Abstract
The pathogenesis of pulmonary hypertension is a complex multifactorial process that involves the remodeling of pulmonary arteries. This remodeling process encompasses concentric medial thickening of small arterioles, neomuscularization of previously nonmuscular capillary-like vessels, and structural wall changes in larger pulmonary arteries. The pulmonary arterial muscularization is characterized by vascular smooth muscle cell (SMC) hyperplasia and hypertrophy. In addition, in uncontrolled pulmonary hypertension, the clonal expansion of apoptosis-resistant endothelial cells leads to the formation of plexiform lesions. Based upon a large number of studies in animal models, the three major stimuli that drive the vascular remodeling process are inflammation, shear stress and hypoxia. Although, the precise mechanisms by which these stimuli impair pulmonary vascular function and structure are unknown, reactive oxygen species (ROS)-mediated oxidative damage appears to play an important role. ROS are highly reactive due to their unpaired valence shell electron. Oxidative damage occurs when the production of ROS exceeds the quenching capacity of the anti-oxidant mechanisms of the cell. ROS can be produced from complexes in the cell membrane (nicotinamide adenine dinucleotide phosphate-oxidase), cellular organelles (peroxisomes and mitochondria), and in the cytoplasm (xanthine oxidase). Furthermore, low levels of tetrahydrobiopterin (BH4) and L-arginine the rate limiting co-factor and substrate for endothelial nitric oxide synthase (eNOS), can cause the uncoupling of eNOS, resulting in decreased NO production and increased ROS production. This review will focus on the ROS generation systems, scavenger antioxidants, and oxidative stress associated alterations in vascular remodeling in pulmonary hypertension.
Keywords: Vascular remodeling, reactive oxygen species, endothelial cells, antioxidants, pulmonary hypertension
Introduction
Pulmonary hypertension (PH) is a progressive disease characterized by an increase in pulmonary vascular resistance (PVR), a mean pulmonary arterial pressure (PAP) >25 mmHg at rest or >30 mmHg during exercise (1). PH constitutes a heterogeneous group of clinical entities sharing similar pathologies that are categorized as pulmonary arterial hypertension, pulmonary venous hypertension, PH associated with hypoxia, and PH associated with chronic thrombotic disease. Histologically, PH is characterized by muscularization of peripheral arteries, medial hypertrophy of muscular arteries, loss of small pre-capillary arteries, and neointima formation. In the later stages of the disease, endothelial cell proliferation leads to the development of plexiform lesions, which are aberrant channels in the obliterated vessel lumen and in the adventitia. The source of the plexiform lesion is unclear but may be due to the clonal expansion of apoptosis-resistant endothelial cells or the proliferation of circulating endothelial progenitor cells at sites of endothelial injury. The vascular changes in PH are collectively referred to as pulmonary vascular remodeling. In this review we will describe the events that underlie the development of PH and the role played by reactive oxygen species in the pathology of this devastating disease.
1. Pulmonary vascular remodeling in pulmonary hypertension
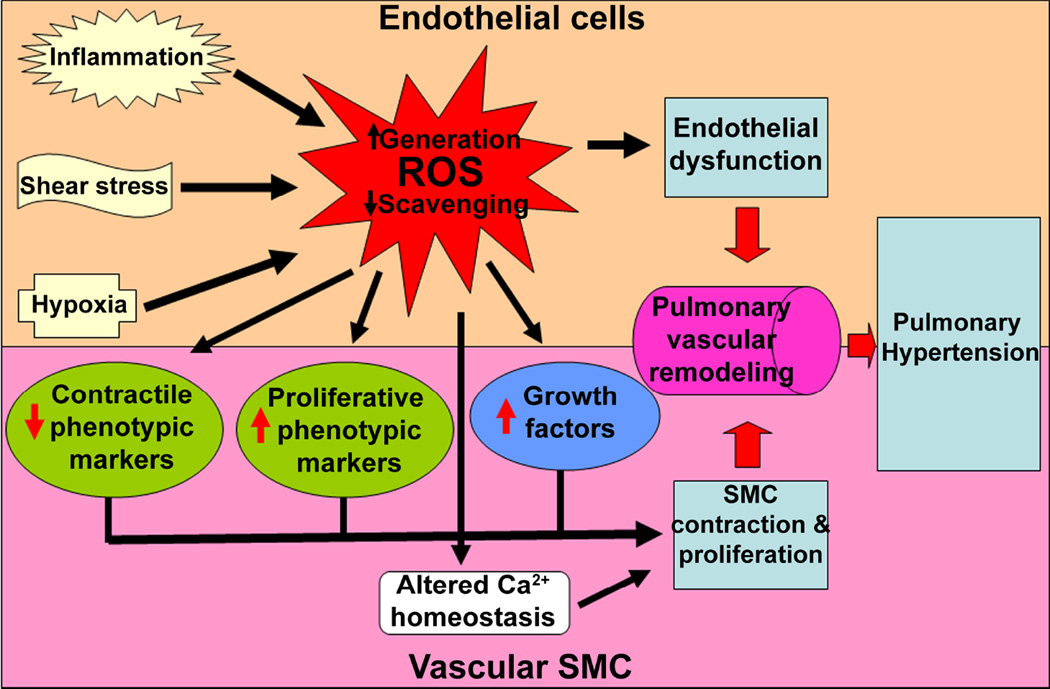
Vascular remodeling is a multi-factorial, multi-cellular process that involves a change in maximal lumen diameter (inward/outward) and alterations in cellular processes such as: cell growth, death, migration, and production and redistribution of extracellular matrix. These lasting alterations in vessel structure contribute to an increase in PVR and PAP. Vascular remodeling is dependent on dynamic interactions between locally generated growth factors, vasoactive substances, and hemodynamic forces. The stimuli responsible for remodeling involve changes in transmural pressure, stretch, shear stress, hypoxia, various mediators (angiotensin II, endothelin (ET)-1, 5-hydroxytryptamine (5-HT), growth factors, and inflammatory cytokines), increased serine elastase activity, and increased production of reactive oxygen species (ROS) (Fig. 1). These stimuli initiate a) endothelial dysfunction, b) smooth muscle cell (SMC) proliferation, and/or c) adventitial abnormalities. However, not all compartments of the vessel are necessarily involved in the development of individual cases of PH.
Figure 1. Schematic view of the pathology of pulmonary hypertension.
The figure summarizes the major pathological events that lead to the development of pulmonary hypertension (PH). In humans, PH is a complex and multifactorial, diffuse disorder of the pulmonary vasculature. However, for the purposes of research and inducing PH in animal models, the etiology of PH can be broadly distinguished into: (a) inflammatory; (b) high shear stress; and (c) hypoxia, even though none of these factors act alone during the progression of the disease. A common mechanism by which these insults provoke vascular dysfunction is by either increasing the production of reactive oxygen species (ROS) or by attenuating the ROS scavenging capability of the cells. Once produced, ROS can influence the growth and morphology of all three layers of the pulmonary vessels. In endothelial cells, ROS promote endothelial proliferation, decrease NO production, and increase the release of vasoactive mediators, leading to endothelial dysfunction. In smooth muscle cells (SMC), oxidative stress caused by ROS induces contraction and a switch to a synthetic phenotype. These SMC alterations are brought about by increasing the intracellular free Ca2+ and decreasing the expression of contractile phenotypic markers, while simultaneously enhancing the expression of proliferative phenotypic markers and growth factors. ROS can also augment the proliferation of fibroblasts in the adventitial layer and their expansion between the endothelium and the internal elastic lamina, known as the neointima (not depicted in the figure). Together, these changes result in vascular remodeling and the development of PH.
1.1. Endothelial cell dysfunction
The endothelium provides a semi-permeable barrier between the vascular and extravascular fluid compartments and is intimately involved in the regulation of vascular tone, growth, and differentiation. In PH, vascular responses to injury caused by hypoxia, increased flow (shear stress), drugs (dexfenfluramine), or toxins (adulterated rapeseed oil) are mediated in part through the disruption of endothelial cell function. The endothelium may respond to specific forms of injury in various ways that can affect the process of vascular remodeling and PVR. For instance, in the chronically hypoxic rat model of PH, endothelial cells proliferate in the main pulmonary arteries much earlier than in the smaller muscular arteries (2). However, under increased shear stress conditions, endothelial cells proliferate mainly in the smaller pulmonary arteries (3). These hyperproliferative endothelial cells exhibit increased numbers/sizes of lamellar structures and organelles, which contribute to their swollen appearance (4,5). In PH, endothelial cells generate less nitric oxide (NO). NO, synthesized largely by endothelial nitric oxide synthase (eNOS) in endothelial cells in the pulmonary vessels, is a vasodilator and suppressor of SMC proliferation. The endothelium also releases other vasoactive mediators, such as ET-1, angiotensin II (Ang-II), thromboxane A2 (TXA2), and growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF) all of which can influence the growth of the underlying smooth muscle layer, resulting in vascular remodeling (6–9). In addition, increased expression and activation of the Tie2 receptor can lead to serotonin mediated SMC contraction and proliferation (10). The endothelial cells of pulmonary hypertensive patients appear to be highly proliferative in response to growth factors and exhibit high rates of glycolysis (11). These disorganized, proliferating endothelial cells form tufts of cellular capillary formations that resemble a vascular plexus and are present within the lumen of dilated aneurysmal thin-walled arteries. These so called plexiform lesions most commonly develop immediately distal to bifurcation sites of small pulmonary arteries (approximately 50 to 300µm) (12). These sites are particularly susceptible to the development of plexiform lesions due to the high levels of fluid shear stress occurring distal to the bifurcations in the pulmonary vessels (3). It has been shown that exposing endothelial cells to a high level of shear stress for up to three weeks using an artificial capillary system results in the development of a highly proliferative phenotype and the occlusion of the intra-luminal space at a much greater rate than endothelial cells exposed to low shear stress for the same duration (3). Further, this hyper-proliferative phenotype appears to be preceded by an initial increase in apoptosis (13).
Previous work has identified increased expression of VEGF (14,15), kinase insert domain-containing receptor (VEGF-R2) (15), angiopoietin-1 (16), 5-lipoxygenase (17), 5-lipoxygenase activating factor (17), ET-1 (18), HOX genes (19), and regulated upon expression normal T-cell expressed and secreted (RANTES) (20) in the lungs of patients with severe PH. Other markers of the severe angioproliferative endothelial cell phenotype in plexiform lesions include the expression of the anti-apoptotic protein, survivin, and the proliferative marker, Ki-67 (3). The endothelial cells in the plexiform lesions also express high levels of the hypoxia inducible factor subunits, HIF-1α and HIF-1β, which together are responsible for the hypoxia dependent induction of VEGF (15). Further, if rats are treated for 3 weeks with the VEGF-R2 blocker, SU5416, under hypoxic conditions, they develop a severe, irreversible, and fatal form of PH that is characterized by the occlusion of pre-capillary pulmonary arteries by clusters of proliferating endothelial cells (21). Treatment with SU5416 also leads to the emergence of a population of apoptosis-resistant hyperproliferative endothelial cells that are thought to form the plexiform lesion (22). However, we still do not fully understand how the plexiform lesion develops in PH. Mutations in the TGF-β receptor II and proapototic, Bax genes have been identified within the endothelial cells of plexiform lesions derived from patients with severe PH (23). Further, in primary pulmonary hypertensive patients, microsatellite mutations have been found in the Bax gene in approximately 20% of plexiform lesions (24). From these data it has been postulated that the down-regulation of Bax and its related genes may contribute to the uncontrolled endothelial cell proliferation within the plexiform lesions. Further, the tumor suppressor gene, peroxisome proliferator-activated receptor (PPAR)-γ, has also been shown to be down-regulated in plexiform lesions of patients with severe PH (25). Thus, it is possible that the modulation of signaling pathways responsible for cell cycle control allows for the monoclonal expansion of endothelial cells from a single cell that has acquired a selective growth advantage (26), leading to the theory that plexiform lesions are actually neoplastic outgrowths. If true, then this suggests that in primary PH gene mutations, rather than local vaso-proliferative factors, play a major role in triggering endothelial cell proliferation.
1.2. Medial Smooth Muscle expansion
The SMC layer undergoes a variety of changes in PH, including hypertrophy, hyperplasia, and extensive matrix protein deposition. SMC hypertrophy is a predominant feature of larger proximal vessels, while smaller resistance vessels undergo hyperplasia (2,27,28). In PH, the SMC contain more prominent endoplasmic reticula and Golgi (28), produce more collagen (types I and IV) and elastin (29), and acquire a more synthetic-, as opposed to a contractile-, phenotype. In the main pulmonary arteries exposed to hypoxia, collagen and elastin are rapidly synthesized and then rapidly removed from these vessel during normoxic recovery (30). As a result, new internal elastic lamina are formed in the hypoxic remodeled vessels, such that their structure comes to resemble that of the resistance vessels of the systemic circulation (31). Landmark studies from Stenmark’s group have also shown that pulmonary vessels are composed of heterogeneous subpopulations of SMC. These subpopulations have marked differences in the phenotype, growth, and matrix-producing capabilities. Therefore, it is possible that the differential proliferative responses or elastogenic responses to hypoxia or vessel wall injury may be attributed to the expansion of specific SMC subpopulations (32). Studies have shown that SMC isolated from the outer media have an augmented proliferative response to hypoxia compared to those isolated from the middle media (33). In addition, the process of muscularization of otherwise non-muscular vessels is a result of the differentiation and hypertrophy of SMC precursor cells and pericytes already present in the vessel wall. Pericytes have been shown to exhibit great plasticity in culture and are capable of differentiating into macrophages, osteoblasts, adipocytes, fibroblasts, and SMCs (34). A recent study found that bone morphogenic protein-4 (BMP-4), TGF-β1, 5-HT, and ET-1 can each induce human pulmonary artery smooth muscle hypertrophy, as evidenced by increases in cell size, protein synthesis, contractile protein expression, and fractional cell shortening (35). SMC hypertrophy is also dependent on the phosphorylation and inhibition of glycogen synthase kinase-3β (GSK-3β) and the activation of p70S6 kinase. In addition, the process of muscularization also requires the migration and differentiation of interstitial fibroblasts (36,37). Thus, overall the prevailing data supports the concept that in PH, SMC growth is stimulated and this correlates with a switch from a contractile to a phenotypic phenotype.
1.3. Changes in the adventitial layer
The adventitial layer surrounding the blood vessel is considered a supporting tissue that is responsible for providing adequate nourishment to the muscle layers of the tunica media. However, the adventitia can also be markedly remodeled in patients with certain forms of collagen vascular diseases associated with severe PH, most notably scleroderma. In PH and other pathologies, fibroblasts from the adventitia can modulate vascular remodeling by modulating several parameters of the SMC layer including phenotypic conversion, proliferation, apoptosis, and migration (38–41). In response to various stimuli, resident fibroblasts can be reprogrammed to have different structural and functional behavior. For example, in experimental models of severe and mild balloon injury, the adventitial fibroblasts can be phenotypically converted into smooth muscle-like cells called myofibroblasts (38–40). Fibroblastscan also be both structurally and functionally heterogeneous (42,43). The activation of fibroblasts by TGF-β1 and TGF-β2 induces a contractile phenotype that correlates with α-SM actin expression and the development of a cytoplasmically enriched microfilamentous system, fibronexus junctions, and other features shared by SMC (44). In PH, the thickening of the adventitia is primarily caused by the proliferation of fibroblasts and myofibroblasts (45). More recent experiments in models of hypercholesterolemia and hypertension have also demonstrated that changes in the adventitial layer precede both intimal and medial remodeling (46). Therefore, under certain conditions, the adventitial compartment may be considered the principal injury sensing tissue of the vessel wall.
1.4. Neointima formation
Neointima formation is intrinsic to severe PH and is characterized by the development of a layer of cells and extracellular matrix between the endothelium and the internal elastic lamina (47,48). Neointima occuring in the small and large arteries are a significant contributor of increased PVR. Neointima are comprised of myofibroblasts expressing the SM markers: α-SM actin and vimentin, but can be distinguished from mature SMC by their lack of highly differentiated SMC markers, such as SM-myosin heavy chain. Additionally, neointimal cells do not express markers of endothelial cells, such as CD31, CD34, or factor VIII (47). It is unknown whether neointimal cells originate by the trans-differentiation of endothelial cells (49), by the migration of SMC from the media, or by the migration of adventitial fibroblasts. However, recent studies using pulse labeling of dividing cells with bromodeoxyuridine have suggested that in injured arteries, proliferating cells from the media and adventitia can migrate to the subendothelial space (50,51). Further, labeled adventitial fibroblasts stably transduced with a lacZ retrovirus were found to be capable of migrating from the adventitia to the media and neointima (40). Unfortunately, the lack of specific markers for fibroblasts and myofibroblasts has hampered a detailed analysis of the phenotypic features of these migrating cells and the time course of their activation during the process of neointima formation. The increased expression and activation of the ROS- generating enzyme system, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been linked to neointima formation in a non-hyperlipidaemic rabbit model of early stage arterial remodeling (52). However this link is controversial as neointima formation is not observed in the chronic hypoxiaor the monocrotaline-induced rat models of PH even though NADPH oxidase activity is increased (53,54). However, when monocrotaline- induced vascular injury is accompanied with high blood flow (shear) induced by pneumonectomy, the rats develop neointima (55,56). This suggests that endothelial injury induced by sustained high levels of shear stress may be an important stimulus for neointima formation and disease progression.
2. Sources of ROS in pulmonary hypertension
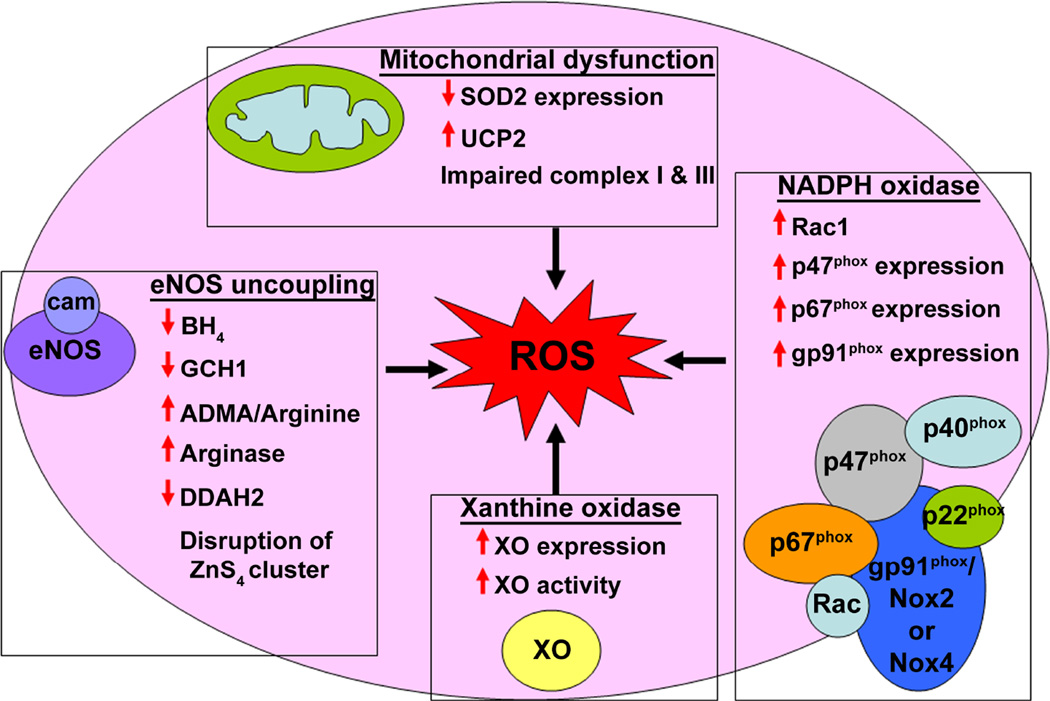
ROS is a term that includes free radicals that are naturally formed as byproducts of oxygen metabolism, such as hydroxyl (OH•), superoxide (O2−), peroxyl (RO2•), lipid peroxyl (LOO•), and non radical species, such as hydrogen peroxide (H2O2), hypochloric acid (HOCl), ozone (O3), and lipid peroxide (LOOH). ROS can also act as intermediates in signaling pathways in much the same manner as classical second messengers. The main ROS involved in cell signaling pathways are O2− and H2O2. Electrically charged O2− is highly reactive and is rapidly dismutated into H2O2, while the enzyme, catalase, decomposes H2O2 to water and oxygen. The low cellular concentrations of O2− (picomolar-nanomolar range) and its limited diffusion enable O2− to acts as an intracellular messenger. In contrast, the high cellular levels of H2O2 (high nanomolar-micromolar range) and its ability to diffuse across hydrophobic membranes allow H2O2 to be regarded as both an intra- and inter- cellular messenger (57). In the lungs, endothelial cells, neutrophils, eosinophils, alveolar macrophages, and alveolar epithelial cells are all major sites of ROS generation. ROS in the pulmonary vasculature can be produced from complexes in the cell membrane, cellular organelles such as, peroxisomes and mitochondria and in the cytoplasm. In addition, low levels of substrates or co-factors, such as L-arginine and tetrahydrobiopterin (BH4) can uncouple eNOS, producing a shift from NO generation to ROS production (58).Several enzymes are now recognized to be involved in generating the increased ROS associated with PH. These include NADPH oxidase, uncoupled NOS, dysfunctional mitochondria, and xanthine oxidase (Fig. 2).
Figure 2. Reactive oxygen species generation in pulmonary hypertension.
The major sources of ROS in the vasculature include uncoupled eNOS, mitochondrial dysfunction, NADPH oxidase, and XO. In PH, all of these sources contribute to the development of oxidative stress. Endothelial NOS uncoupling is mediated by limited L-arginine availability, as a result of increased degradation by arginase upregulation and attenuated synthesis by the downregulation of ASS and ASL, the enzymes responsible for the conversion of L-citrulline to L-arginine. Moreover, a sustained increase in ADMA levels, due to a decrease in DDAH2 activity, competes with L-arginine for binding to eNOS. In addition, in PH, eNOS function is impaired by a decrease in BH4, an essential co-factor for NO generation. GCH1, the rate-limiting enzyme in BH4 biosynthesis, is ubiquinated and targeted for degradation by Hsp70/CHIP. Therefore, the low GCH1 levels, limit the production of BH4. Finally, The disruption of the zinc tetrathiolate (ZnS4) cluster by oxidative attack disrupts the eNOS dimer, which is accompanied by decreased NO generation and increased ROS production. Further, in PH, several markers of mitochondrial dysfunction are observed, including increased levels of uncoupling protein-2 (UCP-2), decreased levels of the mitochondrial antioxidant, superoxide dismutase-2 (SOD2), and the impaired function of complexes I, II, and III of the respiratory chain. Interestingly, ADMA appears to promote these changes in the mitochondria, and also augment mitochondrial ROS generation and decrease ATP production. In addition, several subunits of NADPH oxidase, including p47phox, p67phox, gp91phox, and Rac1, are upregulated in PH, increasing ROS production in the vasculature. Increased levels and activity of XO also contribute to oxidative stress and vascular dysfunction in the early stages of PH.
2.1. NADPH oxidase
Although several enzymes are now recognized to produce ROS; perhaps the most important of these is NADPH oxidase, which is located on the cell membrane, and other organelles, of polymorphonuclear cells, macrophages, vascular SMC, fibroblasts, and endothelial cells (59–62). The NADPH oxidase system was originally studied in cellular innate immunity where it is involved in the destruction of invading bacteria and fungi, and in clearing tissue debris. The NADPH oxidase system is a transmembrane mutimeric protein structure that is capable of producing O2− by catalyzing a one electron reduction of molecular O2, using NADH or NADPH as a donor (63). The NADPH oxidase family consists of seven catalytic homologs: Nox1–5 and Duox1–2, but only Nox1, Nox2, Nox4, and Nox5 are found in the pulmonary vasculature. Apart from the conserved structural properties of the NADPH oxidase enzymes, there are several adapter subunits specific for each member of the Nox family that are critical for enzymatic function. For example, in endothelial cells, SMC, and fibroblasts (64), Nox2, the prototypic Nox, is constitutively associated with p22phox (65–67) and is unstable in the absence of this subunit (68). Upon stimulation, the p47phox subunit becomes phosphorylated which helps recruit the “activator subunit”, p67phox, as well as the p40phox subunit into the Nox2 complex (69). Finally, the small GTPase Rac1 joins the complex by first, directly binding to Nox2 and then subsequently interacting with p67phox (70,71). When the complex is assembled, it is then able to generate O2− by transferring an electron from NADPH in the cytosol to O2 in the luminal or extracellular space (72). Unlike Nox2, the activation of Nox1 requires the interaction with homologues of p47phox and p67phox, NADPH oxidase organizer 1 (NOXO1) and NADPH oxidase activator 1 (NOXA1), respectively (73,74). The other pulmonary vasculature NADPH oxidases, Nox4 and Nox5, seem to be constitutively active and do not require the cytosolic subunits, p47phox, p67phox, p40phox, or GTPase Rac1. However, Nox4 is dependent upon the membrane subunit, p22phox, for its activation, while the role of Rac1 is still controversial (75,76). NADPH oxidase can be activated by growth factors (PDGF, EGF, and TGF-β1), cytokines (tumor necrosis factor-α, interleukin-1, and platelet aggregation factor), mechanical forces (cyclic stretch, laminar-, and oscillatory-shear stress), and metabolic factors (hyperglycemia, hyperinsulinemia, free fatty acids, advanced glycation end products), and G protein–coupled receptor agonists (serotonin, thrombin, bradykinin, ET-1, and Ang II) (77,78) (78,79). Conversely, NADPH oxidase is down-regulated by statins, PPAR agonists, and estradiol (80,81).
NADPH oxidase derived O2− has been shown to play a key role in vascular dysfunction in many different models of PH. In hypoxia-induced PH, Nox2 plays a significant role in ROS generation and endothelial dysfunction (82). Hypoxia also upregulates Nox4 mRNA and protein levels in adventitial fibroblasts (62), and SMC (59), while siRNA mediated silencing of Nox4 reduces ROS and cellular proliferation. In addition, the up-regulation of Nox4 and the subsequent increase in ROS generation attenuates K+(v) channel current in PASMC (83) and promotes Ca2+ influx. However, in pulmonary resistance arteries isolated from hypoxic piglets, only Nox1 and the membrane fraction of p67phox were increased, while Nox4 expression was unchanged (84). Furthermore, in monocrotaline treated rats, Nox4, but not Nox2, is upregulated (85). In lambs with increased PBF, p47phox and Rac1 protein levels are transiently increased, correlating with a significant increase in NADPH oxidase dependent O2− generation at 2- and 4-, but not 8-weeks of age (86). In fetal lambs with a ligated ductus arteriosus, which mimics the human condition of persistent pulmonary hypertension of the newborn (PPHN), an elevation in NADPH oxidase activity and O2− levels correlated with increased mRNA levels for Nox2, Rac1, p47phox, and Nox4, as well as the protein levels of p67phox and Rac1 (87). In PASMC, Nox4 expression is induced via a TGF-β1/Smad 2/3 signaling pathway (88). The subsequent increase in ROS promoted PASMC proliferation, suggesting that Nox4 mediates TGF-β1 induced pulmonary vascular remodeling (88). Nox1 and Nox4 have also been shown to be critical for HIF-2α expression and transcriptional activation (89). Additionally, TGF-β/SMAD signaling can synergize with hypoxia/HIF-1α to promote proliferation (90). Thus, HIF-1α/HIF-2α, TGF-β1, and Nox4 may act synergistically to potentiate pulmonary vascular remodeling. Taken together the available data suggest that NADPH oxidase derived O2− plays an important role in the vascular remodeling in PH.
2.2. Uncoupled NOS
Endothelial NOS is a Ca2+ dependent flavoenzyme that converts L-arginine to L-citrulline to generate NO. This reaction requires the cofactors: Ca2+/calmodulin, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and BH4 (91). In the pulmonary vasculature, NO is normally formed in endothelial cells from eNOS (92). During basal conditions, NO released from the endothelium maintains a low PVR. The acute blockade of NO in rabbits and neonatal piglets has been shown to increase basal PVR (93,94). All three isoforms of NOS i.e. eNOS, neuronal NOS (nNOS), and inducible NOS (iNOS) can generate O2− in conditions of substrate (L-arginine) or cofactor (BH4) deficiency (95). In these conditions, electrons flowing from NADPH through the NOS reductase domain and into to the oxygenase domain are diverted to molecular oxygen to produce O2− rather than to L-arginine to produce NO, therefore making the enzyme dysfunctional under pathological circumstances. The exogenous supplementation of L-arginine and BH4 has a vasodilatory effect in systemic and pulmonary vascular beds and can correct this NOS uncoupling and abnormal endothelium-dependent vasodilation. In patients with PH, L-arginine administration reduces PAP and PVR (96). Decreases in L-arginine, either by enhanced degradation by the enzyme arginase or reduction in L-arginine synthesis can uncouple NOS and drive O2− production. Indeed, in the lungs of lambs with increased PBF, L-arginine levels are decreased as early as 2-weeks after birth (97). Further, these lambs have reduced activities of the arginine recycling enzymes, argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) as well as an increase in arginase activity. These changes correlate with an increase in NOS-derived O2− generation (97). Elevated plasma arginase activity has also been reported in patients with PH secondary to sickle cell disease (98). Furthermore, beyond stimulating eNOS uncoupling, increases in arginase activity can also promote SMC growth and collagen synthesis through the formation of polyamines and L-proline (99).
Asymmetric dimethyl-l-arginine (ADMA), an endogenous, L-arginine analogue, promotes the dissociation of the ferrous-dioxy species from the heme group of NOS, which results in O2− rather than NO production. Increased plasma concentrations of ADMA are associated with oxidative stress within the vasculature and endothelial dysfunction (100). ADMA competes with L-arginine for the substrate binding site on NOS, and therefore, a decrease in the L-arginine:ADMA ratio is an important indicator of reduced NO formation and vascular dysfunction. In a recent study, plasma ADMA was found to be significantly higher and global L-arginine bioavailability significantly lower in patients with PH associated with advanced decompensated heart failure and was also associated with higher PAP and higher central venous pressure (101). Further, in lambs with increased PBF, there is both an increase in ADMA and a reduction in L-arginine (102). Supplementation of these lambs with L-arginine both increased NO production and reduced ROS formation, implicating the L-arginine:ADMA ratio as an important determinant in the development of endothelial function (102,103). ADMA is metabolized by dimethylarginine dimethylaminohydrolase (DDAH) to L-citrulline and dimethylamine. In humans, there are two isoforms of DDAH (DDAH-1 and DDAH-2) that have distinct but overlapping tissue distribution (104). Accumulating evidence suggests that decreased DDAH activity is a key factor in the elevation of ADMA under many pathophysiological conditions (105,106). Indeed, in lambs with increased PBF, the rise in ADMA levels correlated with a decrease in DDAH activity (102). Also, rats with PH and patients suffering from idiopathic pulmonary arterial hypertension (IPAH) also have a marked increase in plasma ADMA levels with a concomitant decrease in DDAH-2 expression at both the mRNA and the protein level with no significant changes in DDAH1 expression (107). Piglets with persistent pulmonary hypertension of the newborn (PPHN) exhibit no changes in DDAH1 expression, but markedly decreased DDAH2 expression (108) resulting in a decrease in total DDAH activity (108). These data suggest that there is a differential regulation of DDAH isoforms during the development of PH and that the dysregulation of DDAH2, rather than DDAH1, may be important for the development of PH (108).
In addition to alterations in arginine metabolism, derangements in the NOS cofactor, BH4, can also modulate O2− generation from eNOS (109). A number of studies have shown that suboptimal concentrations of BH4 induce endothelial dysfunction and O2− generation through eNOS uncoupling (110). BH4 itself is also highly susceptible to oxidative degradation to BH2 (91), and therefore, the oxidative loss of BH4 in response to increased oxidative stress amplifies ROS production by uncoupling of eNOS due to BH4 deficiency. BH4 is generated by two different metabolic pathways: the de novo and the salvage pathways. GTP cyclohydrolase I (GCH1) is the first, rate-limiting enzyme in the de novo pathway of BH4 biosynthesis (111). GCH1 deficient, hph-1 mutant mice develop PH at birth, as indicated by their increased RV/LV+septum ratio secondary to ROS mediated vascular remodeling (112). These mice have low BH4 levels in the lung, elevated ROS levels, and pulmonary arterial medial thickening (112). In lambs with increased PBF, BH4 and GCH1 levels are decreased, which correlates with decreased NO bioavailability (102). Interestingly, the decrease in GCH1 is the result of an ADMA induced ubiquitination and proteasome-dependent degradation of GCH1 (102), indicating the complexity of the interactions in regulating eNOS uncoupling.
All NOS isoforms function as homodimers (113) and an important mediator of the dimeric structure is a region between the eNOS dimer interface known as the zinc tetrathiolate (ZnS4) cluster. The ZnS4 cluster in the oxygenase domain of eNOS is formed by a zinc ion and two cysteine residues (94 and 99) from each monomer and is positioned at equal distance from each heme group. It is thought that the ZnS4 cluster also maintains the integrity of the BH4 binding site of eNOS (114). The maintenance of this dimer interface is key to the enzyme function as mutation of this cluster prevents the binding of zinc, BH4, and L-arginine and eliminates enzyme activity (115). Studies have also shown that the oxidative attack by H2O2 on the ZnS4 cluster disrupts the eNOS dimer, which is accompanied by zinc release and decreased NO generation. However, pre-incubation of eNOS with excess BH4 prevents the destruction of ZnS4 and preserves enzyme activity (116), suggesting that the eNOS dimer collapse may be an important mechanism by which ROS regulate NO bioavailability under conditions of acute oxidative stress. Further, recent data using recombinant eNOS protein purified from E.coli containing disrupted tetrathiolate clusters have shown that, compared to wildtype eNOS, the disruption of the eNOS dimer reduces NO generation to essentially background levels, while significant levels of O2− are still generated (117). However, the role of dimer disruption of eNOS in PH has not been well studied so it is unclear how important this process is to the development of the disease but it warrants further study.
2.3. Mitochondrial dysfunction
Mitochondrial dysfunction is involved in the pathology of a number of diseases including PH and is associated with altered mitochondrial ROS generation. Indeed, mitochondrial dysfunction has been shown to underlie a few documented cases of PH (118–120). Mitochondria are a major source of ROS production in the cardiovascular system. The potential sites of ROS production in the electron transport chain of the mitochondria include the flavin site and ubiquinone reducing site of complex I, the flavin site of complex II, and the ubiquinol oxidizing site in complex III (121). Complexes I and III have been generally shown to produce most of the ROS (122), even though tissue heterogeneity of complexes is an important factor determining the individual role of the complexes in ROS generation. For instance, ROS generation has also been shown to be ablated by inhibiting complex III in neonatal pulmonary artery myocytes exposed to hypoxia (123). In contrast, in the hypoxic mouse lung, the source of the mitochondrial ROS has been localized predominantly to complex II (124). In a monocrotaline-induced rat model of PH, the expression and activity of complex II is increased along with complex II derived ROS (125). Interestingly, PASMC treated with hypoxic media exhibited increased ROS production in the mitochondrial inter-membrane space and decreased ROS in the mitochondrial matrix, suggesting that ROS generation is differentially regulated in the mitochondrial compartments (126).
In PH, the role of mitochondrial derived ROS is both complex and controversial with many competing studies indicating that mitochondrial ROS both increase and decrease during the progression of the disease. For example, decreased levels of mitochondrial derived ROS have also been found in PASMC isolated from human pulmonary hypertensive patients (127). Several studies have also found reduced levels of mitochondrial ROS in the FH rat (128), as well as in hypoxia-, and monocrotaline-induced PH (129). Many researchers have also demonstrated that ROS are generated in proportion to PO2 and that ROS generation with hypoxic pulmonary vasoconstriction (HPV) is actually decreased. These data indicate that inhibiting the mitochondrial electron transport chain with rotenone and antimycin A, results in pulmonary vasoconstriction and a reduction in lung ROS production (130). Using lucigenin-enhanced chemiluminescence, Paky et al. demonstrated that hypoxia attenuated ROS production in the rabbit lung (131). Similarly, in the rat lung, mitochondrial derived ROS increases in direct proportion to an increase in PO2 (132). In cardiomyocytes isolated from the adult guinea pig, hypoxia diminishes mitochondrial-derived H2O2 levels and impairs the basal activity of L-type Ca2+ channel (133).
In PH, mitochondrial derived ROS have been shown to induce pulmonary vascular remodeling by increasing intracellular Ca2+, depolarization/hyperpolarization of the mitochondrial membrane potential (ΔΨm), and PASMC contraction and growth (134). Under hypoxic conditions, mitochondrial derived ROS was shown to be elevated with a concomitant increase in Ca2+ influx and PASMC contraction (135–137). In addition to promoting vascular constriction, mitochondrial derived ROS have been demonstrated to enhance PASMC proliferation (138). In a recent study, hypoxia induced PASMC proliferation was attributed to the opening of mitochondrial K+ATP channels, increased mitochondrial ROS generation, and subsequent activation of the redox sensitive transcription factor, AP-1 (138). The opening of K+ATP channels also inhibited the mitochondrial transition pore, induced depolarization of the ΔΨm, and attenuated cytochrome c release and PASMC apoptosis (138). In contrast, it was shown that in PH and in moderate hypoxia, the ΔΨm was hyperpolarized, mitochondrial derived ROS were decreased, and K+ currents were attenuated, leading to the depolarization of the plasma membrane, Ca2+ influx, and PASMC contraction (139). The activation of NFATc2 by Ca2+ (127) and the inhibition of caspases by K+ contribute to PASMC hypertrophy/proliferation and impaired apoptosis (129,140), respectively. Interestingly, hyperpolarized mitochondria have been documented in PASMC isolated from rodents exposed to chronic hypoxia and monocrotaline (129), FH rats (128), and from human pulmonary hypertensive patients (127). It is hypothesized that the hyperpolarized mitochondria are resistant to apoptosis, which may in part explain the proliferative vascular phenotype (139). In addition, the treatment of PASMC with antioxidants results in the depolarization of the ΔΨm and subsequent apoptosis, suggesting a key role of ROS in mitochondrial membrane hyperpolarization (141). In PH, the increase in mitochondrial ROS may also be attributed to elevated circulating levels of ADMA. In PAEC, ADMA has been shown to induce mitochondrial dysfunction by increasing uncoupling protein-2 (UCP-2) protein levels and mitochondrial ROS in a dose dependent manner, resulting in the reduction in cellular ATP levels (142). Similarly, in lambs with increased PBF have increased ADMA levels (102) and exhibit several markers of mitochondrial dysfunction, such as increased levels of UCP-2, decreased levels of the mitochondrial antioxidant, superoxide dismutase-2 (SOD2), and an increased lactate to pyruvate ratio (143). Thus, overall the data regarding mitochondrial ROS in the development of PH have yet to reach a consensus perhaps due to inherent differences in the models employed or the focus on different cell types (SMC vs. EC) and much work still lies ahead. However, it is fair to conclude that regardless of whether ROS production is increased or decreased, mitochondria play an important role in HPV and likely in the development of PH in general.
2.4. Xanthine oxidase
Xanthine oxidase (XO) is another important source of ROS in the pulmonary vasculature. This molybdenum and iron-containing flavoprotein catalyzes the transformation of hypoxanthine and xanthine to uric acid with concomitant release of O2−/H2O2 as by-products (144). Initially, XO exists in the form of xanthine dehydrogenase (XDH), which is the primary gene product. However, this can be easily converted into XO by reversible thiol oxidation and/or irreversible proteolysis. Therefore, the ratio of XO to XDH in the cell is a critical determinant to assess ROS production by this enzyme (145). The addition of XO or uric acid in cell culture modifies cell growth and proliferation (146), suggesting a role for XO in vascular remodeling. In fact, in rats chronically exposed to hypoxia for 14 days from birth, the XO inhibitor, allopurinol, limited oxidative stress in the lung and attenuated hypoxia-induced vascular remodeling. These hypoxia exposed pups also had increased serum and lung XO activity, increased vascular XO-derived O2− production, and vascular nitrotyrosine formation, which were all prevented by treatment with allopurinol (147). Moreover, in cultured calf PASMC, XO treatment increased the activity of the redox-sensitive transcription factor, Egr-1, induced the phosphorylation of ERK1/2, increased SMC proliferation, and reduced apoptosis (148). In lambs with increased PBF, XO protein levels are significantly increased at 2- and 4-weeks of age but are significantly decreased at 8-weeks. This correlates with an increase in XO dependent O2− generation at 2- and 4-, but not 8-, weeks of age in these lambs (86). In addition, elevated plasma XO levels have been reported in patients with IPAH (149). Collectively, these findings suggest that XO-derived O2− can induce vascular dysfunction, thus impairing pulmonary arterial relaxation and so contribute to the vascular remodeling in PH but that the role of XO may be transient.
3. Oxidative stress in pulmonary vascular remodeling
Oxidative stress describes an imbalance in ROS handling due to either the over-production of ROS and/or decreased capability of antioxidant defenses. ROS play an essential role in the regulation of several physiological and pathophysiological processes. ROS modulates the release of and/or effects of several vasoactive factors, such as ET-1 (150), TXA2, prostacyclin, and PPAR-γ, which can acutely influence vessel tone and, over time, result in vascular remodeling in PH. TXA2, a platelet derived vasoconstrictor, is an important mediator of PH. Its effects are antagonized by the release of prostacyclin from endothelial cells. An imbalance between the release of TXA2 and prostacyclin promotes vascular remodeling in PH (151). NADPH oxidase derived O2− has been associated with a decrease in prostacyclin and an increase in TXA2 levels in monocrotaline-induced PH (85). ET-1, a small 21 polypeptide, produced predominately by the endothelium, is one of the strongest known vasoconstrictors. Increased plasma levels of ET-1 have been demonstrated in patients with PH, and the levels of ET-1 correlated with an increase in PVR (152). Oxidative stress can stimulate both the expression of the ET-1 gene and the secretion of the ET-1 peptide (153). Further, ROS have been shown to increase the mRNA, protein, and activity of ET-1 converting enzyme-1 (ECE-1) (154). ROS can also modulate cellular growth pathways, modulate cellular proliferation, alter transcription factor activity (155), and attenuate apoptosis or regulated cell death (156,157). However, the excessive production of ROS can induce irreversible alterations to cellular lipids, proteins, and DNA, and result in cellular dysfunction or cell death (158,159). ROS generated in the lungs have also been strongly implicated in the pathology of many lung diseases, such as pulmonary emphysema, adult respiratory distress syndrome (ARDS), lung fibrosis, lung transplant rejection, and PH.
3.1. ROS and pulmonary vascular constriction
Pulmonary vasoconstriction is the generation of increased tensile force, which translates into the narrowing of the cross-sectional lumen of the vessel. Pulmonary vasoconstriction contributes to the increase in PVR and, hence, the elevated PAP in PH. Also, as stated above, the increase in PVR and PAP can directly influence SMC hypertrophy and hyperplasia, leading to vessel occlusion (160). Hypoxic pulmonary vasoconstriction (HPV), an adaptive mechanism unique to the lungs, is a major contributor to the development of PH secondary to hypoxic cardiopulmonary diseases and has been extensively detailed in a recent review (161). Although the precise mechanism by which hypoxia causes pulmonary vasoconstriction is still unclear, it is accepted that ROS promote SMC contraction by triggering a rise in cytosolic free Ca2+ concentration and antioxidants abolish the HPV response in isolated rabbit lungs and pulmonary artery myocytes (137,162). Sources of intracellular Ca2+ include mitochondria, inositol 1,4,5-triphosphate (IP3)-sensitive sarcoplasmic reticulum (SR) stores, and ryanodine sensitive SR stores. The majority of studies have identified a central role for ryanodine sensitive SR stores in ROS dependent Ca2+ release (163) and the inhibition of ryanodine receptors abolishes H2O2 induced Ca2+ release in pulmonary artery SMC (PASMC) and vasoconstriction in isolated pulmonary arteries (164,165). However, other studies suggest that mitochondrial derived ROS can also be the trigger that alters intracellular Ca2+ homeostasis and induce vasoconstriction as the inhibition of the mitochondrial electron transport chain and ROS production attenuates intracellular Ca2+ and HPV in isolated rat lungs and pulmonary artery myocytes, (137). H2O2 can also activate phospholipase C in a concentration dependent manner, which leads to the conversion of phosphatidylinositol 4,5-bisphosphate to IP3 and diacylglycerol, triggering the release of Ca2+ from IP3-sensative intracellular stores (166). In PH, transient receptor potential (TRPC) genes, which encode store-operated and receptor-operated cation channels, are upregulated in PASMC and these have also been shown to play an important role in Ca2+ elevation (167,168). The increased expression of acid-sensing ion channel 1 (ASIC1) in PH also promotes intracellular Ca2+ accumulation through store operated Ca2+ entry (SOCE) (169). In addition to increased intracellular Ca2+ release, ROS also promote Ca2+ influx. In PH, the decreased expression and/or functioning of redox sensitive voltage gated K+ channels (Kv1.5, Kv2.1) contributes to a sustained elevation in Ca2+ through the activation of voltage-gated L-type Ca2+ channels (170,171).
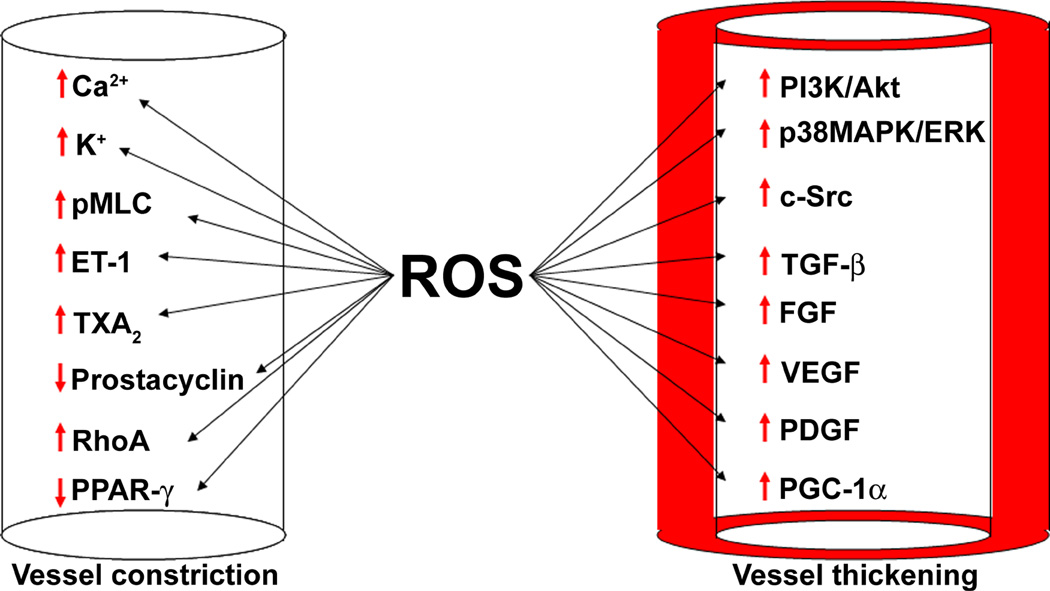
Increases in intracellular Ca2+ stimulates SMC contraction through the calmodulin dependent activation of myosin light chain kinase (MLCK) and the phosphorylation of the myosin light chain (MLC) of myosin II, which stimulates its ATPase activity (172). In addition, the small GTPase, RhoA, and its downstream effector, Rho kinase, can increase the phosphorylation of MLC by inhibiting MLC phosphatase. In the rat hypoxic model of PH, the RhoA/Rho kinase pathway mediates an increase in basal pulmonary vascular tone (173), and the Rho kinase inhibitor, fasudil, decreases PAP and PVR in patients with severe PH (174). ROS trigger the translocation of Rho kinase from the nucleus to the cytosol in pulmonary artery SMC, leading to enhanced MLC phosphorylation (175). In PH, ROS has been shown to activate RhoA and RhoA/Rho kinase dependent Ca2+ sensitization and vasoconstriction (150,176). Therefore, it is evident that ROS play an important role in regulating both intracellular release and Ca2+ entry into the cells. Although, when the levels of ROS reach pathologic levels, this can trigger an exaggerated pulmonary vasoconstriction response in the pulmonary vessel. The available literature indicates that ROS can mediate an increase in pulmonary vasoconstriction and therefore can increase PVR. However, vasoconstriction is only a short term and early modulator of PVR. During the course of PH, structural adaptation of resistance vessels in the pulmonary vasculature is necessary to maintain elevated PVR and PAP for prolonged periods. Also, the vasoconstriction may well contribute to an increase in pulmonary arterial thickness. Indeed, some of the vasoconstrictor substances released during the course of the disease, such as ET-1 and serotonin, serve as growth factors for SMC, independent of their effects on vascular tone (177,178). Thus, the mechanisms by which ROS contribute to the development of vascular dysfunction and remodeling in PH can b broadly divided into two major categories: factors affecting vascular tone and factors influencing vessel wall thickness. However, there is no clear demarcation separating these two mechanisms, as factors affecting tone may also contribute to vascular proliferation (Fig. 3).
Figure 3. Reactive oxygen species signaling in pulmonary hypertension.
The vasoactive mediators that contribute to the development of pulmonary vascular remodeling and PH can be broadly divided into two major categories: factors affecting vascular tone and factors influencing vessel wall thickening. ROS influence the generation of several of these factors. In PH, ROS decrease the expression and/or function of redox sensitive voltage gated K+ channels (Kv1.5, Kv2.1), leading to elevations in intracellular K+ and Ca2+ influx through the activation of voltage-gated L-type Ca2+ channels. In turn, Ca2+ induces SMC contraction through the calmodulin dependent activation of myosin light chain kinase (MLCK) and the subsequent phosphorylation of the myosin light chain (MLC). ROS also promote the activation of the small GTPase, RhoA, and its downstream effector, Rho kinase, which increase the phosphorylation of MLC through the inhibition of MLC phosphatase. In addition, ROS promote pulmonary vessel constriction by enhancing the production of endothelin-1 (ET-1) and thromboxane A2 (TXA2), while attenuating the levels of vasodilators, such as prostacyclin and peroxisome proliferator-activated receptors-γ (PPAR-γ). The role of ROS in mediating pulmonary SMC proliferation and vessel thickening is more complex. Oxidative stress induces the expression of several growth factors, such as transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), and platelet-derived growth factor (PDGF). In addition, ROS also activate p38MAPK and ERK1/2, which promote proliferation. The MAPK signaling pathway is also activated by pp60Src, through ROS mediated activation. Further, ROS stimulate Akt1, which promotes SMC proliferation through the upregulation of PGC-1α. Together, the dysregulation of these constrictive and proliferative factors by ROS promotes the vascular remodeling seen in PH.
3.2. ROS mediated increase in pulmonary vascular thickness
Under physiological conditions, the thickness and mass of the pulmonary arterial wall is maintained through a delicate balance between the proliferation and apoptosis of fibroblasts, SMC, and endothelial cells. The disturbance of this balance in favor of proliferation results in the thickening of the pulmonary arterial wall, leading to the narrowing and eventual obliteration of the vessel lumen. This process also decreases pulmonary vascular compliance, eventually resulting in the development of PH (179). The factors affecting SMC proliferation are diverse and complex. However, ROS are important mediators of the proliferative reponse in pulmonary vascular cells (180). ROS increase the expression and/or activation of multiple factors involved in cellular growth. These include PI3K/Akt, p38MAPK, c-Src, TGF-β1, VEGF, PDGF, FGF-2, nuclear factor of activated T cells (NFAT) and peroxisome proliferator activated receptor gamma co-activator 1α (PGC-1α). All these factors have been implicated in the vascular remodeling that occurs in PH (181–185). These mitogens also exert their proliferative effects on PASMC, PAEC, and fibroblasts at least in part via ROS (180,186–188). For example, in human lung fibroblasts and bovine PAEC, TGF-β increases H2O2 (188,189); in return, H2O2 enhances the activity of TGF-β1 (190). In addition, growth factors can also stimulate ROS generation. For example, in human PASMC, TGF-β1 induces the expression of Nox4, increasing NADPH oxidase derived O2−.
The MAPKs are a family of serine/threonine kinases that regulate cellular growth and apoptosis. There are 4 main MAPKs, including extracellular signal–regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNKs, also termed SAPKs), p38 MAPKs, and big MAPK-1. In SMC, H2O2 can activate p38 MAPK (191), JNK (192), and big MAPK-1 (193). In addition, XO derived ROS have been shown to promote the cellular proliferation and vascular remodeling by inducing the phosphorylation of ERK1/2 and the subsequent up-regulation of the redox-sensitive transcription factor, early growth response-1 (Egr-1) in pulmonary arteries (148). The bone morphogenetic protein (BMP) can also activate ERK, p38MAPK, and JNK (194). The activation of ERK increases the expression of matrix metalloproteinase (MMP)-9, which has been shown to correlate with the severity of PH in various animal models (195–197). In pulmonary hypertensive arteries, TGF-β2 and TGF-β3 have also been shown to increase the production of extracellular matrix molecules, such as elastin (198,199). Thus, the activation of these kinases by BMP and TGF-β is thought to be critical to the development of vascular remodeling in PH (194). ROS have also been shown to be involved in regulating mitochondrial biogenesis via the transcription of the mitochondrial biogenesis regulator, PGC-1α. Although this is a complex process, as the effect of ROS on PGC-1α expression differs depending on the level of ROS within the cell. Low levels of ROS reduce PGC-1α mRNA while elevated levels of H2O2 induce PGC-1α transcription indirectly, via AMPK activation (200). PGC-1α has been shown to be upregulated in PASMC under hypoxic conditions via PI3 kinase/Akt signaling and promote hypoxia-induced proliferation of PASMC through the up-regulation of proliferating cell nuclear antigen (PCNA), cyclinA, cyclinE, and mitochondrial biogenesis (201). In addition, Akt, a serine/threonine kinase that plays a key role in glucose metabolism, apoptosis, proliferation, cell migration and protein synthesis (202) is stimulated in chronic intermittent hypoxia induced PH via Nox derived ROS leading to the muscularization of the distal pulmonary arteries (203). Exogenous H2O2 has also been shown to activate Akt in SMC (204) while serotonin stimulates PASMC proliferation through ROS induced phosphorylation and activation of Akt (205). It has also been demonstrated that ROS dependent activation of the proto-oncogene, Ras, induces recruitment of PI3-kinase to Ras, an event required for Akt activation (206). ROS have also been shown to activate the Ca2+ dependent transcription factor, NFATc3 (207). The nuclear localization and activation of NFATc3 increases pulmonary arterial wall thickness and promote vascular remodeling (208,209). Thus, ROS play a significant role in the vascular remodeling during the development of PH.
3.3 ROS and growth factor signaling
ROS are also involved in growth factor signaling. For example, H2O2 induces tyrosine phosphorylation and activation of the EGF-R as this can be inhibited by antioxidants (210). While H2O2 (211), hypoxia (212,213), mechanical stretch, and shear stress (214) have been shown to induce PDGF expression. In a recent study, it was determined that c-Src, a proto-oncogene and a tyrosine-protein kinase is an important signaling molecule which forms a complex with the EGF-R (215), and activates MAPK (216). In mouse fibroblasts, H2O2 activates c-Src (216) and stimulates proliferation. The expression of the SMC mitogen, FGF-2, is also regulated by ROS (217). In PASMC, Nox derived ROS increase the activity of the FGF-2 promoter through the activation of the redox sensitive transcription factor, HIF-1α (217). The increases in FGF-2 expression can also be stimulated by factors known to increase ROS signaling in PASMC, such as FGF-2 itself, ET-1, and TGF-β1 and is inhibited by Nox inhibitor, apocynin (217). Furthermore, FGF-2 increases PASMC proliferation through the activation of Nox, PI3-kinase, and Akt (217). FGF-1 and -2 was also found to increase the expression of the ET-1 subtype A receptor (ET-AR) in PASMC (218). In a lamb model of PH secondary to increased PBF, FGF-2 mRNA and protein levels are increased in serum, lung tissue, and isolated pulmonary arteries at 4-weeks of age (7). In addition, cyclic stretch and laminar shear stress, two types of mechanical forces that are increased in these lambs, are able to increase FGF-2 promoter activity in both PASMC and PAEC, suggesting that FGF-2 plays a significant role in vascular remodeling under conditions of high PBF (7).
VEGF and its receptors have also been shown to be involved in the pathogenesis of vascular remodeling in PH. VEGF, an endothelial cell specific angiogenic mitogen, is associated with the abnormal endothelial cell proliferation and disordered angiogenesis in plexiform lesions. In severe PH, VEGF expression is increased within the pulmonary vasculature, including plexiform lesions (15,219–221), secondary to increased oxidative stress in PAEC (222) and PASMC (223). VEGF expression is also upregulated via a TGFβ-1 dependent activation of NADPH oxidase in PASMC subjected to cyclic stretch (224). Although the isoform VEGF-A has been proposed to play a protective role (225), VEGF-B appears to exacerbate vascular remodeling, as VEGF-B knockout mice exposed to chronic hypoxia exhibit significantly less pulmonary vascular remodeling compared to wild type mice (226). VEGF binds to two high-affinity tyrosine kinase receptors located in the endothelium: fms-like tyrosine kinase (Flt-1; VEGFR-1) and kinase inert domain–containing receptor (KDR; VEGFR-2). In the lungs of hypertensive patients, VEGFR-1 mRNA expression is increased in the endothelial cells of arteries adjacent to plexiform lesions (219,221), while VEGFR-2 mRNA is elevated in the endothelial cells within the plexiform lesion (219). Taken together, the data available data strongly support an important role for ROS in promoting vascular remodeling in PH and this occurs, at least in part, through the stimulation of growth factor signaling.
4. ROS Scavenging Systems in the pulmonary vasculature
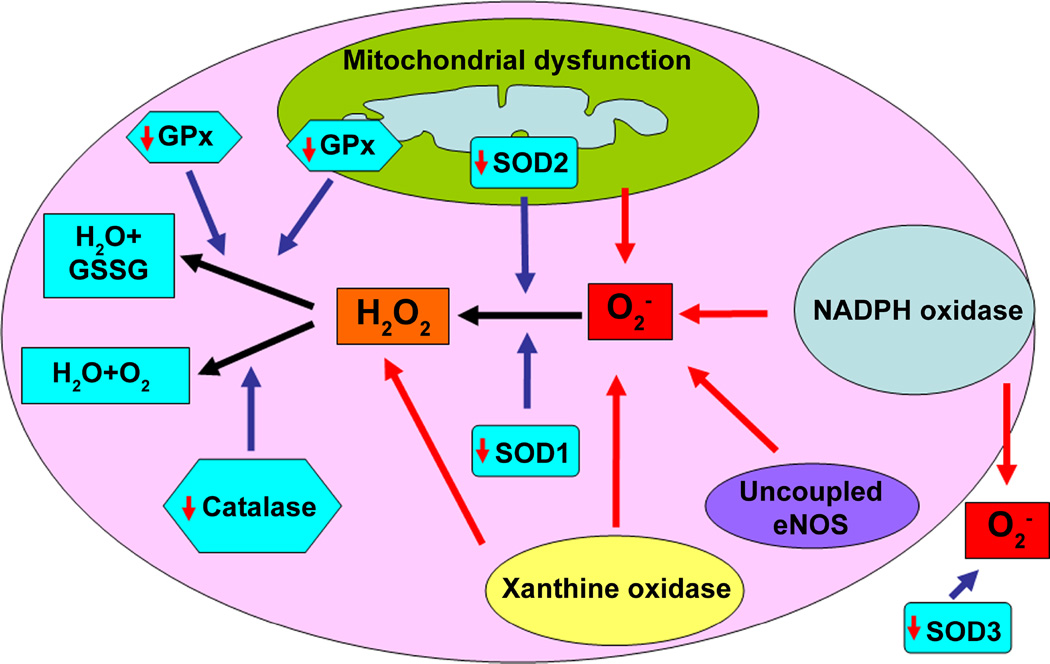
Antioxidant enzymes play a critical role in the regulation of the oxidant levels in the vasculature, and their dysregulation has been implicated in the pathology of systemic and pulmonary hypertension (227). The major vascular enzymatic antioxidants are SOD, catalase, and glutathione peroxidase (GPx) (228–230) (Fig. 4). The role of catalase and superoxide dismutase (SOD) has been extensively studied in PH. There are three types of SOD: Cu/ZnSOD (SOD1) in the cytoplasm, MnSOD (SOD2) in the mitochondria, and an extracellular EC-SOD (SOD3) that all serve to catalyze the rapid conversion of O2− into H2O2, whereas catalase decomposes H2O2 into O2 and H2O (227). Similarly, GPx decomposes H2O2 into H2O and in the process two molecules of glutathione (GSH) are oxidized to glutathione disulfide (GSSG).
Figure 4. Reactive oxygen species scavenging in pulmonary hypertension.
ROS are scavenged by antioxidant systems to preserve the cellular redox homeostasis and to prevent oxidative damage to cellular proteins, lipids, and DNA. The major vascular enzymatic antioxidants are SOD, catalase, and GPx. There are three types of SOD: cytoplasmic Cu/ZnSOD (SOD1), mitochondrial MnSOD (SOD2), and extracellular EC-SOD (SOD3) that catalyze the rapid conversion of O2− into H2O2. In PH, there is a decrease in the expression and/or activity of all three SOD isoforms, increasing O2− levels. The H2O2 that is produced by SOD can be enzymatically reduced in the cytoplasm by catalase and GPx. Catalase decomposes H2O2 to O2 and H2O, while GPx requires two molecules of GSH to reduce one molecule of H2O2 to two molecules of H2O and in the process GSH is oxidized to GSSG. Catalase activity/expression is altered in PH, while the expression/activity of GPx is decreased in many forms of PH. The result of reduced ROS quenching by these antioxidants adds to the oxidative stress observed in PH.
4.1. Superoxide dismutases in pulmonary hypertension
SOD is a universally expressed enzyme and all the three isoforms are expressed in the lung. In PH of different etiologies, the expression and/or activity of these isoforms is altered, which contributes to oxidative stress and vascular remodeling. For example, in an ovine ductal ligation model of PPHN, total SOD activity is decreased in the pulmonary arteries after 9 days of ligation (231). SOD1 protein levels are also diminished in resistance pulmonary arteries from hypoxia induced pulmonary hypertensive piglets (232). In the monocrotaline rat model of PH, RV tissue homogenates have decreased SOD1 and SOD2 mRNA expression (125). In addition, SOD2 protein levels and activity are attenuated in the FH rat, in the plexiform lesions of pulmonary arteries from human PH patients, and in PAEC isolated from patients with IPAH (128,233–235). Furthermore, SOD3 activity is decreased in PASMC isolated from PPHN lambs (236) and in PASMC exposed to hyperoxic or exogenous H2O2 (236). The decrease in SOD3 activity in these cells is associated with increased SOD3 protein thiol oxidation. SOD3 activity is also decreased in vivo in PPHN lambs (236). In a developmental study of lambs with PH secondary to increased PBF, SOD1 levels were increased at 4- and 8- compared to 2-weeks of age, while they remain constant throughout the 8-week period in control lambs (237). These results correlate with in vitro data where SOD1 expression is increased in endothelial cells and porcine arterioles exposed to shear stress (238). Therefore, the developmental increases in SOD1 expression in lambs with increased PBF may be due to increased shear forces. In lambs with increased PBF there is also a transient reduction in SOD2 and SOD3 protein levels at 2-weeks of age, but this decrease is not sustained during development (237).
The effects of SOD in PH have been extensively study by modulating levels both in vitro and in vivo. A recent study has shown an important role of SOD3 in the pathogenesis of pulmonary vascular remodeling associated with PH secondary to bleomycin exposure (239). In this study, transgenic mice over-expressing SOD3 in the lung were protected against pulmonary artery medial wall thickening, adventitial collagen deposition, intimal and medial cellular proliferation, and pulmonary artery intimal thickening after the administration of bleomycin (239). The overexpression of SOD3 attenuated RVSP and increased survival (239). Intratracheal viral mediated overexpression of SOD3 in monocrotaline treated rats attenuated RVSP, RV/LV+septum weight, medial wall thickness, muscularization of microvessels, PCNA positive PASMC, and vascular ROS (240). Similarly, in a model of hypoxia induced PH, transgenic mice with lung specific overexpression of SOD3 were protected against elevations in RVSP, RV/LV+septum weight, and pulmonary vascular wall thickening (241). Relaxation/contraction curves using pulmonary arteries isolated from these mice demonstrated increased relaxation to NO donors (241). Conversely, SOD3 knockout significantly exacerbates the hypoxia-induced elevations in RVSP, RV/LV+septum weight, and fully muscularized arterioles. Interestingly the ablation of SOD3 does not cause differences in these parameters under normoxic conditions (242). Also, monocrotaline caused a significantly greater increase in RVSP, RV/LV+septum weight, and fully muscularized arterioles in SOD3 loss of function mutant rats (E124D). Again these parameters were not different in untreated wild-type and SOD3(E124D) rats (242). This suggests that the loss of endogenous SOD3 does not stimulate the development of PH under physiological conditions but under conditions of excessive stress, endogenous SOD3 protects the lung. Other studies have also implicated reductions in SOD2 in the development of PH. The depletion of SOD2 in PASMC, using an siRNA approach, leads to the appearance of hyperpolarized mitochondria, low H2O2 production, low caspase 3 activity, and increased proliferation (234). In contrast, the siRNA mediated knockdown of SOD2 in endothelial cells leads to an increase in HIF-1α levels, a decrease in NO production, and endothelial dysfunction (235) while a SOD mimetic prevents the development of PH in the FH rat. Interestingly, these studies revealed that the decreased expression of SOD2 in the FH rat is due to the hypermethylation of the SOD2 promoter (234).
Similarly, the administration SOD1 to pulmonary arteries isolated from PPHN lambs enhances their relaxation to exogenous NO (243). Further, the intratracheal administration of SOD1 in these lambs selectively decreases PAP and enhances the pulmonary vasodilator effects of inhaled NO (243). Systemic oxygenation is also improved (244). In these lambs, SOD1 delivery increases eNOS expression/function, enhances GCH1 expression and BH4 levels, and decreases ROS generation (245). Overall, it is likely that the vasoprotective role of SOD in PH not only involves ROS scavenging but also increased NO bioavailability.
4.2. Catalase in pulmonary hypertension
Catalase is mainly located in cellular peroxisomes and the cytosol. It contains a molecule of Fe3+ at its active site and catalyzses the decomposition of H2O2 to H2O and O2. Catalase is very effective during high levels of oxidative stress and has the highest turnover number of all enzymes: each molecule can degrade 40 million molecules of H2O2 in 1 sec. The regulation of catalase levels/activity in PH is variable amongst different etiologies. In monocrotaline-treated rats, catalase activity in lung tissue homogenates has been shown to increase (246). In the same study, an SO2 donor further enhanced plasma catalase activity, which correlated with decreased medial hyperterophy, reduced small/medium pulmonary artery thickening, and attenuated elastin staining compared to monocrotaline treatment alone (246). In patients with COPD and PH, serum activities of catalase and SOD are not different to the controls (247). Similarly, in patients with idiopathic PH, catalase activity is not altered (248). However, in lambs with PH secondary to increased PBF, catalase levels and activity are transiently decreased, suggesting that the loss of catalase activity may be involved in the early stages of the disease process (237). Treatment with exogenous catalase has also been shown to have a protective effect on lung function in PH. For example, in PASMC isolated from PPHN lambs, PEG-catalase treatment restored SOD3 activity (236). Similarly, the addition of PEG-catalase to isolated pulmonary arteries from PPHN lambs normalizes the vasodilator responses to exogenous NO (249). While in vivo, a single intratracheal dose of PEG-catalase to PPHN lambs enhanced SOD3 activity, reduced O2− levels, and improved oxygenation (236). In a rat model of hyperoxia induced PH, the intratracheal injection of liposome-encapsulated catalase during the O2 exposure prevented chronic vascular and parenchymal damage due to oxygen toxicity (250). Together, the available data suggest that reductions in catalase activity may be an early event in the progression of PH and that H2O2 can also stimulate O2− levels. However, the utility of enhancing catalase activity in humans with PH is unclear.
4.3. Glutathione Peroxidase in pulmonary hypertension
GPx is a selenium containing enzyme that exists in eight isoforms. GPx1 is the most abundant isoform and is found in the cytoplasm of many mammalian tissues. GPx requires 2 molecules of glutathione (GSH) to reduce 1 molecule of H2O2 to 2 molecules of H2O and in the process GSH is oxidized to GSSG. The role of GPx in the pathogenesis of PH is far from clear, as only limited information is available. GPx has been shown to be important in protecting the lungs of fetal rabbits against birth related oxidative stress (251). A compensatory and adaptive elevation of GPx activity, as well as increases in GSH, has also been found in rats exposed to monocrotaline (246). However, human studies suggest that GPx levels and activity are decreased in PH. For example, in patients with primary PH, GPx activity in the plasma is decreased, although erythrocytes had elevated GPx activity. This disparity was greatest in patients with severe cardiac insufficiency and PH (252). Further, patients with COPD and secondary PH have lower GPx activity in erythrocytes. This appears to be a specific affect on GPx, as erythrocyte activities of SOD and catalase are not disrupted (247). Another study examining the role of GPx in idiopathic PH has also demonstrated that GPx activities are decreased in peripheral lung tissue (248). However, another study evaluating the BALF of idiopathic PH patients found that GPx activity was unchanged, although GSH levels were increased (253). These inconsistencies may be explained by the presence of different oxidative stress levels in lung tissue and BALF. However, a more likely explanation is that the regulation of GPx activity may depend upon the duration and severity of the oxidative stress with acute elevations in ROS triggering compensatory increases in GPx activity, while chronic exposures to ROS may overwhelm the ability of the lung to maintain this elevated level.
5. ROS and vascular remodeling in animal models of pulmonary hypertension
The pulmonary circulation has large vascular reserves and compliance; and so, patients with PH tend to present at later stages of the disease when the process of vascular dysfunction is already far advanced. As a result, it is very difficult to study the natural course of the development of the vascular lesions in humans. Thus, much of our understanding of vascular dysfunction is the result of investigations in animal models of PH.
5.1. Exposure of rodents to chronic hypoxia
A wide variety of animal species are employed to study hypoxia- induced PH, although rats and mice are the most commonly used. Compared to other models of PH, the pathology in this model is very predictable and reproducible. However, the degree of response to hypoxia varies according to age, sex, species, and strain (254). Hypoxia is normally induced in these animals by exposing them to 10%–12% oxygen under either normobaric or hypobaric conditions compared to control animals exposed to 21% oxygen (255,256). The duration of the exposure to hypoxia varies from a day to several weeks and therefore may cause acute-, intermittent-, or chronic hypoxia- induced PH. Acutely, hypoxia will induce HPV, thereby increasing PVR, while prolonged exposure can induce pulmonary arterial remodeling, right ventricular (RV) hypertrophy, and right heart failure secondary to the resultant PH. In the human condition, the large proximal pulmonary arteries have a marked increase in the thickness of the media and the adventitia. In rats, however, the adventitial thickening is early and massive, whereas thickening of the media is slow (257). Several studies have shown that the medial thickening of pulmonary arteries is attributed to the proliferation of less differentiated SM-like cells in response to hypoxic exposure (32,258). In contrast, the morphological changes in the distal pulmonary arteries are more complex. In some species, the SMC from the distal pulmonary arteries are resistant to growth in response to hypoxia (259). In rats and mice, however, the SMC in the distal pulmonary arteries proliferate in response to hypoxia. In these rodents, hypoxia induces the expression of growth factors, such as PDGF and TGF-β, which promote distal arterial muscularization (260). In addition, the inhibition of ROS production has been demonstrated to suppress the signaling pathways that stimulate proliferation, suggesting the vital role of oxidative damage in hypoxia- induced PH (260). The most characteristic pulmonary vascular change that occurs with hypoxia is the neomuscularization of otherwise non-muscular pre-capillary arteries in the alveolar wall (261,262).
Other characteristic cellular changes in the chronic hypoxic models include, hypertrophy and hyperplasia of the endothelial cells and thickening of the sub-endothelial space (262) and the conversion of the SMC to a synthetic phenotype. The adventitial fibroblasts also proliferate and exhibit increased levels of contractile- and extracellular matrix-proteins as well as enhanced NADPH oxidase derived ROS; all of which can directly affect medial SMC tone and growth (262,263). Inflammation also appears to play a significant role in the hypoxia-induced remodeling process in at least some strains of rats (264). It is of interest to note that the fawn-hooded (FH) rats develop more severe PH and remodeling than other strains with exposure to hypoxia and represent the most severe spectrum of hypoxia- induced PH in rodents (128). In contrast, the exposure of mice to chronic hypoxia is associated with only minimal medial thickening of muscular resistance vessels and a brief increase in SMC proliferation (265). FH rats have more immature lungs with a decreased number of alveoli at birth (266) and exhibit increased ET-1 production, which may account for their heightened pressure and remodeling responses to hypoxia (267). However, FH rats are prone to develop systemic hypertension, which is usually not a feature of human PH disease.
5.2. Monocrotaline induced pulmonary arterial hypertension
PH can be induced in rats through the administration of monocrotaline as a single, subcutaneous injection of 60 mg/kg (268). In rats, this results in pathological alterations within the lung and heart similar to that observed in humans with PH. Monocrotaline is a phytotoxin (269) and is first modified in the liver to the active, electrophile monocrotaline pyrrole (270), which has a half-life of about 3s in aqueous environments near neutral pH (271). The stabilization of the monocrotaline pyrrole by red blood cells facilitates its subsequent transport to the lung (272). The circulatory proximity of the liver to the lung endothelium, the increased thymidine uptake, the decreased 5-HT clearance, and the extravasculature leakage of large macromolecules in the pulmonary capillaries demonstrate that monocrotaline intoxication targets the pulmonary endothelium (269,273). In vitro experiments have demonstrated that monocrotaline pyrrole can impair endothelial barrier function (274), inhibit cell proliferation (275), prolong cell cycle arrest in the G2-M phase (276), and promote apoptosis (277). Apoptosis occurs in rat pulmonary artery endothelial cells (PAEC) following the in vivo administration of monocrotaline (278). The muscularization of nonmuscularized and muscularized arterioles also occurs and is detectable on days 3 and 7 post injection, respectively and reaches significance on days 10 and 14, respectively (279). Elevated levels of ROS are also observed in the lung and RV, and antioxidants have been shown to attenuate the progression of PH in this model (240).
5.3. The Sugen-hypoxia model of pulmonary arterial hypertension
This model is developed by subcutaneously injecting adult male Sprague-Dawley rats with a VEGFR-2 antagonist, SU5416 (20 mg/kg), followed by exposure to chronic hypoxia (10% O2, 3 weeks) (280). The rats are then returned to normoxia (21% O2) for an additional 10 to 11 weeks (280). Using this protocol, the rats develop severe PH accompanied by a pulmonary arteriopathy strikingly similar to that observed in human severe PH. Rats also display high right ventricular systolic pressure (RVSP) and severe RV hypertrophy (280). The pulmonary arteries also exhibit various forms of vascular remodeling, including medial wall hypertrophy, various degrees of neointimal thickening: eccentric, concentric, and concentric laminar, and two different patterns of complex plexiform lesions: stalk-like and aneurysm-like (280). The administration of an endothelin A receptor blocker, BQ123, and RhoA kinase inhibitors, fasudil or Y-27632, can prevent spontaneous vasoconstriction (281). Although the role of ROS in the development of PH in this model has not yet been studied, the use of hypoxia to induce the pulmonary vascular dysfunction in these rats suggests that the oxidative stress will likely play a central role in the vascular remodeling.
5.4. Chronic intrauterine pulmonary hypertension due to partial ligation of the ductus arteriosus
PH in newborns is characterized by elevated PVR, resulting in severe hypoxemia upon birth (282). Although the mechanisms contributing to PH in children are poorly understood, clinical and experimental studies suggest that chronic PH in utero leads to the failure of the normal transition to a low PVR and high pulmonary blood flow (PBF) environment at birth (282,283). In fetal lambs, chronic intrauterine PH due to partial ligation of the ductus arteriosus causes right-to-left shunting across the foramen ovale and ductus arteriosus. This is characterized with marked elevation of intrauterine PAP, RV hypertrophy, lung structural changes, abnormal pulmonary vasoreactivity, and the failure to achieve the normal decline in PVR at birth (283,284). This experimental model of PH is also characterized by the attenuation of pulmonary vasodilation in response to small increases in fetal PO2 (283) and the impairment of endothelium-dependent vasodilation to acetylcholine (285). After 9 days of ductal ligation, these lambs have increased superoxide (O2−) levels localized to the adventitia and SMC of hypertensive vessels (231). In addition, the activity of superoxide dismutase (SOD), an antioxidant, is significantly decreased in the arteries from ligated lambs without associated changes in SOD protein expression (231). NADPH oxidase is a potential source of ROS in this model, as the levels of p67phox, a subunit of the NADPH oxidase complex, is significantly increased in the pulmonary arteries from the ligated lung as early as 2 days (231).
5.5. A lamb model of congenital heart disease with increased pulmonary blood flow
This model is established by placing an 8-mm graft between the ascending aorta and main pulmonary artery in the late-gestation fetal lamb (286). Following spontaneous delivery, these shunted lambs display morphological and physiological features that mimic the human disease. At 1 month of age, they have dramatically elevated PAP associated with an increase in PBF, systemic blood flow, and left and right atrial pressures (286). Shunted lambs also have an increase in pulmonary vessel numbers associated with the elevated expression of VEGF, VEGFR-1, VEGFR-2 (8), TGF-β1, and its ALK-1 receptor (9). These lambs also have a selective impairment of endothelium-dependent pulmonary vasodilation in both intact and isolated pulmonary arteries (287). There are early alterations in the ET-1 cascade that result in increased ET-1 production, increased ET-1-mediated vasoconstriction, and decreased vasodilation (288). These shunted lambs also have elevated O2− levels, which correlate with increased expression of NADPH oxidase subunits, Rac1 and p47phox (238). Moreover, the flavoprotein inhibitor, diphenyliodonium (DPI), reduces O2− levels in lung sections prepared from shunted-, but not age matched control-lambs (238). In addition, shunted lambs exhibit eNOS uncoupling that is associated with a low BH4/BH2 ratio (238) and reduced L-arginine levels (102). Further, the treatment of shunted lambs with antioxidants restores endothelial dependent vasodilation (243). In addition, the chronic administration of L-carnitine, preserves endothelial function in these lambs (289). Together these results suggest that oxidative stress plays a significant role in the development of PH in shunted lambs.
5.6. A lamb model of rebound pulmonary hypertension
Several studies have noted a potentially life-threatening increase in PVR on acute withdrawal of inhaled NO (290,291). This rebound pulmonary hypertension is manifested by an increase in PVR, compromised cardiac output, severe hypoxemia, or a combination of these pathologies (290,291). This has been modeled in the lamb, where inhaled NO (40ppm) is delivered in nitrogen into the inspiratory limb of the ventilator and continued for 24h (292). Inhaled NO transiently decreases the mean PAP and left PVR (293). However, during the 24h exposure, PAP returns to pre-inhaled NO values (293) and upon discontinuation of inhaled NO, there is a rapid increase in both mean PAP and left PVR (292). Fluoresecent microscopy studies have shown that these lambs have increased O2− and peroxynitrite levels (294) associated with decreased NOS activity (292) and increased ET-1 levels, suggestive of vascular dysfunction (238,294). However, NOS activity (292) is preserved, O2− and peroxynitrite levels are not increased, and no increase in PVR is observed upon the withdrawal of inhaled NO when the lambs are treated with polyethylene glycol-conjugated SOD (PEG-SOD) (238,294). Again these data implicate enhanced ROS in the pathology of this disease.
6. Anti-oxidant therapeutic strategies for the management of vascular dysfunction in pulmonary hypertension
PH and its associated vascular remodeling have no targeted treatment strategies that satisfactorily promote the survival of patients. Even with the development of novel therapies, targeted at specific signaling pathways, it is still not clear that long term survival of patients has been significantly impacted. There is no evidence that current therapies will allow patients to improve their survival past the first decade of their disease process. For instance, Bosentan, a dual endothelin receptor antagonist, has been shown to improve 6-minute walk test distance and improve dyspnea in the BREATHE-1 study on humans (295). However, due to its potential risk for serious liver injury and birth defects, its use has not gained momentum. In addition, pulmonary artery vasodilators, such as epoprostenol, iloprost, and treprostinil, are FDA approved prostanoids that are currently available for the treatment of PH. Chronic intravenous treatment with epoprostenol has been shown to slow the progression of the disease and improve the walk test, however, its short half life, side effects, and complicated delivery system (chronic indwelling and infected catheters) have been an impediment in its broader use. Therefore, the identification of new therapeutic strategies to treat patients with PH is still ongoing. As mentioned in this review and as evidenced by several human and animal model studies, increased oxidative stress plays an important role in the development of vascular remodeling in PH, and therapies targeted at the major ROS generators have been initiated. The inhibition of NADPH oxidase with apocynin has been shown to significantly improve oxygenation, decrease the contractility to norepinephrine, increase NOS activity, and enhance pulmonary artery relaxation to NO donors in PPHN lambs (296). Recently, phase I clinical trials have been conducted to investigate the effects of inhaled apocynin in reducing ROS levels in mild asthmatic patients. Apocynin was found to significantly decrease ROS levels in the exhaled breath condensate of patients with mild asthma. No adverse events were observed throughout the study (297). These human and animal studies suggest that targeting the NADPH oxidase system may have potential clinical utility in abrogating ROS generation and improving NO bioavailability in PH.
Mitochondria are another potentially important target for the treatment of vascular dysfunction in PH, as they regulate both apoptosis and vascular tone. Dichloroacetate (DCA), a metabolic modulator that increases mitochondrial oxidative phosphorylation, has been shown to prevent and reverse established monocrotaline-induced PH by inducing mitochondria-dependent apoptosis and reversing the downregulation of Kv1.5 in the medial layer of resistance pulmonary arteries (129). DCA is well tolerated by patients with moderate or severe PH and is currently under phase 1 clinical trials. Another antioxidant that is currently in phase 1 clinical trials for the treatment of PH is coenzyme Q-10 (CoQ10). This oil-soluble, vitamin-like substance primarily found in the mitochondria is present in all eukaryotic cells. CoQ10 plays an important role in ATP generation due to its involvement in the electron transport chain of the mitochondrion. CoQ10 undergoes continuous oxidation and reduction enabling it to function as both an electron carrier from the mitochondrial complex I and II to complex III and an antioxidant. The antioxidant property of CoQ10 stems from the fact that it readily gives up one or both electrons. A recent meta-analysis of 12 published clinical trials of CoQ10 for hypertension concluded that CoQ10 has the potential to lower systemic blood pressure in hypertensive patients without significant sideeffects (298).
Besides the increase in ROS generation in PH, the loss or attenuation of antioxidant enzymes also play a significant role in increased oxidative stress in pulmonary vascular remodeling. Therefore, the replacement of these antioxidants is an important component of overall antioxidant therapy. SOD and catalase are the prime candidates for antioxidant replacement therapy. These enzymes can be either delivered exogenously or endogenously upregulated. Owing to their large size and rapid elimination, the exogenous administration of SOD and catalase is not an effective delivery system. However, the use of liposomes as a delivery tool to entrap the enzymes can improve bioavailability and increase enzyme activity. In addition, the covalent conjugation of polyethylene glycol (PEG) to SOD and catalase increases the enzymatic circulatory half-life and reduces protein immunogenicity. Chemical modifications and genetic manipulations of SOD and catalase have also been proposed in order to provide more effective delivery to in vitro cultured cells. In contrast, the endogenous upregulation of these enzymes provides more sustainable effects. Several compounds, such as stanozol (299), extract from Terminalia arjuna (300), retinol (301), and malathion (302), have been found to enhance endogenous SOD and catalase levels. More recently, a dietary supplement called Protandim, which is composed of extracts from five widely used medicinal plants (Bacopa monniera, Silybum marianum, Withania somnifera, Camellia sinensis, and Curcuma longa), has been shown to increase SOD and catalase levels and decrease lipid peroxidation in healthy human subjects (303). However, in a second human clinical study, a 7-day treatment with Protandim did not attenuate pulmonary oxidative stress or alveolar permeability in recovering alcoholics (304). Thus, although the use of antioxidant therapy is appealing, the results in humans have been les than promising. Most likely this is due to a number of parameters that will need to be optimized for therapeutic benefits to occur. First, the limitations in maintaining the antioxidant at levels to exert a biologic effect must be overcome by perhaps using recombinant proteins with greater activity or resistance to degradation. Second, delivery strategies must be developed that will specifically target the lung and the correct cell type in the lung. More radically perhaps, the whole strategy of using non-specific antioxidants may need to be rethought. Although there is no large antioxidant study in humans with PH, there are in other forms of lung injury. It is well established that there is an antioxidant imbalance in the lungs of patients with ALI (305). However, despite this imbalance the results using antioxidants in clinical trials have been disappointing (306). In a large randomized, double blind, placebocontrolled, prospective trial comparing N-acetylcysteine or oxothiazolidine to placebo patients with ARDS, there was no mortality difference between the three groups, although the duration of the lung injury was reduced (307). Dietary supplementation with the fish oil, γ-linoleic acid, and antioxidants also shortened the duration of mechanical ventilation and the number of organ failures in ARDS patients but did not reduce mortality (308). Further, the Omega arm of the NHLBI funded multi-center EDEN-Omega clinical trial was stopped for futility. A number of reasons have been proposed for the failure of these antioxidant trials: the best antioxidants were not used, the dose was not high enough, or that the trials have not lasted long enough (309). However, we propose that the reason these trials have failed is that cells require a certain redox balance for optimal health and an overwhelming antioxidant therapy results in this balance shifting towards a reducing environment that also induces pathological cellular responses. Further, we propose that successful clinical trials will require a strategy that will selectively target the pathways/proteins involved in the pathology, preserve physiological signaling, and minimize potential deleterious effects in normal cells. However, for this strategy to be feasible, a much greater fundamental understanding of PH will be required.
Conclusion
In conclusion, it is evident that both scientific and clinical studies implicate the disruption of enzymatic systems that produce and scavenge ROS in the pathogenesis and the progression of vascular remodeling in PH. Although, current therapies have shown high efficacy in reducing PAP, increasing right-heart function, and improving exercise tolerance, they have largely been unable to increase survival. Therefore, newer therapeutic approaches are needed to combat the high mortality associated with PH. In this review, we have highlighted studies that have shown that attenuating eNOS uncoupling, directly inhibiting NADPH oxidase and XO, restoring mitochondrial function, and scavenging ROS may hold promise in reversing the disease process. The potential of antioxidant therapy should, however, be approached cautiously; as although most of the animal data show beneficial effects of antioxidants in the attenuation of vascular pathology in PH, human clinical trials involving antioxidants have not shown promising benefits in reversing pulmonary pathology. One of the major reasons behind these failures has been that the duration of the trials has not been proportionate to the severity of the disease. It may be impossible to reverse the vascular remodeling due to several decades of oxidative insult by only a few days or months of antioxidant therapy. Therefore, the lack of positive outcomes in the clinical trials does not negate the central role of oxidative stress in pulmonary vascular remodeling in PH. Rather, these results provide an opportunity to further evaluate optimal antioxidant strategies, the ideal study population, and the appropriate trial duration.
Acknowledgements
Supported in part by grants HL60190 (to SMB), HL67841 (to SMB), HL084739 (to SMB), HD057406 (to SMB), HL61284 (to JRF), all from the National Institutes of Health, a Transatlantic Network Development Grant from the LeDucq Foundation (to SMB and JRF), a BGIA from the AHA (SS), as well as Seed- (SS) and Programmatic (SMB)-Awards from the GHSU Cardiovascular Discovery Institute.
References
- 1.Gaine SP, Rubin LJ. Lancet. 1998;352(9129):719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 2.Meyrick B, Reid L. The American journal of pathology. 1979;96(1):51–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Cool CD, Groshong SD, Oakey J, Voelkel NF. Chest. 2005;128(6 Suppl):565S–571S. doi: 10.1378/chest.128.6_suppl.565S. [DOI] [PubMed] [Google Scholar]
- 4.Meyrick B, Reid L. Laboratory investigation; a journal of technical methods and pathology. 1980;42(6):603–615. [PubMed] [Google Scholar]
- 5.Meyrick B, Reid L. Laboratory investigation; a journal of technical methods and pathology. 1978;38(2):188–200. [PubMed] [Google Scholar]
- 6.Dschietzig T, Richter C, Bartsch C, Bohme C, Heinze D, Ott F, Zartnack F, Baumann G, Stangl K. Biochemical and biophysical research communications. 2001;289(1):245–251. doi: 10.1006/bbrc.2001.5946. [DOI] [PubMed] [Google Scholar]
- 7.Wedgwood S, Devol JM, Grobe A, Benavidez E, Azakie A, Fineman JR, Black SM. Pediatric research. 2007;61(1):32–36. doi: 10.1203/01.pdr.0000250013.77008.28. [DOI] [PubMed] [Google Scholar]
- 8.Mata-Greenwood E, Meyrick B, Soifer SJ, Fineman JR, Black SM. American journal of physiology. 2003;285(1):L222–L231. doi: 10.1152/ajplung.00388.2002. [DOI] [PubMed] [Google Scholar]
- 9.Mata-Greenwood E, Meyrick B, Steinhorn RH, Fineman JR, Black SM. American journal of physiology. 2003;285(1):L209–L221. doi: 10.1152/ajplung.00171.2002. [DOI] [PubMed] [Google Scholar]
- 10.Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. American journal of respiratory and critical care medicine. 2006;174(9):1025–1033. doi: 10.1164/rccm.200602-304OC. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Proc. Natl. Acad. Sci. U. S. A. 2007;104(4):1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. The American journal of pathology. 1999;155(2):411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Faseb J. 2005;19(9):1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 14.Tuder RM, Groves B, Badesch DB, Voelkel NF. The American journal of pathology. 1994;144(2):275–285. [PMC free article] [PubMed] [Google Scholar]
- 15.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. J Pathol. 2001;195(3):367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 16.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. The New England journal of medicine. 2003;348(6):500–509. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- 17.Wright L, Tuder RM, Wang J, Cool CD, Lepley RA, Voelkel NF. American journal of respiratory and critical care medicine. 1998;157(1):219–229. doi: 10.1164/ajrccm.157.1.9704003. [DOI] [PubMed] [Google Scholar]
- 18.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. The New England journal of medicine. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 19.Golpon HA, Geraci MW, Moore MD, Miller HL, Miller GJ, Tuder RM, Voelkel NF. The American journal of pathology. 2001;158(3):955–966. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lhermine A, Marfaing-Koka A, Simonneau G, Emilie D, Humbert M. American journal of respiratory and critical care medicine. 2002;165(4):534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 21.Voelkel NF, Tuder RM. J Clin Invest. 2000;106(6):733–738. doi: 10.1172/JCI11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakao S, Tatsumi K, Voelkel NF. Respiratory research. 2009;10:95. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeager ME, Golpon HA, Voelkel NF, Tuder RM. Chest. 2002;121(3 Suppl):61S. [PubMed] [Google Scholar]
- 24.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Circulation research. 2001;88(1):E2–E11. doi: 10.1161/01.res.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 25.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Circulation research. 2003;92(10):1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 26.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. Clinics in chest medicine. 2001;22(3):405–418. doi: 10.1016/s0272-5231(05)70280-x. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie JC, Clancy J, Jr, Klein RM. Blood vessels. 1984;21(2):80–89. [PubMed] [Google Scholar]
- 28.Meyrick B, Reid L. The American journal of pathology. 1980;100(1):151–178. [PMC free article] [PubMed] [Google Scholar]
- 29.Prosser IW, Stenmark KR, Suthar M, Crouch EC, Mecham RP, Parks WC. The American journal of pathology. 1989;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- 30.Poiani GJ, Tozzi CA, Yohn SE, Pierce RA, Belsky SA, Berg RA, Yu SY, Deak SB, Riley DJ. Circ Res. 1990;66(4):968–978. doi: 10.1161/01.res.66.4.968. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins N, McLoughlin P. J Anat. 2002;201(4):335–348. doi: 10.1046/j.1469-7580.2002.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Circulation research. 1997;81(6):940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- 33.Dempsey EC, Frid MG, Aldashev AA, Das M, Stenmark KR. Canadian journal of physiology and pharmacology. 1997;75(7):936–944. [PubMed] [Google Scholar]
- 34.Hirschi KK, D'Amore PA. Cardiovascular research. 1996;32(4):687–698. [PubMed] [Google Scholar]
- 35.Deng H, Hershenson MB, Lei J, Anyanwu AC, Pinsky DJ, Bentley JK. Am J Physiol Lung Cell Mol Physiol. 298(6):L793–L803. doi: 10.1152/ajplung.00108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones R. Am J Pathol. 1992;141(6):1491–1505. [PMC free article] [PubMed] [Google Scholar]
- 37.Jones R, Jacobson M, Steudel W. American journal of respiratory cell and molecular biology. 1999;20(4):582–594. doi: 10.1165/ajrcmb.20.4.3357. [DOI] [PubMed] [Google Scholar]
- 38.Zalewski A, Shi Y. Arteriosclerosis, thrombosis, and vascular biology. 1997;17(3):417–422. doi: 10.1161/01.atv.17.3.417. [DOI] [PubMed] [Google Scholar]
- 39.Faggin E, Puato M, Zardo L, Franch R, Millino C, Sarinella F, Pauletto P, Sartore S, Chiavegato A. Arteriosclerosis, thrombosis, and vascular biology. 1999;19(6):1393–1404. doi: 10.1161/01.atv.19.6.1393. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Circulation. 2000;101(12):1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 41.Gutterman DD. The American journal of physiology. 1999;277(4 Pt 2):H1265–H1272. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- 42.Koyama E, Leatherman JL, Shimazu A, Nah HD, Pacifici M. Dev Dyn. 1995;203(2):152–162. doi: 10.1002/aja.1002030204. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt-Graff A, Desmouliere A, Gabbiani G. Virchows Arch. 1994;425(1):3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- 44.Gabbiani G. Cardiovascular research. 1998;38(3):545–548. doi: 10.1016/s0008-6363(98)00065-0. [DOI] [PubMed] [Google Scholar]
- 45.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Physiology (Bethesda, Md. 2006;21:134–145. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 46.Herrmann J, Samee S, Chade A, Rodriguez Porcel M, Lerman LO, Lerman A. Arterioscler Thromb Vasc Biol. 2005;25(2):447–453. doi: 10.1161/01.ATV.0000152606.34120.97. [DOI] [PubMed] [Google Scholar]
- 47.Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. American journal of respiratory and critical care medicine. 2000;162(4 Pt 1):1577–1586. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 48.Botney MD, Parks WC, Crouch EC, Stenmark K, Mecham RP. The Journal of clinical investigation. 1992;89(5):1629–1635. doi: 10.1172/JCI115759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beranek JT. The Journal of thoracic and cardiovascular surgery. 2001;121(4):820–821. doi: 10.1067/mtc.2001.113931. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Circulation. 1996;94(7):1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 51.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Circulation. 1996;93(12):2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 52.Paravicini TM, Gulluyan LM, Dusting GJ, Drummond GR. Circ Res. 2002;91(1):54–61. doi: 10.1161/01.res.0000024106.81401.95. [DOI] [PubMed] [Google Scholar]
- 53.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, Weissmann N. Free Radic. Biol. Med. 2012;52(6):1033–1042. doi: 10.1016/j.freeradbiomed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Hypertension. 2009;54(3):668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Botney MD. American journal of respiratory and critical care medicine. 1999;159(2):361–364. doi: 10.1164/ajrccm.159.2.9805075. [DOI] [PubMed] [Google Scholar]
- 56.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. The American journal of pathology. 1997;151(4):1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 57.Schroder E, Eaton P. Current opinion in pharmacology. 2008;8(2):153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BSS, Karoui H, Tordo P, Pritchard KA. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. 1998 doi: 10.1073/pnas.95.16.9220. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Circulation research. 2007;101(3):258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 60.Babior BM. IUBMB Life. 2000;50(4–5):267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- 61.Vignais PV. Cell Mol Life Sci. 2002;59(9):1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. Antioxidants & redox signaling. 2008;10(10):1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 63.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H Oxidase : Role in Cardiovascular Biology and Disease. 2000 doi: 10.1161/01.res.86.5.494. In. [DOI] [PubMed] [Google Scholar]
- 64.Paravicini TM, Touyz RM. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 65.Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. The Biochemical journal. 2007;403(1):97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. J Biol Chem. 2004;279(44):45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 67.Kawahara T, Ritsick D, Cheng G, Lambeth JD. The Journal of biological chemistry. 2005;280(36):31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 68.Parkos CA, Dinauer MC, Jesaitis AJ, Orkin SH, Curnutte JT. Blood. 1989;73(6):1416–1420. [PubMed] [Google Scholar]
- 69.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. The Journal of biological chemistry. 1998;273(27):16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 70.Diebold BA, Bokoch GM. Nature immunology. 2001;2(3):211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 71.Lapouge K, Smith SJ, Walker PA, Gamblin SJ, Smerdon SJ, Rittinger K. Molecular cell. 2000;6(4):899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- 72.Touyz RM, Yao G, Schiffrin EL. Arterioscler Thromb Vasc Biol. 2003;23(6):981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 73.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Free Radic Biol Med. 2006;41(2):193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 74.Cheng G, Lambeth JD. Gene. 2005;356:118–126. doi: 10.1016/j.gene.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Cellular signalling. 2006;18(1):69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 76.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Am J Physiol Renal Physiol. 2003;285(2):F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 77.Bayraktutan U, Blayney L, Shah AM. Arterioscler Thromb Vasc Biol. 2000;20(8):1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 78.Li JM, Shah AM. J Biol Chem. 2002;277(22):19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 79.Takeya R, Sumimoto H. Antioxidants & redox signaling. 2006;8(9–10):1523–1532. doi: 10.1089/ars.2006.8.1523. [DOI] [PubMed] [Google Scholar]
- 80.Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Biochemical and biophysical research communications. 2000;269(3):713–717. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- 81.Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Circulation. 2001;104(15):1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 82.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, Muller B. British journal of pharmacology. 2006;148(5):714–723. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, Weissmann N. Free radical biology & medicine. 52(6):1033–1042. doi: 10.1016/j.freeradbiomed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. American journal of physiology. 2009;297(4):L596–L607. doi: 10.1152/ajplung.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seta F, Rahmani M, Turner PV, Funk CD. PloS one. 6(8):e23439. doi: 10.1371/journal.pone.0023439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma S, Kumar S, Wiseman DA, Kallarackal S, Ponnala S, Elgaish M, Tian J, Fineman JR, Black SM. Vascular pharmacology. 53(1–2):38–52. doi: 10.1016/j.vph.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. American journal of physiology. 2009;297(1):L184–L195. doi: 10.1152/ajplung.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. American journal of physiology. 2006;290(4):L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 89.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. The Journal of biological chemistry. 2007;282(11):8019–8026. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- 90.Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. The Journal of biological chemistry. 2001;276(42):38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 91.Yokoyama M, Hirata K. Cardiovasc Res. 2007;73(1):8–9. doi: 10.1016/j.cardiores.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Parviz M, Bousamra M, 2nd, Chammas JH, Birks EK, Presberg KW, Jacobs ER, Nelin LD. Ann Thorac Surg. 1999;67(2):522–527. doi: 10.1016/s0003-4975(98)01141-2. [DOI] [PubMed] [Google Scholar]
- 93.Adnot S, Raffestin B, Eddahibi S. Respir Physiol. 1995;101(2):109–120. doi: 10.1016/0034-5687(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 94.Lipsitz EC, Weinstein S, Smerling AJ, Stolar CJ. Journal of pediatric surgery. 1996;31(1):137–140. doi: 10.1016/s0022-3468(96)90336-x. [DOI] [PubMed] [Google Scholar]
- 95.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehta S, Stewart DJ, Langleben D, Levy RD. Circulation. 1995;92(6):1539–1545. doi: 10.1161/01.cir.92.6.1539. [DOI] [PubMed] [Google Scholar]
- 97.Sharma S, Kumar S, Sud N, Wiseman DA, Tian J, Rehmani I, Datar S, Oishi P, Fratz S, Venema RC, Fineman JR, Black SM. Vascular pharmacology. 2009;51(5–6):359–364. doi: 10.1016/j.vph.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Jama. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maarsingh H, Dekkers BG, Zuidhof AB, Bos IS, Menzen MH, Klein T, Flik G, Zaagsma J, Meurs H. Eur Respir J. 38(2):318–328. doi: 10.1183/09031936.00057710. [DOI] [PubMed] [Google Scholar]
- 100.Sydow K, Munzel T. Atherosclerosis. 2003;4(4):41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 101.Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, Thomas JD, Moravec CS, Hazen SL, Tang WH. Journal of the American College of Cardiology. 59(13):1150–1158. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun X, Fratz S, Sharma S, Hou Y, Rafikov R, Kumar S, Rehmani I, Tian J, Smith A, Schreiber C, Reiser J, Naumann S, Haag S, Hess J, Catravas JD, Patterson C, Fineman JR, Black SM. American journal of respiratory cell and molecular biology. 45(1):163–171. doi: 10.1165/rcmb.2009-0467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aggarwal S, Gross CM, Kumar S, Datar S, Oishi P, Kalkan G, Schreiber C, Fratz S, Fineman JR, Black SM. Journal of cellular physiology. 226(12):3104–3113. doi: 10.1002/jcp.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P. The Biochemical journal. 1999;343(Pt 1):209–214. [PMC free article] [PubMed] [Google Scholar]
- 105.Sasaki A, Doi S, Mizutani S, Azuma H. American journal of physiology. 2007;292(6):L1480–L1487. doi: 10.1152/ajplung.00360.2006. [DOI] [PubMed] [Google Scholar]
- 106.Wilcken DE, Sim AS, Wang J, Wang XL. Molecular genetics and metabolism. 2007;91(4):309–317. doi: 10.1016/j.ymgme.2007.04.017. discussion 308. [DOI] [PubMed] [Google Scholar]
- 107.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, Aigner C, Fink L, Muyal JP, Weissmann N, Grimminger F, Seeger W, Schermuly RT. Faseb J. 2005;19(9):1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 108.Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Circulation. 2003;107(8):1195–1201. doi: 10.1161/01.cir.0000051466.00227.13. [DOI] [PubMed] [Google Scholar]
- 109.Alp NJ, Channon KM. Arterioscler Thromb Vasc Biol. 2004;24(3):413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt TS, Alp NJ. Clin Sci (Lond) 2007;113(2):47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 111.Auerbach G, Nar H. Biological chemistry. 1997;378(3–4):185–192. [PubMed] [Google Scholar]
- 112.Belik J, McIntyre BA, Enomoto M, Pan J, Grasemann H, Vasquez-Vivar J. Free radical biology & medicine. 51(12):2227–2233. doi: 10.1016/j.freeradbiomed.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Forstermann U. Pflugers Arch. 459(6):923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 114.Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Cell. 1998;95(7):939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 115.Forstermann U, Munzel T. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 116.Fonseca FV, Ravi K, Wiseman D, Tummala M, Harmon C, Ryzhov V, Fineman JR, Black SM. DNA and cell biology. 29(3):149–160. doi: 10.1089/dna.2009.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, Fulton D, Black SM. J. Endocrinol. 210(3):271–284. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barclay AR, Sholler G, Christodolou J, Shun A, Arbuckle S, Dorney S, Stormon MO. Journal of inherited metabolic disease. 2005;28(6):1081–1089. doi: 10.1007/s10545-005-4484-x. [DOI] [PubMed] [Google Scholar]
- 119.Venditti CP, Harris MC, Huff D, Peterside I, Munson D, Weber HS, Rome J, Kaye EM, Shanske S, Sacconi S, Tay S, DiMauro S, Berry GT. Journal of inherited metabolic disease. 2004;27(6):735–739. doi: 10.1023/B:BOLI.0000045711.89888.5e. [DOI] [PubMed] [Google Scholar]
- 120.Sproule DM, Dyme J, Coku J, de Vinck D, Rosenzweig E, Chung WK, De Vivo DC. Journal of inherited metabolic disease. 2008 doi: 10.1007/s10545-007-0735-3. [DOI] [PubMed] [Google Scholar]
- 121.Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. The Journal of biological chemistry. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duchen MR. The Journal of physiology. 1999;516(Pt 1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gong Y, Yi M, Fediuk J, Lizotte PP, Dakshinamurti S. Free radical biology & medicine. 48(7):882–894. doi: 10.1016/j.freeradbiomed.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 124.Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, Braun-Dullaeus RC, Kummer W. American journal of physiology. 2003;284(5):L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- 125.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Cardiovascular research. 2007;75(4):770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 126.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Circulation research. 106(3):526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. Circulation. 2006;113(22):2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 129.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Circulation research. 2004;95(8):830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 130.Archer SL, Huang J, Henry T, Peterson D, Weir EK. Circulation research. 1993;73(6):1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 131.Paky A, Michael JR, Burke-Wolin TM, Wolin MS, Gurtner GH. J Appl Physiol. 1993;74(6):2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- 132.Freeman BA, Crapo JD. The Journal of biological chemistry. 1981;256(21):10986–10992. [PubMed] [Google Scholar]
- 133.Hool LC, Arthur PG. Circulation research. 2002;91(7):601–609. doi: 10.1161/01.res.0000035528.00678.d5. [DOI] [PubMed] [Google Scholar]
- 134.Firth AL, Yuill KH, Smirnov SV. American journal of physiology. 2008;295(1):L61–L70. doi: 10.1152/ajplung.90243.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Free radical biology & medicine. 2008;45(9):1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waypa GB, Chandel NS, Schumacker PT. Circulation research. 2001;88(12):1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 137.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Circulation research. 2002;91(8):719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 138.Hu HL, Zhang ZX, Chen CS, Cai C, Zhao JP, Wang X. American journal of respiratory cell and molecular biology. 42(6):661–666. doi: 10.1165/rcmb.2009-0017OC. [DOI] [PubMed] [Google Scholar]
- 139.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. American journal of physiology. 2008;294(2):H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 140.Krick S, Platoshyn O, Sweeney M, Kim H, Yuan JX. American journal of physiology. 2001;280(4):C970–C979. doi: 10.1152/ajpcell.2001.280.4.C970. [DOI] [PubMed] [Google Scholar]
- 141.Wedgwood S, Black SM. American journal of physiology. 2003;285(2):L305–L312. doi: 10.1152/ajplung.00382.2002. [DOI] [PubMed] [Google Scholar]
- 142.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. American journal of physiology. 2008;294(6):C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, Fineman JR, Black SM. American journal of physiology. 2008;294(1):L46–L56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW. Cell. 1981;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- 145.Granger DN. The American journal of physiology. 1988;255(6 Pt 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 146.Rao GN, Corson MA, Berk BC. The Journal of biological chemistry. 1991;266(13):8604–8608. [PubMed] [Google Scholar]
- 147.Jankov RP, Kantores C, Pan J, Belik J. American journal of physiology. 2008;294(2):L233–L245. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 148.Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, Stenmark KR, Nozik-Grayck E. PloS one. 6(11):e27531. doi: 10.1371/journal.pone.0027531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gabrielli LA, Castro PF, Godoy I, Mellado R, Bourge RC, Alcaino H, Chiong M, Greig D, Verdejo HE, Navarro M, Lopez R, Toro B, Quiroga C, Diaz-Araya G, Lavandero S, Garcia L. Journal of cardiac failure. 17(12):1012–1017. doi: 10.1016/j.cardfail.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 150.Jernigan NL, Walker BR, Resta TC. American journal of physiology. 2008;295(3):L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Farber HW, Loscalzo J. The New England journal of medicine. 2004;351(16):1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 152.Stewart DJ, Levy RD, Cernacek P, Langleben D. Annals of internal medicine. 1991;114(6):464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 153.Cheng TH, Shih NL, Chen SY, Loh SH, Cheng PY, Tsai CS, Liu SH, Wang DL, Chen JJ. Journal of molecular and cellular cardiology. 2001;33(10):1805–1814. doi: 10.1006/jmcc.2001.1444. [DOI] [PubMed] [Google Scholar]
- 154.Lopez-Ongil S, Saura M, Zaragoza C, Gonzalez-Santiago L, Rodriguez-Puyol M, Lowenstein CJ, Rodriguez-Puyol D. Free radical biology & medicine. 2002;32(5):406–413. doi: 10.1016/s0891-5849(01)00822-x. [DOI] [PubMed] [Google Scholar]
- 155.John M.C G, Barry H. Free Radicals and Antioxidants in the Year 2000: A Historical Look to the Future. 2000 doi: 10.1111/j.1749-6632.2000.tb06182.x. In. [DOI] [PubMed] [Google Scholar]
- 156.Kinnula VL, Crapo JD. Superoxide Dismutases in the Lung and Human Lung Diseases. 2003 doi: 10.1164/rccm.200212-1479SO. In. [DOI] [PubMed] [Google Scholar]
- 157.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. 2000 doi: 10.1152/ajplung.2000.279.6.L1005. In. [DOI] [PubMed] [Google Scholar]
- 158.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. The International Journal of Biochemistry & Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 159.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Chemico-Biological Interactions. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 160.Hishikawa K, Nakaki T, Marumo T, Hayashi M, Suzuki H, Kato R, Saruta T. The Journal of clinical investigation. 1994;93(5):1975–1980. doi: 10.1172/JCI117189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Physiological reviews. 92(1):367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Weissmann N, Winterhalder S, Nollen M, Voswinckel R, Quanz K, Ghofrani HA, Schermuly RT, Seeger W, Grimminger F. American journal of physiology. 2001;280(4):L638–L645. doi: 10.1152/ajplung.2001.280.4.L638. [DOI] [PubMed] [Google Scholar]
- 163.Favero TG, Zable AC, Abramson JJ. The Journal of biological chemistry. 1995;270(43):25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 164.Lin MJ, Yang XR, Cao YN, Sham JS. American journal of physiology. 2007;292(6):L1598–L1608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 165.Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Free radical biology & medicine. 2008;45(10):1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 166.Wang XT, McCullough KD, Wang XJ, Carpenter G, Holbrook NJ. The Journal of biological chemistry. 2001;276(30):28364–28371. doi: 10.1074/jbc.M102693200. [DOI] [PubMed] [Google Scholar]
- 167.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Circulation research. 2004;95(5):496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 168.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Circulation research. 2006;98(12):1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 169.Jernigan NL, Herbert LM, Walker BR, Resta TC. American journal of physiology. 302(6):C931–C940. doi: 10.1152/ajpcell.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Circulation. 1998;98(14):1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 171.Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. J Appl Physiol. 2001;90(6):2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- 172.Somlyo AP, Somlyo AV. Nature. 1994;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 173.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. American journal of physiology. 2004;287(4):L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 174.Doggrell SA. Expert opinion on investigational drugs. 2005;14(9):1157–1159. doi: 10.1517/13543784.14.9.1157. [DOI] [PubMed] [Google Scholar]
- 175.Knock GA, Snetkov VA, Shaifta Y, Connolly M, Drndarski S, Noah A, Pourmahram GE, Becker S, Aaronson PI, Ward JP. Free radical biology & medicine. 2009;46(5):633–642. doi: 10.1016/j.freeradbiomed.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. American journal of physiology. 298(2):L232–L242. doi: 10.1152/ajplung.00276.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. J Appl Physiol. 1994;77(3):1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- 178.Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. The Journal of clinical investigation. 2000;105(11):1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rubin LJ. The New England journal of medicine. 1997;336(2):111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 180.Wedgwood S, Dettman RW, Black SM. American journal of physiology. 2001;281(5):L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 181.Arcot SS, Fagerland JA, Lipke DW, Gillespie MN, Olson JW. Growth factors (Chur, Switzerland) 1995;12(2):121–130. doi: 10.3109/08977199509028958. [DOI] [PubMed] [Google Scholar]
- 182.Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. American journal of respiratory and critical care medicine. 1998;158(6):1920–1928. doi: 10.1164/ajrccm.158.6.9804076. [DOI] [PubMed] [Google Scholar]
- 183.Katayose D, Ohe M, Yamauchi K, Ogata M, Shirato K, Fujita H, Shibahara S, Takishima T. The American journal of physiology. 1993;264(2 Pt 1):L100–L106. doi: 10.1152/ajplung.1993.264.2.L100. [DOI] [PubMed] [Google Scholar]
- 184.Perkett EA, Badesch DB, Roessler MK, Stenmark KR, Meyrick B. American journal of respiratory cell and molecular biology. 1992;6(1):82–87. doi: 10.1165/ajrcmb/6.1.82. [DOI] [PubMed] [Google Scholar]
- 185.Gillespie MN, Rippetoe PE, Haven CA, Shiao RT, Orlinska U, Maley BE, Olson JW. The American review of respiratory disease. 1989;140(5):1463–1466. doi: 10.1164/ajrccm/140.5.1463. [DOI] [PubMed] [Google Scholar]
- 186.Lee SL, Wang WW, Moore BJ, Fanburg BL. Circulation research. 1991;68(5):1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- 187.Lee SL, Wang WW, Fanburg BL. Faseb J. 2001;15(7):1324–1325. doi: 10.1096/fj.00-0431fje. [DOI] [PubMed] [Google Scholar]
- 188.Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Faseb J. 2000;14(12):1741–1748. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- 189.Thannickal VJ, Fanburg BL. The Journal of biological chemistry. 1995;270(51):30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 190.Barcellos-Hoff MH, Dix TA. Molecular endocrinology (Baltimore, Md. 1996;10(9):1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 191.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. The Journal of biological chemistry. 1998;273(24):15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 192.Yoshizumi M, Abe J, Haendeler J, Huang Q, Berk BC. The Journal of biological chemistry. 2000;275(16):11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- 193.Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. The Journal of biological chemistry. 1996;271(28):16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- 194.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Human molecular genetics. 2002;11(13):1517–1525. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- 195.Cho A, Graves J, Reidy MA. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(12):2527–2532. doi: 10.1161/01.atv.20.12.2527. [DOI] [PubMed] [Google Scholar]
- 196.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, Ermert M, Weissmann N, Rose F, Guenther A, Walmrath D, Seeger W, Grimminger F. American journal of respiratory and critical care medicine. 2004;169(1):39–45. doi: 10.1164/rccm.200302-282OC. [DOI] [PubMed] [Google Scholar]
- 197.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Samidurai A, Pullamsetti S, Weissmann N, Schudt C, Ermert L, Seeger W, Grimminger F. Circulation research. 2004;94(8):1101–1108. doi: 10.1161/01.RES.0000126050.41296.8E. [DOI] [PubMed] [Google Scholar]
- 198.Botney MD, Bahadori L, Gold LI. The American journal of pathology. 1994;144(2):286–295. [PMC free article] [PubMed] [Google Scholar]
- 199.Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. American journal of respiratory cell and molecular biology. 2002;26(2):183–188. doi: 10.1165/ajrcmb.26.2.4666. [DOI] [PubMed] [Google Scholar]
- 200.Irrcher I, Ljubicic V, Hood DA. American journal of physiology. 2009;296(1):C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 201.Rao J, Li J, Liu Y, Lu P, Sun X, Sugumaran PK, Zhu D. Molecular and cellular biochemistry. doi: 10.1007/s11010-012-1313-z. [DOI] [PubMed] [Google Scholar]
- 202.Coffer PJ, Jin J, Woodgett JR. The Biochemical journal. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. American journal of respiratory cell and molecular biology. 2009;40(5):601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. The Journal of biological chemistry. 1999;274(32):22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 205.Liu Y, Fanburg BL. American journal of respiratory cell and molecular biology. 2006;34(2):182–191. doi: 10.1165/rcmb.2005-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Deora AA, Win T, Vanhaesebroeck B, Lander HM. The Journal of biological chemistry. 1998;273(45):29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- 207.Ke Q, Li J, Ding J, Ding M, Wang L, Liu B, Costa M, Huang C. American journal of physiology. 2006;291(2):L257–L264. doi: 10.1152/ajplung.00007.2006. [DOI] [PubMed] [Google Scholar]
- 208.Bierer R, Nitta CH, Friedman J, Codianni S, de Frutos S, Dominguez-Bautista JA, Howard TA, Resta TC, Bosc LV. American journal of physiology. 301(6):L872–L880. doi: 10.1152/ajplung.00405.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.de Frutos S, Spangler R, Alo D, Bosc LV. The Journal of biological chemistry. 2007;282(20):15081–15089. doi: 10.1074/jbc.M702679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang D, Yu X, Cohen RA, Brecher P. The Journal of biological chemistry. 2000;275(16):12223–12230. doi: 10.1074/jbc.275.16.12223. [DOI] [PubMed] [Google Scholar]
- 211.Montisano DF, Mann T, Spragg RG. J Appl Physiol. 1992;73(6):2255–2262. doi: 10.1152/jappl.1992.73.6.2255. [DOI] [PubMed] [Google Scholar]
- 212.Liu Y, Cox SR, Morita T, Kourembanas S. Circulation research. 1995;77(3):638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 213.Kourembanas S, Hannan RL, Faller DV. The Journal of clinical investigation. 1990;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fisher AB, Chien S, Barakat AI, Nerem RM. American journal of physiology. 2001;281(3):L529–L533. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 215.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. The Journal of biological chemistry. 1998;273(15):8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 216.Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. The Journal of biological chemistry. 1997;272(33):20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- 217.Black SM, DeVol JM, Wedgwood S. American journal of physiology. 2008;294(1):C345–C354. doi: 10.1152/ajpcell.00216.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li P, Oparil S, Sun JZ, Thompson JA, Chen YF. J Appl Physiol. 2003;95(2):643–651. doi: 10.1152/japplphysiol.00652.2002. discussion 863. [DOI] [PubMed] [Google Scholar]
- 219.Hirose S, Hosoda Y, Furuya S, Otsuki T, Ikeda E. Pathology international. 2000;50(6):472–479. doi: 10.1046/j.1440-1827.2000.01068.x. [DOI] [PubMed] [Google Scholar]
- 220.Geiger R, Berger RM, Hess J, Bogers AJ, Sharma HS, Mooi WJ. The Journal of pathology. 2000;191(2):202–207. doi: 10.1002/(SICI)1096-9896(200006)191:2<202::AID-PATH608>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 221.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. American journal of respiratory and critical care medicine. 2001;164(10 Pt 1):1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 222.Irwin DC, McCord JM, Nozik-Grayck E, Beckly G, Foreman B, Sullivan T, White MT, Crossno JJ, Bailey D, Flores SC, Majka S, Klemm D, van Patot MC. Free radical biology & medicine. 2009;47(1):55–61. doi: 10.1016/j.freeradbiomed.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Biological chemistry. 2004;385(3–4):249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 224.Black SM, Grobe A, Mata-Greenwood E, Noskina Y. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5053–5056. doi: 10.1109/IEMBS.2004.1404397. [DOI] [PubMed] [Google Scholar]
- 225.Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. American journal of respiratory cell and molecular biology. 1998;18(6):768–776. doi: 10.1165/ajrcmb.18.6.2980. [DOI] [PubMed] [Google Scholar]
- 226.Wanstall JC, Gambino A, Jeffery TK, Cahill MM, Bellomo D, Hayward NK, Kay GF. Cardiovascular research. 2002;55(2):361–368. doi: 10.1016/s0008-6363(02)00440-6. [DOI] [PubMed] [Google Scholar]
- 227.Wassmann S, Wassmann K, Nickenig G. Hypertension. 2004;44(4):381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 228.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Hypertension. 2006;48(3):473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 229.Sindhu RK, Ehdaie A, Farmand F, Dhaliwal KK, Nguyen T, Zhan CD, Roberts CK, Vaziri ND. Biochim Biophys Acta. 2005;1743(1–2):86–92. doi: 10.1016/j.bbamcr.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 230.Sui H, Wang W, Wang PH, Liu LS. Blood Press. 2005;14(6):366–372. doi: 10.1080/08037050500210781. [DOI] [PubMed] [Google Scholar]
- 231.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Circulation research. 2003;92(6):683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 232.Fike CD, Aschner JL, Zhang Y, Salvemini D, Kaplowitz MR. Chest. 2005;128(6 Suppl):555S–556S. doi: 10.1378/chest.128.6_suppl.555S. [DOI] [PubMed] [Google Scholar]
- 233.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. American journal of respiratory and critical care medicine. 2004;169(6):764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 234.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Circulation. 121(24):2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, Tuder RM. The American journal of pathology. 176(3):1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Antioxidants & redox signaling. 15(6):1497–1506. doi: 10.1089/ars.2010.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. American journal of physiology. 2007;293(4):L960–L971. doi: 10.1152/ajplung.00449.2006. [DOI] [PubMed] [Google Scholar]
- 238.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. American journal of physiology. 2006;290(6):L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 239.Van Rheen Z, Fattman C, Domarski S, Majka S, Klemm D, Stenmark KR, Nozik-Grayck E. American journal of respiratory cell and molecular biology. 44(4):500–508. doi: 10.1165/rcmb.2010-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. American journal of respiratory and critical care medicine. 2008;177(2):219–226. doi: 10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 241.Ahmed MN, Zhang Y, Codipilly C, Zaghloul N, Patel D, Wolin M, Miller EJ. Molecular medicine (Cambridge, Mass. 18(1):38–46. doi: 10.2119/molmed.2011.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, Xu Y, Tang Q, Hu D, Somani A, Geurts AM, Ostertag E, Bache RJ, Weir EK, Chen Y. Hypertension. 58(2):303–309. doi: 10.1161/HYPERTENSIONAHA.110.166819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. American journal of respiratory and critical care medicine. 2001;164(5):834–839. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 244.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. American journal of respiratory and critical care medicine. 2006;174(12):1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. American journal of physiology. 2008;295(6):L979–L987. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jin HF, Du SX, Zhao X, Wei HL, Wang YF, Liang YF, Tang CS, Du JB. Acta Pharmacol Sin. 2008;29(10):1157–1166. doi: 10.1111/j.1745-7254.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 247.Joppa P, Petrasova D, Stancak B, Dorkova Z, Tkacova R. Wiener klinische Wochenschrift. 2007;119(13–14):428–434. doi: 10.1007/s00508-007-0819-y. [DOI] [PubMed] [Google Scholar]
- 248.Masri FA, Comhair SA, Dostanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, Erzurum SC. Clinical and translational science. 2008;1(2):99–106. doi: 10.1111/j.1752-8062.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. American journal of physiology. 2005;289(4):L660–L666. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Thibeault DW, Rezaiekhaligh M, Mabry S, Beringer T. Pediatric pulmonology. 1991;11(4):318–327. doi: 10.1002/ppul.1950110408. [DOI] [PubMed] [Google Scholar]
- 251.Frank L, Groseclose EE. Pediatric research. 1984;18(3):240–244. doi: 10.1203/00006450-198403000-00004. [DOI] [PubMed] [Google Scholar]
- 252.Irodova NL, Lankin VZ, Konovalova GK, Kochetov AG, Chazova IE. Bulletin of experimental biology and medicine. 2002;133(6):580–582. doi: 10.1023/a:1020238026534. [DOI] [PubMed] [Google Scholar]
- 253.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. American journal of respiratory and critical care medicine. 1998;158(3):917–923. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]
- 254.Rabinovitch M, Konstam MA, Gamble WJ, Papanicolaou N, Aronovitz MJ, Treves S, Reid L. Circulation research. 1983;52(4):432–441. doi: 10.1161/01.res.52.4.432. [DOI] [PubMed] [Google Scholar]
- 255.Maruyama J, Jiang BH, Maruyama K, Takata M, Miyasaka K. Chest. 2004;126(6):1919–1925. doi: 10.1378/chest.126.6.1919. [DOI] [PubMed] [Google Scholar]
- 256.Sluiter I, van Heijst A, Haasdijk R, Kempen MB, Boerema-de Munck A, Reiss I, Tibboel D, Rottier RJ. Experimental and molecular pathology. 93(1):66–73. doi: 10.1016/j.yexmp.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 257.Darlington DN, Jones RO, Marzella L, Gann DS. J Appl Physiol. 1995;78(6):2025–2032. doi: 10.1152/jappl.1995.78.6.2025. [DOI] [PubMed] [Google Scholar]
- 258.Frid MG, Aldashev AA, Nemenoff RA, Higashito R, Westcott JY, Stenmark KR. Arteriosclerosis, thrombosis, and vascular biology. 1999;19(12):2884–2893. doi: 10.1161/01.atv.19.12.2884. [DOI] [PubMed] [Google Scholar]
- 259.Archer SL. The Journal of laboratory and clinical medicine. 1996;127(6):524–529. doi: 10.1016/s0022-2143(96)90142-0. [DOI] [PubMed] [Google Scholar]
- 260.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. American journal of respiratory cell and molecular biology. 42(4):482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yamagishi S, Imaizumi T. International journal of tissue reactions. 2005;27(3):125–135. [PubMed] [Google Scholar]
- 262.Reid LM. The American review of respiratory disease. 1989;140(5):1490–1493. doi: 10.1164/ajrccm/140.5.1490. [DOI] [PubMed] [Google Scholar]
- 263.Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, Fink L, Schmidt S, Krick S, Camenisch G, Gassmann M, Seeger W, Hanze J. Faseb J. 2002;16(12):1660–1661. doi: 10.1096/fj.02-0420fje. [DOI] [PubMed] [Google Scholar]
- 264.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. American journal of physiology. 2009;297(2):L238–L250. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dempsey EC, Wick MJ, Karoor V, Barr EJ, Tallman DW, Wehling CA, Walchak SJ, Laudi S, Le M, Oka M, Majka S, Cool CD, Fagan KA, Klemm DJ, Hersh LB, Gerard NP, Gerard C, Miller YE. The American journal of pathology. 2009;174(3):782–796. doi: 10.2353/ajpath.2009.080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Le Cras TD, Kim DH, Gebb S, Markham NE, Shannon JM, Tuder RM, Abman SH. The American journal of physiology. 1999;277(4 Pt 1):L709–L718. doi: 10.1152/ajplung.1999.277.4.L709. [DOI] [PubMed] [Google Scholar]
- 267.Zamora MR, Stelzner TJ, Webb S, Panos RJ, Ruff LJ, Dempsey EC. The American journal of physiology. 1996;270(1 Pt 1):L101–L109. doi: 10.1152/ajplung.1996.270.1.L101. [DOI] [PubMed] [Google Scholar]
- 268.Altiere RJ, Olson JW, Gillespie MN. The Journal of pharmacology and experimental therapeutics. 1986;236(2):390–395. [PubMed] [Google Scholar]
- 269.Wilson DW, Segall HJ, Pan LC, Lame MW, Estep JE, Morin D. Critical reviews in toxicology. 1992;22(5–6):307–325. doi: 10.3109/10408449209146311. [DOI] [PubMed] [Google Scholar]
- 270.Mattocks AR. Nature. 1968;217(5130):723–728. doi: 10.1038/217723a0. [DOI] [PubMed] [Google Scholar]
- 271.Mattocks AR, Jukes R. Chemico-biological interactions. 1990;75(2):225–239. doi: 10.1016/0009-2797(90)90120-c. [DOI] [PubMed] [Google Scholar]
- 272.Pan LC, Lame MW, Morin D, Wilson DW, Segall HJ. Toxicology and applied pharmacology. 1991;110(2):336–346. doi: 10.1016/s0041-008x(05)80016-x. [DOI] [PubMed] [Google Scholar]
- 273.Meyrick BO, Reid LM. The American journal of pathology. 1982;106(1):84–94. [PMC free article] [PubMed] [Google Scholar]
- 274.Taylor DW, Wilson DW, Lame MW, Dunston SD, Jones AD, Segall HJ. Toxicology and applied pharmacology. 1997;143(1):196–204. doi: 10.1006/taap.1996.8083. [DOI] [PubMed] [Google Scholar]
- 275.Hoorn CM, Wagner JG, Roth RA. Toxicology and applied pharmacology. 1993;120(2):281–287. doi: 10.1006/taap.1993.1113. [DOI] [PubMed] [Google Scholar]
- 276.Thomas HC, Lame MW, Wilson DW, Segall HJ. Toxicology and applied pharmacology. 1996;141(1):319–329. doi: 10.1006/taap.1996.0289. [DOI] [PubMed] [Google Scholar]
- 277.Thomas HC, Lame MW, Dunston SK, Segall HJ, Wilson DW. Toxicology and applied pharmacology. 1998;151(2):236–244. doi: 10.1006/taap.1998.8458. [DOI] [PubMed] [Google Scholar]
- 278.Jones PL, Rabinovitch M. Circulation research. 1996;79(6):1131–1142. doi: 10.1161/01.res.79.6.1131. [DOI] [PubMed] [Google Scholar]
- 279.Meyrick B, Reid L. The American journal of pathology. 1979;94(1):37–50. [PMC free article] [PubMed] [Google Scholar]
- 280.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Circulation. 121(25):2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 281.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Circulation research. 2007;100(6):923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 282.Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. The Journal of pediatrics. 1976;89(4):626–630. doi: 10.1016/s0022-3476(76)80405-2. [DOI] [PubMed] [Google Scholar]
- 283.Abman SH, Shanley PF, Accurso FJ. The Journal of clinical investigation. 1989;83(6):1849–1858. doi: 10.1172/JCI114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Morin FC., 3rd Pediatric research. 1989;25(3):245–250. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 285.McQueston JA, Kinsella JP, Ivy DD, McMurtry IF, Abman SH. The American journal of physiology. 1995;268(1 Pt 2):H288–H294. doi: 10.1152/ajpheart.1995.268.1.H288. [DOI] [PubMed] [Google Scholar]
- 286.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. Circulation. 1995;92(3):606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 287.Reddy VM, Wong J, Liddicoat JR, Johengen M, Chang R, Fineman JR. The American journal of physiology. 1996;271(2 Pt 2):H562–H570. doi: 10.1152/ajpheart.1996.271.2.H562. [DOI] [PubMed] [Google Scholar]
- 288.Black SM, Bekker JM, Johengen MJ, Parry AJ, Soifer SJ, Fineman JR. Pediatric research. 2000;47(1):97–106. doi: 10.1203/00006450-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 289.Sharma S, Sun X, Kumar S, Rafikov R, Aramburo A, Kalkan G, Tian J, Rehmani I, Kallarackal S, Fineman JR, Black SM. Free radical biology & medicine. 53(2):216–229. doi: 10.1016/j.freeradbiomed.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Atz AM, Adatia I, Wessel DL. The Annals of thoracic surgery. 1996;62(6):1759–1764. doi: 10.1016/s0003-4975(96)00542-5. [DOI] [PubMed] [Google Scholar]
- 291.Lavoie A, Hall JB, Olson DM, Wylam ME. American journal of respiratory and critical care medicine. 1996;153(6 Pt 1):1985–1987. doi: 10.1164/ajrccm.153.6.8665066. [DOI] [PubMed] [Google Scholar]
- 292.Sheehy AM, Burson MA, Black SM. The American journal of physiology. 1998;274(5 Pt 1):L833–L841. doi: 10.1152/ajplung.1998.274.5.L833. [DOI] [PubMed] [Google Scholar]
- 293.Thelitz S, Bekker JM, Ovadia B, Stuart RB, Johengen MJ, Black SM, Fineman JR. The Journal of thoracic and cardiovascular surgery. 2004;127(5):1285–1292. doi: 10.1016/j.jtcvs.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 294.Oishi P, Grobe A, Benavidez E, Ovadia B, Harmon C, Ross GA, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. American journal of physiology. 2006;290(2):L359–L366. doi: 10.1152/ajplung.00019.2005. [DOI] [PubMed] [Google Scholar]
- 295.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. The New England journal of medicine. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 296.Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, Russell JA, Steinhorn RH. American journal of physiology. 302(6):L616–L626. doi: 10.1152/ajplung.00064.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, Pawliczak R. Experimental lung research. 38(2):90–99. doi: 10.3109/01902148.2011.649823. [DOI] [PubMed] [Google Scholar]
- 298.Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, Watts GF. Journal of human hypertension. 2007;21(4):297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- 299.Pey A, Saborido A, Blazquez I, Delgado J, Megias A. The Journal of steroid biochemistry and molecular biology. 2003;87(4–5):269–277. doi: 10.1016/j.jsbmb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 300.Karthikeyan K, Bai BR, Gauthaman K, Sathish KS, Devaraj SN. Life sciences. 2003;73(21):2727–2739. doi: 10.1016/s0024-3205(03)00671-4. [DOI] [PubMed] [Google Scholar]
- 301.Dal-Pizzol F, Klamt F, Benfato MS, Bernard EA, Moreira JC. Free radical research. 2001;34(4):395–404. doi: 10.1080/10715760100300331. [DOI] [PubMed] [Google Scholar]
- 302.Ahmed RS, Seth V, Pasha ST, Banerjee BD. Food Chem Toxicol. 2000;38(5):443–450. doi: 10.1016/s0278-6915(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 303.Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. Free radical biology & medicine. 2006;40(2):341–347. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 304.Burnham EL, McCord JM, Bose S, Brown LA, House R, Moss M, Gaydos J. American journal of physiology. 302(7):L688–L699. doi: 10.1152/ajplung.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1998;11(3):745–757. [PubMed] [Google Scholar]
- 306.Conner BD, Bernard GR. Clin. Chest Med. 2000;21(3):563–587. doi: 10.1016/s0272-5231(05)70167-2. [DOI] [PubMed] [Google Scholar]
- 307.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. Chest. 1997;112(1):164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 308.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. Crit. Care Med. 1999;27(8):1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 309.Steinhubl SR. The American journal of cardiology. 2008;101(10A):14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]