Abstract
Objective
Our group and others have shown that serial intra-lesional injections of common warts with skin testing reagents such as Candida, mumps and Trichophyton are effective in regressing injected and non-injected warts. Anti-HPV T-cell responses appear to be induced. The goal of this study was to understand the mechanisms of how Candida skin testing reagent enhances immune responses.
Methods
The following immunological features were studied to understand how Candida induces immune responses in healthy subjects: (1) proliferative capacity of T-cells upon exposure to Candida through monocyte-derived human Langerhans cells (LCs) measured using alamarBlue, (2) cytokine (IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-23Ap19, IFN-γ, and TNF-α) expression upon Candida stimulation of LCs by quantitative reverse transcription (qRT)-PCR and cytokine secretion by ELISA, (3) expression of pattern recognition receptors (PRRs) known to associate with Candida albicans (DC-SIGN, dectin-1, dectin-2, galectin-3, mincle, mannose receptor, Toll-like receptors 1, 2, 4, 6, and 9) on LCs by qRT-PCR, (4) role of dectin-1 in IL-12 production by antibody blocking, and (5) induction of Th1, Th2, and/or Th17 responses by intracellular cytokine staining of CD4 cells exposed to Candida pulsed LCs for IFN-γ, IL-4, and IL-17A.
Results
T-cell proliferation upon stimulation with Candida-pulsed LCs was significantly higher compared to proliferation in the absence of Candida (p=0.004). The most frequently expressed cytokine in stimulated LCs was IL-12p40 mRNA, and IL-12p40 and IL-12p70 were also detected at protein levels. All other cytokine mRNAs examined were detected in the following order of decreasing frequency: IL23Ap19, IFN-γ, IL-1 β, IL-6, IL-8, and IL-10. LCs expressed all PRRs examined. Anti-dectin-1 inhibited IL-12p40 mRNA production upon Candida stimulation of LCs from some healthy subjects. IFN-γ secretion was increased and IL-4 secretion was decreased in CD4 cells of a few healthy subjects, but IL-17A was essentially unchanged upon Candida treatment.
Conclusions
Proliferation of T-cells in a substantial majority of healthy subjects can be demonstrated with Candida stimulation. We show Th1 promotion and dectin-1 stimulation of LCs as potential mechanisms in some healthy subjects.
1. Introduction
Several studies have shown treatment of warts with Candida, mumps, and/or Trichophyton skin test reagent injection to be effective in not only resolving treated warts but also distant untreated warts [1–6]. Other studies have also shown the effectiveness of Candida skin test reagent injection immunotherapy in the pediatric populations [1, 7]. In a recently completed Phase I investigational new drug study (NCT00569231) in which the largest wart was treated with Candin® (Allermed, San Diego, CA), a colorless extract of Candida albicans, our group reported complete resolution of the treated warts in 82% (nine of 11) of the subjects, and complete resolution of distant untreated warts in 75% (six of eight) of the subjects [6]. Furthermore, T-cell responses to the HPV 57 L1 peptide were detected in 67% (six of nine) of the complete responders. Because these recall antigens are derived from very different organisms, cross-reactivity of their antigenic determinants with HPV would be very unlikely. Several lines of evidence support the notion that Candida binds pattern recognition receptors (PRRs) and activates innate and adaptive immune responses [8–17]. Candida albicans can activate multiple host PRRs including DC-SIGN [8], dectin-1 [9], dectin-2 [15], galectin-3 [18], mannose receptor [8], mincle [17], and some Toll-like receptors (TLRs) [12, 13, 16, 19, 20]. Dectin-1 is a particularly important candidate receptor since its activation can mediate the differentiation of human monocytes into dendritic cells [14].
More recently, Zielinski et al. have reported that Candida albicans-specific T helper cells produced IL-17 and IFN-γ, and that IL-1β, IL-23, and IL-6 contributed to the induction of Th17 differentiation [21]. Furthermore, most individuals have been shown to have skin resident memory T-cells that can respond to Candida albicans through IL-17 and IFN-γ production [22]. The goal of this study was to elucidate the mechanisms of how Candin enhances immune responses by studying its ability to induce T-cell proliferation, investigating cytokine secretions by LCs and CD4 T-cells, and examining involvement of various PRRs.
2. Methods
2.1 Subjects
Whole blood samples were collected from healthy volunteers (n = 12), and were centrifuged to concentrate the buffy coat layer. Alternatively, source leukocytes were collected by apheresis from blood donors (n = 9, Key Biologics, LLC, Memphis, TN). Peripheral blood mononuclear cells (PBMCs) were isolated using ficoll-hypaque density gradient, and were cryopreserved. The study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences, and written informed consents were obtained.
2.2 T-cell proliferation assay using alamarBlue
PBMCs were thawed and monocytes were negatively selected using a commercially available kit (Monocyte Isolation Kit II, Miltenyi Biotec, Auburn, CA). Monocytes were converted to Langerhans cells (LCs) using GM-CSF, IL-4 and TGF-β1 for 7 days as described by Fahey and colleagues [23]. PBMCs from the same subjects were thawed on day 7, and CD3+CD25− population was selected using a pan T-cell isolation kit II (Milenyi Biotec) to which biotinylated anti-CD25 antibody (Miltenyi Biotec) was added to remove regulatory T-cells. One hundred and fifty thousand T-cells and 3 × 103 LCs were plated per well of a 96-well flat bottom plate in 100 μl of Yssel's media (Gemini Bioproducts Inc., Woodland, CA) containing 5% human serum. Wells with media only, cells only (T-cells and LCs), cells and Candin at 150 μl/ml, and cells with tetanus toxoid (500 ng/ml, EMD Milipore, Billerica, MA) were set up in 6 replicates. After 7 days of incubation, 10% of volume in each well was replaced with alamarBlue (Life Technologies, Grand Island, NY), and the plates were incubated for 6 hours. Mean fluorescence was measured (530 nm excitation and 590 nm emission wavelengths) using Synergy-2 multi plate reader (US BioTek, Seattle, WA).
In selected experiments, LC purity was determined with using anti-CD1a-FITC antibody (eBioscience, San Diego, CA), anti-Langerin-PE antibody (Beckman-Coulter, Brea, CA), anti-E-Cadherin-PerCP710 antibody (eBioscience), or relevant isotype controls. T-cells were stained with anti-human CD3-FITC, anti-human CD25-PerCP-Cy5.5, or relevant isotype controls (all from eBiosciences). Data were acquired using FACS Fortessa (BD Biosciences, San Jose, CA), and were analyzed using FACS Diva and CellQuest Pro softwares (BD Biosciences) at the University of Arkansas for Medical Sciences Microbiology and Immunology Flow Cytometry Core Laboratory.
2.3 Quantitative reverse transcription (qRT)-PCR analysis of LCs treated with Candin
LCs were prepared as described above, and 1 × 106 LCs each were treated with Candin (10, 50, and 150 μl/ml). For each experiment, one or more PRR agonists were used as positive controls: lipopolysaccharide (LPS, InvivoGen, San Diego, CA) at 100 ng/ml, zymosan (InvivoGen) at 10 μg/ml, mannan (Sigma-Aldrich, St. Louis, MO) at 150 μg/ml, HIV-1 gp120 protein (MyBioSource, San Diego, CA) at 10 μg/ml, and/or lipoteichoic acid (LTA-SA, InvivoGen) at 1 μg/ml. Wells with media only served as a negative control. Cells were harvested at 8 and 24 hours for RNA extraction using RNeasy kit (Qiagen, Valencia, CA). RNA samples were treated with RNase-free DNase I (Promega, Madison, WI), and were reverse transcribed with SuperScript III enzyme (Life Technologies). RT reactions were diluted to 200 μl and 2.5 μl used for quantitative PCR analysis. qRT-PCR was performed in duplicate for IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-23Ap19, IFN-γ, and TNF-α using an iQ-SYBR mix (Bio-Rad, Hercules, CA) in a CFX96 Real Time System (Bio-Rad). In some experiments, the expressions of PRRs known to associate with Candida albicans (DC-SIGN, dectin-1, dectin-2, galectin-3, mincle, mannose receptor, TLRs 1, 2, 4, 6, and 9) were examined in addition to the cytokines. All primers were designed using Beacon Design software (Bio-Rad, Table 1). The threshold cycles were normalized to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression, and were calculated as fold change over untreated LCs at 8 hour.
Table 1.
Primers used for qRT-PCR
| Description | Gene name | Accession no. | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|---|
| DC-SIGN, CD 209 | hDCSIGN | NM_001144899.1 | TGC AGT CTT CCA GAA GTA ACC GCT | TGT TGG GCT CTC CTC TGT TCC AAT |
| C-type lectin domain family 7, member A (CLEC7A) | hDectin1 | NM_197947.2 | TGC TTG GTA ATA CTG GTG ATA G | GGT TGA CTG TGG TTC TCT T |
| C-type lectin domain family 6, member A (CLEC6A) | hDectin2 | NM_001007033 | AAC ACA GAA GCA GAG CAG AAT | TCC AGA AGA CTA TTG AAG CAC ATT |
| Lectin, galactoside-binding, soluble, 3 (LGAL3) | hGalectin3 | NM_001177388.1 | TGT GCC TTA TAA CCT GCC TTT GCC | TTC TGT TTG CAT TGG GCT TCA CCG |
| Glyceraldehyde-3-phosphate dehydrogenase | hGAPDH | NM_002046.4 | GGA CCT GAC CTG CCG TCT AG | GTA GCC CAG GAT GCC CTT GA |
| interferon, gamma | hlFN-γ | NM_000619.2 | TGT GGA GAC CAT CAA GGA AGA C | TGC TTT GCG TTG GAC ATT CAA G |
| Interleukin 10 | hlL-10 | NM_000572.2 | GGG TTG CCA AGC CTT GTC TG | CGC CGT AGC CTC AGC CTG |
| Interleukin 12B | hlL-12p40 | NM_002187.2 | GGA GTG CCA GGA GGA CAG TG | GTT CTT GGG TGG GTC AGG TTT G |
| Interleukin 1, beta | hlL-1β | NM_000576.2 | CAG GGA CAG GAT ATG GAG CAA C | CAC GCA GGA CAG GTA CAG ATT C |
| Interleukin 23 alpha subunit p19 (IL23A) | hlL23A p19 | NM_016584.2 | AGT GTG GAG ATG GCT GTG ACC | GGG CTA TCA GGG AGC AGA GAA G |
| Interleukin 6 (interferon, beta 2) | hlL-6 | NM_000600.3 | GTA GTG AGG AAC AAG CCA GAG C | GGC ATT TGT GGT TGG GTC AGG |
| Interleukin 8 | hlL-8 | NM_000584.3 | GAC CAC ACT GCG CCA ACA C | AAA CTT CTC CAC AAC CCT CTG C |
| Lymphocyte antigen 96 (LY96) | hMD2 | NM_001195797.1 | TGC CGA GGA TCT GAT GAC | GAA TTA GGT TGG TGT AGG ATG AC |
| C-type lectin domain family 4, member E (CLEC4E) | hMincle | NM_014358.2 | TCA GAA TAC CGG TGT GGC CTT TCT | TGG TTA CAG CCT GTT TGG AGC TGA |
| Mannose receptor, Ctype2 | hMRC2 | NM_006039.4 | AGC AAC GTC ACC AAA GAA ACG CAG | AGA ACT GTG CCT CTG ACC ACT TCA |
| Toll-Like Receptor 1/6* | hTLR1 or TLR 6 | NM_003263.3 or NM_006068.4 | ATG TGG CAG CTT TAG CAG CCT TTC | TCT GGA AGA AAT CAG CCG ATG GGT |
| Toll-Like Receptor 2 | hTLR2 | NM_003264 | TGC TGC CAT TCT CAT TCT | CAC TCC AGG TAG GTC TTG |
| Toll-Like Receptor 4 | hTLR4 | NM_138557 | CGT GCT GGT ATC ATC TTC AT | GGT AAG TGT TCC TGC TGA G |
| Toll-Like Receptor 9 | hTLR9 | NM_017442.3 | ATC TGC ACT TCT TCC AAG GCC TGA | AAG GCC AGG TAA TTG TCA CGG AGA |
| Tumor Necrosis Factor alpha | hTNF-α | NM_000594.3 | GGG GTG GAG CTG AGA GAT AAC C | ACG GCG ATG CGG CTG ATG |
The same primers were used to analyze TLR 1 and 6 amplifying a 100% homologous region between the two genes.
2.4 ELISA assays for IL-12p40 and IL-12p70 proteins
Selected culture supernatants from experiments with LCs treated with Candin were analyzed for the presence of IL-12p40 protein using the OptEIA™ kit (BD, Franklin Lakes, NJ) and/or IL-12p70 protein using the IL-12p70 High Sensitivity ELISA kit (eBioscience).
2.5 Antibody blocking using anti-dectin-1 antibody
The role of dectin-1 in IL-12p40 mRNA production was evaluated by performing antibody blocking of LCs treated with Candin. LCs were prepared, and qRT-PCR assays for IL-12p40 were performed as described above. To demonstrate the antibody's ability to block, anti-dectin-1 antibody at 20 μg/ml (R&D Systems, Minneapolis, MN) or an isotype control antibody (mouse IgG2b) was added to zymosan treated LCs and LCs were harvested 5 hours later. Candin (150 μl/ml) treated LCs were harvested at 24 hours along with those treated with anti-dectin-1 antibody and the isotype control antibody. The threshold cycles were normalized to GAPDH expression, and fold changes over untreated LCs at 5 or 8 hours were calculated.
2.6 Intracellular cytokine staining
The methods were adapted from those described by Zielinski et al. [21]. LCs were prepared as described above. On day 7, PBMCs from the same subjects were thawed and negatively selected for CD4 T-cells (CD4 T-cell isolation kit II, Miltenyi Biotec). Five hundred thousand CD4 T-cells and 1 × 104 LCs were plated per well of a 24-well flat bottom plate in 500 μl of Yssel's media containing 5% human serum with or without Candin (150 μl/ml). After a 6 day incubation, phorbol 12-myristate 13-acetate (PMA, 200 nM, Sigma-Aldrich) and ionomycin (1 μg/ml, Sigma-Aldrich) were added. After 2 hours, Brefeldin A (10 μg/ml, eBiosciences, San Diego, CA) was added. After an additional 2 hours, the CD4 T-cells were permeabilized/fixed, and stained intracellularly with fixable viability dye eFluor 450®, anti-human IFN-γ phycoerythrin, anti-human IL-17A PerCP-Cy5.5, and anti-human IL-4 allophycocyanin (all reagents and antibodies from eBioscience). Cells stained with isotype control antibodies served as a negative control. Ten thousand events were acquired using FACS Fortessa (BD Bioscience), and percentages of CD4 T-cells producing IFN- γ, IL-17A, and IL-4 were enumerated using FACS Diva software (BD Biosciences).
3. Results
3.1 T-cell proliferation induced by Candin
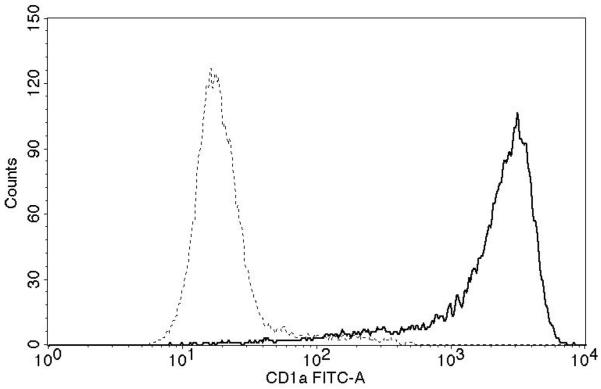
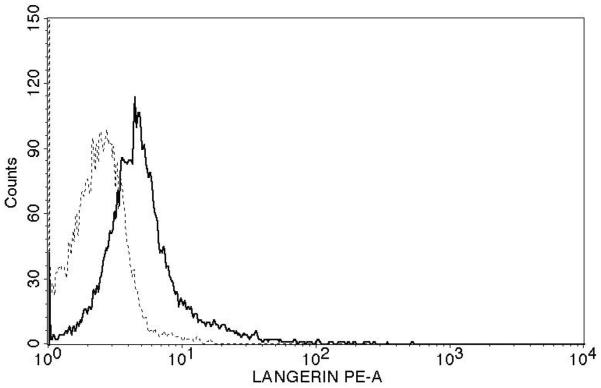
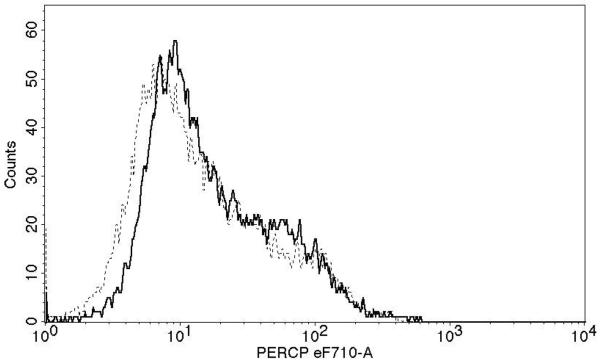
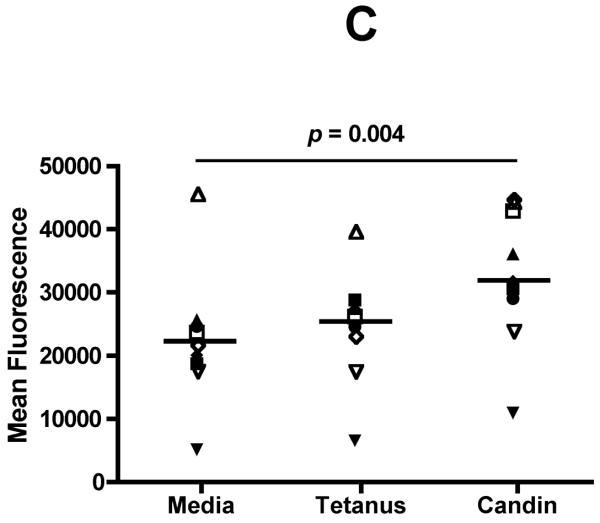
Successful conversion to a LC phenotype was demonstrated by the expression of CD1a, Langerin, and E-cadherin using FACS analysis (Fig. 1A). Of nine subjects examined, CD3 T-cells (Fig. 1B) from seven (78%) demonstrated measurable proliferation (≥ 5,000 increases in mean fluorescence) when treated with Candin-pulsed LCs (increases in mean fluorescence of 11,811-, 10,419-, 5,770-, 11,638-, 19,218-, 6,283-, and 23,033 respectively). CD3 T-cells from two subjects (22%) demonstrated proliferation when stimulated with tetanus toxoid-pulsed LCs (10,003 and 7,255)(Fig. 1C). Mean fluorescence for the Candin group (32,753) was significantly higher than that of media control (22606)(paired t-test, p=0.004).
Fig. 1.
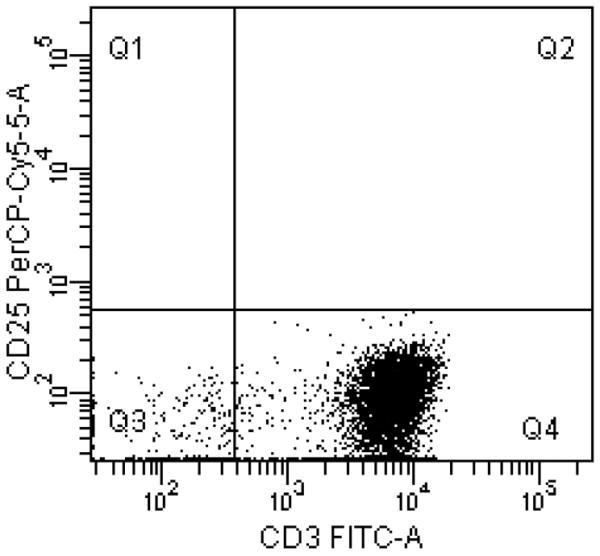
A, Surface expressions of CD1a, Langerin, and E-cadherin (solid lines) demonstrate successful conversion of monocytes into LCs. Dotted lines represent staining with isotype controls. B, A representative example of purified CD3+CD25−T-cell population in Q4 with purity of 95.1%. C, T-cell proliferation measured using alamarBlue. The mean of six replicates from each individual subject is plotted. The bar represents the mean of the group. P values were calculated using paired t-test.
3.2 Multiple cytokines are expressed by LCs exposed to Candin with IL-12 being the most frequently detected
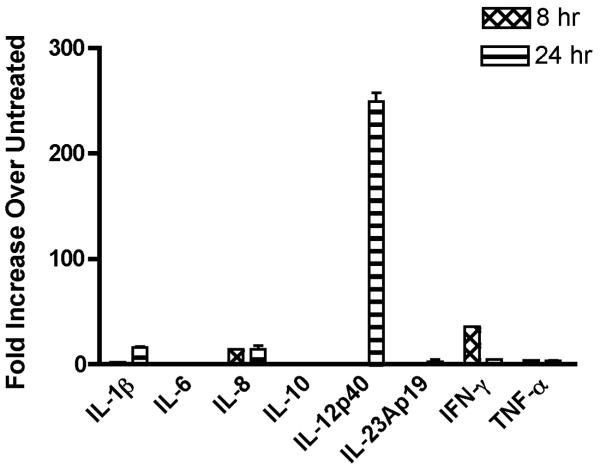
PBMC samples from 8 subjects were obtained twice at least one month apart (i.e., 2 experiments per subject), and mRNA expression of cytokines (Table 1) upon Candin stimulation was examined by qRT-PCR (Fig. 2, Table 2). The amplifications of the intended cDNAs were confirmed by DNA sequencing of gel-purified products from selected experiments. The mean number of cytokines expressed per experiment was 3.2, and IL-12p40 was the most commonly induced cytokine (≥ 5 fold over untreated), and was detected in 15 of 16 experiments. The next most commonly expressed cytokine was IL23Ap19 (10 experiments) followed by IFN-γ (9 experiments), IL-qβ (7 experiments), IL-6 (2 experiments), IL-8 (2 experiments), and IL-10 (2 experiments).
Fig. 2.
A representative example of qRT-PCR results of monocyte-derived human Langerhans cells treated with Candin (150 μl/ml). The bars represent SD.
Table 2.
A summary of qRT-PCR results for the three most commonly detected cytokines
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood draw | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Cytokine, Candin, time | ||||||||||||||||
|
| ||||||||||||||||
| IL-12p40, 50μl/ml, 8h | 85 | 75 | 183 | 6 | 5 | 5 | 6 | |||||||||
| IL-12p40, 50μl/ml, 24h | 5 | 8 | 109 | 48 | 289 | 11 | 10 | 6 | 83 | |||||||
| IL-12p40, 150μl/ml, 8h | 55 | 6 | 6 | 83 | 47 | 39 | 6 | 5 | ||||||||
| IL12-p40, 150μl/ml, 24h | 249 | 35 | 198 | 5 | 26 | 165 | 11 | 58 | 7 | 32 | ||||||
|
| ||||||||||||||||
| IL-23Ap19, 50μl/ml, 8h | ||||||||||||||||
| IL-23Ap19, 50μl/ml, 24h | 6 | 5 | ||||||||||||||
| IL23Ap19, 150μl/ml, 8h | 6 | 9 | 5 | |||||||||||||
| IL23Ap19, 150μl/ml, 24h | 19 | 6 | 6 | 5 | 6 | 7 | 7 | 35 | 23 | 5 | ||||||
|
| ||||||||||||||||
| IFN-γ, 50μl/ml, 8h | 46 | 8 | 1252 | 13 | 40 | |||||||||||
| IFN-γ, 50μl/ml, 24h | 14 | 25 | 143 | 17 | 365 | |||||||||||
| IFN-γ, 150μl/ml, 8h | 36 | 28 | 15 | 18 | 81 | 5 | ||||||||||
| IFN-γ, 150μl/ml, 24h | 5 | 6 | 7 | 48 | ||||||||||||
Fold increases of ≥ 5 are shown. Each subject was tested twice with blood samples drawn at least 1 month apart.
Supernatants from above experiments were analyzed by ELISA for the presence of IL12p40 protein. The amounts detected in media only wells was subtracted from Candin treated (10, 50 or 150 μl/ml) wells. IL12p40 protein was detected in 13 of 46 samples tested and ranged from 2.4 to 932.4 pg/ml. IL12p70 protein was analyzed only in samples treated with Candin at 150 μl/ml, and the amount in corresponding untreated samples were also subtracted. IL12p70 was detected in 5 of 14 samples treated with Candin (range 3 to 62 ng/ml).
3.3 Candin induced expression of PRRs by LCs
RNAs extracted from samples treated with Candin (n = 6) were also analyzed for the expression of PRRs (Table 1). All PRRs examined were amplified at 24 hours in all 6 individuals. When expression over untreated (≥ 5 fold over untreated) was analyzed, expression of DC-SIGN and TLR 9 was upregulated in 2 subjects (5 to 67 fold and 7 to 13 fold respectively), and expression of mincle and mannose receptor was upregulated in 1 subject (9 fold and 5 fold respectively).
3.4 Dectin-1 is utilized in some subjects in IL-12 production
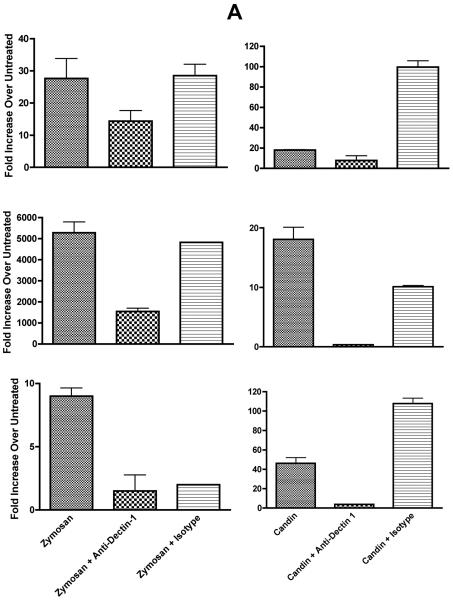
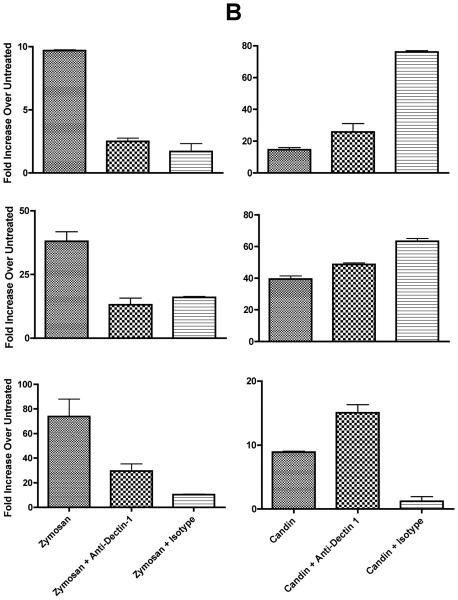
We proceeded to investigate the role of dectin-1 since zymosan was the only positive control reagent which almost always resulted in IL-12p40 production by qRT-PCR (data not shown), and IL-12p40 was the most commonly expressed cytokines in Candin-treated LCs. Of 11 subjects examined, all showed ≥ 5 fold increase over untreated (range 6 to 5,282 fold) with zymosan all of which were blocked by the anti-dectin-1 antibody [≥ 50% blocking in 9 subjects, 40% in one, and 33% in one[24]]. Six of 11 subjects (55%) demonstrated IL-12p40 production with Candin treatment (Fig. 3), and more than 50% blocking by anti-dectin-1 was shown in three subjects (Fig. 3A) indicating that dectin-1 has a role in IL-12 production upon Candin stimulation in some individuals.
Fig. 3.
A, In three healthy subjects with positive Candin responses, antibody blocking with anti-dectin-1 antibody was demonstrated suggesting the role of dectin-1 in the signaling pathway resulting in IL-12 production by LCs. B, In three other healthy subjects with positive Candin responses, no blocking with anti-dectin-1 antibody was observed suggesting that dectin-1 does not have a role in IL-12 secretion in these individuals. The values are IL-12p40 fold increase over untreated ± SD.
3.5 Intracellular cytokine expression in Candin treated LCs
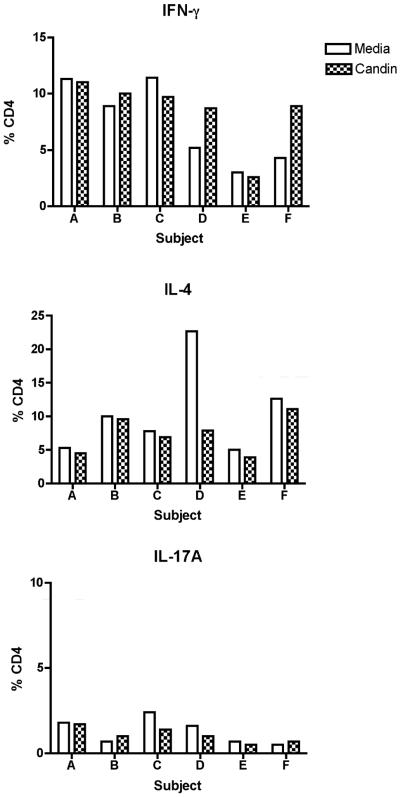
In seven healthy subjects, intracellular secretion of IFN-γ (Th1), IL-4 (Th2), and IL-17A (Th17) after stimulation of CD4 T-cells with Candin exposed LCs were examined (Fig. 4). Small increases in IFN- γ in Candin treated cells compared to media control were seen in two subjects (D and F in the top panel of Fig. 4). A decrease in IL-4 secretion was also seen in D. Minimal changes were observed in the IL-17A levels. Therefore, in some subjects a small increase in Th1 response, and a decrease in Th2 response were observed in response to Candin stimulation of LCs.
Fig. 4.
Intracellular cytokine staining examining expressions of IFN-γ, IL-4, and IL-17A by CD4 T-cells from six healthy subjects stimulated with LCs exposed to Candin.
4. Discussion
Traditionally, recall antigens which typically include a panel of Candida, mumps, and Trichophyton were used as a control to indicate an intact cellular immunity when patients were being tested for Tuberculosis by placement of PPD intradermally. T-cell mediated inflammation would become evident in 24 to 48 hours [25]. A number of studies have demonstrated that recall antigen injections can also be used to treat common warts [1–5, 7]. When proliferative capacity of T-cells exposed to Candin was examined in this study, proliferation was observed in seven of nine (78%) of healthy subjects. This proportion is similar to the percentage of people known to be skin test positive for Candida (75%) [25], and similar to the proportion of subjects who demonstrated complete resolution of Candin-treated common warts (82%) [6]. Therefore, it is tempting to speculate that Candin would have immune-enhancing effects in 70 to 80% of the population.
In recent years, numerous PRRs, including TLRs [26, 27], retinoic acid inducible gene-I like receptors [28], C-type lectin receptors [29, 30], nucleotide-binding and oligomerization-like receptors [28], and scavenger receptors [31], have been described, and their role in inducing innate and potentially adaptive immunity has been studied. A number of unrelated findings support the notion that recall antigens may work through PRRs to impart immune enhancing effects. When challenged with Candida albicans, mice deficient in myeloid differentiation factor 88, a universal TLR adaptor protein, demonstrated impaired survival compared to wild-type mice [16]. Such impairment corresponded not only with deficient production of Th1 cytokines, but also with greatly diminished frequencies of IFN-γ–producing CD4 and CD8 T-cells. A particular polymorphism in TLR 1 has been associated with impaired TLR 1 function, and predisposition to candidemia in humans suggesting that TLR 1 has an important role in fighting Candida infection [20]. Also, some evidence suggests that Candida albicans may work through TLR 2 [16], TLR 4 [13], and TLR 6 [12]. Mice deficient in TLR 2 or TLR 4 had impaired defense against Candida albicans infection. In TLR 2-deficient mice (TLR2−/−), in vitro production of TNF-α and macrophage inhibitory protein-2 (MIP-2) by macrophages was reduced. In TLR 4-defective C3H/HeJ mice, production of CXC chemokines KC and MIP-2 were decreased. The defects in both mouse strains resulted in decreased neutrophil recruitment. On the other hand, TLR 6-deficient mice (TLR6−/−) did not have increased susceptibility to Candida infections; rather, these mice demonstrated defective production of IL-10 and increased production of IFN-γ, resulting in imbalanced Th1 and Th2 responses. Involvement of TLR 9 has also been studied with results suggesting the DNA from Candida albicans activates TLR 9-mediated signaling pathways in a manner independent of the unmethylated CpG motif [19].
C-type lectin receptors include mannose receptors [32], mannose-binding lectins [33], DC-SIGN and related receptors [33], and β-glucan receptors (such as dectin-1) [34, 35]. Dectin-1 has an important role in the recognition of pathogenic fungi (Candida albicans, Aspergillus fumigatus, Pneumocystis carinii) and also has been shown to collaborate with TLR2 in the activation of NF-κB [9]. The extracellular domain of dectin-2 has been shown to bind to the hyphal components of Candida albicans, Microsporum audouinii, and Trichophyton rubrum [15]. Galectin-3, a lectin, interacts with β-1,2 mannosides on Candida albicans [36]. Cambi et al. have demonstrated that DC-SIGN and mannose receptors specifically mediate the binding and internalization of Candida albicans by human dendritic cells [8]. Another C-type lectin, mincle, plays an essential and non-redundant role in Candida-induced TNF-α induction in mouse macrophages [17]. In order to obtain a clue as to which PRR(s) may be working to transmit the signals from Candin, the presence of PRRs known to bind to components of Candida albicans were studied. All PRRs examined were detected. In addition, we examined the amplification of these PRRs upon Candin stimulation since increased PRR expression is sometimes associated with activation [37, 38]. A few receptors (DC-SIGN, mincle, monocyte receptor, and TLR-9) were increased in expression upon Candin treatment in very few healthy subjects. Therefore, this exercise was not helpful in identifying the relevant PRR(s). Among the number of PRR agonists used in qRT-PCR analyses, zymosan was the only agonist which resulted in the expression of IL-12p40 from LCs of almost all individuals. Thus, we proceeded to investigate the role of dectin-1. Blocking with anti-dectin-1 antibody demonstrated that dectin-1 seems to be utilized in some (three of six) individuals who responded to Candin with increased IL-12p40 (Fig. 3). Dectin-1 and TLR 2 collaborate in the activation of NF-κB [9], and dectin-1 can also independently mediate NFAT activation in dendritic cells leading to induction of key inflammatory mediators including IL-12p70 [39]. Therefore, it would be interesting to investigate whether Candin has any role in NF-κB and NFAT activation in the future.
IL-12p40 and IL-12p70 were also detected at a protein level, which would suggest that dectin-1-mediated effect of Candin is Th1 promoting. Candida albicans has been shown to have both Th1 and Th17 promoting effects [21, 22]; however, we did not observe expression of cytokines such as IL-1b, IL-6, and IL-23 by Candin-treated LCs that have been shown to lead to IL-17 secretion [21].
When common warts were treated with Candin, the largest wart was injected every three weeks. In addition to the treated warts which completely regressed in 82% of the patients (nine of 11), 75% of first distant warts (six of eight) and 100% of second distant warts (six of six) completely resolved suggesting the induction of HPV-specific immunity [6]. Indeed, T-cell responses to HPV 57 L1 peptide 380–412 were detected in 67% of complete responders. In this study, we further characterized the T-cell responses to Candin by staining CD4 T-cells intracellularly for IFN-γ, IL-4, and IL-17A. IFN-γ was detected in higher percentages of CD4 T-cells in two of seven healthy subjects examined upon Candin treatment, and the percentage of IL-4 secreting CD4 T-cells was decreased in one subject. Although we may anticipate Candin treatment of common warts to be effective in 70% to 80% of individuals, there may be multiple underlying mechanisms for its effectiveness. In some individuals, promotion of Th1 response possibly through a pathway involving dectin-1 may have an important role.
Since Candida albicans is known to contain multiple PRR agonists [8–19], at least their subset is expected to be contained in Candin. Synergic effects of use of multiple agonists in inducing T-cell responses have been reported [40, 41]. Ahonen et al. reported CD8 T-cell responses that were 10 to 20 times greater with TLR 7 and CD40 agonists combined as a vaccine adjuvant. Synergy with CD40 agonist was also shown with agonists for TLR 2/6, 3, 4, and 9. The CD8 T-cell expansion was dependent on B7-mediated co-stimulation and on type I IFN [40]. Further investigation by the same group showed that regulated expression of CD70 on dendritic cells was behind the CD8 T-cell expansion [41]. It is difficult to obtain an FDA approval for a vaccine using a novel adjuvant. Candida skin test reagents such as Candin are already FDA approved, and they have been in clinical use for decades. Therefore, the use of such reagents as vaccine adjuvants is expected to gain more expeditious FDA approval. We are now investigating the potential of Candin to enhance immune response as a vaccine adjuvant for a peptide-based human papillomavirus therapeutic vaccine, and have opened enrollment (NCT01653249). The results of the current study suggest that Candin may also work well as a vaccine adjuvant since T-cell proliferative activity was shown in 78% of healthy subjects tested, and since promotion of Th1 response appeared to be the mechanism in at least some individuals.
Highlights
Most healthy subjects showed T-cell proliferation with Candid-pulsed Langerhans cells.
Interleukin 12 was the most frequently secreted cytokine by Candida-pulsed Langerhans cells.
Dectin-1 appears to be utilized in IL-12 expression in some individuals.
Table 3A.
IL-12p40 Protein Expressed by LCs upon Candin Stimulation
| Subject-Blood Draw # | Candin dose (μl/ml) | IL12p40 (pg/ml) |
|---|---|---|
| 1-1 | 50 | 18.2 |
| 150 | 75.6 | |
| 1-2 | 50 | 37.2 |
| 150 | 11.6 | |
| 2-1 | 10 | |
| 50 | ||
| 150 | ||
| 2-2 | 10 | |
| 50 | ||
| 150 | 47.6 | |
| 3-1 | 10 | |
| 50 | ||
| 150 | ||
| 3-2 | 10 | |
| 50 | 2.4 | |
| 150 | ||
| 4-1 | 10 | |
| 50 | ||
| 150 | ||
| 4-2 | 10 | |
| 50 | ||
| 150 | ||
| 5-1 | 10 | |
| 50 | 57.9 | |
| 150 | ||
| 5-2 | 10 | 932.4 |
| 50 | 746.9 | |
| 150 | ||
| 6-1 | 10 | |
| 50 | ||
| 150 | ||
| 6-2 | 10 | |
| 50 | ||
| 150 | ||
| 7-1 | 10 | |
| 50 | 22.6 | |
| 150 | ||
| 7-2 | 10 | |
| 50 | ||
| 150 | ||
| 8-1 | 10 | |
| 50 | ||
| 150 | ||
| 8-2 | 10 | 20.2 |
| 50 | 34.7 | |
| 150 | 36.7 |
Any detectable amounts at 24 hours are shown.
Values from untreated wells have been subtracted from Candin treated wells.
Table 3B.
IL-12p70 Protein Expressed by LCs upon Candin Stimulation
| Subject-Blood Draw # | Candin dose (μl/ml) | IL12p70 (pg/ml) |
|---|---|---|
| 2-1 | 150 | |
| 2-2 | 150 | 0.055 |
| 3-1 | 150 | |
| 3-2 | 150 | 0.062 |
| 4-1 | 150 | 0.003 |
| 4-2 | 150 | 0.049 |
| 5-1 | 150 | |
| 5-2 | 150 | 0.057 |
| 6-1 | 150 | |
| 6-2 | 150 | |
| 7-1 | 150 | |
| 7-2 | 150 | |
| 8-1 | 150 | |
| 8-2 | 150 |
Any detectable amounts at 8 hours are shown.
Values from untreated wells have been subtracted from Candin treated wells.
Acknowledgements
This study was supported by grants from NIH (R01 CA143130, UL1TR000039, and P20GM103625), the American Cancer Society (RSG-06-180-01-MBC), UAMS College of Medicine (Intramural Gant for an Individual Pilot Study), and the National Natural Science Foundation of China (No. 81101989).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests Mayumi Nakagawa is an inventor named in the patent application for the peptide-based HPV therapeutic vaccine mentioned in the discussion section (U. S. Serial No. 12/286,822).
References
- [1].Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]
- [2].Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- [3].Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: a safe and effective therapy. J Drugs Dermatol. 2004;3:263–5. [PubMed] [Google Scholar]
- [4].Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol. 2001;137:451–5. [PubMed] [Google Scholar]
- [5].Phillips RC, Ruhl TS, Pfenninger JL, Garber MR. Treatment of warts with Candida antigen injection. Arch Dermatol. 2000;136:1274–5. doi: 10.1001/archderm.136.10.1274-a. [DOI] [PubMed] [Google Scholar]
- [6].Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O'Bryan K, et al. Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol. 2010;146:1431–3. doi: 10.1001/archdermatol.2010.350. [DOI] [PubMed] [Google Scholar]
- [7].Maronn M, Salm C, Lyon V, Galbraith S. One-year experience with candida antigen immunotherapy for warts and molluscum. Pediatr Dermatol. 2008;25:189–92. doi: 10.1111/j.1525-1470.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- [8].Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–9. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- [11].Ku JK, Kwon HJ, Kim MY, Kang H, Song PI, Armstrong CA, et al. Expression of Toll-like receptors in verruca and molluscum contagiosum. J Korean Med Sci. 2008;23:307–14. doi: 10.3346/jkms.2008.23.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008;52:118–23. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- [13].Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–9. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- [14].Nisini R, Torosantucci A, Romagnoli G, Chiani P, Donati S, Gagliardi MC, et al. beta-Glucan of Candida albicans cell wall causes the subversion of human monocyte differentiation into dendritic cells. J Leukoc Biol. 2007;82:1136–42. doi: 10.1189/jlb.0307160. [DOI] [PubMed] [Google Scholar]
- [15].Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–66. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- [16].Villamon E, Gozalbo D, Roig P, O'Connor JE, Fradelizi D, Gil ML. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7. doi: 10.1016/j.micinf.2003.09.020. [DOI] [PubMed] [Google Scholar]
- [17].Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–13. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- [18].Jouault T, El Abed-El Behi M, Martinez-Esparza M, Breuilh L, Trinel PA, Chamaillard M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–87. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- [19].Miyazato A, Nakamura K, Yamamoto N, Mora-Montes HM, Tanaka M, Abe Y, et al. Toll-like receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect Immun. 2009;77:3056–64. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, et al. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934–43. doi: 10.1093/infdis/jir867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- [22].Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–84. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fahey LM, Raff AB, Da Silva DM, Kast WM. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J Immunol. 2009;182:2919–28. doi: 10.4049/jimmunol.0803645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Inovio Product Pipeline: VGX-3100: Theraeputic Vaccine for Cervical Dysplasia/Cancer. [Google Scholar]
- [25].Esch RE, Buckley CE., 3rd A novel Candida albicans skin test antigen: efficacy and safety in man. J Biol Stand. 1988;16:33–43. doi: 10.1016/0092-1157(88)90027-3. [DOI] [PubMed] [Google Scholar]
- [26].Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13:859–75. doi: 10.1634/theoncologist.2008-0097. [DOI] [PubMed] [Google Scholar]
- [27].Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [28].Creagh EM, O'Neill LAJ. TLRs, NLRs and RLRs: a trinity of pathogen sensor that cooperate in innate immunity. TRENDS in Immunology. 2006;27:352–7. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [29].Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- [30].Weis W, Taylor M, Drickamer K. The C-type lectin superfamily in the immune system. Immunological Review. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- [31].Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cellular Microbiology. 2009;11:1160–9. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- [32].Stahl P, Ezekowitz R. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–5. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- [33].Jack D, Klein N, Turner M. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunological Review. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- [34].Brown G, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413 doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- [35].Brown G, Herre J, Williams DL, Willment J, Marshall A, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. Journal of Experimental Medicine. 2003;197:1119. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jouault T, Behi MEA-E, Matinez-Esparza M, Breuilh L, Trinel P-A, Chamaillard M, et al. Specific Recognition of Candida albicans by Macrophages Requires Galectin-e to Discriminate Saccharomyces cerevisiae and Needs Association with TLR2 for Signaling. Journal of Immunology. 2006;177:4679–87. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- [37].Biswas I, Garg I, Singh B, Khan GA. A key role of toll-like receptor 3 in tissue factor activation through extracellular signal regulated kinase 1/2 pathway in a murine hypoxia model. Blood Cells Mol Dis. 2012;49:92–101. doi: 10.1016/j.bcmd.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [38].Sinha S, Guo Y, Thet S, Yuan D. IFN type I and type II independent enhancement of B cell TLR7 expression by natural killer cells. J Leukoc Biol. 2012;92:713–22. doi: 10.1189/jlb.0212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–15. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- [40].Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–84. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–72. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]