Abstract
RAS mutations or its activation by upstream receptor tyrosine kinases are frequently associated with poor response of carcinomas to chemotherapy. The 18 kDa propeptide domain of lysyl oxidase (LOX-PP) released from the secreted precursor protein (Pro-LOX) has been shown to inhibit RAS signaling and the transformed phenotype of breast, pancreatic, lung, and prostate cancer cells in culture, and formation of tumors by Her-2/neu-driven breast cancer cells in a mouse xenograft model. Here, we tested the effects of LOX-PP on MIA PaCa-2 pancreatic cancer cells, driven by mutant RAS. In MIA PaCa-2 cells in culture, LOX-PP attenuated the ERK and AKT activities and decreased the levels of the NF-κB p65 and RelB subunits and cyclin D1, which are activated by RAS signaling. In mouse xenograft growth, LOX-PP reduced growth of tumors by these pancreatic cancer cells, and the nuclear levels of the p65 NF-κB subunit and cyclin D1 proteins. While biological agents attenuate tumor growth when used alone, often they have additive or synergistic effects when used in combination with chemotherapeutic agents. Thus, we next tested the hypotheses that LOX-PP sensitizes pancreatic and breast cancer cells to the chemotherapeutic agent doxorubicin. Purified LOX-PP enhanced the cytotoxic effects of doxorubicin in pancreatic and breast cancer cells, as judged by ATP production, Cell Death ELISA assays, caspase 3 activation, PARP cleavage, and Annexin V staining. Thus, LOX-PP potentiates the cytotoxicity of doxorubicin on breast and pancreatic cancer cells, warranting additional studies with a broader spectrum of current cancer treatment modalities.
Keywords: LYSYL OXIDASE, PANCREATIC CANCER, BREAST CANCER, DOXORUBICIN, APOPTOSIS
Most of the currently used cancer-targeting drugs derive their “specificity” for cancerous tissues from the fact that cancer cells are typified by aberrant rates of cellular growth. This often leads to injury of non-targeted tissues, in particular of tissues that by their very nature display higher proliferation rates such as the lining of the gut, stomach, esophagus, and hair follicle of the skin. Therefore, most chemotherapeutic drugs are given at suboptimal doses to minimize undesired side effects. Targeting cancers with sub-optimal doses carries an additional risk—selection of cancer cell populations that have a growth advantage under the treatment conditions. Development of such drug-resistant cancers that no longer respond to these treatments can have dire consequences for the patients. These tumors are frequently typified by accumulation of mutations in proto-oncogenes such as RAS or in upstream receptor tyrosine kinases, leading to their constitutive activation, or tumor suppressor genes such as TP53 and PTEN leading to their inactivation. Multiple approaches are currently underway to enhance the efficacy of these drugs. One approach aims to potentiate the effects of the chemotherapeutic drugs by combining with a targeted agent that is well tolerated yet capable of lowering the threshold for chemotherapy-induced cell death, thereby enhancing killing of the cancer cells while minimizing toxicity.
Lysyl oxidase (LOX) (protein-6-oxidase; EC 1.4.3.13) is the key extracellular enzyme that promotes collagen and elastin cross-linking, which is required for the biosynthesis of functional extracellular matrices. In addition, the LOX gene was isolated as the “ras recision” gene (rrg) with ability to inhibit the transforming activity of the RAS oncogene in NIH 3T3 fibroblasts in culture [Contente et al., 1990; Kenyon et al., 1991]. The ability of the LOX gene to inhibit tumor formation by gastric cancer cells was also shown in a mouse model [Kaneda et al., 2004]. The LOX gene encodes a 50 kDa lysyl oxidase proenzyme (termed Pro-LOX), which is secreted into the extracellular environment where it is processed by proteolytic cleavage to the active 32 kDa LOX enzyme and an 18 kDa amino-terminal propeptide (LOX-PP). Of note, the tumor suppressor activity of the LOX gene resides specifically in the propeptide domain, and not in the LOX enzyme [Palamakumbura et al., 2004]. Importantly, the tumor suppressor effects of LOX-PP are seen in carcinomas of the breast, pancreas, lung, and prostate, in which RAS signaling is constitutively active either directly by the presence of mutated/oncogenic RAS genes (pancreas and lung) or indirectly by the activation of upstream receptor tyrosine kinases such as Her-2/neu or fibroblast growth factor receptor (breast and prostate) [Min et al., 2007; Wu et al., 2007; Palamakumbura et al., 2009]. RAS-induced signaling molecules/pathways that are inhibited by LOX-PP include ERK, phosphatidylinositol-3-kinase (PI3K)/AKT, NF-κB and its downstream targets BCL-2 and cyclin D1 [Guttridge et al., 1999]. Re-introduction of BCL-2 into H1299 lung cancer or PANC-1 pancreatic cancer cells expressing LOX-PP restored the transformed phenotype, suggesting that BCL-2 is an essential target [Wu et al., 2007]. The epithelial to mesenchymal transition (EMT) in breast cancer cells mediated by oncogenic Her-2/neu is reverted by LOX-PP, as judged by upregulation of E-cadherin, γ-catenin, and estrogen receptor (ER)α, reduced levels of Snail and vimentin, and by decreased ability to migrate or to form branching colonies in Matrigel [Min et al., 2007]. Fibronectin-mediated integrin activation—known to be involved in growth factor receptor cross-talk—is downregulated by LOX-PP as well [Zhao et al., 2009]. In vivo, LOX-PP expression leads to reduced formation of tumors by Her-2/neu-driven breast cancer cells in a nude mouse xenograft model [Min et al., 2007]. A single nucleotide polymorphism (SNP) (rs1800449) G473A, resulting in an Arg158Gln substitution in a highly conserved region in the LOX-PP domain, was recently shown to significantly impair the ability of LOX-PP to inhibit RAS signaling pathways driven by Her-2/neu in breast cancer cells and formation of tumors in mice [Min et al., 2009]. These findings corroborate the anti-cancer function of LOX-PP. Here, we have assessed the tumor suppressor activity of LOX-PP in pancreatic xenografts and report that LOX-PP is a potent inhibitor of pancreatic tumor growth in vivo. Furthermore, we show that while LOX-PP does not induce cell death by itself, it has the potential to enhance death induced by the chemotherapeutic drug doxorubicin in both pancreatic and breast cancer cells.
MATERIALS AND METHODS
ANTIBODIES AND REAGENTS
The antibodies used were from the following sources. Cell Signaling: phosphorylated AKT (Ser473), phosphorylated ERK1/2 (Thr202 Tyr204), and caspase 3; Santa Cruz Biotechnology: p65, Lamin B, and RelB; BD Biosciences: cleaved PARP; Sigma: cyclin D1 and β-actin; Invitrogen: V5 epitope. Doxorubicin was purchased from Sigma and dissolved in water. Recombinant LOX-PP was expressed and purified as described [Vora et al., 2010]. Charcoal stripped (CS)-FBS was purchased from Invitrogen.
CELL CULTURE AND TREATMENT CONDITIONS
MIA PaCa-2 pancreatic cancer cells were obtained from and grown as described by the American Type Culture Collection (ATCC). The MIA PaCa-2 cell line was established from pancreatic tumor tissue from a 65-year-old Caucasian male. NF639, MDA-MB-231, and Hs578T cells were described elsewhere [Wang et al., 2007]. HEK293T cells were cultured described [Kirsch et al., 1998].
DNA CONSTRUCTS AND RETROVIRAL INFECTION
The cDNA fragments encoding murine Pro-LOX and LOX-PP containing a carboxy-terminal V5/His tag were cloned in the retroviral vector pCXbsr, which contains a constitutive cytomegalovirus (CMV) promoter (generously provided by Tsuyoshi Akagi, OBI, Osaka, Japan) were described previously [Min et al., 2007]. Retrovirus stocks were generated by co-transfecting HEK293T cells with the amphotropic (pCLAmpho) vector (Imgenex) and either empty vector (EV) pCXbsr or pCXbsr-LOX-PP. After 48 h, MIA PaCa-2 cells were infected with filtered culture supernatant from the HEK293T cells containing CXbsr-EV or CXbsr-LOX-PP viruses that was supplemented with 6 μg/ml polybrene. Infected cells were selected with 10 μg/ml blasticidin to generate pools of stable infectants of LOX-PP and EV control cells, and stable cell populations isolated.
IMMUNOBLOT ANALYSIS
For preparation of whole cell protein extracts (WCEs), cells were incubated in lysis buffer (50 mmol/L Tris (pH 7.6), 150 mmol/L NaCl, 1% Triton X-100) plus protease inhibitor cocktail and phosphatase inhibitors (20 mmol/L NaPP, 10 mmol/L NaF, and 1 mmol/L Na3VO4). Protein concentrations were determined using the detergent-compatible protein assay kit (Bio-Rad). Samples (50 μg) were subjected to immunoblotting as described [Min et al., 2007]. To isolate nuclear proteins, washed cells were lysed by suspension in ice-cold lysis buffer (10 mmol/L Tris (pH 7.6), 10 mmol/L KCl, 1 mmol/L MgCl2) plus dithiothreitol (DTT) at 1 mM, 1% Nonidet P40, and protease inhibitor cocktail (Roche Diagnostics) for 15 min. Lysis was verified by crystal violet staining. The nuclei were pelleted by centrifugation for 4 min at 2,500 rpm at 4°C, and the supernatant was discarded. The nuclear pellets were washed once in lysis buffer without detergent, and proteins were extracted in RIPA lysis buffer (50 mmol/L Tris–HCl (pH 7.5), 150 mmol/L NaCl, 1% sodium lauryl sarcosine, 1% Nonidet P40, 0.1% SDS, 1 mmol/L EDTA) plus 1 mM DTT, and protease inhibitor cocktail as above. The extracts were briefly sonicated, followed by centrifugation for 30 min at 14,000g at 4°C, and the supernatant containing the nuclear proteins was quantitated.
To detect expression of recombinant proteins in cell culture medium, 1 or 2 ml of 10 ml culture medium were subjected to immunoprecipitation using a V5 antibody (Invitrogen) and protein A-sepharose (Invitrogen). Immunoblot analysis was performed using anti-V5 antibody followed by incubation with protein A conjugated to horseradish peroxidase (HRP) as described [Min et al., 2007].
XENOGRAFT EXPERIMENTS
NCrnu/nu nude mice were purchased from Taconic Laboratories (Albany, NY) at 7–9 weeks of age. Mice were housed in a two-way barrier at the Boston University School of Medicine Transgenic mouse facility in accordance with the regulations of the American Association for the Accreditation of Laboratory Animal Care. MIA PaCa-2 cells were infected with CXbsr-EV or CXbsr-LOX-PP viruses. After 16 h, cells were injected subcutaneously (4 × 106 per injection) in both flanks (EV, left; LOX-PP, right) of nude mice (n = 8). Tumor size was measured with calipers and tumor volumes were calculated using the following formula: . All mice were sacrificed on day 42. Tumors were dissected, weighed, and snap frozen in liquid nitrogen. Protein extracts were prepared by homogenizing frozen tumor specimens as described above. Equal amounts of protein were subjected to immunoblotting (see above). Tumor weights between groups EV and LOX-PP were compared using two-tailed, paired Student’s t-test. P <0.05 was considered statistically significant.
CELL VIABILITY ASSAY
Cells were seeded, in quadruplicate, at 1,000 cells/well into 96-well plates and incubated overnight. Cells were then treated with LOX-PP at either 0, 2, or 4 μg/ml in DMEM–2% CS-FBS for 24 h. Subsequently, doxorubicin was added at the indicated dose and the cultures incubated for another 24 h. Cell viability was determined by measuring ATP production using an ATPlite one step luminescence ATP detection assay system (Perkin Elmer) as recommended by the manufacturer.
CELL DEATH ELISA
Cells were seeded, in triplicate, at 1,000 cells/well into 96-well plates. Following overnight incubation, cultures were pre-treated with LOX-PP at 4 μg/ml for 24 h in DMEM–2% CS-FBS. Subsequently, cultures were either left untreated or treated with doxorubicin at 1 μM for another 24 h. Cell death was measure using Cell Death ELISA assay (Roche Diagnostics) according to the manufacturer’s instructions.
ANNEXIN V FLOW CYTOMETRY ANALYSIS
Cells were seeded at 8 × 104 cells/well into six-well plates. After overnight incubation, cells were pre-treated with LOX-PP at 4 μg/ml for 24 h in DMEM–2% CS-FBS. Subsequently, cells were either left untreated or treated with doxorubicin at 1 μM for another 24 h. Cells were collected and subjected to Annexin V staining (Beckman Coulter) and analyzed by flow cytometry (FACScalibur, Becton Dickinson).
RESULTS
RECOMBINANT LOX-PP ATTENUATES ERK AND AKT PHOSPHORYLATION IN MIA PaCa-2 CELLS
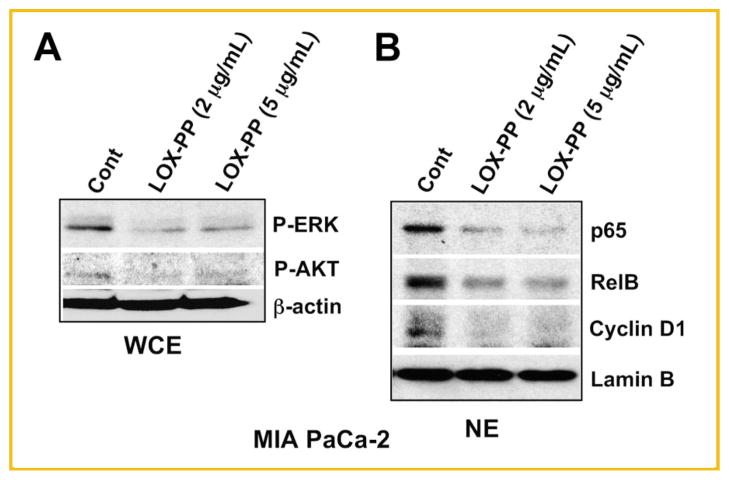
Previously we demonstrated that ectopically expressed LOX-PP inhibits RAS-induced activation of the MAPK/ERK and PI3K/AKT pathways of PANC-1 pancreatic cancer cells [Wu et al., 2007]. To determine whether these findings can be extended to an additional pancreatic cell line, we selected MIA PaCa-2 cells and tested recombinant LOX-PP. MIA PaCa-2 cells were treated with 2 or 5 μg/ml LOX-PP in DMEM–0.5% FBS for 24 h and then stimulated with 10% FBS for 30 min. WCEs were collected and subjected to analysis of ERK and AKT phosphorylation using immunoblotting. Both concentrations of LOX-PP substantially reduced ERK and AKT activation compared to control cell lysates (Fig. 1A). Pancreatic cancer cell growth is characterized by ERK/AKT-signaling-mediated activation of the classic and alternative NF-κB pathways [Nishina et al., 2009] leading to increased nuclear levels of p65, RelB, and cyclin D1, product of a known NF-κB target gene. A substantial reduction in nuclear levels of p65, RelB, and cyclin D1 were detected when MIA PaCa-2 cells were treated with 2 or 5 μg/ml LOX-PP in DMEM–0.5% FBS for 24 h and then stimulated with 10% FBS for 16 h (Fig. 1B). Thus, LOX-PP reduces RAS signaling mediated by the AKT and ERK pathways in the pancreatic cell line MIA PaCa-2 in vitro.
Fig. 1.
Purified recombinant LOX-PP attenuates the ERK and AKT kinase activities and downstream signaling targets in MIA PaCa-2 pancreatic cells. A: MIA PaCa-2 cells were treated with 2, 5 μg/ml LOX-PP or water control in DMEM–0.5% FBS for 24 h and then stimulated with 10% FBS for 30 min and WCEs subjected to Western blot analysis for phosphorylated ERK (P-ERK) and AKT (P-AKT), or β-actin, which confirmed equal loading. B: MIA PaCa-2 cells were treated with LOX-PP at the indicated concentrations in DMEM–0.5% FBS for 24 h and then stimulated with 10% FBS for 16 h and nuclear extracts (NEs) were analyzed for the expression of p65, RelB, cyclin D1, or Lamin B.
LOX-PP EXPRESSION IN MIA PaCa-2 CELLS ATTENUATES TUMOR GROWTH IN A XENOGRAFT MODEL
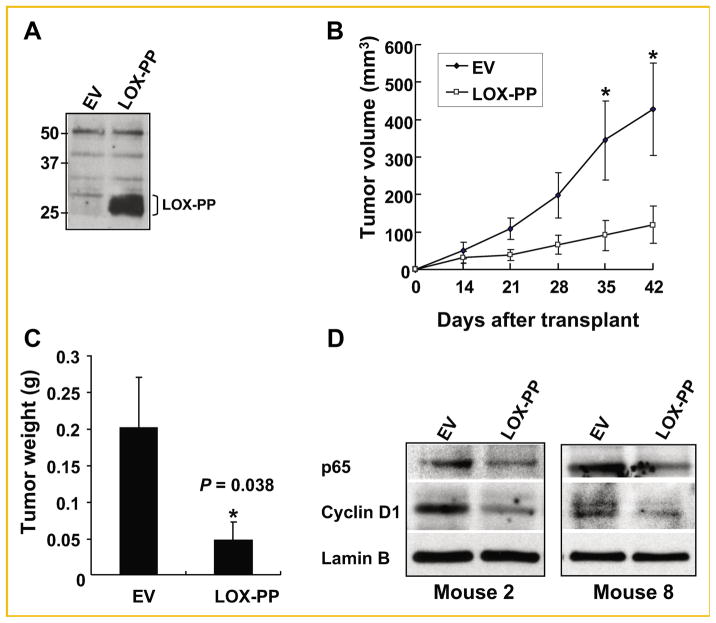
To determine whether LOX-PP can reduce tumor formation of pancreatic cancer cells in vivo, we performed xenograft experiments by subcutaneously injecting populations of MIA PaCa-2 cells 16 h post-infection with either control virus (CXbsr-EV) or virus expressing LOX-PP (CXbsr-LOX-PP). LOX-PP expression in the supernatant of infected MIA PaCa-2 cells was confirmed before performing the xenograft experiment (Fig. 2A). The mobility of LOX-PP on SDS–PAGE observed is similar to what was seen in media samples taken from NF639 cells ectopically expressing LOX-PP [Min et al., 2007]. Nude mice (n = 8) were injected in the left or right flanks with the infected MIA PaCa-2 cells (4 × 106) described above. Rapid tumor growth was noted for the EV-infected populations (Fig. 2B). In contrast, the tumors resulting from the LOX-PP-infected MIA PaCa-2 cells began to grow at a slower rate and were notably smaller throughout the observation period of 42 days, with highly significant differences reached on days 35 and 42 (Fig. 2B). Remarkably, the average tumor weight for LOX-PP xenografts was 76% lower in comparison to the EV group on day 42 (P = 0.038; Fig. 2C). Western blots of nuclear cell fractions directly derived from the tumors indicate that the levels of the NF-κB p65 subunit and of cyclin D1 were substantially and significantly lower in tumors derived from MIA PaCa-2-LOX-PP populations compared to control tumors (Fig. 2D), consistent with the in vitro data obtained with MIA PaCa-2 cells (Fig. 1) and with PANC-1 cells [Wu et al., 2007]. Similar data were obtained with extracts from other mice (data not shown). Thus, LOX-PP suppresses RAS-driven pathways and strongly inhibits tumor formation by MIA PaCa-2 cells in vivo.
Fig. 2.
LOX-PP suppresses pancreatic tumor formation by MIA PaCa-2 cells in vivo. MIA PaCa-2 cells infected with CXbsr-EV or CXbsr-LOX-PP viruses were subcutaneously injected (4 × 106 cells per injection) in both flanks (EV, left; LOX-PP, right) of NCrnu/nu nude mice (n = 8). A: Expression of LOX-PP was confirmed by immunoprecipitation and immunoblotting as described in the Materials and Methods Section before injection of cells. B: Tumor volumes were measured as described in the Materials and Methods Section and plotted as a function of days after injection (transplant). Bars represent SEM; *statistically significant differences. C: Average tumor weights were determined on day 42. Bars represent SEM (P = 0.038). D: Nuclear extracts of tumors of MIA PaCa-2-LOX-PP and MIA PaCa-2-EV xenografts from Mouse 2 and 8 were analyzed by immunoblotting for expression of the NF-κB subunit p65, cyclin D1, and Lamin B.
LOX-PP INCREASES SENSITIVITY OF MIA PaCa-2 CELLS TO KILLING BY DOXORUBICIN
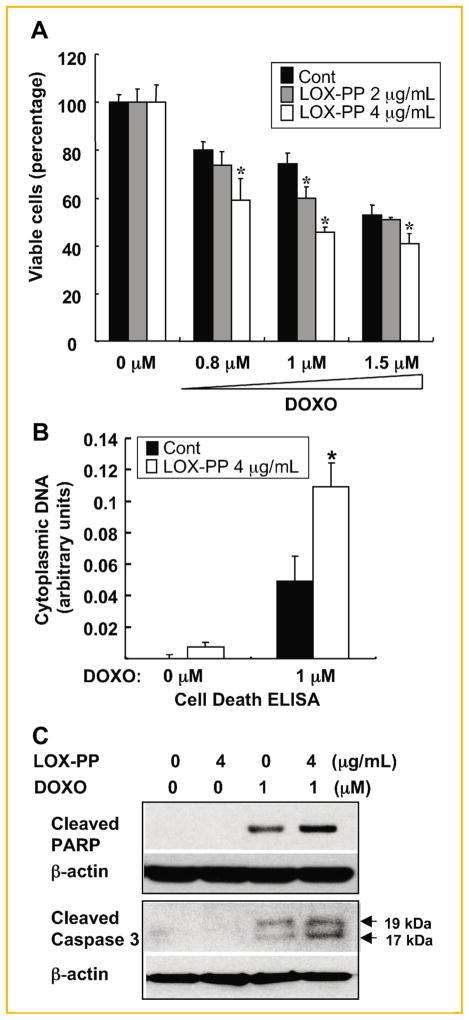
In order to gain insights into possible mechanisms of action of LOX-PP to inhibit pancreatic tumor formation, the effects of LOX-PP on the cytotoxicity of doxorubicin towards MIA PaCa-2 cells were assessed in vitro using a cell viability ATP production assay. MIA PaCa-2 cells were pre-treated with purified LOX-PP at 2 or 4 μg/ml, or water as control, in DMEM–2% CS-FBS for 24 h. The cultures were then incubated in the presence of doxorubicin at 0, 0.8, 1, and 1.5 μM for another 24 h. Doxorubicin treatment alone decreased the number of viable MIA PaCa-2 cells in a dose-dependent fashion, for example, ~80% survival at 0.8 μM down to ~50% at 1.5 μM (Fig. 3A). The combination of doxorubicin with LOX-PP further decreased viable cells compared to doxorubicin alone, with the higher dose of LOX-PP yielding a statistically significant enhancement of killing at all doses of doxorubicin (Fig. 3A). The cytotoxic effects were also quantified by using a Cell Death ELISA assay, which measures histone-associated DNA fragments in the cytoplasm. MIA PaCa-2 cells were pre-treated with LOX-PP at 4 μg/ml or water as control for 24 h in DMEM–2% CS-FBS and then incubated in the presence of 0 or 1 μM doxorubicin for an additional 24 h. The combined treatment of LOX-PP at 4 μg/ml with doxorubicin versus doxorubicin alone led to a 2.5-fold increase in apoptosis (Fig. 3B). Thus, LOX-PP enhances the sensitivity of MIA PaCa-2 cells to killing by doxorubicin.
Fig. 3.
LOX-PP enhances doxorubicin-mediated killing of MIA PaCa-2 cells in culture. A: MIA PaCa-2 cells were pre-treated with 2 or 4 μg/ml LOX-PP or water as control in DMEM–2% CS-FBS for 24 h, and then the indicated amounts of doxorubicin were added for an additional 24 h. Cell viability was determined by measuring ATP production. The value obtained with 0 μM doxorubicin was set to 100%. Data represent mean ± SD of quadruplicate samples (these experiments have been repeated in a similar manner at least two times and data from a representative experiment shown). P values were calculated using Student’s t-test, *P <0.05. B: Cells were treated with 0 or 4 μg/ml LOX-PP for 24 h and then with 0 or 1 μM doxorubicin for an additional 24 h. Cell Death ELISA assays were performed as described in the Materials and Methods Section. Data represent mean ± SD of triplicate samples. P values were calculated using Student’s t-test. C: MIA PaCa-2 cells were treated either with doxorubicin (1 μM) or LOX-PP (4 μg/ml) individually or in combination as described above and WCEs analyzed by immunoblotting for cleaved PARP and caspase 3 expression, and for β-actin, which confirmed equal loading.
It is known that doxorubicin induces cell death mainly through a mitochondria-mediated apoptosis pathway [Xu et al., 2007]. Here we assessed the effects of LOX-PP on doxorubicin-induced killing by measuring caspase 3 and PARP cleavage, two important downstream molecules in the apoptosis pathways. MIA PaCa-2 cells were pre-treated with LOX-PP at 4 μg/ml or water as control for 24 h and then treated with doxorubicin at 0 or 1.0 μM for another 24 h. The resulting WCEs were subjected to immunoblotting. As expected, treatment with doxorubicin alone induced caspase 3 and PARP cleavage (Fig. 3C). Importantly, the combination of LOX-PP with doxorubicin substantially enhances cleavage of caspase 3 and PARP when compared with doxorubicin alone, consistently with the Cell Death ELISA results (Fig. 3B).
LOX-PP INCREASES SENSITIVITY OF Her-2/neu-DRIVEN NF639 BREAST CANCER CELLS TO DOXORUBICIN
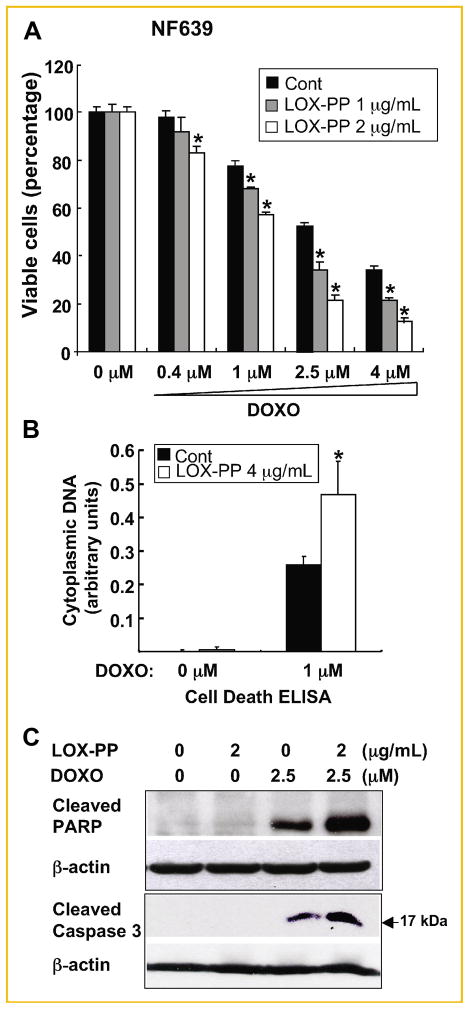
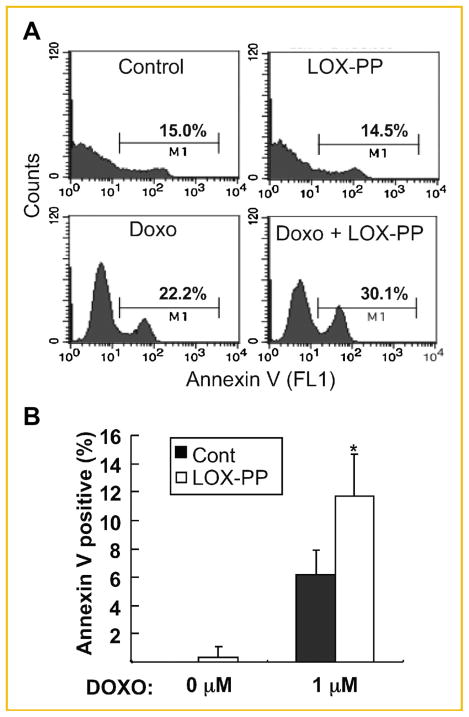
Previously, we observed that while LOX-PP can inhibit growth of murine NF639 breast cancer cells driven by Her-2/neu, which signals via RAS, but failed to induce substantial killing of these cells in vitro [Min et al., 2007]. Thus, we next asked whether LOX-PP increases doxorubicin-mediated cytotoxicity of these breast cancer cells. NF639 cells were pre-treated with 1 or 4 μg/ml LOX-PP or with water as control for 24 h and subsequently treated with 0, 0.4, 1, 2.5, or 4 μM doxorubicin for another 24 h and analyzed for the cell viability using the ATP production assay (Fig. 4A). Doxorubicin treatment caused a dose-dependent decrease in viable cells with only ~40% live cells left at 4 μM doxorubicin. The combination of LOX-PP with doxorubicin greatly enhanced the killing by doxorubicin alone (Fig. 4A). Only ~10% of NF639 cells were viable upon treatment with the combination of 4 μM doxorubicin and 4 μg/ml LOX-PP. An approximate 2-fold increase in apoptosis was measured in the Cell Death ELISA assay, performed as described above, with the combined treatment of 4 μg/ml LOX-PP with 1 μM doxorubicin compared with 1 μM doxorubicin alone (Fig. 4B). Similarly, the combination of LOX-PP with doxorubicin substantially enhanced cleavage of caspase 3 and PARP compared with doxorubicin alone (Fig. 4C). Lastly, apoptosis was further quantified via measurement of Annexin V-positive cells by flow cytometry analysis (Fig. 5A). The combined treatment of LOX-PP with doxorubicin caused an ~2-fold increase in the Annexin V-positive cell population compared to doxorubicin alone (Fig. 5B). Taken together, these data indicated that LOX-PP sensitizes NF639 cells to doxorubicin-induced apoptosis.
Fig. 4.
LOX-PP enhances doxorubicin-induced apoptosis of Her-2/neu-driven NF639 breast cancer cells. A: Cells, pre-treated with 1 or 4 μg/ml LOX-PP for 24 h, were then treated with the indicated amounts of doxorubicin for an additional 24 h. Cell viability was determined by measuring ATP production. Data represent mean ± SD of quadruplicate samples. P values were calculated using Student’s t-test, *P <0.05. B: NF639 cells were treated with 4 μg/ml LOX-PP or water control for 24 h and subsequently with 0 or 1 μM doxorubicin for another 24 h. Apoptosis was determined by Cell Death ELISA assays as described in Figure 3B. Data represent mean ± SD of triplicate samples. P values were calculated using Student’s t-test, *P <0.05. C: NF639 cells were treated with 2 μg/ml LOX-PP and 2.5 μM doxorubicin individually or in combination as described in Figure 3C and analyzed by immunoblotting of WCEs for cleaved PARP and caspase 3 and for β-actin expression.
Fig. 5.
LOX-PP increases doxorubicin-induced Annexin V-positive cell population in NF639 cells. NF639 cells pre-treated with 4 μg/ml LOX-PP or water as control for 24 h were subsequently treated with 0 or 1 μM doxorubicin for another 24 h. A: Apoptosis was evaluated by Annexin V staining using flow cytometry. Histograms depicting percent apoptotic cells (M1) for each treatment condition are shown. B: Data are presented as mean ± SD of three independent experiments.
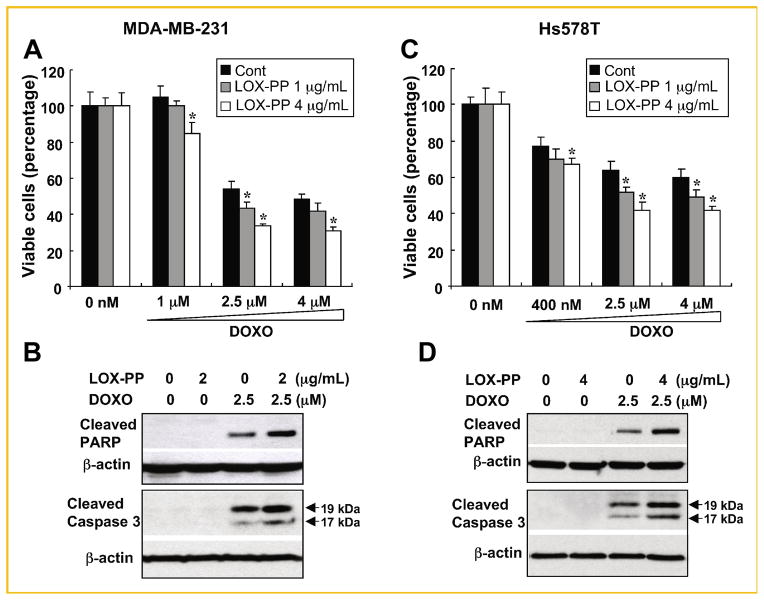
LOX-PP INCREASES SENSITIVITY OF MDA-MB-231 AND Hs578T BREAST CANCER CELLS TO DOXORUBICIN
To investigate whether LOX-PP enhances doxorubicin-mediated cell killing of human breast cancer cells, we subjected two aggressive, ER-negative breast cancer cells lines to similar treatment with LOX-PP and/or doxorubicin. Pre-treatment with LOX-PP increased the sensitivity of MDA-MB-231 and Hs578T cells to doxorubicin-induced cell death. In both cell lines, LOX-PP further decreased cell viability compared to doxorubicin alone (Fig. 6A, C). Consistently, LOX-PP treatment enhanced PARP and caspase 3 cleavage compared with doxorubicin alone (Fig. 6B, D). Furthermore, pre-treatment with LOX-PP significantly increased Annexin V-positive cell population compared to doxorubicin alone (data not shown). Taken together, these data indicated that LOX-PP sensitizes highly malignant ER-negative breast cancer cells to doxorubicin treatment.
Fig. 6.
LOX-PP enhances doxorubicin-induced apoptosis in human ER-negative MDA-MB-231 and Hs578T breast cancer cells. A, C: Cells were pre-treated with 1 or 4 μg/ml LOX-PP for 24 h and then with the indicated doses of doxorubicin for an additional 24 h. Cell viability was determined by measuring ATP production. Data represent mean ± SD of quadruplicates. P values were calculated using Student’s t-test, *P <0.05. B, D: MDA-MB-231 and Hs578T breast cancer cells were treated with the indicated amounts of LOX-PP and doxorubicin (2.5 μM) individually or in combination as described in Figure 3C and WCEs analyzed by immunoblotting for cleaved PARP and caspase 3 expression, and β-actin for loading control.
DISCUSSION
Here we show for the first time that LOX-PP enhances the cytotoxic effects of doxorubicin in two different epithelial cancer cells: pancreatic and breast cancer and inhibits growth of pancreatic cancer cells in a subcutaneous mouse tumor model. LOX-PP enhanced cleavage of caspase 3 and PARP, and induced an increase in Annexin V-positive cells when used in combination with the chemotherapeutic drug doxorubicin. Doxorubicin is one of the most important and effective chemotherapeutic agents for breast cancer and activates apoptosis via the mitochondrial intrinsic pathway in several different cancers [Kemp et al., 2001]. As shown here, single agent treatment with LOX-PP acted in a cytostatic manner similar to trastuzumab, which directly targets the Her-2/neu receptor and is used in the clinic for treatment of Her-2/neu-positive metastatic breast cancers [Pietras et al., 1998]. Though trastuzumab alone does not kill cancer cells, in combinatorial use with chemotherapeutic agents such as doxorubicin, it enhances responsiveness to chemotherapy and overall survival of patients [Nahta et al., 2004]. Our findings suggest the potential usefulness of LOX-PP in the setting of combinatorial treatments for breast and pancreatic cancers.
It is currently unknown by which mechanism(s) LOX-PP cooperates with doxorubicin in promoting cell death. The inhibition of growth of pancreatic xenograft tumors was associated with reduced p65 levels and attenuated cell-cycle activity as determined by reduced cyclin D1. Similarly, we showed attenuation of the MAPK/ERK and AKT pathway with downregulation of the downstream targets NF-κB and cyclin D1 in cells in culture (Fig. 1) in accordance with previous studies in PANC-1 cells [Wu et al., 2007]. Moreover, we have shown that LOX-PP attenuated the PI3K/AKT and NF-κB survival pathways and the proliferative MAPK/ERK pathway in breast and prostate cancer cells [Min et al., 2007; Palamakumbura et al., 2009]. In PANC-1 pancreatic cancer cells in culture, we previously demonstrated that LOX-PP strongly reduces BCL-2 expression [Wu et al., 2007]. Therefore, these data suggest that the combined effects of LOX-PP on these proliferation and prosurvival pathways enhanced the sensitivity of pancreatic and breast cancer cells to doxorubicin. This is consistent with reports of enhanced sensitivity to chemotherapy and radiation in breast, lung, and brain cancer cells in which NF-κB has been rendered inactive by the use of parthenolides, small molecule inhibitors or the IκB-alpha super-repressor [Yamagishi et al., 1997; Wang et al., 1999; Patel et al., 2000; Sweeney et al., 2005]. Thus, one major mechanism of enhancing the sensitivity of cells to doxorubicin might be the potent negative effect of LOX-PP on NF-κB, which frequently prevents apoptosis by activating prosurvival genes [Monks et al., 2004]. More recently, we have shown that LOX-PP attenuates fibronectin-mediated activation of FAK and p130Cas/BCAR1 [Zhao et al., 2009]. High expression levels of p130Cas have been associated with tamoxifen and doxorubicin resistance in human breast cancer [Tikhmyanova et al., 2010]. It appears that p130Cas-mediated resistance to tamoxifen as well as doxorubicin occurs through its effects on proliferation and survival, for example, by altering the balance of the BCL-2 family proteins [Ta et al., 2008; Soni et al., 2009]. Therefore, a second mechanism might involve altering the balance of the BCL-2 family proteins towards proapoptotic members.
In in vivo xenograft experiments, LOX-PP reduced the burden of MIA PaCa-2 cell-derived tumors by ~75% (Fig. 2), comparable to previous breast cancer cell xenografts (~60%) [Min et al., 2007]. MIA PaCa-2 cells are mutant for TP53 and RAS like PANC-1 cells [Wu et al., 2007]. This is important because human pancreatic cancers are characterized by accumulation of a large number of mutations, including gene deletions, amplifications, or point mutations [Maitra and Hruban, 2008]. Among the most frequent ones are mutated RAS and TP53, which render these tumors resistant to treatment with chemotherapy. To date, single-agent gemcitabine is almost exclusively used as first-line treatment of advanced pancreatic adenocarcinomas although with limited success. Multiple clinical trials in which gemcitabine was combined with existing or new agents such as cisplatin, oxaliplatin, 5-fluorouracil have failed to demonstrate significant improvement in almost all cases [Stathis and Moore, 2010].
Among the newer approaches to treating pancreatic cancers are the combined use of cytotoxic agents with inhibitors designed to target specific molecules, that is, the insulin-like growth factor-1 receptor, the epidermal growth factor receptor 2 (Her-2/neu), and matrix metalloproteinases (MMPs) [Merl et al., 2010; Stathis and Moore, 2010]. Benefits of theses treatments are still marginal, though the efficacy of some of these agents has been demonstrated for other types of carcinomas such as carcinomas of the lung and breast [Slamon et al., 2001; Feld et al., 2006]. Based on these disappointing results, it has been suggested that further drug developments should aim to target a broad number of downstream mediators or nodal point within signaling cascades [Stathis and Moore, 2010]. Therefore, in light of the broad inhibitory activities of LOX-PP [Min et al., 2007; Wu et al., 2007; Zhao et al., 2009; Palamakumbura et al., 2009] our findings are encouraging and warrant further exploration of LOX-PP as potential treatment modality for pancreatic cancer used in combination with chemotherapeutic agents. Doxorubicin has been widely studied in pancreatic cancer cells in vitro [Arlt et al., 2001] and one recent trial investigated the combined effect of continuous doxorubicin, 5-fluorouracil, and mitomycin-C as second-line treatment in gemcitabine pre-treated pancreatic and biliary tract cancers [Lee et al., 2009]. It was found that this treatment regiment constitutes a safe and feasible salvage therapy for gemcitabine-resistant pancreatic cancers.
In summary, our findings that LOX-PP sensitizes breast cancer cells to doxorubicin treatment suggest that LOX-PP could be a potential novel treatment used in combination with doxorubicin for breast cancer and potentially for pancreatic cancer.
Acknowledgments
These studies were supported by grants from the NIH R01 CA106468 (K.H.K), R01 CA82742 (G.E.S., P.C.T., and K.H.K.), and R01 CA143108 (G.E.S., K.H.K., and P.C.T.).
Footnotes
The authors declare no conflict of interest.
References
- Arlt A, Vorndamm J, Breitenbroich M, Folsch UR, Kalthoff H, Schmidt WE, Schafer H. Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20:859–868. doi: 10.1038/sj.onc.1204168. [DOI] [PubMed] [Google Scholar]
- Contente S, Kenyon K, Rimoldi D, Friedman RM. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science. 1990;249:796–798. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- Feld R, Sridhar SS, Shepherd FA, Mackay JA, Evans WK. Use of the epidermal growth factor receptor inhibitors gefitinib and erlotinib in the treatment of non-small cell lung cancer: A systematic review. J Thorac Oncol. 2006;1:367–376. [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda A, Wakazono K, Tsukamoto T, Watanabe N, Yagi Y, Tatematsu M, Kaminishi M, Sugimura T, Ushijima T. Lysyl oxidase is a tumor suppressor gene inactivated by methylation and loss of heterozygosity in human gastric cancers. Cancer Res. 2004;64:6410–6415. doi: 10.1158/0008-5472.CAN-04-1543. [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Sun S, Gurley KE. p53 induction and apoptosis in response to radio- and chemotherapy in vivo is tumor-type-dependent. Cancer Res. 2001;61:327–332. [PubMed] [Google Scholar]
- Kenyon K, Contente S, Trackman PC, Tang J, Kagan HM, Friedman RM. Lysyl oxidase and rrg messenger RNA. Science. 1991;253:802. doi: 10.1126/science.1678898. [DOI] [PubMed] [Google Scholar]
- Kirsch KH, Georgescu MM, Hanafusa H. Direct binding of p130(Cas) to the guanine nucleotide exchange factor C3G. J Biol Chem. 1998;273:25673–25679. doi: 10.1074/jbc.273.40.25673. [DOI] [PubMed] [Google Scholar]
- Lee S, Oh SY, Kim BG, Kwon HC, Kim SH, Rho MH, Kim YH, Rho MS, Jeong JS, Kim HJ. Second-line treatment with a combination of continuous 5-fluorouracil, doxorubicin, and mitomycin-C (conti-FAM) in gemcitabine-pretreated pancreatic and biliary tract cancer. Am J Clin Oncol. 2009;32:348–352. doi: 10.1097/COC.0b013e31818c08ff. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merl MY, Abdelghany O, Li J, Saif MW. First-line treatment of metastatic pancreatic adenocarcinoma: Can we do better?. Jop; Highlights from the “2010 ASCO Annual Meeting.”; Chicago, IL, USA. June 4–8, 2010; 2010. pp. 317–20. [PubMed] [Google Scholar]
- Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, Sonenshein GE. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–1112. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, Spicer DB, Rosenberg L, Palmer JR, Sonenshein GE. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009;69:6685–6693. doi: 10.1158/0008-5472.CAN-08-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks NR, Biswas DK, Pardee AB. Blocking anti-apoptosis as a strategy for cancer chemotherapy: NF-kappaB as a target. J Cell Biochem. 2004;92:646–650. doi: 10.1002/jcb.20080. [DOI] [PubMed] [Google Scholar]
- Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- Nishina T, Yamaguchi N, Gohda J, Semba K, Inoue J. NIK is involved in constitutive activation of the alternative NF-kappaB pathway and proliferation of pancreatic cancer cells. Biochem Biophys Res Commun. 2009;388:96–101. doi: 10.1016/j.bbrc.2009.07.125. [DOI] [PubMed] [Google Scholar]
- Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–40600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- Palamakumbura AH, Vora SR, Nugent MA, Kirsch KH, Sonenshein GE, Trackman PC. Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene. 2009;28:3390–3400. doi: 10.1038/onc.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, Gelfanov V, Boswell SH, Goulet RJ, Jr, Sledge GW, Jr, Nakshatri H. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–4169. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Soni S, Lin BT, August A, Nicholson RI, Kirsch KH. Expression of a phosphorylated p130(Cas) substrate domain attenuates the phosphatidyli-nositol 3-kinase/Akt survival pathway in tamoxifen resistant breast cancer cells. J Cell Biochem. 2009;107:364–375. doi: 10.1002/jcb.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, Sheridan C, Campbell RA, Murry DJ, Badve S, Nakshatri H. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- Ta HQ, Thomas KS, Schrecengost RS, Bouton AH. A novel association between p130Cas and resistance to the chemotherapeutic drug adriamycin in human breast cancer cells. Cancer Res. 2008;68:8796–8804. doi: 10.1158/0008-5472.CAN-08-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora SR, Guo Y, Stephens DN, Salih E, Vu ED, Kirsch KH, Sonenshein GE, Trackman PC. Characterization of recombinant lysyl oxidase propeptide. Biochemistry. 2010;49:2962–2972. doi: 10.1021/bi902218p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhou JY, Tainsky MA, Wu GS. Evidence that tumor necrosis factor-related apoptosis-inducing ligand induction by 5-Aza-2′-deoxycytidine sensitizes human breast cancer cells to adriamycin. Cancer Res. 2007;67:1203–1211. doi: 10.1158/0008-5472.CAN-06-2310. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Miyakoshi J, Takebe H. Enhanced radiosensitivity by inhibition of nuclear factor kappa B activation in human malignant glioma cells. Int J Radiat Biol. 1997;72:157–162. doi: 10.1080/095530097143374. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Min C, Vora S, Trackman PC, Sonenshein GE, Kirsch KH. The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of FAK and p130CAS in breast cancer cells. J Biol Chem. 2009;284:1385–1393. doi: 10.1074/jbc.M802612200. [DOI] [PMC free article] [PubMed] [Google Scholar]