Abstract
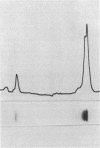
The genes for the large and small subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase from the cyanobacterium Anacystis nidulans were subcloned into plasmid pUC9. After induction, both genes were expressed in Escherichia coli and the subunits were assembled into an active holoenzyme. The enzyme was purified from E. coli to high specific activity and was found to contain equimolar amounts of large and small subunits. The assembly of the hexadecameric ribulose bisphosphate carboxylase/oxygenase in E. coli should provide the basis for studies on the mechanism of assembly and the role of small subunits in catalysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Abel K. M. Kinetics and subunit interactions of ribulose bisphosphate carboxylase-oxygenase from the cyanobacterium, Synechococcus sp. J Biol Chem. 1981 Aug 25;256(16):8445–8451. [PubMed] [Google Scholar]
- Andrews T. J., Ballment B. Active-site carbamate formation and reaction-intermediate-analog binding by ribulosebisphosphate carboxylase/oxygenase in the absence of its small subunits. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3660–3664. doi: 10.1073/pnas.81.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T. J., Ballment B. The function of the small subunits of ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1983 Jun 25;258(12):7514–7518. [PubMed] [Google Scholar]
- Asami S., Takabe T., Akazawa T., Codd G. A. Ribulose 1,5-bisphosphate carboxylase from the halophilic cyanobacterium Aphanothece halophytica. Arch Biochem Biophys. 1983 Sep;225(2):713–721. doi: 10.1016/0003-9861(83)90082-6. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Incharoensakdi A., Takabe T., Takabe T., Akazawa T. Heterologous hybridization of ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) restores the enzyme activities. Biochem Biophys Res Commun. 1985 Jan 31;126(2):698–704. doi: 10.1016/0006-291x(85)90241-4. [DOI] [PubMed] [Google Scholar]
- Jordan D. B., Chollet R. Subunit dissociation and reconstitution of ribulose-1,5-bisphosphate carboxylase from Chromatium vinosum. Arch Biochem Biophys. 1985 Feb 1;236(2):487–496. doi: 10.1016/0003-9861(85)90651-4. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Quivey R. G., Jr, Tabita F. R. Cloning and expression in Escherichia coli of the form II ribulose 1,5-bisphosphate carboxylase/oxygenase gene from Rhodopseudomonas sphaeroides. Gene. 1984 Nov;31(1-3):91–101. doi: 10.1016/0378-1119(84)90198-7. [DOI] [PubMed] [Google Scholar]
- Robison P. D., Martin M. N., Tabita F. R. Differential effects of metal ions on Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase and stoichiometric incorporation of HCO3- into a cobalt(III)--enzyme complex. Biochemistry. 1979 Oct 16;18(21):4453–4458. doi: 10.1021/bi00588a001. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Caruso P., Whitman W. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal Biochem. 1978 Feb;84(2):462–472. doi: 10.1016/0003-2697(78)90064-7. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Colletti C. Carbon dioxide assimilation in cyanobacteria: regulation of ribulose, 1,5-bisphosphate carboxylase. J Bacteriol. 1979 Nov;140(2):452–458. doi: 10.1128/jb.140.2.452-458.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. One-step isolation of microbial ribulose-1,5-diphosphate carboxylase. Arch Microbiol. 1974;99(3):231–240. doi: 10.1007/BF00696237. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Stevens S. E., Jr, Gibson J. L. Carbon dioxide assimilation in blue-green algae: initial studies on the structure of ribulose 1,5-bisphosphate carboxylase. J Bacteriol. 1976 Feb;125(2):531–539. doi: 10.1128/jb.125.2.531-539.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T., Nishimura M., Akazawa T. Presence of two subunit types in ribulose-1,5-bisphosphate carboxylase from blue-green algae. Biochem Biophys Res Commun. 1976 Jan 26;68(2):537–544. doi: 10.1016/0006-291x(76)91179-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]