Abstract
Tremor is the most common movement disorder presenting to an outpatient neurology practice and is defined as a rhythmical, involuntary oscillatory movement of a body part. The authors review the clinical examination, classification, and diagnosis of tremor. The pathophysiology of the more common forms of tremor is outlined, and treatment options are discussed. Essential tremor is characterized primarily by postural and action tremors, may be a neurodegenerative disorder with pathologic changes in the cerebellum, and can be treated with a wide range of pharmacologic and nonpharmacologic methods. Tremor at rest is typical for Parkinson’s disease, but may arise independently of a dopaminergic deficit. Enhanced physiologic tremor, intention tremor, and dystonic tremor are discussed. Further differential diagnoses described in this review include drug- or toxin-induced tremor, neuropathic tremor, psychogenic tremor, orthostatic tremor, palatal tremor, tremor in Wilson’s disease, and tremor secondary to cerebral lesions, such as Holmes’ tremor (midbrain tremor). An individualized approach to treatment of tremor patients is important, taking into account the degree of disability, including social embarrassment, which the tremor causes in the patient’s life.
Keywords: Tremor, essential tremor, Parkinson’s disease, dystonia, pathophysiology
Tremor is defined as a rhythmical, involuntary oscillatory movement of a body part that is produced by alternating contractions of reciprocally innervated muscles.1,2 It is the most commonly encountered movement disorder symptom, and is frequently evaluated and treated in family medicine, internal medicine, emergency medicine, and of course in neurology practices.3,4 When assessing a patient with tremor, the phenomenology on the tremor, the presence or absence of other neurologic signs or symptoms, and the possible modifying influence of medications or alcohol are important factors to be determined. The patient’s history and a targeted neurologic examination will usually suffice to diagnose the cause of the tremor.
A wide array of treatment modalities are available for tremor, and most depend on the type or the underlying cause of the tremor. Treatment is tailored individually, taking into account the objectively measurable tremor severity, the degree of disability or impairment experienced by the patient, including embarrassment in social situations, as well as the patient’s preference among the various treatment options.5 The majority of patients with tremor have relatively mild symptoms and some may benefit from reassurance alone. The overall effectiveness of pharmacologic treatments of tremor unfortunately remains mediocre, and patients frequently decide to discontinue such treatments. A fraction of patients with tremor has such severe symptoms that surgical procedures, such as deep brain stimulation (DBS), may be necessary. In this article, we provide the clinician with a review of the assessment, pathophysiology, and treatment of the more common forms of tremor.
PATHOPHYSIOLOGY OF TREMOR
Progress has been achieved in mapping tremors to certain structures or pathways in the nervous system, even though the exact pathophysiology of tremor is still incompletely understood. Two basic principles have been postulated in tremorogenesis. One emphasizes a functional hyperexcitability and rhythmic oscillation of neuronal loops in the absence of structural changes. This hyperexcitability has been studied with neurophysiologic techniques in humans and animals, and modeled in dynamic mathematical paradigms.6 Complete reversibility of some tremor symptoms after alcohol ingestion or with medication has been interpreted as evidence for an overwhelmingly or exclusively functional disturbance. The second principle is that of a permanent structural pathology with signs of neurodegeneration. This concept has more recently received renewed attention after systematic pathologic studies of patients with essential tremor revealed characteristic pathologic changes.4
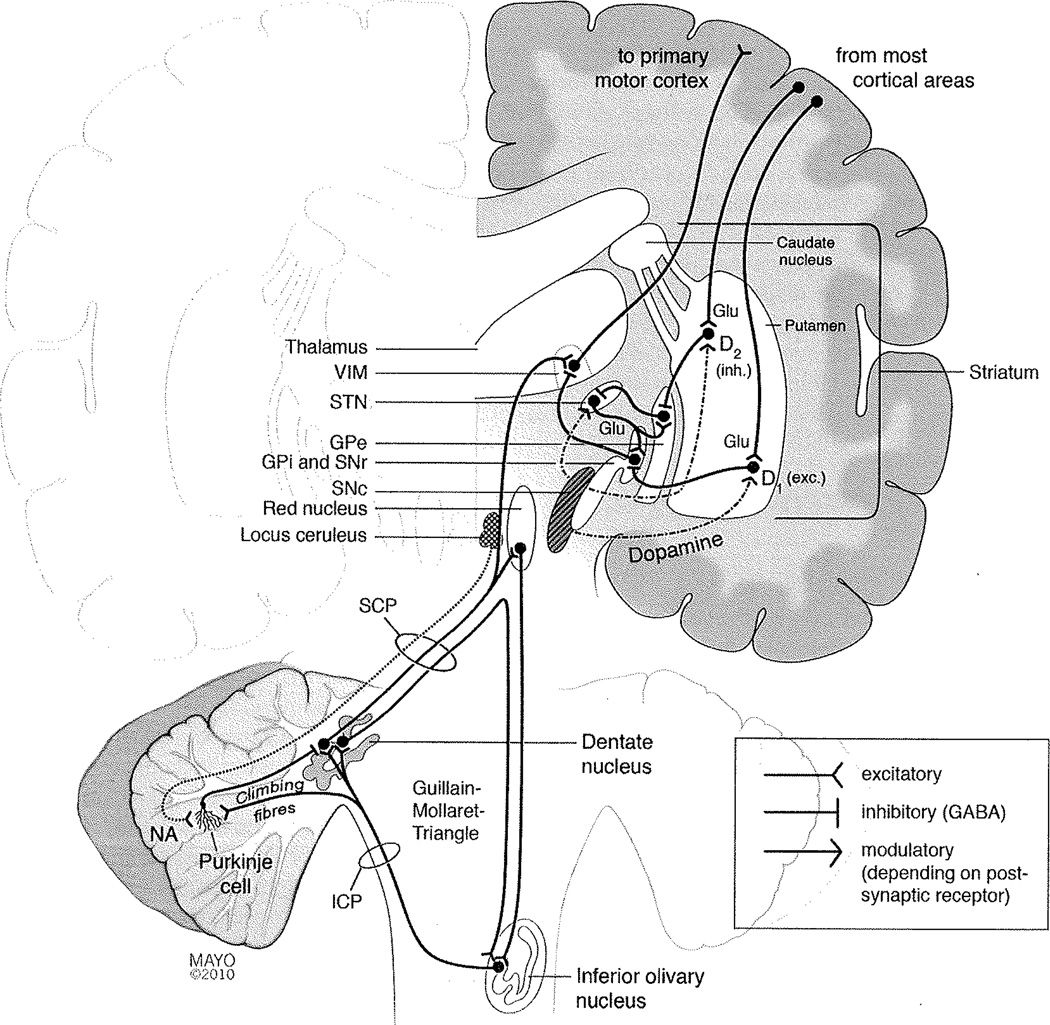
Two sets of neuronal networks are of particular importance (Fig. 1). One is the corticostriatothalamocortical hap through the basal ganglia, whose physiologic task is the integration of different muscle groups for complex movement programs. This loop also ensures that an ongoing movement program will not be terminated or disturbed by minor or irrelevant external influences. The other circuit involves the red nucleus, inferior olivary nucleus (ION), and the dentate nucleus, forming the triangle of Guillain and Mollaret (Guillain-Mollaret triangle). This circuit’s main physiologic task is to fine-tune voluntary precision movements. Among its components, probably the ION plays the most important role in the genesis of tremor. The neurons of the ION receive their input from the red nucleus, and project as climbing fibers to Purkinje cells in the cerebellar cortex. The individual ION neurons are connected by gap junctions and can thereby act as a synchronized neuronal ensemble.7 In healthy individuals, ION neurons exhibit regular oscillatory depolarizations mediated by calcium-channels.8 These oscillations serve an important physiologic purpose as pacemakers in the timely processing and temporal coordination of the cerebellar modulation of precision movements as well as in cerebellar motor learning.7 A line of evidence suggests that such synchronized oscillations of ION neurons also are involved in the genesis of tremor. The β-carboline alkaloids harmine, harmaline, and tetrahydroharmine from the Harmal plant (Peganum harmala, “Syrian Rue”), increase ION neuron excitability. Although the seeds and roots of this plant also have hallucinogenic and antinociceptive properties, and have been used as an entheogen for many centuries,9 a transient cerebellar syndrome with dysmetria and nystagmus as well as intention and postural tremor were documented after ingestion of high doses.10 This effect is also seen in animals, and in fact harmaline is frequently used to model essential tremor in animals.11 In such animals, harmaline-induced tremors are abolished by lesioning the ION, emphasizing harmaline’s impact on the triangle of Guillain and Mollaret.12 In addition to chemical substances, structural lesions affecting this circuit can cause tremor. Lesions damaging afferents to the ION are responsible for symptomatic palatal tremor associated with reactive hypertrophic degeneration of the ION.13 Hypertrophy of the ION is also seen in the rare syndrome of progressive ataxia and palatal tremor, further implicating ION in tremorogenesis.14
Figure 1.
Schematic and simplified synopsis of the brain regions and pathways involved in tremorogenesis. See text for details. (D1, Dopamine receptor type 1; D2, dopamine receptor type 2; exc., excitatory; GABA, γ-amino butyric acid; Glu, glutamate; GPe, external globus pallidus; GPi, internal globus pallidus; ICP, inferior cerebellar peduncle; inh., inhibitory; SCP, superior cerebellar peduncle; SNc, substantia nigra, pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; VIM, ventrointermediate nucleus of thalamus.)
GENERAL APPROACH TO AN OUTPATIENT WITH TREMOR
Tremor Phenomenology, Terminology, and Classification
Assessment of a patient with tremor starts with the characterization of the tremor phenomenology, which narrows down the differential diagnosis and often can establish a diagnosis. Tremors can be classified according to various parameters (Table 1). The most important parameter for tremor evaluation is describing when the tremor occurs in relation to movements or position of the affected body part, distinguishing between tremor at rest (rest or resting tremor) and action tremor. This distinction helps in grouping tremors according to their pathophysiology and etiology, which in turn is highly relevant for choosing the most promising treatment option.
Table 1.
Parameters for the Clinical Characterization of Tremor
| Tremor at rest—action tremor |
| Location (affected body part) |
| Frequency |
| Rhythmicity |
| Amplitude |
| Exacerbating or alleviating factors |
| Other neurologic signs or symptoms |
Tremor at rest denotes a tremor in a body part that is not voluntarily moved or maintained in a certain position against gravity, and typically occurs in Parkinson’s disease (PD). The other main tremor type, action tremor, includes all tremor manifestations in body parts that are not at rest, and comprises postural tremor and kinetic tremor (Table 2). In most cases, action tremor can be easily distinguished from tremor at rest. However, patients with PD may display a tremor that reoccurs when arms are maintained stretched out for some seconds (reemerging tremor). From a physiologic standpoint, this can be considered a tremor at rest, as the body part has been held motionless in this position for a period of time. Thus, tremor that reemerges after a short period should not be classified as true postural tremor.
Table 2.
Common Types of Tremor
| Type | Characteristics |
|---|---|
| Rest tremor (tremor at rest) | Tremor in a body part that is not voluntarily activated and is completely supported against gravity (includes reemerging tremor, see text). |
| Action tremor | Any tremor that is produced by voluntary contraction of muscle |
| Postural tremor | Tremor that is present while voluntarily maintaining a position against gravity. Rarely, the tremor may specifically occur in certain positions, but not in others (position-specific tremor). |
| Kinetic tremor | Tremor that occurs during any voluntary movement |
| Unspecified kinetic tremor | During nongoal-directed movements |
| Intention tremor | During goal-directed movements |
| Task-specific kinetic tremor | Appears only, or becomes markedly exacerbated, during specific activities |
| Isometric tremor | Occurs as a result of muscle contraction against a rigid stationary object |
Adapted from Bhidayasiri R. Differential diagnosis of common tremor syndromes. Postgrad Med J 2005;81(962):756–762, with permission from BMJ Publishing Group Ltd.
Other tremor characteristics are the location or distribution in the different body parts, the tremor frequency, the presence of exacerbating or alleviating factors, and the presence of other neurologic signs or symptoms (Table 1). These characteristics are rarely specific for a certain cause of tremor. For example, essential tremor may or may not improve after alcohol ingestion, but also other types of tremor may improve with alcohol.15
TREMOR FREQUENCY
The frequency of a tremor can be approximated by observation with the naked eye, and more accurately measured with surface electromyography. The most often encountered tremors have frequencies between 4 and 12 Hz.1 Tremor in PD usually has a slower frequency of between 3 and 5 Hz, and essential tremor and enhanced physiologic tremor range from 5 to 10 Hz. However, although there may be general differences in average tremor frequency among different types of tremors, the frequencies overlap considerably between different disorders. Thus, the exact determination of tremor frequency rarely adds decisive new information when the cause of a tremor in an individual patient is uncertain. Exceptions are unusually fast or slow tremor frequencies, which may help to establish a correct diagnosis. Tremor frequencies below 4 Hz occur in PD, cerebellar disease, Holmes’ tremor (midbrain tremor), drug-induced or palatal tremors.1 Primary orthostatic tremor has a high frequency of 12 to 18 Hz.1 On the other hand, the clinical appearances of these syndromes are often characteristic enough for an accurate diagnosis without measuring tremor frequency.
TREMOR TERMINOLOGY
The nomenclature of tremors is not standardized and sometimes confusing, and some terms may have different meanings. Strictly speaking, the term “intention tremor” only denotes rhythmic, oscillatory movements. However, the term is also sometimes used to describe more irregular, ataxic movements. Both represent a disturbance in the fine-tuning of goal-directed movements and point toward the cerebellum or its inflow- and outflow tracts. Similarly, the expression “dystonic tremor” usually stands for arrhythmic (i.e., the intervals between the movements are not equal) and/or irregular (i.e., amplitudes vary from one movement to the next) movements that are thus, not “tremor” according to its definition. However, both intention tremor and dystonic tremor are included in this review, as they are important differential diagnoses along with “true” tremors, and are commonly referred to as “tremors.” We prefer the term “intention tremor” to “cerebellar tremor,” as other types of tremor also involve the cerebellum (see below). Patients may display several types of tremor and it can be challenging to separate the single components. A general rule is to name the predominant tremor after the position in which the largest amplitude occurs. A diagnostic problem may arise when action tremor persists at rest. If an action tremor persists with the same amplitude during rest, by convention the tremor is considered an action tremor.1
Interview and Clinical Examination of Patients with Tremor
When assessing a patient with tremor, the type of tremor (Table 2) is characterized and other manifestations of a possible underlying neurologic disorder are actively sought. A thorough neurologic examination of a patient presenting with tremor includes the following:
Tremor at rest may be seen when observing the patient with the affected body part neither voluntarily activated nor supported against gravity. It can become more pronounced when the patient is concentrating on other tasks, e.g., when walking or during a conversation. Postural tremor that was not seen in other parts of the examination can become visible when the patient holds the upper extremities in an outstretched position with the hands supine, prone, and in the wing position (i.e., with the index fingers pointing at each other in front of the thorax but not touching). Irregular hand or finger movements in these positions are not tremor. Sudden loss of muscle tone with a sudden drop of a finger or hand, succeeded by a corrective movement back to the initial position, indicates negative myoclonus. Intention tremor is characterized by overshooting movements of increasing amplitude when approaching a goal. It can be elicited in goal-directed activities, such as finger-to-nose, heel-to-shin, and toe-to-finger movements. Observing a patient while drawing (e.g., Archimedes spirals) or writing is often helpful: Action tremor is increased during writing or drawing, and a task-specific tremor may become obvious. In PD, there usually is no tremor during writing, but other signs can be seen, such as increasing micrographia and slow movements. Pouring water from one cup into another shows the degree of disability due to kinetic tremor in a practical situation.
Important clues about an underlying neurologic disorder in patients presenting with a tremor can be found during the examination of the cranial nerves, speech, gait, balance, and muscle tone. Eye movement abnormalities may suggest cerebellar disease, and Kayser-Fleischer rings are specific for impaired copper homeostasis, although their absence does not exclude Wilson’s disease. Torticollis, blepharospasm, or orofacial twitching may indicate dystonia. These signs can be very mild, in which case the patient may not be aware of any disturbance. Several movement disorders affect the fine-tuned movements of the tongue, where possible abnormal findings include fasciculations or slowness of tongue movements. Slow and irregular speech with increased separation of syllables or explosive sounds may indicate cerebellar dysarthria. Dystonia can manifest as spasmodic dysphonia, with effortful, jerky, strained sounds in the adductor type of spasmodic dysphonia, or a breathy, whispering voice with sudden breaks in the abductor type of spasmodic dysphonia. Typical parkinsonian or cerebellar gait may be noted, and muscular rigidity in combination with a tremor at rest is typical for PD, whereas spasticity may develop in multiple sclerosis.
As with other movement disorder symptoms, the severity of a patient’s tremor may wax and wane considerably over time, and is influenced by the patient’s emotional state. Although the opposite may be true, generally action tremors will be more severe during an office visit (which usually is accompanied by some uneasiness or anxiety), and tremors at rest will become less obvious or not visible at all. Thus, observations made during a short office visit may be misleading, and information from the patient (or proxy) is important.
Identifying Drugs and Toxins that May Cause Tremor
The list of medications and toxins that can cause tremor is long. A comprehensive history must include all medications that a patient is taking, as well as possible exposure to toxins. Table 3 summarizes the most common medications and toxins that cause tremor. If in doubt, reports of the given drug inducing and/or exacerbating tremor should be sought. For most of these medications, tremor is a dose-dependent side effect and will disappear as the dose is decreased or the medication discontinued. In a patient who is treated with lithium or valproate sodium and who develops tremor, the serum concentrations of these substances should be determined. Some individuals may consume coffee, tea, or other stimulants in unusually high amounts, which can be a sufficient explanation for pronounced tremors. Hyperthyroidism and hypoglycemia may also cause tremor.
Table 3.
Common Causes of Medication- or Toxin-Induced Tremors
| Class of Medication or Toxin | Examples |
|---|---|
| Beta-adrenergic agonists | Terbutaline, metaproterenol, isoetharine, epinephrine (adrenaline) |
| Antidepressants | Bupropion, lithium, tricyclic antidepressants |
| Neuroleptics | Haloperidol |
| Anticonvulsants | Valproate sodium |
| Dopamine agonists | Amphetamine |
| Heavy metals | Mercury, lead, arsenic, bismuth |
| Xanthines or derivatives | coffee, tea, theophylline, cyclosporine |
Ancillary Testing in the Assessment of Tremor Patients
In the outpatient setting, the clinical features and neurologic examination findings are the most important assessment tools in evaluating patients with tremor. Extensive laboratory testing is usually not necessary. For routine evaluation, thyroid function tests are performed in most or all patients with tremor to exclude hyperthyroidism. In patients under 55 years, serum and urine tests for Wilson’s disease may be indicated. Serum ceruloplasmin as well as serum and urine copper levels can exclude Wilson’s disease with reasonable sensitivity, but when they give ambiguous or negative results and a clinical suspicion of Wilson’s disease remains, other test methods need to be considered.16–18 Further studies are warranted in individual patients where a rare cause of the tremor is suspected, but these will rarely be used in the initial workup of a tremor outpatient.
Nonpharmacologic Treatment
Various nonpharmacologic treatment options for tremor are available, most of which are not specific for a tremor of a certain etiology. Coping strategies form an integral part in the care of a tremor patient. Simple advice may sometimes be helpful, such as avoiding the use of a computer mouse or laser pointer, which magnify tremor movements, in situations where this is embarrassing. The patient can be encouraged to inform others openly about his or her propensity to tremors and about their benign nature. Some patients may seek medical advice because of concerns that the tremor may be the first sign of a severe disorder such as PD, amyotrophic lateral sclerosis, or a brain tumor. Such patients may not need medical treatment, but feel comfortable after reassurance that their tremor does not herald a more severe disorder. Counseling should include stressing the benign natural course of a particular tremor when appropriate. Agents that are suspected to cause or worsen a tremor should be removed whenever possible. Nonpharmacologic symptomatic treatment options include the use of larger utensil handles or wrist weights, or occupational assessments and advice.19,20 Positive effects of biofeedback, acupuncture, and whole body sound wave vibration therapy on tremor have been reported.21–23
SPECIFIC TYPES OF TREMOR
This section describes the types of tremor that are most commonly encountered in an outpatient setting and rarer, but important differential diagnoses that require specific treatment.
Physiologic Tremor
Slight, usually bilateral postural or kinetic action tremor, particularly in the hands and fingers, is a normal phenomenon and does not indicate a disorder (physiologic tremor). Physiologic tremor is more intense in situations of stress or anxiety, after strenuous physical work or exercise, or after ingestion of caffeine or other stimulants. More pronounced cases of easily visible and usually reversible tremor without evidence of neurologic disease reflect enhanced physiologic tremor1. Usually, nonpharmacologic treatment options are sufficient. However, for some patients, even mild physiologic tremor can lead to large degrees of embarrassment and functional disability, such as the violinist at a decisive audition, or any professional giving an important presentation. The use of a β-blocker (e.g., propranolol) before such a situation may alleviate the tremor, but the optimal dose should be found prior to the important event.
Essential Tremor
Essential tremor is the most common form of tremor, and probably the most common movement disorder in general. Unfortunately, there is no uniformly accepted definition of essential tremor. Widely used definitions are those developed by the Movement Disorder Society’s Tremor Investigation Group and those used in the Washington Heights-Inwood genetic study, but several others exist.1,24–27 However, the percentage of individuals fulfilling different commonly used diagnostic criteria for essential tremor has shown considerable variation.26 The Movement Disorder Society’s Tremor Investigation Group defines essential tremor as a bilateral, largely symmetric postural or kinetic tremor involving hands and forearms that is visible and persistent, and in which there is no other explanation for the tremor.1 Additional or isolated head tremor is compatible with essential tremor as long as there is no abnormal head posturing.1 In view of the difficulties in applying essential tremor diagnostic criteria, it may be reasonable to consider treating individuals even if they do not strictly fulfill these criteria. Probably, essential tremor reflects a clinical syndrome rather than a single disease entity.
Only about half of essential tremor patients report a positive family history, which means that the term “familial tremor” is not congruent with essential tremor.26 Essential tremor may involve the voice, but not in isolation, and only rarely affects the legs. Although the diagnostic criteria require “largely symmetric” tremor, 50% of 487 consecutive individuals diagnosed with essential tremor at Mayo Clinic had asymmetrical disease, most of them with greater tremor severity on their dominant side.26 A tremor strictly confined to an ipsilateral arm and leg (hemibody tremor is less likely essential tremor and more likely secondary to a structural lesion.28 Essential tremor usually has a frequency of 5 to 10 Hz and no latency to onset. Symptom severity often increases over time, but the progression can be very slow.26,29 The majority of patients do not show accompanying neurologic signs or symptoms, but occasionally instability or more distinct cerebellar signs may be found during examination, especially in long-standing tremor.30
Several lines of evidence suggest that cerebellar function is disturbed in essential tremor. This is consistent with clinical observations of cerebellar signs, such as slight dysmetria, an ataxic gait, or a component of intention tremor within a subgroup of patients with essential tremor.30,31 Until a few years ago, essential tremor was considered a non-degenerative disorder resulting from abnormal excitability alone. More recently, relatively slight but distinct pathologic changes in essential tremor have been studied systematically.4 Different patterns of pathologic appearance were distinguishable. In one group of essential tremor patients, there were pathologic changes within the cerebellar cortex. These included the loss of Purkinje cells, rounded swellings of their axons (visible microscopically as “torpedoes”) and dendrites, a disturbed micro-architecture of the cerebellar cortex with heterotopic Purkinje cells displaced into the molecular layer, and unusually dense and tangled basket cell plexus (“hairy baskets”).4 A pathologically distinct second group of essential tremor patients had Lewy bodies in the locus ceruleus (but not in other structures, as in PD).4 The noradrenergic cells of the locus ceruleus terminate in the branches of the widely ramified Purkinje cell dendrites. Purkinje cells are γ-amino-butyratergic cells that exert an inhibitory effect on the neurons of the dentate nucleus. Cell loss in the locus ceruleus leads to decreased noradrenergic stimulation of Purkinje celis, which reduces their inhibitory effect on the dentate nucleus and the other components of the triangle of Guillain and Mollaret. This mechanism is analogous to the severe action tremor characteristic of spinocerebellar ataxia type 2 (SCA 2), whose pathologic correlate is the preferential degeneration of Purkinje cells.32 Efferent fibers of the cerebellar dentate nucleus also project to the ventrointermediate nucleus of thalamus (VIM). These more recent pathologic findings, taken together with the higher incidence of essential tremor observed in relatives of individuals with other neurodegenerative disorders, such as PD and possibly a common genetic background,33–35 have led to suggestions that essential tremor in fact also is a neurodegenerative disorder.
TREATMENT OF ESSENTIAL TREMOR
A variety of treatment options for essential tremor are available today which makes it possible, but also necessary, to select the most appropriate solution for the individual patient. The patient’s subjective experience of the tremor’s severity and the degree of impairment and disability that it causes in the patient’s life are more important than the objective assessment during the patient’s clinic visit. Such assessment may be difficult. Studies have shown that on average, physical and mental quality of life measures are lower in essential tremor patients compared with healthy individuals.36,37 Nevertheless, a considerable number of essential tremor patients have a low degree of impairment or disability and little emotional suffering from their disorder. The nonpharmacologic treatment options outlined above are considered for all patients with essential tremor.
Pharmacologic treatment may be utilized either intermittently or daily and is most effective at reducing limb tremor in essential tremor. In the absence of contraindications, propranolol or primidone are both recommended as first-line choices.15,38–40 Propranolol may be effective typically in doses of 40 to 240 mg/day. It may be prudent to obtain an electrocardiogram prior to starting propranolol, to assess for significant bradycardia, and to be cognizant of a β-blocker’s potential to induce orthostatic hypotension, especially in older patients. Primidone is not approved for the treatment of essential tremor in many countries (including the United States), but widely considered effective. It should be initiated gingerly; perhaps 12.5 mg daily, then titrated upward slowly to the lowest, effective dose, which is usually between 50 and 750 mg daily (divided into twice-daily or thrice-daily dosing). If propranolol or primidone do not provide satisfactory tremor relief, guidelines unanimously recommend the combination of propranolol plus primidone. Gabapentin, topiramate, or lorazepam are considered second- and third-line drugs.15,39,40 Clozapine or botulinum toxin injections may provide relief to patients not responding to the options above, but both have disadvantages.39,41 Clozapine confers a risk of agranulocytosis and necessitates checking regular blood cell counts. Botulinum toxin remains expensive, needs to be administered repeatedly, and there is a risk for weakness in the body parts treated. Overall, the pharmacologic treatment efficacy of essential tremor is unfortunately low. A reduction of the tremor’s severity by 75% is considered a good response, and only 40 to 50% of patients will benefit from pharmacologic treatment.3,38 The tremor will rarely disappear completely or in all situations, and thus physician and patient need to be aware that the goal of treatment is a noticeable reduction in tremor severity, not utter freedom from symptoms. A recent study that included 528 essential tremor patients found that almost one-third of patients discontinued treatment within the first year.4 This fraction was similar for those with mild or more severe tremor, and the result was largely ascribed to the inadequacy of medical treatment options.4
In one study, alcohol ingestion was more efficacious at alleviating the tremor of essential tremor than propranolol or primidone, but some patients experience a rebound worsening of tremor when the alcohol’s effect wanes.42 There are concerns about alcohol dependence and abuse, but studies addressing this issue have led to conflicting results.15 Alcohol may not be acceptable to a patient for personal, cultural, or religious reasons. Surgical treatment with deep brain stimulation (DBS) of a target within or near the VIM (Fig. 1) can improve essential tremor in patients who do not respond satisfactorily to other treatment modalities, and has a good short-term and long-term effect.43 Maximal motor improvement of motor symptoms and minimal side effects were achieved by targeting DBS at the cerebellothalamic tracts in the subthalamic area rather than the thalamus itself.44
Tremor in Parkinson’s Disease
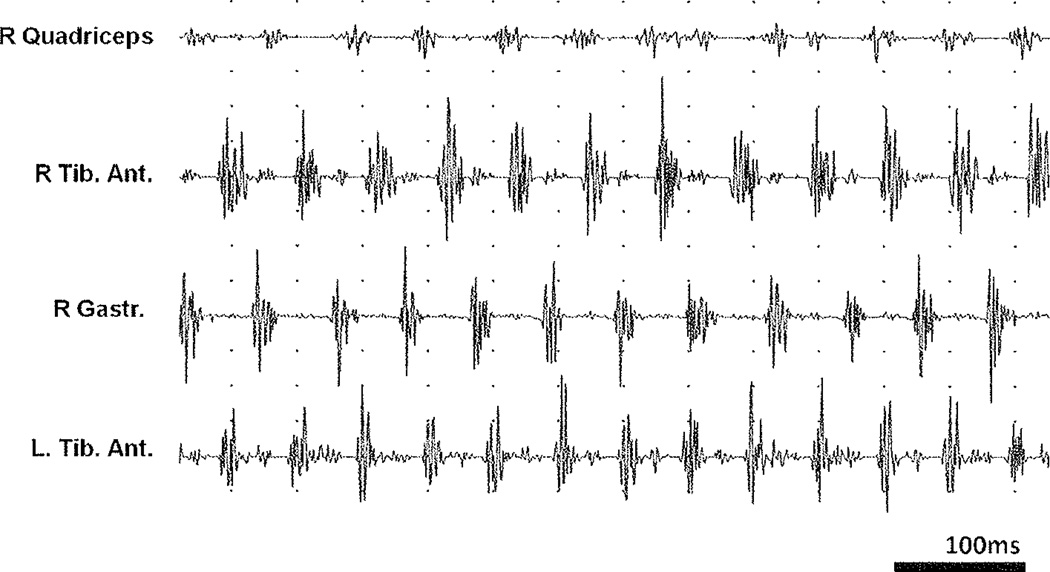
Tremor is one of the cardinal features of PD and was described in ancient Indian descriptions of the “kampavata” illness, which probably corresponds to the modern definition of PD.45 Tremor is often the presenting feature of PD, but it is not a necessary feature for this diagnosis, and ~25% of patients with PD in fact never develop tremor (akinetic-rigid form). Furthermore, tremor may diminish in later stages of the disease, when bradykinesia becomes more prominent.46 The typical and rather complex movements of parkinsonian rest tremor (Fig. 2) indicate PD with high specificity. They include agonist and antagonist activation alternating in a precisely tuned manner, often leading to a stereotypical series of movements, such as the typical pill-rolling tremor.
Figure 2.
Complex nature of tremor at rest in Parkinson’s disease (PD). Electrophysiologic surface recording study. The surface electromyography (EMG) recordings of a 67-year-old man with PD reveal a rhythmic activity with a frequency of ~5 Hz in all muscles studied. All recordings shown were performed simultaneously. The antagonists, anterior tibial and gastrocnemius muscles, are activated in shifted phases, and a slight electrical activity was detected in the quadriceps musculature on the same side. The frequency in the electrophysiologic activity on the left and on the right side differs slightly, with 13 activations in the left anterior tibial muscle, but only 12 in the right side during the period represented in the figure. This indicates that tremors originate in separate circuits in the left and right sides, and the overall picture underscores the central origin and complex nature.
There are descriptions of patients with only a rest tremor who do not subsequently develop PD, and the term monosymptomatic rest tremor has been suggested when this situation has persisted for at least 2 years.1 However, a reduced putaminal fluorodopa uptake has been found in some patients with monosymptomatic rest tremor, suggesting they may have subclinical parkinsonian syndromes.47
Diagnostic difficulties can arise when a patient only has tremor and no other signs and symptoms are found, when no tremor is visible during the office visit, or when other forms of tremor coexist.1 Rarely, PD patients may only have a kinetic tremor. Thus, a diagnosis of PD should never be based solely on tremor, but requires the presence of the other cardinal symptoms of PD, notably, bradykinesia.
Tremor in PD is often more difficult to alleviate than the hypokinetic PD manifestations (bradykinesia and rigidity).48 The tremor is not as responsive to dopaminergic therapy as the hypokinesias, or may not improve with medical treatment at all.48 An analysis of what is known about the pathogenesis of PD tremor may help explain this discrepancy. The pathologic hallmark of PD is the loss of dopamine-producing neurons in the substantia nigra pars compacta (SNc), especially its ventrolateral portion, which projects to the putamen.48,49 This induces a dopaminergic deficit in the striatum, where these neurons form synapses on neurons belonging to two distinct classical corticostriatothalamocortical circuits, known as the indirect and direct pathways (Fig. 1). Dopaminergic neurons project to striatal cells that form part of the indirect pathway. These are equipped with inhibitory D2 receptors. Thus, dopamine exerts an inhibitory net effect on the indirect pathway loop, and the dopaminergic deficit of PD reduces this inhibition. Dopamine also acts on the excitatory D1 receptors found on inhibitory striatopallidal pathway cells of the direct pathway. More recent findings also show that the anatomic connections between the brainstem nuclei are more complex than previously appreciated. Cortical neurons that activate the STN without any relay in the basal ganglia have been identified, the hyperdirect pathway.50,51 Furthermore, the “striatofugal” neurons from the striatum to the internal globus pallidus (GPi), which form part of the classical direct pathway, at least in nonhuman primates also send collaterals to the external globus pallidus (GPe).52,53 This means that the classical direct and indirect pathways are closely interwoven. Furthermore, feedback neurons from the GPe to the striatum as well as from GPe to GPi have been discovered in different mammals.54
Several intriguing findings argue against the striatonigral dopaminergic deficit directly causing PD tremor. The extent of dopamine deficiency and the degree of disease progression correlate well with the severity of rigidity and bradykinesia, but not with tremor.54–56 In statistical analyses of PD patients’ symptoms, tremor occurred independently from the other cardinal features.57 Through DBS electrodes, high-frequency oscillations were recorded from the STN in PD patients with tremor, and likewise, these oscillations correlated with akinesia and rigidity, but not with tremor.56,58 Rigidity and bradykinesia improved after the injection of GABA agonist muscimol into the pallidum, but simultaneously rest tremor deteriorated.59 In view of these findings, it has been postulated that tremor in PD results from a compensatory mechanism downstream of the disturbed basal ganglia activity.54 Another possibility is that tremor, analogous to many other signs and symptoms of PD,60 may be another consequence of the neurodegenerative changes that underlie PD, independent from the direct cause of bradykinesia or rigidity.
TTREATMENT OF TREMOR IN PARKINSON’S DISEASE
Available treatment options include dopaminergic agents, anticholinergics, β blockers, and DBS. Levodopa and dopamine agonists alleviate parkinsonian symptoms including tremor in some patients, but often tremor control is not satisfactory. Although frequently discussed, there is no convincing data showing that dopamine agonists lead to greater improvement of tremor than levodopa. The clinical trials that were performed with this question in mind either did not directly compare a dopamine agonist to levodopa61–65; did not asses tremor as primary outcome but in post hoc analyses62,65; or the recorded effect sizes, even though statistically significant, were small.63,64 It also remains uncertain whether the addition of a dopamine agonist to levodopa may lead to small improvements in tremor. In general, levodopa remains the antiparkinsonian medication producing maximal motor benefit in PD patients, with the fewest side effects. In patients younger than 60 years of age, dopamine agonists may be considered as there is some evidence for a possibly lower risk for dyskinesias in later stages of the disease, compared with when treatment was initiated with levodopa.66 More recently, 10-year follow-up data from a multicenter cohort found no such difference.67
Beta-blockers have a documented effect also in parkinsonian tremor, but may increase the orthostatic hypotension that often develops in PD, which can have serious consequences. However, in 2003 a Cochrane review of four studies could not determine whether β-blocker therapy is effective and safe for the treatment of tremor in PD and warned against bradykinesias as a side effect.68
Anticholinergic drugs were formerly used for the treatment of PD. The rationale behind their use is that the dopaminergic deficit in PD leads to a relative excess of acetylcholine in the striatum, and that anticholinergic drugs can restore a balance on a lower level of both transmitters. In fact, experience shows that anticholinergics can improve tremor in PD. However, there are no modern studies on their use, and side effects can be dramatic. Nevertheless, some authorities recommend anticholinergics as one of several treatment options for younger patients with tremor-dominant PD who do not respond to other medications.69 DBS targeting the subthalamic nucleus or VIM, stations in the indirect pathway, has been shown to be effective.70,71
Other Causes of Tremor
Many other disorders may be encountered in an outpatient clinical practice that can cause tremor or movement disorders with a similar appearance.
NEUROPATHIC TREMOR
Tremor can be a presenting or predominant sign of polyneuropathies or other lesions of peripheral nerves (neuropathic tremor). In particular, immunoglobulin-mediated forms, such as IgM-neuropathy or chronic inflammatory demyelinating polyneuropathy, may be associated with tremor. Usually, these disorders develop subacutely within weeks to months; thus, the temporal profile of tremor development is unique. On neurologic examination, other signs of peripheral neuropathy will be present. Serum electrophoresis, electrophysiologic studies, cerebrospinal fluid analysis, and sometimes nerve biopsy, can help establish a diagnosis.72 These disorders need to be diagnosed in a timely manner as they may be treatable with immunosuppressive therapies, such as corticosteroids, intravenous immunoglobulin, cyclophosphamide, or plasma exchange. The cause of excessive immunoglobulin production is usually monoclonal gammopathy of unknown significance, but a certain amount of screening tests are conducted to exclude plasmocytoma, amyloidosis, or lymphoreticular malignancy.73
TREMOR FROM CEREBRAL OR BRAINSTEM SYNDROMES
Lesions of cerebral structures implicated in tremorogenesis (Fig. 1) may cause tremor. The lesions may be a consequence of trauma, stroke, tumors, infection, or other disorders. The history and temporal development can provide clues, as these tremors develop within a shorter time.74 Treatment of the underlying disorder is paramount. Symptomatic treatments of the tremor include similar options as for other types of tremor.
Holmes’ tremor (midbrain tremor, rubral tremor, cerebellar outflow tremor) has an unusual appearance of combined rest and intention tremor, often localized to one upper extremity, associated with ipsilateral dysmetria and dysdiadochokinesia.6 Some patients may have postural tremor, often primarily in proximal muscles, as an additional feature.1 The frequency is generally below 4.5 Hz and may be irregular. Holmes’ tremor occurs as a consequence of a lesion damaging both the dopaminergic and the cerebellothalamic/cerebelloolivary systems.6,75 It does not appear simultaneously with the lesion, but after a delay of 1 to 24 months. Brainstem stroke and trauma are the most common causes. As the dopaminergic system is involved in most cases, treatment with levodopa should be attempted.75 Drugs used for the treatment of essential tremor may also be effective, and DBS in VIM has proven beneficial.6
DYSTONIC TREMOR
Dystonic tremor is a focal and mainly postural/kinetic tremor in an individual with dystonia. The tremor may occur in the same body part as the dystonia, or in different areas. Both the frequency and amplitude are often irregular and variable. A typical example is dystonic head tremor in a patient with torticollis. Diagnosis rests on finding other signs or symptoms of dystonia, bearing in mind that some symptoms, such as mild blepharospasm, a subtle voice change of spasmodic dysphonia, or a slight torticollis, may be easily missed as important clues by both the physician and the patient. Responsiveness to sensory tricks (gestes antagonistiques) indicates a dystonic tremor. Many dystonias are hereditary, and signs and symptoms of dystonia in a relative may help establish a correct diagnosis. The precise relationship of dystonic tremor to dystonia has been debated. It has been suggested that dystonic movements may be tremulous in nature, or that dystonic tremor results from a more or less conscious attempt of the patient to restore normal body position against the permanent dystonic muscle contraction.6 No intrinsic oscillatory rhythm has been found or proposed, in line with the lack of rhythmicity generally observed in dystonic tremor. In dystonia, activity in the lentiform nuclei (putamen, external and internal globus pallidus) at rest is generally disturbed, and there are cortical abnormalities in areas of the sensorimotor circuitry.76 Botulinum toxin injections can ameliorate dystonia and dystonic tremor, and are accepted as the treatment of choice.39 In patients where botulinum toxin is insufficient or impracticable, dystonic tremor can be treated with functional lesioning of the common outflow from the basal ganglia through the GPi and VIM, for example, by DBS.77 Task-specific tremor, such as tremor that only occurs when writing (primary writing tremor) or when performing other specific tasks, may be a form of dystonic tremor.78
PSYCHOGENIC TREMOR
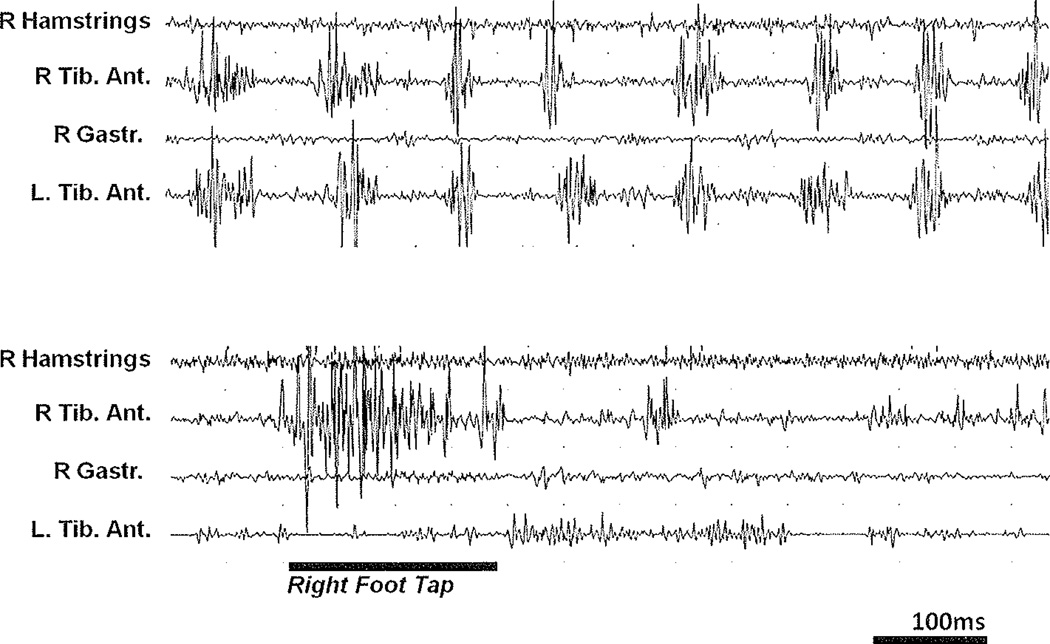
As most other movement disorder signs, tremor or tremor-like movements may have a psychogenic cause. Differentiating psychogenic tremor from tremor that has a somatic etiology can be very challenging, and misdiagnosis is not uncommon. These difficulties arise because the spectrum of somatic tremor types is wide and as their symptoms usually fluctuate in severity, depending on the subject’s emotional state among other factors. Several clues may suggest a psychogenic cause.79,80 Sudden onset, spontaneous remission, or larger variations of amplitude and frequency are unusual in somatic tremor. A psychogenic tremor may become less severe or disappear on distraction, for example, with alternate finger tapping or mental concentration on serial 7s, or on suggestion – by applying a vibrating tuning fork to a patient’s forehead and informing the patient (wrongly) that this can stop the tremor. Similar information about hyperventilation may lead to tremor exacerbation.80 Entrainment refers to the change in frequency of a psychogenic tremor in adaptation to voluntary movements, such as a regular movement in the contralateral limb (Fig. 3). Loading a tremorous limb with a weight changes the tremor frequency in physiologic tremor, but not in essential tremor, or psychogenic tremor, and often increases the in amplitude of a psychogenic tremor.6,81
Figure 3.
Entrainment of psychogenic tremor. Electrophysiologic surface recording study. A 28-year-old woman had developed tremor in her legs 3 months previously. The surface EMG recordings shown here were recorded with the patient standing. There is nearly simultaneous and rhythmic activity in both anterior tibial muscles, but the length, amplitude, and shape of the single bursts is less regular than in Figure 2. Simultaneous contractions in both limbs (upper row) often indicate voluntary activation.87 There is only a very slight antagonist activity simultaneous with a reflex-like tonic activity in all muscles. When the patient was asked to slowly tap down her right foot (lower row, black bar), the rhythmic activity almost completely abates (entrainment), suggesting a psychogenic cause for this patient’s tremor.
INTENTION TREMOR
Intention tremor results when the antagonist activation that normally stops a goal-directed movement as the goal is approached, is inappropriately sized or timed. It often indicates a lesion in the dentate nucleus or its outflow tract through the superior cerebellar peduncle. Underlying causes include multiple sclerosis, spinocerebellar ataxias, and other degenerative, metabolic, or neoplastic disorders affecting these cerebellar structures. Treatment is often not satisfactory, but low doses of benzodiazepines can improve the situation, and promising results of DBS treatment have been reported.82
TREMOR IN WILSON’S DISEASE
All types of tremor can be the presenting sign of Wilson’s disease, with postural and/or rest tremor being most common. The typical proximal “wing beating tremor” is often missing in the early stages of the disease. In addition, the other features of the clinical phenotype can vary greatly.18 Dysarthria and subtle personality changes are early signs in about half of patients, whereas the more well-known corneal Kayser-Fleischer and changes on magnetic resonance imaging (MRI) can be difficult to detect or absent.17 The disease is rare and follows an autosomal recessive pattern of inheritance. More than 150 different mutations have been described in the ATP7B gene responsible for Wilson’s disease. Unfortunately, a normal serum ceruloplasmin level does not rule out Wilson’s disease with certainty; given the potential for causative treatment, at least one more means of investigation such as elevated 24-hour copper urine should probably be obtained if ceruloplasmin is normal.16 A molecular genetic diagnosis is most sensitive, but is a laborious and costly effort as the known mutations are spread out over the gene’s 21 exons.
PRIMARY ORTHOSTATIC TREMOR
Primary orthostatic tremor has a peculiar and highly characteristic clinical picture with high frequency (12–18 Hz) tremor that occurs in the legs of a person when erect and causes postural instability. The high tremor frequency leads to a partial fusion of the single muscle contractions, and it can be easier to hear the contractions through a stethoscope applied to thigh or calf muscles. The sound has been compared with that of a helicopter.83 Treatment options include clonazepam, primidone,84 benzodiazepines, and gabapentin.85
PALATAL TREMOR
Palatal tremor mainly affects the soft palate, but may include other cranial musculature.13 It can be caused by a lesion in the brainstem; for example, through infarction, hemorrhage or trauma, that affects the rostral parts of the triangle of Guillain and Mollaret.86 In other patients, the cause remains enigmatic. Olivary hypertrophy results from a degenerative process and can be detected on an MRI scan. Patients can be unimpaired by the tremor, but may experience a disturbing clicking sound generated by activation of muscles in proximity of the eustachian tube.13
CONCLUSION
Tremor can be a complaint or sign indicating various underlying neurologic disorders. Progress has been made regarding our understanding of its pathophysiology, and the available options to treat tremor patients have increased. Nevertheless, important questions remain unanswered, such as the exact cause or origin of tremors. There clearly is a need for more effective treatments for most forms of tremor, and the underlying neurologic disorders that cause tremor.
ACKNOWLEDGMENTS
We thank Jay A. Van Gerpen, Department for Neurology, Mayo Clinic, for providing the images and patient descriptions for Figs. 2 and 3, and for revising the manuscript; Margaret A. McKinney, Media Support Services, Mayo Clinic, for the graphic design of Fig. 1; and Meinie Seelen, Lund University, Sweden, for proofreading the manuscript. AP received funding from Swedish Parkinson’s Academy, The Research Foundation of the Swedish Parkinson’s Disease Association, AFA Insurance Sweden, Lund University Research Fund, and The Royal Physiographic Society in Lund. ZKW is partially supported by the NS40256, NS057567, AG017216, NS070276, Mayo Clinic Florida Research Committee CR program, and a gift from Carl Edward Bolch, Jr. and Susan Bass Bolch.
REFERENCES
- 1.Deuschl G, Bain P, Brin M Ad Hoc Scientific Committee. Consensus statement of the Movement Disorder Society on Tremor. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 2.Zesiewicz TA, Hauser RA. Phenomenology and treatment of tremor disorders. Neurol Clin. 2001;19(3):651–680. doi: 10.1016/s0733-8619(05)70039-6. vii vii. [DOI] [PubMed] [Google Scholar]
- 3.Thanvi B, Lo N, Robinson T. Essential tremor-the most common movement disorder in older people. Age Ageing. 2006;35(4):344–349. doi: 10.1093/ageing/afj072. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9(6):613–622. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, Rios E. Embarrassment in essential tremor: prevalence, clinical correlates and therapeutic implications. Parkinsonism Relat Disord. 2009;15(7):535–538. doi: 10.1016/j.parkreldis.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elble R. The pathophysiology of tremor. In: Watts RL, Koller WC, editors. Movement Disorders: Neurologic Principles and Practice. 2nd ed. New York: McGraw Hill; 2004. pp. 481–492. [Google Scholar]
- 7.Van Der Giessen RS, Koekkoek SK, van Dorp S, et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58(4):599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Llinás R, Yarom Y. Oscillatory properties of guinea-pig-inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monsef HR, Ghobadi A, Iranshahi M, Abdollahi M. Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test. J Pharm Pharm Sci. 2004;7(1):65–69. [PubMed] [Google Scholar]
- 10.Frison G, Favretto D, Zancanaro F, Fazzin G, Ferrara SD. A case of beta-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Sci Int. 2008;179(2–3):e37–e43. doi: 10.1016/j.forsciint.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Miwa H, Hama K, Kajimoto Y, Kondo T. Effects of zonisamide on experimental tremors in rats. Parkinsonism Relat Disord. 2008;14(1):33–36. doi: 10.1016/j.parkreldis.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Wilms H, Sievers J, Deuschl G. Animal models of tremor. Mov Disord. 1999;14(4):557–571. doi: 10.1002/1531-8257(199907)14:4<557::aid-mds1004>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol. 2000;21(6):1073–1077. [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE. Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain. 2004;127(Pt 6):1252–1268. doi: 10.1093/brain/awh137. [DOI] [PubMed] [Google Scholar]
- 15.Hess CW, Saunders-Pullman R. Movement disorders and alcohol misuse. Addict Biol. 2006;11(2):117–125. doi: 10.1111/j.1369-1600.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer RF. Wilson’s disease. Semin Neurol. 2007;27(2):123–132. doi: 10.1055/s-2007-971173. [DOI] [PubMed] [Google Scholar]
- 17.Ghika J, Vingerhoets F, Maeder P, Borruat F-X, Bogousslavsky J, et al. EMC-Neurologie. 2004;1:481–511. [Google Scholar]
- 18.Mak CM, Lam CW. Diagnosis of Wilson’s disease: a comprehensive review. Crit Rev Clin Lab Sci. 2008;45(3):263–290. doi: 10.1080/10408360801991055. [DOI] [PubMed] [Google Scholar]
- 19.Meshack RP, Norman KE. A randomized controlled trial of the effects of weights on amplitude and frequency of postural hand tremor in people with Parkinson’s disease. Clin Rehabil. 2002;16(5):481–492. doi: 10.1191/0269215502cr521oa. [DOI] [PubMed] [Google Scholar]
- 20.McGruder J, Cors D, Tiernan AM, Tomlin G. Weighted wrist cuffs for tremor reduction during eating in adults with static brain lesions. Am J Occup Ther. 2003;57(5):507–516. doi: 10.5014/ajot.57.5.507. [DOI] [PubMed] [Google Scholar]
- 21.Lundervold DA, Poppen R. Biobehavioral intervention for older adults coping with essential tremor. Appl Psychophysiol Biofeedback. 2004;29(1):63–73. doi: 10.1023/b:apbi.0000017864.06525.eb. [DOI] [PubMed] [Google Scholar]
- 22.Shulman LM, Wen X, Weiner WJ, et al. Acupuncture therapy for the symptoms of Parkinson’s disease. Mov Disord. 2002;17(4):799–802. doi: 10.1002/mds.10134. [DOI] [PubMed] [Google Scholar]
- 23.King LK, Almeida QJ, Ahonen H. Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. NeuroRehabilitation. 2009;25(4):297–306. doi: 10.3233/NRE-2009-0528. [DOI] [PubMed] [Google Scholar]
- 24.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic J, Beach J, Pandolfo M, Patel PI. Familial essential tremor in 4 kindreds. Prospects for genetic mapping. Arch Neurol. 1997;54(3):289–294. doi: 10.1001/archneur.1997.00550150047015. [DOI] [PubMed] [Google Scholar]
- 26.Whaley NR, Putzke JD, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: phenotypic expression in a clinical cohort. Parkinsonism Relat Disord. 2007;13(6):333–339. doi: 10.1016/j.parkreldis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Deuschl G, Elble R. Essential tremor—neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24(14):2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 28.Benito-León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2(12):666–678. doi: 10.1038/ncpneuro0347. quiz 2p, 691. [DOI] [PubMed] [Google Scholar]
- 29.Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77(11):1235–1237. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9(2):193–196. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- 31.Deuschl G, Wenzelburger R, Löffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123(Pt.8):1568–1580. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- 32.Lastres-Becker I, Rüb U, Auburger G. Spinocerebellar ataxia 2 (SCA2) Cerebellum. 2008;7(2):115–124. doi: 10.1007/s12311-008-0019-y. [DOI] [PubMed] [Google Scholar]
- 33.Spanaki C, Plaitakis A. Essential tremor in Parkinson’s disease kindreds from a population of similar genetic background. Mov Disord. 2009;24(11):1662–1668. doi: 10.1002/mds.22655. [DOI] [PubMed] [Google Scholar]
- 34.Vilariño-Güell C, Ross OA, Wider C, et al. LINGO1 rs9652490 is associated with essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(2):109–111. doi: 10.1016/j.parkreldis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilarino-Guell C, Wider C, Ross OA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 11(4):401–408. doi: 10.1007/s10048-010-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006;21(8):1114–1118. doi: 10.1002/mds.20884. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HV, Ngian V, Cordato D, Shen Q, Chan DK. Quality of life in a random sample of community dwelling older patients with essential tremor. Acta Neurol Scand. 2007;116(5):289–292. doi: 10.1111/j.1600-0404.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 38.Lyons KE, Pahwa R. Pharmacotherapy of essential tremor: an overview of existing and upcoming agents. CNS Drugs. 2008;22(12):1037–1045. doi: 10.2165/0023210-200822120-00006. [DOI] [PubMed] [Google Scholar]
- 39.Zesiewicz TA, Elble R, Louis ED, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64(12):2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 40.Elble RJ. Tremor: clinical features, pathophysiology, and treatment. Neurol Clin. 2009;27(3):679–695. v–vi, v–vi. doi: 10.1016/j.ncl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Simpson DM, Blitzer A, Brashear A, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70(19):1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain PG, Findley LJ, Thompson PD, et al. A study of hereditary essential tremor. Brain. 1994;117(Pt 4):805–824. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- 43.Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18(2):163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- 44.Herzog J, Hamel W, Wenzelburger R, et al. Kinematic analysis of thalamic versus subthalamic neurostimulation postural and intention tremor. Brain. 2007;130(Pt 6):1608–1625. doi: 10.1093/brain/awm077. [DOI] [PubMed] [Google Scholar]
- 45.Gourie-Devi M, Ramu MG, Venkataram BS. Treatment of Parkinson’s disease in ‘Ayurveda’ (ancient Indian system of medicine): discussion paper. J R Soc Med. 1991;84(8):491–492. doi: 10.1177/014107689108400814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elizan TS, Sroka H, Maker H, Smith H, Yahr MD. Dementia in idiopathic Parkinson’s disease. Variables associated with its occurrence in 203 patients. J Neural Transm. 1986;65(3–4):285–302. doi: 10.1007/BF01249089. [DOI] [PubMed] [Google Scholar]
- 47.Brooks DJ, Playford ED, Ibanez V, et al. Isolated tremor and disruption of the nigrostriatal dopaminergic system: an 18F-dopa PET study. Neurology. 1992;42(8):1554–1560. doi: 10.1212/wnl.42.8.1554. [DOI] [PubMed] [Google Scholar]
- 48.Carr J. Tremor in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8(4):223–234. doi: 10.1016/s1353-8020(01)00037-2. [DOI] [PubMed] [Google Scholar]
- 49.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 50.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalambo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43(2):111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 51.Nambu A. A new approach to understand the pathophysiology of Parkinson’s disease. J Neurol. 2005;252(Suppl 4):IV1–IV4. doi: 10.1007/s00415-005-4002-y. [DOI] [PubMed] [Google Scholar]
- 52.Lévesque M, Parent A. The striatofugal fiber system in primates: a revaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci U S A. 2005;102(33):11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadjar A, Brotchie JM, Guigoni C, et al. Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J Neurosci. 2006;26(34):8653–8661. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22(4):387–393. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- 55.Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson’s disease best reflects the nigrostriatal lesion? J Ann Neurol. 1997;41(1):58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- 56.Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations m PD: can they be the cause of bradykinesia and akinesia? Exp Neurol. 2009;219(1):58–61. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Stochl J, Boomsma A, Ruzicka E, Brozova H, Blahus P. On the structure of motor symptoms of Parkinson’s disease. Mov Disoid. 2008;23(9):1307–1312. doi: 10.1002/mds.22029. [DOI] [PubMed] [Google Scholar]
- 58.Kühn AA, Tsui A, Aziz T, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215(2):380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Penn RD, Kroin JS, Reinkensmeyer A, Corcos DM. Injection of GABA-agonist into globus pallidus in patient with Parkinson’s disease. Lancet. 1998;351(9099):340–341. doi: 10.1016/S0140-6736(05)78336-7. [DOI] [PubMed] [Google Scholar]
- 60.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 61.Pogarell O, Gasser T, van Hilten JJ, et al. Pramipexole in patients with Parkinson’s disease and marked drug resistant tremor: a randomised, double blind, placebo controlled multi-centre study. J Neurol Neurosurg Psychiatry. 2002;72(6):713–720. doi: 10.1136/jnnp.72.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrag A, Keens J, Warner J Ropinirole Study Group. Ropioirole for the treatment of tremor in early Parkinson’s disease. Eur J Neurol. 2002;9(3):253–257. doi: 10.1046/j.1468-1331.2002.00392.x. [DOI] [PubMed] [Google Scholar]
- 63.Navan P, Findley LJ, Jeffs JA, Pearce RK, Bain PG. Double-blind, single-dose, cross-over study of the effects of pramipexole, pergolide, and placebo on rest tremor and UPDRS part III in Parkinson’s disease. Mov Disord. 2003;18(2):176–180. doi: 10.1002/mds.10320. [DOI] [PubMed] [Google Scholar]
- 64.Navan P, Findley LJ, Jeffs JA, Pearce RK, Bain PG. Randomized, double-blind, 3-month parallel study of the effects of pramipexole, pergolide, and placebo on Parkinsonian tremor. Mov Disord. 2003;18(11):1324–1331. doi: 10.1002/mds.10538. [DOI] [PubMed] [Google Scholar]
- 65.Möller JC, Eggert KM, Unger M, Odin P, Chaudhuri KR, Oertel WH. Clinical risk-benefit assessment of dopamine agonists. Eur J Neurol. 2008;15(Suppl 2):15–23. doi: 10.1111/j.1468-1331.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 66.van Hilten JJ, Ramaker CC, Stowe R, Ives NJ. Bromocriptine versus levodopa in early Parkinson’s disease. Cochrane Database Syst Rev. 2007;4:CD002258. doi: 10.1002/14651858.CD002258.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katzenschlager R, Head J, Schrag A, Ben-Shlomo Y, Evans A, Lees AJ. Parkinson’s Disease Research Group of the United Kingdom. Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology. 2008;71(7):474–480. doi: 10.1212/01.wnl.0000310812.43352.66. [DOI] [PubMed] [Google Scholar]
- 68.Crosby NJ, Deane KH, Clarke CE. Beta-blocker therapy for tremor in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(1):CD003361. doi: 10.1002/14651858.CD003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segrell ND, Granérus A-K, Holmberg B, et al. [Accessed January 10, 2011];[Swedish Guidelines for the Diagnosis and Treatment of Parkinson’s Disease. Revised version #3 2009; Available at: http://www.swemodis.se/images/Dokument/riktlinjer2009.pdf.
- 70.Krack P, Pollak P, Limousin P, Benazzouz A, Benabid AL. Stimulation of subthalamic nucleus alleviates tremor in Parkinson’s disease. Lancet. 1997;350(9092):1675. doi: 10.1016/s0140-6736(97)24049-3. [DOI] [PubMed] [Google Scholar]
- 71.Follett KA, Weaver FM, Stern M, et al. CSP 468 Study Group. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 72.Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352(13):1343–1356. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 73.Ropper AH, Gorson KC. Neuropathies associated with paraproteinemia. N Engl J Med. 1998;338(22):1601–1607. doi: 10.1056/NEJM199805283382207. [DOI] [PubMed] [Google Scholar]
- 74.Netravathi M, Pal PK, Ravishankar S, Indira Devi B. Electrophysiological evaluation of tremors secondary to space occupying lesions and trauma: correlation with nature and sites of lesions. Parkinsonism Relat Disord. 2010;16(1):36–41. doi: 10.1016/j.parkreldis.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Gajos A, Bogucki A, Schinwelski M, et al. The clinical and neuroimaging studies in Holmes’ tremor. Acta Neurol Scand. 2010;122(5):360–366. doi: 10.1111/j.1600-0404.2009.01319.x. [DOI] [PubMed] [Google Scholar]
- 76.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9(3):222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 77.Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5(2):320–330. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bain PG, Findley LJ, Britton TC, et al. Primary writing tremor. Brain. 1995;118(Pt 6):1461–1472. doi: 10.1093/brain/118.6.1461. [DOI] [PubMed] [Google Scholar]
- 79.Bhidayasiri R. Differential diagnosis of common tremor syndromes. Postgrad Med J. 2005;81(962):756–762. doi: 10.1136/pgmj.2005.032979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenney C, Diamond A, Mejia N, Davidson A, Hunter C, Jankovic J. Distinguishing psychogenic and essential tremor. J Neurol Sci. 2007;263(1–2):94–99. doi: 10.1016/j.jns.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 81.Deuschl G, Köster B, Lücking CH, Scheldt C. Diagnostic and pathophysiological aspects of psychogenic tremors. Mov Disord. 1998;13(2):294–302. doi: 10.1002/mds.870130216. [DOI] [PubMed] [Google Scholar]
- 82.Freund HJ, Barnikol UB, Nolte D, et al. Subthalamic-thalamic DBS in a case with spinocerebellar ataxia type 2 and severe tremor-A unusual clinical benefit. Mov Disord. 2007;22(5):732–735. doi: 10.1002/mds.21338. [DOI] [PubMed] [Google Scholar]
- 83.Brown P. New clinical sign for orthostatic tremor. Lancet. 1995;346(8970):306–307. doi: 10.1016/s0140-6736(95)92190-7. [DOI] [PubMed] [Google Scholar]
- 84.Britton TC, Thompson PD, van der Kamp W, et al. Primary orthostatic tremor: further observations in six cases. J Neurol. 1992;239(4):209–217. doi: 10.1007/BF00839142. [DOI] [PubMed] [Google Scholar]
- 85.Raethjen J, Deuschl G. Tremor. Ther Umsch. 2007;64(1):35–40. doi: 10.1024/0040-5930.64.1.35. [DOI] [PubMed] [Google Scholar]
- 86.Sharma P, Eesa M, Poppe AY, Goyal M. Teaching Neuro-Image: posttraumatic palatal tremor. Neurology. 2008;71(13):e30. doi: 10.1212/01.wnl.0000326578.18675.3a. [DOI] [PubMed] [Google Scholar]
- 87.Raethjen J, Kopper F, Govindan RB, Volkmann J, Deuschl G. Two different pathogenetic mechanisms in psychogenic tremor. Neurology. 2004;63(5):812–815. doi: 10.1212/01.wnl.0000137012.35029.6b. [DOI] [PubMed] [Google Scholar]