007. JAMA. 2011;305(11):1106–1112 [DOI] [PubMed] [Google Scholar]
- 25. Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2287–2293 [DOI] [PubMed] [Google Scholar]
- 26. Johnson KC, Miller AB, Collishaw NE, et al. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tob Control. 2011;20(1):e2. [DOI] [PubMed] [Google Scholar]