Abstract
Glycosaminoglycans (GAGs) isolated from brittlestars, Echinodermata class Ophiuroidea, were characterized, as part of attempts to understand the evolutionary development of these polysaccharides. A population of chondroitin sulfate/dermatan sulfate (CS/DS) chains with a high overall degree of sulfation and hexuronate epimerization was the major GAG found, whereas heparan sulfate (HS) was below detection level. Enzymatic digestion with different chondroitin lyases revealed exceptionally high proportions of di- and trisulfated CS/DS disaccharides. The latter unit appears much more abundant in one of four individual species of brittlestars, Amphiura filiformis, than reported earlier in other marine invertebrates. The brittlestar CS/DS was further shown to bind to growth factors such as fibroblast growth factor 2 and to promote FGF-stimulated cell signaling in GAG-deficient cell lines in a manner similar to that of heparin. These findings point to a potential biological role for the highly sulfated invertebrate GAGs, similar to those ascribed to HS in vertebrates.
Keywords: brittlestar, chondroitin sulfate, dermatan sulfate, fibroblast growth factor 2
Introduction
Glycosaminoglycans (GAGs) are ubiquitous, linear polysaccharides of repeating disaccharide units composed of hexosamine and hexuronic acid residues that have been conserved throughout the evolution from invertebrates (e.g., planarians) to vertebrates (e.g., humans) (Cassaro and Dietrich 1977; Medeiros et al. 2000). The hexosamine moiety is glucosamine in heparan sulfate (HS)/heparin and hyaluronan, and galactosamine in chondroitin sulfate (CS) and dermatan sulfate (DS). The hexuronic acid is either d-glucuronate (GlcA) or its C5-epimer l-iduronate (IdoA). Except for hyaluronan, the GAG chains carry sulfate groups at various positions of the sugar rings. The sulfated GAGs show enormous structural variability and microheterogeneity (Casu and Lindahl 2001).
Invertebrate CS/DS can remain unmodified such as chondroitin in Caenorhabditis elegans (Yamada et al. 1999) or become extensively modified through sulfation and epimerization, as in ascidian CS/DS (Pavão et al. 1998). Sulfate groups may occupy unusual positions, such as the 3-O-sulfate group in the unique -GlcA(3S)β1-3GalNAc(6S)β1- disaccharide unit in king crab and squid cartilage (Kinoshita et al. 1997). Fucosylation in sea cucumber (Vieira and Mourão 1988) and sea urchins (Yamada et al. 2011), two other echinoderms, and glucosylation in squid cartilage (Habuchi et al. 1977; Kinoshita-Toyoda et al. 2004) further extend the GAG heterogeneity. Conservation of GAG structures during evolution is ascribed to their interaction with proteins (e.g., growth factors, cytokines and morphogens) involved in essential processes of animal development and homeostasis.
In the present study, we have investigated sulfated GAGs in echinoderms that provide attractive models with regard to structure and biological function. Echinoderms are deuterostomes, an average of 70% of echinoderm genes having human homolog (Sea Urchin Genome Sequencing Consortium 2006). The echinoderms comprise five classes: Echinoidea (sea urchins), Asteroidea (sea stars, commonly called starfish), Holothuroidea (holothurians, commonly called sea cucumbers), Ophiuroidea (brittlestars) and Crinoidea (sea lilies). Brittlestars are stellate echinoderms with a central, flattened disc sharply set off from the long segmented arms. They are found in most parts of the world, from the Arctic and Antartic to the tropics. Brittlestars are common in many shallow-water marine habitats, and are dominant in many parts of the deep sea. Most brittlestars have the capacity to autotomize their arms at any intersegmental level (Wilkie 2001) followed by regeneration, and they may therefore contribute to our understanding of tissue/organ regeneration at molecular and cellular levels. Owing to the shared ancestry of echinoderms with chordates, findings from such models are likely to promote mammalian regeneration research (Dupont and Thorndyke 2007). As a first step toward regeneration studies, we have identified and characterized CS/DS from a mixed population of brittlestars and from individual brittlestar species (Amphiura filiformis, Amphiura chiajei, Ophiothrix fragilis and Ophiocomina nigra). Exceptionally highly sulfated GAG species were discovered. These polysaccharides were assessed for protein binding and ability to promote cell signaling by a prototypic fibroblast growth factor (FGF), FGF2, thus attempting to model potential biological function.
Results
Characterization of GAGs isolated from mixed brittlestar species
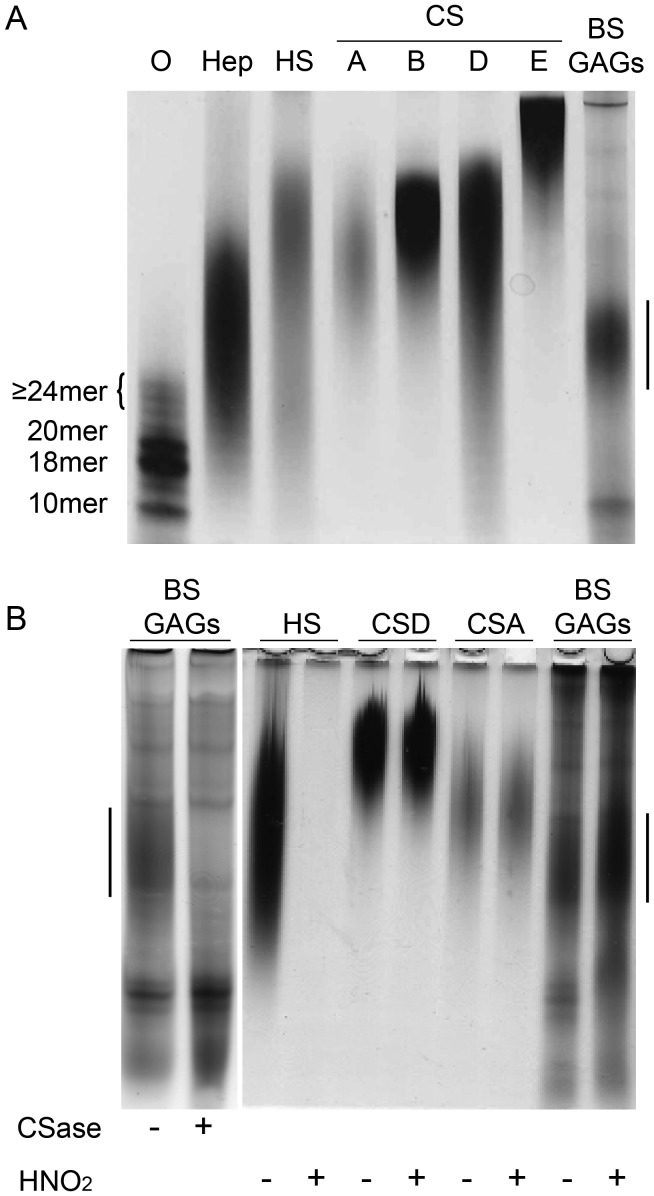
GAGs were isolated from a mixed population of brittlestars by proteolytic digestion followed by separation of polyanionic components through anion-exchange chromatography using a linear salt gradient. To discriminate GAGs from other polyanionic structures, the effluents were tested colorimetrically for hexuronic acid. Pink-staining uronic acid-containing components emerged at an ionic strength in the range 0.8–1.6 M NaCl and were pooled. Material emerging at either lower (<0.8 M NaCl) or higher ionic strength (>1.6 M NaCl) yielded a light brownish color suggesting lack of uronic acid. To determine the size of the uronic acid-positive polyanionic components, effluents were subjected to native Tris-borate-ethylenediaminetetraacetic acid (EDTA)-polyacrylamide gel electrophoresis (TBE-PAGE) separating polysaccharides according to size and charge (Cowman et al. 1984; Hampson and Gallagher 1984), and stained with Alcian blue/silver. A major population of polydisperse Alcian blue/silver positive components was observed along with some faster and slower migrating components. The electrophoretic mobility of the major brittlestar band (vertical bar in Figure 1) was higher than those of various CS reference samples (≥20 kDa), but approximately similar to that of heparin from bovine lung (∼11–12 kDa, Figure 1A).
Fig. 1.
Susceptibility of brittlestar GAGs to enzymatic and chemical cleavage. (A) Purified mixed-population brittlestar polysaccharides (BS GAGs) along with size-defined heparin oligosaccharides (O: 10-mer, 18-mer, 20-mer and ≥24-mer [elution position marked with a bracket]), heparin (Hep), HS and CSs A, B, D and E were separated by native TBE-PAGE. (B) GAGs (500 ng) were treated by CSase ABC (CSase) or nitrous acid at pH 1.5 (HNO2) and separated by TBE-PAGE as indicated. Treated (+) and mock-treated samples (buffer without enzyme and “neutralized” nitrous acid, respectively) (−) are indicated. Gels were stained by Alcian blue/silver for sulfated GAGs. The vertical bar in each gel indicates the major brittlestar GAG further purified for protein-binding and cell stimulation assays.
In order to identify brittlestar GAGs, samples were subjected to selective chemical (nitrous acid at pH 1.5) and enzymatic cleavage (chondroitinase [CSase] ABC). Nitrous acid selectively cleaves HS/heparin at N-sulfated glucosamine units (Casu and Lindahl 2001). The brittlestar material (BS GAGs) resisted nitrous acid treatment, as did CSA and CSD, whereas the positive control sample HS was degraded by this treatment (Figure 1B). In contrast, the major brittlestar component was eliminated by digestion with CSase ABC, whereas the minor bands appeared unaffected (Figure 1B). The brittlestar polysaccharides thus contained a major proportion of CSase ABC-sensitive GAGs.
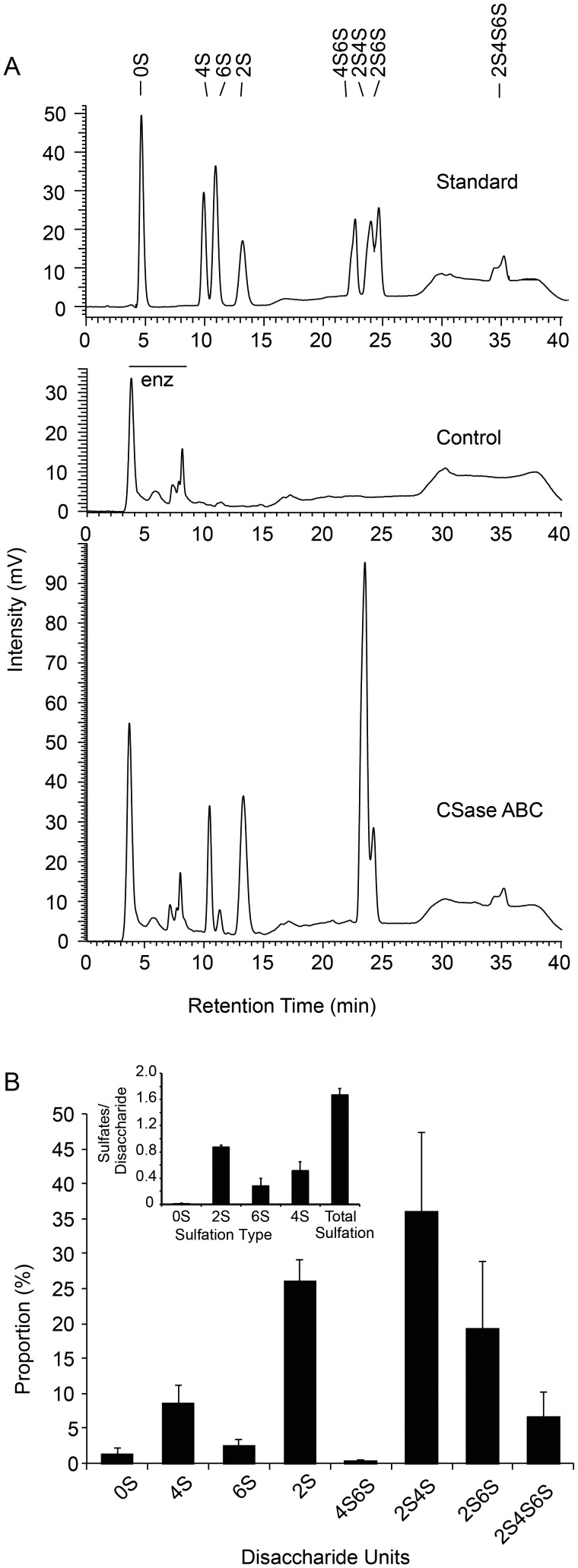
The mixed-population brittlestar GAGs were further characterized by identification of disaccharides generated by CSase ABC digestion. Polysaccharides were isolated either by the approach described above or by a mini-scale version (see “Materials and methods” section) and exhaustively digested with CSase ABC and the resultant disaccharides were separated by reverse-phase ion-pairing (RPIP) chromatography (Figure 2A). Essentially no nonsulfated disaccharide (elution at 5.0 min; 0S) and relatively modest amounts of the monosulfated disaccharides ΔHexA1-3GalNAc(4S) (where ΔHexA stands for the 4,5 unsaturated hexuronate unit) (4S) and ΔHexA(2S)1-3GalNAc (2S) (eluted after 10–15 min) were detected. The dominant disaccharide species in these brittlestar samples co-eluted with the di-O-sulfated disaccharide standards ΔHexA(2S)1-3GalNAc(4S) (2S4S) and ΔHexA(2S)1-3GalNAc(6S) (2S6S) which emerged after 23.5 and 24.5 min, respectively. Small amounts of the rare tri-O-sulfated disaccharide ΔHexA(2S)1-3GalNAc(4,6S) (2S4S6S) emerged at ∼35 min. All the peaks coincided with commercial standard ΔCS disaccharides. The signals were quantified against known amounts of these standards to yield the composition for brittlestar CS/DS shown in Figure 2B. An average of ∼1.7 sulfate groups per disaccharide unit was calculated (Figure 2B inset), in reflection of the abundant di-/trisulfated disaccharide species (Figure 2B).
Fig. 2.
Composition of brittlestar GAGs. (A) The top panel represents a chromatogram of standard 4,5-unsaturated CS/DS disaccharides (20 ng each) after separation by RPIP-HPLC with postcolumn detection (marked “Standard”) containing the commercially available disaccharide units ΔHexA1-3GalNAc (0S); ΔHexA1-3GalNAc(4S) (4S); ΔHexA1-3GalNAc(6S) (6S); ΔHexA(2S)1-3GalNAc (2S); ΔHexA1-3GalNAc(4,6S) (4S6S); ΔHexA(2S)1-3GalNAc(4S) (2S4S); ΔHexA(2S)1-3GalNAc(6S) (2S6S); ΔHexA(2S)1-3GalNAc(4,6S) (2S4S6S). Nota bene: The tri-O-sulfated 2S4S6S appears as a characteristic double peak of constant ratio. In the middle panel, a typical chromatogram of 500 ng of a mock-treated brittlestar GAGs with heat-inactivated enzyme (“Control”) and in the bottom panel of CSase ABC-digested GAGs (“CSase ABC”) are presented. The horizontal bar indicates reactive contaminants detected in a sample treated with heat-inactivated enzyme (enz). (B) The various disaccharides retrieved from mixed brittlestar GAGs were quantified and the relative proportion of each disaccharide unit plotted (average of 4 independent samples, error bars indicate standard deviation [SD]). (Inset) The overall degree of sulfation at each position and total sulfation was calculated based on disaccharide composition gained in (B).
Digestion of the residual GAG-fraction, depleted of the CSase ABC-sensitive CS/DS-population, with heparin lyases yielded no peaks on subsequent RPIP-high-pressure (or high-performance) liquid chromatography (HPLC) (data not shown), suggesting that brittlestar GAGs do not contain any significant amounts of HS/heparin. Fucosylation of CS/DS is a modification commonly observed in echinoderms (sea urchin, sea cucumber and feather star) (Erlinger et al. 1993; Mourão et al. 1996; Tapon-Bretaudière et al. 2002). We, therefore, analyzed the GAG-containing pools for the presence of fucose modification. Colorimetric determination of the uronic acid-positive diethylaminoethyl (DEAE) pools from mixed echinoderms indicated a fucose content of <0.25 mol fucose/mol disaccharide, while a similar pool from A. filiformis fell below detection limit (<0.05 mol/mol disaccharide). By using mild acid hydrolysis followed by CSase ABC treatment, no further increase in CSase ABC-sensitive structures was seen (data not shown), suggesting that the brittlestar CS/DS lacks fucose substituents.
Species-specific composition of galactosaminoglycans
As the mixed population of brittlestars contained several species, we sought to characterize GAGs from the individual brittlestar species, A. filiformis, A. chiajei, O. fragilis and O. nigra. The GAGs from these species were isolated by the mini-scale approach followed by enzymatic digestion similar to the brittlestar mixture and analysis by RPIP-HPLC. The same disaccharides were apparent as in the CS/DS of the mixed brittlestar population, yet at widely different species-specific proportions (Table I). Most remarkably, A. filiformis showed a high proportion (∼40%) of the tri-O-sulfated disaccharide (2S4S6S) (Table I, Supplementary data, Figure S1A), while all the four species contained abundant 2,4-di-O-sulfated disaccharide unit (2S4S) (∼25–50%). Furthermore, A. filiformis and A. chiajei additionally contained large amounts of the 2,6-di-O-sulfated disaccharide (2S6S) unit (∼15–25%). Overall, A. filiformis CS/DS, the most highly sulfated species displayed an overall sulfation degree of ∼2.4 sulfate groups/disaccharide unit (Table I). To ensure completeness of CSase ABC action, the same sample was also analyzed by size exclusion column mass spectrometry (SEC-MS). Mono-, di- and tri-O-sulfated disaccharides were detected, whereas no larger oligosaccharide structures were observed, confirming that chains were completely susceptible to CSase ABC action in these samples (Supplementary data, Figure S2).
Table I.
Disaccharides obtained by exhaustive enzymatic cleavage of CS/DS-chains from different brittlestars
| Mixed species, n = 4 | A. filiformis, n = 3 | A. chiajei, n = 2 | O. fragilis, n = 2 | O. nigra, n = 2 | |
|---|---|---|---|---|---|
| Disaccharide unitsa | |||||
| ΔHexA-GalNAc (0S) | 1.3 (±1.0) | 0.0 (±0.0) | 1.1 (±0.8) | 3.7 (±2.6) | 1.2 (±0.7) |
| ΔHexA-GalNAc4S (4S) | 8.8 (±2.7) | 2.2 (±0.1) | 7.6 (±5.3) | 12.1 (±1.0) | 13.8 (±3.7) |
| ΔHexA-GalNAc6S (6S) | 2.4 (±0.9) | 2.1 (±0.2) | 6.5 (±2.5) | 0.7 (±0.3) | 5.5 (±1.5) |
| ΔHexA2S-GalNAc (2S) | 26.0 (±3.1) | 1.6 (±0.1) | 10.9 (±0.1) | 40.9 (±0.4) | 23.1 (±2.3) |
| ΔHexA-GalNAc4,6S (4S6S) | 0.3 (±0.2) | 0.8 (±0.0) | 0.2 (±0.2) | 0.5 (±0.1) | 0.3 (±0.5) |
| ΔHexA2S-GalNAc4S (2S4S) | 36.0 (±11.2) | 27.2 (±0.2) | 52.5 (±3.6) | 33.8 (±1.2) | 43.6 (±1.1) |
| ΔHexA2S-GalNAc6S (2S6S) | 19.2 (±9.8) | 24.4 (±0.3) | 15.9 (±2.1) | 6.4 (±0.4) | 6.4 (±1.6) |
| ΔHexA2S-GalNAc4,6S (2S4S6S) | 6.5 (±3.8) | 41.7 (±0.3) | 5.3 (±2.4) | 1.9 (±0.2) | 6.0 (±0.3) |
| Sulfation degreeb | |||||
| 2-O-sulfate | 87.6 (±2.3) | 95.0 (±0.1) | 84.6 (±3.8) | 83.0 (±1.8) | 79.2 (±2.5) |
| 4-O-sulfate | 28.3 (±11.2) | 69.0 (±0.3) | 27.9 (±2.6) | 9.5 (±0.1) | 18.3 (±3.2) |
| 6-O-sulfate | 51.2 (±13.7) | 71.9 (±0.3) | 65.6 (±0.5) | 48.2 (±2.1) | 63.7 (±4.7) |
| Total sulfate | 167.1 (±9.6) | 236.0 (±0.2) | 178.1 (±6.9) | 140.7 (±3.8) | 161.2 (±1.0) |
aDisaccharides were generated by CSase ABC-digestion of extracts from the microscale isolation approach and analyzed by RPIP-HPLC as described under the “Materials and methods” section. All disaccharides contain a 4,5-unsaturated hexuronic acid (ΔHexA) unit. The shortened abbreviation used in figures and text is indicated in bracket behind each disaccharide. Values are given in mol % of total disaccharides as calculated from peak areas relative to known amounts of standard disaccharides. The values are the mean ± SD of n samples.
bThe degree and type of sulfation are indicated per 100 disaccharide units calculated from disaccharides species in footnote “a”.
Domain organization of brittlestar CS/DS
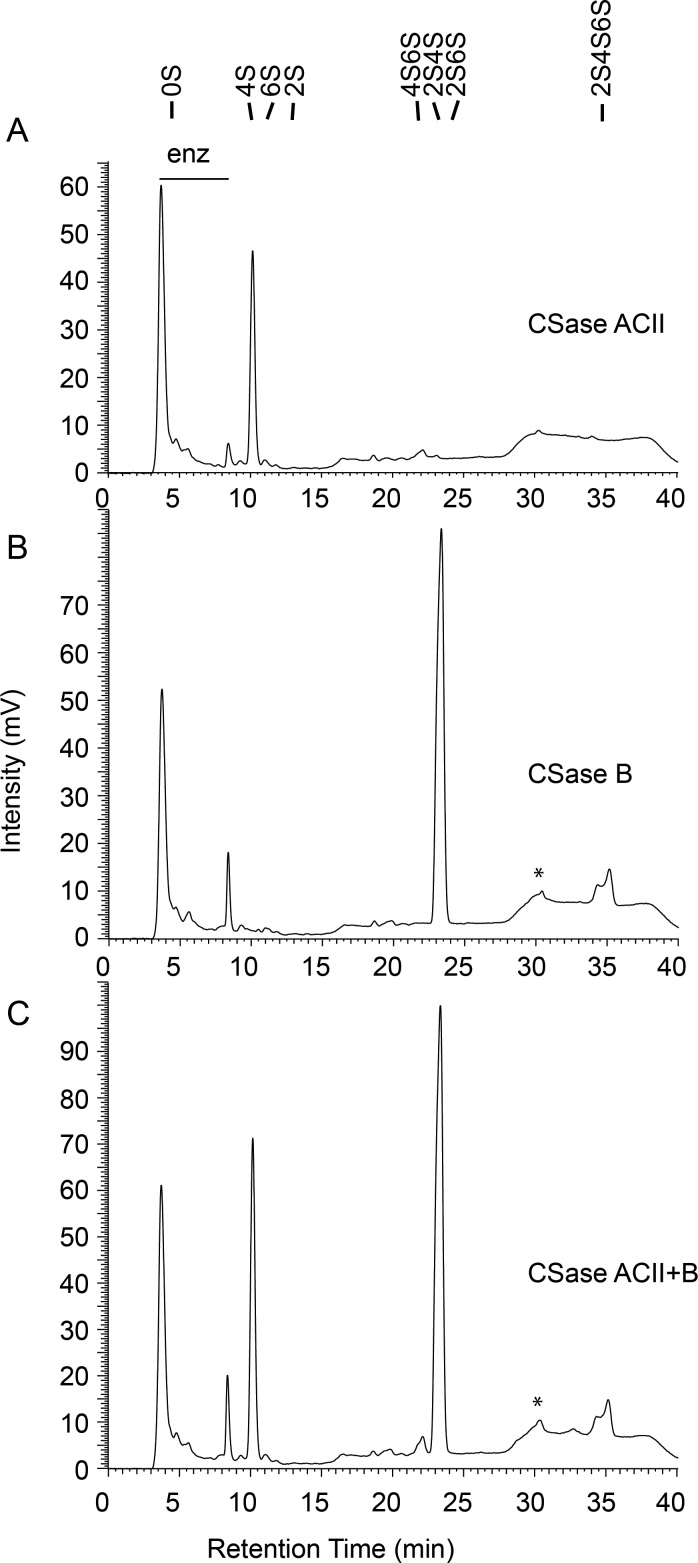
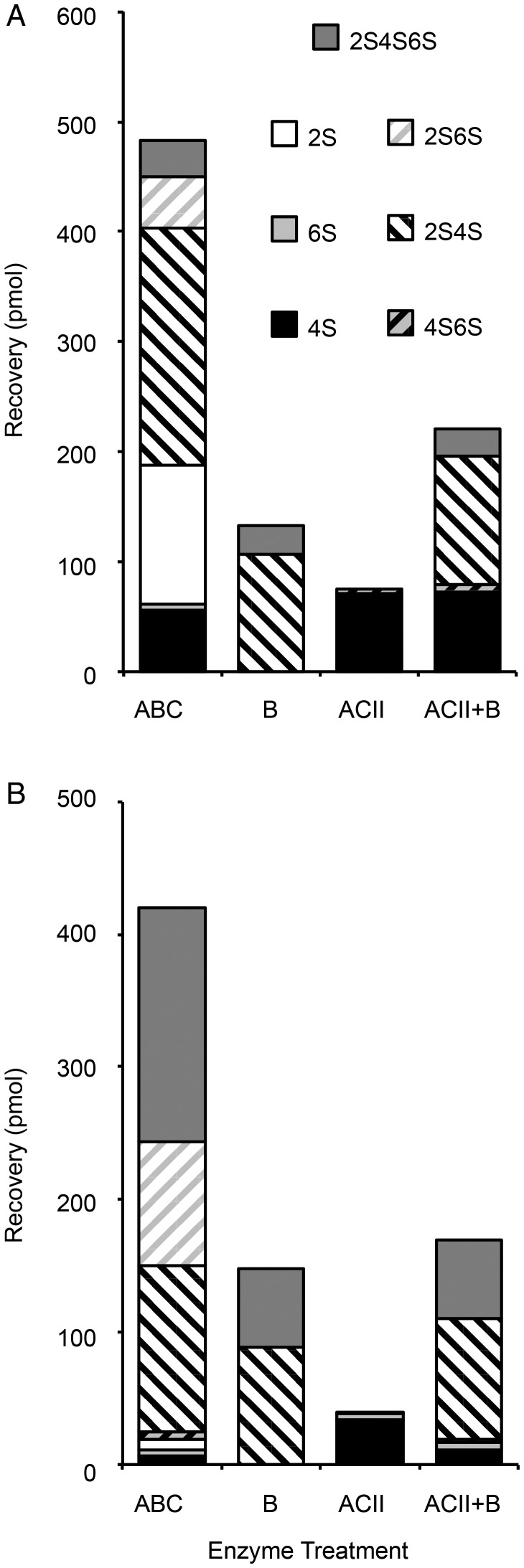
We next characterized the GAGs from the mixed brittlestar population and A. filiformis regarding distribution of the various disaccharide units. The eliminative cleavage by CSase ABC results in formation of a Δ4,5-double bond of hexuronic acid at the nonreducing end, such that GlcA- and IdoA-containing target sequences give rise to disaccharide products with the same unsaturated hexuronate unit (Yamagata et al. 1968) as the distinctive configuration at C5 of target hexuronate residues is lost (Figure 2A). We, therefore, used more restrictive chondroitin lyases, specific to non-epimerized structures (CSase ACII) (Figure 3A) and epimerized structures (CSase B) (Figure 3B), respectively, that allowed us also to retrieve information related to chain organization. Compositional data are quantitative only in case of complete digestion of a chain into disaccharides, whereas resistant glycosidic linkages will give rise to oligosaccharides larger than disaccharides, with corresponding loss of signal intensity (only the reducing end of each oligosaccharide, independent of its size, reacts with the postcolumn reagent). Comparison of disaccharide recoveries from equal amounts of substrate treated with different enzymes allowed us to determine the proportion of the susceptible disaccharide units and establish their approximate arrangement in the chains. Digestion of the mixed-population brittlestar CS/DS with CSase ACII, an exoglycosidase, resulted in one major peak emerging at the position of 4S corresponding to ∼10% of the total chain material and a small peak co-eluting with the 4S6S standard (Figures 3A and 4A, CSase ACII). Thus, these disaccharides must have been arranged in a contiguous sequence amenable to enzyme attack from the nonreducing end. Digestion with CSase B alone, an endoglycosidase specifically cleaving the glycosidic linkage to C4 of IdoA, yielded half of the 2,4-O-sulfated (2S4S) and essentially all of the tri-O-sulfated disaccharides (2S4S6S) (Figures 3B and 4A, CSase B) compared with treatment with CSase ABC (Figures 2A and 4A), suggesting that the parent disaccharide units contained IdoA and were arranged in a contiguous manner, a DS chain domain. Some disaccharide building blocks recovered in the CSase ABC digest, however, were resistant to either CSase ACII or CSase B treatment (compare Figure 2A, CSase ABC, with Figure 3A and B, CSase ACII and CSase B). To test whether the combined action of ACII and B would result in release of these remaining structures, a digestion was performed with both enzymes together (Figure 3C, CSase ACII + CSase B). The combined treatment released essentially all disaccharides also observed after the individual treatments, i.e., 4S, 2S4S and 2S4S6S, but none of the residual saccharides, indicating that the remaining chain structures did not contain alternating structures of CSase ACII and CSase B sensitive units (Figure 4A). As only marginal amounts of tetrasaccharides were detected in any of the chromatograms (Figure 3B and C, marked with asterix), these results suggest that the remaining non-cleaved disaccharide units were aligned in longer oligosaccharides resistant to both CSase ACII and CSase B.
Fig. 3.
Sensitivity of brittlestar GAGs to selective CSase treatment. Equal amounts (500 ng) of isolated GAGs were digested by either CSase ABC (see Figure 2A), ACII (A), B (B) or ACII + B (C) and the products were separated by RPIP-HPLC as indicated. The peak appearing after 4 min originates from the digestion buffer while the minor peaks emerging between 5 and 9 min are due to contaminants in the respective buffer/enzyme preparations (marked with bar “enz”). A peak eluting at ∼30 min typically containing tetrasaccharides is indicated with an asterix. Standard disaccharides are as indicated in Figure 2.
Fig. 4.
Recovery of disaccharides from brittlestar GAGs digested by different CSases. Equal aliquots (500 ng) of mixed-population brittlestar (A) and A. filiformis (B) GAGs were subjected to different enzyme treatments as described in Figure 3 and recoveries of different disaccharides after complete or selective CSase treatments were recorded as indicated. Monosulfated disaccharides are indicated with filled boxes by sulfate group codes (4S: black, 6S: light grey, 2S: white), disulfated disaccharides are striped in shades of the corresponding component sulfates (4S6S: black/light gray; 2S4S: white/black, 2S6S: white/light gray), trisulfated disaccharides (2S4S6S) are marked as filled dark gray boxes. One of three similar experiments is shown for each GAG sample.
The insight into CS/DS chain organization gained from the mixed population of brittlestars prompted us to analyze domain features in the most highly sulfated CS/DS, isolated from A. filiformis. Separate and combined enzymatic digestions of A. filiformis GAGs were performed and quantified, in analogy with mixed brittlestar GAGs (Figure 4B, Supplementary data, Figure S1B). In case of A. filiformis, individual treatment with CSase B resulted in essentially similar recovery of 2,4-di-O-sulfated disaccharides (2S4S) when compared with CSase ABC treatment (Figure 4B), whereas only ∼40% of the tri-O-sulfated disaccharides (2S4S6S) were released by either CSase B alone or in combination with CSase ACII. As in the mixed brittlestar population, none of the 2,6-di-O-sulfated disaccharides (2S6S) was sensitive to either of the individual enzymes and only released by CSase ABC (Figure 4B).
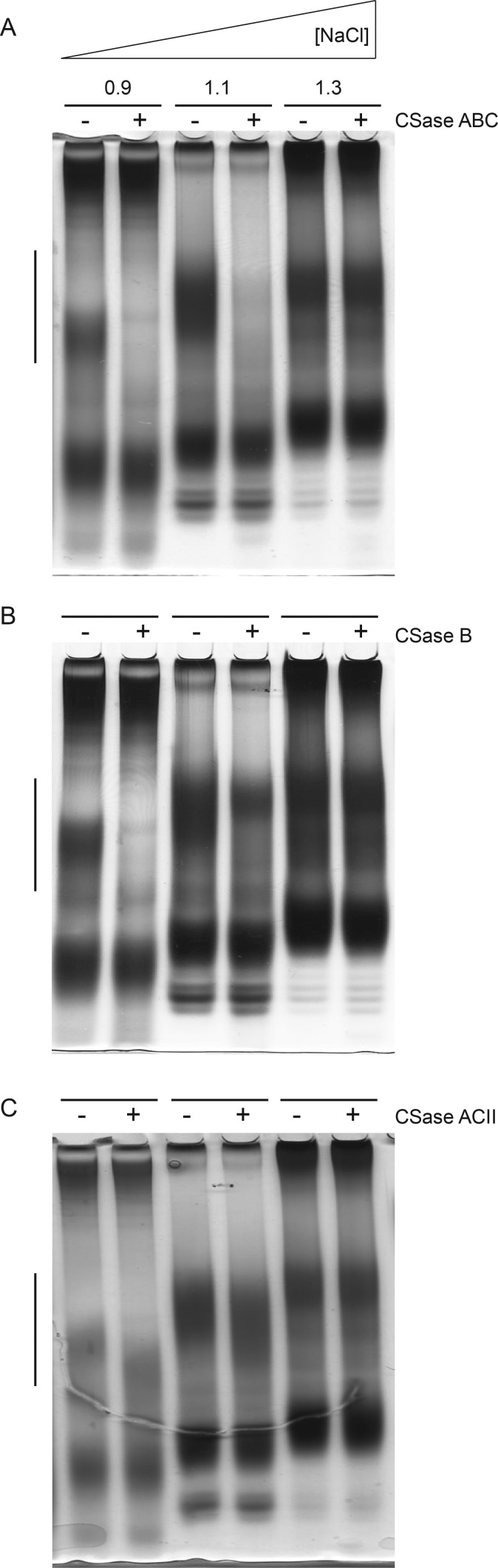
To analyze domain distribution among the CS/DS population, A. filiformis GAGs were subfractionated by DEAE-development by a step-wise increase of ionic strength. Separation of the different DEAE-effluent pools by native TBE-PAGE revealed various polydisperse bands typical of GAGs (and other polyanionic structures) (Figure 5, Supplementary data, Figure S3). The A. filiformis specific CS/DS-species were identified by CSase ABC treatment of an equal aliquot of each DEAE-pool followed by separation by TBE-PAGE (Figure 5A). Predominant bands in the 0.9 M- and the 1.1 M-pools were the only CSase ABC-sensitive components detected by elimination of the respective band from the gel. In the low (0.3 and 0.5 M; not shown)- and high-salt pools (1.3 M), essentially no cleared zones were manifest upon CSase ABC treatment, suggesting the absence of CS/DS in these pools and instead indicating the presence of other types of Alcian blue/silver positive polyanionic structures.
Fig. 5.
Identification of CSase-sensitive GAGs from A. filiformis arms. CSase-sensitive fractions of GAGs isolated from arms were subjected to CSase ABC (A), CSase B (B) or CSase ACII (C) and separated by TBE-PAGE followed by staining with Alcian blue/silver staining. The migration range of CSase-sensitive molecules is indicated by a bar to the left. Mock-digested control samples (“−”) were run parallel to enzyme-treated (“+”) aliquots. Fractions from the DEAE anion-exchange column (eluted by the indicated salt concentration [NaCl]) containing CSase-sensitive glycans are shown.
In parallel, these samples were treated by CSase B and CSase ACII, respectively. Also with these enzymes the same bands in the 0.9 M-and the 1.1 M-fraction were susceptible to cleavage. The target component in the 0.9 M-fraction was completely eliminated by CSase B digestion, as was most of the corresponding component of the 1.1 M-fraction (Figure 5B). However, a minor but significant portion of the latter component appeared resistant to the enzyme through repeated experiments. CSase ACII, selective for non-epimerized CS-structures acted on both pools by shifting the migration distance of the susceptible chains (Figure 5C). Implications of these findings with regard to the domain organization of brittlestar CS/DS are outlined in “Discussion”.
Effect of brittlestar CS/DS on FGF2 binding and cell signaling
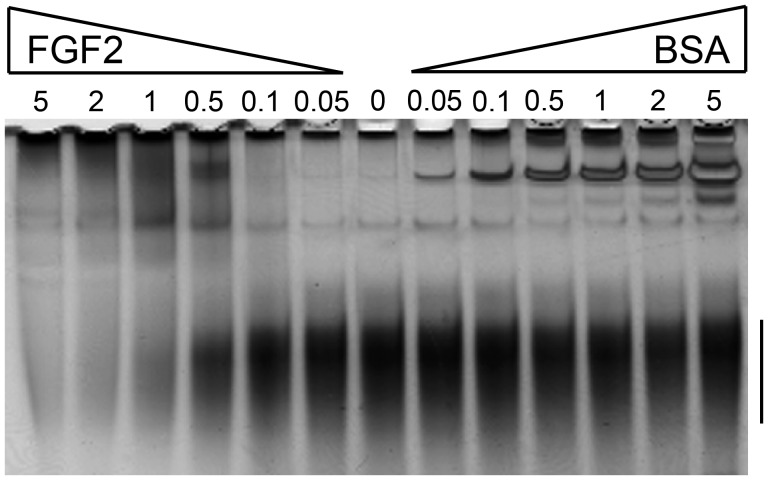
Considering the structural properties of brittlestar CS/DS and the lack of detectable HS, we wondered whether these polysaccharides would be able to bind proteins in a manner similar to HS. We, therefore, isolated the CSase ABC-sensitive polysaccharides from mixed brittlestar species using preparative native PAGE and tested the ability of these CS/DS chains to bind growth factors such as FGFs and vascular endothelial growth factor (VEGF)-A165, as well as the anticoagulant protease inhibitor antithrombin (AT) or the recognized CS/DS binding chemokine platelet factor 4 (PF4) (Petersen et al. 1999; Trowbridge and Gallo 2002). CS/DS was preincubated with various amounts of proteins to enable binding, and the mixtures were then separated by native TBE-PAGE. As migration of GAGs in TBE-PAGE depends on chain length and negative charges of the polysaccharides (Cowman et al. 1984; Hampson and Gallagher 1984), interaction with proteins will reduce migration of the GAGs (in analogy to DNA-gel shift assays) (Misevic 1989). Complex formation between protein and GAGs did indeed result in reduced migration of the polysaccharide chains and thus in a gel shift when compared with unbound GAG chains that move freely (Figure 6).
Fig. 6.
Interaction of brittlestar GAGs with FGF2 analyzed by gel-shift assay. PAGE-purified mixed-population brittlestar CS/DS (500 ng) was incubated with increasing amounts (in μg) of FGF2 and BSA as indicated above the gel and the samples were separated by TBE-PAGE. GAGs were visualized by staining with Alcian blue/silver. The migration range of unbound, free GAGs is indicated by a bar to the right.
Significant binding of brittlestar GAGs to FGF2 was observed at ≥0.5 μg protein, i.e., at approximately equimolar concentration of brittlestar CS/DS and FGF2, increasing FGF/GAG ratios resulting in progressive depletion of free GAG chains (Figure 6). A rough assessment of conditions leading to ∼50% GAG complexing pointed to low micromolar affinity. Similar shifts in GAG mobility upon protein binding were observed with FGF1 and VEGF-A165 (Supplementary data, Figure S4). On the other hand, neither VEGF-A165b nor AT affected the gel mobility of brittlestar CS/DS indicating poor binding (Supplementary data, Figure S4), whereas PF4 demonstrated binding to the brittlestar GAGs (Supplementary data, Figure S5).
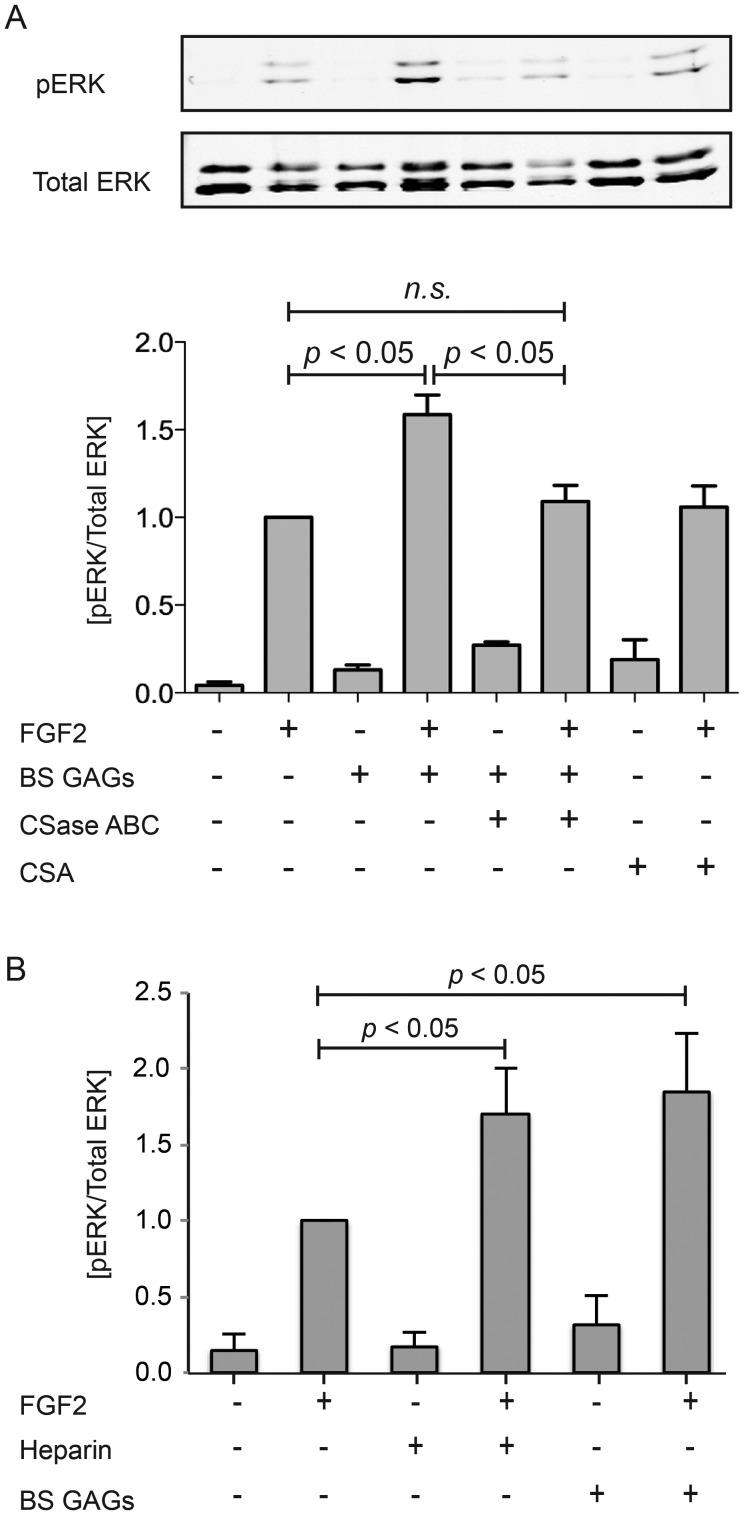
GAGs not only facilitate protein binding and sequestration but also participate in cell signaling (Pye et al. 1998; Ornitz 2000; Casu and Lindahl 2001; Jastrebova et al. 2010). We, therefore, investigated the effect of purified brittlestar CS/DS on FGF2-induced cell signaling. Phosphorylated extracellular signal-regulated kinase (ERK)1/2, a downstream effector of the mitogen activated protein kinase pathway, was measured in Chinese hamster ovary (CHO) 745 cells stimulated by FGF2. CHO 745 cells lack endogenous GAGs and can, therefore, be tested with exogenous GAGs from various sources in combination with FGF2. Minimum baseline activity was observed in unstimulated cells (Figure 7) and cells treated with PAGE-purified brittlestar CS/DS (Figure 7A) or heparin (Figure 7B) alone. Cell signaling in cells stimulated with FGF2 was significantly potentiated (∼1.7-fold) by concomitant addition of either heparin (Figure 7B) or brittlestar CS/DS at similar low concentrations, whereas the low-sulfated CSA, or CSase ABC-digested brittlestar CS/DS were inefficient (Figure 7A).
Fig. 7.
Cell co-stimulation with FGF and brittlestar GAGs. GAG-deficient CHO 745 cells were incubated with FGF2 (10 ng/mL) alone or in combination with PAGE-purified brittlestar GAGs (5 ng/mL), CSA (5 ng/mL) or CSase ABC-digested brittlestar GAGs (5 ng/mL) for 30 min as indicated (A). After cell lysis, equal amounts of protein extracts were separated by SDS–PAGE followed by western blotting with anti-pERK and anti-totalERK antibodies as described in the “Materials and methods” section (representative blots in upper panel). The signal intensities were plotted as ratio of pERK/total extracellular signal-regulated kinase (average of 4 independent experiments) with the signal by FGF stimulation alone after 30 min set as 1 (lower panel). (B) GAG-deficient CHO 745 cells were incubated with FGF2 (10 ng/mL) alone or in combination with either PAGE-purified brittlestar GAGs (5 ng/mL) or heparin (5 ng/mL) for 30 min as indicated and analyzed in an analogous fashion. Statistical significance (four independent experiments) was calculated using student's “t-test” and is indicated by P < 0.05.
Discussion
Marine animals are known to contain sulfated polysaccharides with unique structural features (Cassaro and Dietrich 1977; Nader et al. 1984; Medeiros et al. 2000; Volpi and Maccari 2009; Kozlowski et al. 2011). Sulfated GAGs have important roles in vertebrate biology, and have been implicated in diverse processes such as neurite outgrowth (Deepa et al. 2002; Nandini et al. 2005), angiogenesis (Tapon-Bretaudière et al. 2002) and wound healing (Penc et al. 1998). Although studies of GAGs have been carried out in a few classes of Echinodermata (e.g., Echinoidea and Holothuroidea) (Mourão et al. 1996; Tapon-Bretaudière et al. 2002), the Ophiuroidea, e.g., brittlestars are of particular interest because of their marked ability to regenerate severed limbs (Wilkie 2001), a process potentially subject to GAG-dependent cell signaling. Anticipating such regeneration studies, we report here the structural characterization of brittlestar GAGs.
Our results identify CS/DS as the major GAG in brittlestars, while no HS-related components were detected. This is a remarkable finding since HS and heparin are known to appear at the beginning of the eumetazoan lineage and are conserved throughout evolution of multicellular organisms (Medeiros et al. 2000; Kozlowski et al. 2011; Yamada et al. 2011). Indeed, HS/heparin has been identified in other Echinoidea such as sea urchin (Lovtrup-Rein and Lovtrup 1984; Bergeron et al. 2011), and various other invertebrates (Cassaro and Dietrich 1977), but has, to the best of our knowledge, not been reported in brittlestars. The occurrence of HS/heparin in trace amounts cannot be excluded, but would at most account for ∼1% of the total GAGs. CS/DS chain modifications such as fucosylation seen in some echinoderms (e.g., sea urchin and sea cucumber) (Mourão et al. 1996; Tapon-Bretaudière et al. 2002) were lacking in brittlestar GAGs as the chains were susceptible to CSase ABC. This enzyme is capable of cleaving essentially all types of CS/DS chains, but only provided that they lack atypical substituents (e.g., fucose residues) (Sugahara and Yamada 2000). The brittlestar GAGs showed no additional components in CSase digests after mild acid hydrolysis to remove potential fucose substituents. Minimal amounts of fucose could be detected by colorimetric assessment of GAG-containing fractions in mixed brittlestar samples still containing other polyanionic polymers of unidentified structures, whereas none was detected in A. filiformis samples.
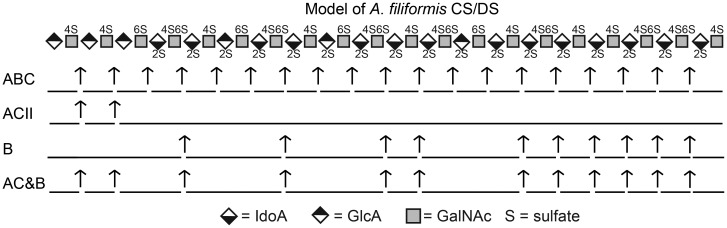
Disaccharide analysis of CS/DS isolated from the mixed population of brittlestars after CSase ABC treatment showed ∼1.7 sulfate groups per disaccharide unit (Figure 2B inset, Table I), which is remarkably high compared with mammalian CS/DS (Casu 1991). Moreover, A. filiformis CS/DS displayed an even higher degree of sulfation, ∼2.4 sulfate groups/disaccharide unit (Table I). This sulfate content is exceptional also in relation to CS/DS from other known marine vertebrates and invertebrates (Yamada et al. 2011), and comparable to that of heparin. CSase ABC-generated disaccharides yield information on the relative contents of different disaccharide units within a chain population. However, the β-eliminative cleavage of CSase ABC results in unsaturated disaccharides with Δ4,5-double bonds in nonreducing-terminal hexuronic acid units, and hence in loss of the distinctive C5-configuration of GlcA- and IdoA-containing target sequences (Yamagata et al. 1968). Instead, information about the arrangement of different disaccharide units along the chain was obtained using CSases with more restricted cleavage specificities. The 2-O-sulfated and 2,4-di-O-sulfated disaccharide units generally contain IdoA, a characteristic component of DS chains generated by C5-epimerization of GlcA precursor units. In order to identify epimerized (IdoA-containing) and non-epimerized (GlcA-containing) disaccharide units, largely confined to different chain domains (Malmström et al. 2012), we used CSase B and CSase ACII, respectively. CSase B is an endoglycosidase that preferentially cleaves IdoA-containing DS structures (Linhardt et al. 2006), whereas CSase ACII is an exoglycosidase specific to GlcA-containing structures and will not attack an IdoA unit or a GlcA sulfated at C2 (Hiyama and Okada 1975). Di-O- and tri-O-sulfated disaccharide units susceptible to release by CSase ABC thus resist CSase ACII cleavage if they are located at internal positions of the chain, separate from the nonreducing terminus, or else if they contain IdoA or 2-O-sulfated GlcA units (Sugahara et al. 1996). Resistant to CSase B are galactosaminidic linkages to GlcA units, or to IdoA if the linkage adjacent GalNAc residues lack 4-O-sulfation (Michel et al. 2004). Based on this information, recoveries of the various disaccharides following selective lyase digestions (Figure 4) can be interpreted in terms of disaccharide distribution along a GAG chain. A model compatible with such data relating to CS/DS from A. filiformis features variably sulfated and epimerized domains (Figure 8) somewhat akin to those in HS chains (Lindahl and Li 2009). Notably, the entire population of CS/DS chains contain a nonreducing-terminal ACII-sensitive domain as demonstrated by digestion of differently sulfated subfractions (Figure 5C). Whether this model would be representative of the entire CS/DS pool remains somewhat unclear, in view of the CSase ABC-sensitive but CSase B-resistant component in the 1.1 M-fraction (Figure 5B).
Fig. 8.
Schematic illustration of a tentative chain structure of A. filiformis GAG. The model (reducing terminus to the right) describes a hypothetical distribution consistent with the data presented based on recovery of disaccharide units (Figure 4) from chains subjected to selective CSase digestions (as indicated to the left). Potential cleavage sites for the respective enzymes are marked by arrows, and the resulting di- or larger oligosaccharide products indicated by bars of corresponding length. Symbols (diamond with black upper and lower parts for GlcA and IdoA, respectively; squares for GalNAc) represent the sugar units and sulfate modifications (S) are indicated by position.
The DS structures identified in brittlestar GAGs are in accord with previous demonstrations of 2S4S disaccharides in mammals (Bao et al. 2004) and invertebrates, e.g., ascidians (Pavão et al. 1998). However, the proportion of the trisulfated disaccharide unit, 2S4S6S, in A. filiformis CS/DS exceeds those found in other brittlestar species (Table I), in any other previously examined marine invertebrate (including the clam Scapharca inaequivalvis [Volpi and Maccari 2009) or hagfish notochord (Nandini et al. 2004]), or to our knowledge, in any other galactosaminoglycan described. An evolutionary view on GAG structure reveals that marine invertebrates have exceedingly oversulfated GAGs, along with other modifications adding to structural complexity (Yamada et al. 2011). The high degree of sulfation has been tentatively explained in terms of adaptation to the habitat of these organisms, i.e., a surrounding of high salinity (Sampaio and Nader 2006; Yamada et al. 2011).
The biological roles of GAGs in development and homeostasis, including their participation in cell signaling depend on their ability to interact with proteins (Casu and Lindahl 2001; Bishop et al. 2007; Malavaki et al. 2008; Lindahl and Li 2009). IdoA units are conformationally flexible and thus promote interactions of HS (Casu 1991) as well as DS (Nandini et al. 2004) with various proteins. We, therefore, tested whether brittlestar (mixed species) CS/DS could bind proteins known to interact with HS. The gel mobility shift assay used to assess GAG-protein binding demonstrated that brittlestar CS/DS is capable of binding FGF1, FGF2, VEGF-A165 and PF4 (Figure 6; Supplementary data, Figures S4 and S5). These findings are in accord with those of earlier studies of growth factor binding to CS/DS hybrid structures isolated from invertebrates (Nandini et al. 2004). On the other hand, neither VEGF-A165b, a VEGF-A splice variant lacking HS-binding motif (Cébe Suarez et al. 2006), nor AT affected the gel mobility of brittlestar CS/DS indicating poor binding ability (Supplementary data, Figure S4). The lack of AT interaction is at variance with previous reports on AT binding to sulfated galactosaminoglycans in echinoderma (Mourão et al. 1996). This discrepancy presumably reflects the lack of sulfated fucose substituents in brittlestar CS/DS, previously shown to promote AT recognition (Mourão et al. 1996; Mulloy et al. 2000). Also, AT binding to heparin/HS depends on a highly specific sulfation pattern (Casu and Lindahl 2001). Cell stimulation with brittlestar CS/DS further demonstrates an FGF-co-receptor capacity of these GAGs that appears similar to that of heparin, pointing to a possible role of brittlestar GAGs in cell-regulatory mechanisms (Figure 7). Notably, CSA containing approximately one sulfate group/disaccharide unit failed to elicit a response to FGF2. These results are in accord with the notion that growth factors, here represented by a prototypic FGF, bind to sulfated domains in various GAGs that mediate effective signal transduction, and thus elicit specific cellular response (Reiland and Rapraeger 1993; Penc et al. 1998; Sperinde and Nugent 1998). They also support previous conclusions regarding the relatively low specificity of FGF2-GAG interactions (Kreuger et al. 2005; Jastrebova et al. 2010). Several growth factors and neuropeptides have been suggested to be active in tissue formation and regeneration of brittlestars, and among them are members of the bone morphogenetic family and potentially also FGFs (Thorndyke and Candia Carnevali 2001; Bannister et al. 2008; Burns et al. 2011). Potential roles of brittlestar GAGs could, therefore, be anticipated in presentation of morphogen/growth factor gradients as well as stimulation of cells in regeneration processes. These animals may thus be useful model organisms in studies of structure—function relationships for highly sulfated GAGs in relation to important biological processes such as wound healing and tissue regeneration.
Materials and methods
Collection of brittlestars
A mixed population and four individual species of brittlestars (Echinodermata, Ophiroidea) were collected living in the vicinity of the Sven Lovén Center for Marine Sciences, Kristineberg (Fiskebäckskil, Sweden). Sediment containing A. filiformis and A. chiajei was collected at 25–40 m depth, using a Petersen mud grab. Individuals were immediately sampled from the sediment cores by gentle rinsing to avoid breaking arms. Ophiocomina nigra and Ophiothrix fragilis were collected on rocky bottom using a dredge. Animals were frozen in liquid nitrogen before isolation of GAGs.
Isolation of GAGs
Brittlestar species (∼5 g whole animals) were homogenized in cold acetone and left for 24 h. The solvent was removed by filtration over Whatman No. 3MM paper, and the pellet was suspended in a minimal volume of water. Methanol and chloroform were added to a final ratio of 3:8:4 (H2O:CH3OH:CHCl3 v/v) under stirring and samples were left at room temperature overnight. The solvent mixture was removed by filtration as above, and the residue was washed twice with ethanol and dried at room temperature. The defatted tissue powder was suspended in protease digestion buffer (0.1 M Tris/HCl, pH 8.0, 2 mM CaCl2, 3% ethanol) at 100 mg/mL, and protease Type XIV (Sigma-Aldrich, Stockholm, Sweden) was added (5 mg/g tissue). After incubation at 55°C for 24 h, another aliquot of protease was added and the incubation continued for another 24 h. The incubation was terminated by heating the sample at 96°C for 15 min, and the suspension was centrifuged at 2000 × g for 15 min. Nucleic acid in the supernatant was digested by adding 125 units of benzonase (Merck, Darmstadt, Germany), along with MgCl2 to a final concentration of 2 mM, followed by incubation at 37°C for 24 h. The reaction was stopped as above and the supernatant was loaded onto a column of 5 mL packed DEAE-Sephacel gel (GE Healthcare Biosciences, Uppsala, Sweden) equilibrated in 50 mM Tris/HCl, pH 7.5, 0.2 M NaCl. The column was washed with one bed volume of equilibration buffer and three volumes of 50 mM NaAcetate, pH 4.5, 0.2 M NaCl to eliminate neutral carbohydrates. Polyanionic GAGs adsorbed to the column were eluted by use of a linear salt gradient of NaCl from 0.2 to 2 M applied in 50 mM NaAcetate, pH 4.5 (15 column volumes total). The salt gradient was monitored by conductivity measurement of the effluent fractions. Fractions were collected and an aliquot tested for hexuronic acid content (Blumenkrantz and Asboe-Hansen 1973), and positive fractions were pooled, dialyzed against H2O in dialysis bags with cut-off (3000 MWCO) and dried.
Polysaccharides from A. filiformis arms were isolated in an analogous manner except the DEAE-Sephacel column in step-wise mode with 6 column volumes each of 0.1, 0.3, 0.5, 0.7, 0.9, 1.1, 1.3, 1.5, 1.7, 1.9 and 2 M NaCl in 50 mM NaAcetate, pH 4.5. The effluents of each salt concentration were combined and desalted by dialysis.
Mini-scale isolation of GAGs
Mixed and individual species of brittlestars were dried by lyophilization and ground tissue (10 mg) suspended in 0.5 mL of protease buffer (50 mM Tris/HCl, pH 8, 1 mM CaCl2, 1% Triton X-100) containing 0.8 mg/mL protease (Type XIV) was incubated at 55°C for 24 h with end-over-end mixing essentially as described (Ledin et al. 2004). A second aliquot of protease was added after 20 h. After heat inactivation of the protease, the sample was adjusted to 2 mM MgCl2 and benzonase (12 mU) was added. The sample was incubated at 37°C for 2 h, heat inactivated, adjusted to a final sodium chloride concentration of 0.1 M and centrifuged at 10,000 × g for 10 min. For purification of GAGs from sample digests, 0.15 mL columns of DEAE-Sephacel were prepared in Bio-Rad 5-mL columns. The columns were primed by washing with six column volumes of elution solution (2 M NaCl) and six column volumes of loading buffer (50 mM Tris/HCl, pH 8, 0.1 M NaCl). The supernatants of the sample extracts were applied, and the columns were washed successively with six volumes of loading buffer, six volumes of wash buffer (50 mM NaAcetate, pH 4, 0.1 M NaCl) and five volumes of 0.1 M NaCl. Elution of GAGs was achieved by adding six volumes of 2 M NaCl. Effluents were collected, concentrated in a speed vac and adjusted to 200 μL. Samples were desalted on PD MiniTrap™ G-25 columns (GE Healthcare Biosciences) before further analysis.
Colorimetric quantification of samples
GAGs were quantified by colorimetric determination of hexuronic acid using the meta-hydroxy-diphenyl method (Blumenkrantz and Asboe-Hansen 1973) with GlcA as a standard. A factor of 3 was arbitrarily employed to convert values into saccharide mass.
Fucose was determined by the procedure of Dische and Shettles (1948) using methyl-fucose (Sigma-Aldrich) as a standard.
Enzymatic digestion of GAGs
Isolated GAGs (10 μg) after anion-exchange chromatography were digested with CSase ABC isolated from Proteus vulgaris (Seikagaku Corp., Tokyo, Japan), a chondroitin lyase with broad substrate specificity, as follows. Dried material was dissolved in a final volume of 100 µL of 20 mM Tris/Acetate buffer, pH 8.0, containing 20 mU of CSase ABC and incubated at 37°C for 3 h. The reaction was terminated by heat inactivation at 96°C for 10 min and the sample centrifuged at 10,000 × g for 10 min. An aliquot of the supernatant was transferred to an HPLC tube for disaccharide analysis. CS/DS-depleted GAG chains were further purified by another round of DEAE chromatography to remove CS/DS disaccharides and digested with heparin lyases (IBEX Pharmaceuticals, Inc., Montreal, Canada) before HS-disaccharide analysis as described (Ledin et al. 2004). GAGs isolated by the small-scale approach from 10 mg dry tissue were digested in an analogous manner after the first DEAE with CSase ABC at 37°C for 3 h and all sample analyzed by RPIP-HPLC as described under ‘Disaccharide analysis’.
Isolated GAGs (0.5 μg) were also digested with either 20 mU CSase AC II isolated from Arthrobacter auresence (Seikagaku Corp.) or CSase B isolated from Flavobacterium heparinum (Iduron, Manchester, GB) in a final volume of 100 µL 50 mM Tris/HCl, 30 mM NaAcetate, pH 8.0, and 50 mM Tris/HCl, pH 7.5, 4 mM CaCl2, respectively, at 37°C for 3 h. Combined digestion with CSase ACII and CSase B yielded identical results in either 20 mM Tris/Acetate buffer, pH 8.0, or 50 mM Tris/HCl, pH 7.5, 4 mM CaCl2 at 37°C for 3 h. Reactions were terminated by boiling and samples prepared for disaccharide analysis and separation by TBE-PAGE.
Disaccharide analysis
CS and HS disaccharide analysis after either chondroitin lyase or heparin lyase digestion, respectively, was performed by RPIP-HPLC as described (Ledin et al. 2004). Signals were quantified against known amounts of commercially available, standard disaccharides analyzed in parallel runs. Disaccharide standards (Δ4,5-unsaturated CS disaccharides [1 nonsulfated, 3 mono-O-sulfated, 3 di-O-sulfated and 1 tri-O-sulfated disaccharide as indicated in Figure 2] and Δ4,5-unsaturated HS disaccharides [1 nonsulfated, 3 monosulfated, 3 disulfated and 1 trisulfated]) were obtained from Iduron and Sigma, respectively.
Mass spectrometry
CSase ABC cleavage products were analyzed for di- and oligosaccharides by SEC liquid chromatography/mass spectrometry as reported previously (Shi and Zaia 2009; Staples et al. 2010). In short, gel-purified CS/DS (∼3 μg) were digested with CSase ABC and separated by a Superdex Peptide column (3.2 mm × 300 mm) (GE Healthcare Biosciences) in 12.5 mM formic acid, pH 4.4 (adjusted by ammonium hydroxide), 10% acetonitrile, at isocratic flow (16 μL/min) delivered by a Waters Acquity UPLC system. The effluent was coupled to an Applied Biosystem Sciex QSTAR mass spectrometer through a TurboIonSpray interface operating in negative polarity mode.
Chemical treatment of GAGs
N-sulfated glucosamine residues in HS/heparin are sensitive to cleavage by nitrous acid at pH 1.5 (Shively and Conrad 1976). To assess the presence of these GAGs, brittlestar GAGs (1 μg) along with control HS and heparin were dissolved in 200 μL of freshly prepared barium nitrite (500 mM of nitrite), adjusted to pH 1.5 with 1 M sulfuric acid and incubated at RT for 10 min. Reaction mixtures were neutralized with sodium carbonate (2 M) and precipitated by ethanol/NaAcetate. Samples were dissolved in 200 μL H2O and desalted. Effluent fractions from the PD-MiniTrap™ G-25 columns between 0.6 and 1.4 mL were collected. The resulting cleavage products were assessed by TBE-PAGE as described in the section ‘Tris-borate-EDTA-polyacrylamide gel electrophoresis’. For control purposes, an aliquot of brittlestar GAGs was treated for the same period with neutralized deamination reagent and subjected to desalting.
Brittlestar GAGs were tested for the presence of fucose modification by mild acid treatment as described (Mourão et al. 1996) with slight modifications. Briefly, 20 μg isolated GAG was subjected to mild acid hydrolysis in 100 μL of 75 mM H2SO4 maintained at 96°C for a period of 30 min. Hydrolyzates were neutralized to pH 7.0 by addition of ice cold 0.1 M NaOH and desalted on PD-MiniTrap™ G-25 columns. Desalted samples and control CSD from shark cartilage, either untreated or digested with CSase ABC, were analyzed by RPIP-HPLC and by TBE-PAGE (described in the section ‘Tris-borate-EDTA-polyacrylamide gel electrophoresis’).
Tris-borate-EDTA-polyacrylamide gel electrophoresis
Desalted brittlestar GAGs were separated by TBE-PAGE on a 15% native polyacrylamide gel and stained with ammoniacal silver after prestaining with Alcian blue as described (Pelkonen et al. 1988; Lyon and Gallagher 1990). Heparin from bovine lung (Lindahl et al. 1965), HS from swine intestine (a gift from G. van Dedem Diosynth, Oss, The Netherlands), CSA from bovine trachea and CSB from porcine intestinal mucosa (Sigma-Aldrich), CSD from shark cartilage and CSE from squid cartilage (Seikagaku Corp.) and size-defined heparin oligosaccharides (Spillmann et al. 1998) were used for comparison. Stained gels were scanned for documentation.
Brittlestar GAGs were purified from nonfixed polyacrylamide gels by excising the CSase ABC-sensitive band by comparison with an Alcian/silver-stained lane from the same gel and re-swollen back to identical size in H2O. Thereafter, the sliced gel was extracted in water overnight. The gel extract was lyophilized, reconstituted in H2O and desalted by dialysis against H2O (molecular weight cut-off 3000 Da). An aliquot of the PAGE-purified GAG was tested for purity by control treatment with CSase ABC and renewed separation by TBE-PAGE. PAGE-purified GAG was further used in all interaction and cell stimulation assays (described in later sections).
Gel mobility shift assay
Protein-GAG interactions were tested by incubating PAGE-purified brittlestar GAGs (0.5 μg GAG [by colorimetric hexuronic acid determination]) with increasing amounts of human FGFs 1 and 2 (PeproTech, Inc., NJ), mouse vascular endothelial growth factor A165 or a splice variant devoid of HS binding (VEGF-A165 and 165b, a kind gift of Kurt Ballmer-Hofer, Paul Scherrer Institute, Switzerland) (Cébe Suarez et al. 2006), AT, PF4 and bovine serum albumin (BSA) (from Sigma) (0.05–5 μg) in 15 μL of Tris-buffered saline (TBS: 50 mM Tris/HCl, pH 7.0, 150 mM NaCl) at RT for 1 h. Thereafter, samples were separated by TBE-PAGE.
Cell activation assay
Chinese hamster ovary cells pgsA-745 (CHO 745) deficient in endogenous HS and CS synthesis due to the absence of xylosyltransferase were generously supplied by J. Esko (University of California, San Diego, La Jolla, CA) (Esko et al. 1985). These cells were activated with FGF2 as described (Jastrebova et al. 2010). CHO 745 cells (5 × 104) were cultured in 6-well plates (Nunc) for 48 h in Ham's F-12 medium containing 10% fetal bovine serum (Invitrogen, Carlstadt, CA), penicillin G (0.6%) and streptomycin sulfate (0.5%) (SVA, Uppsala, Sweden) and then starved in serum-free medium for 24 h. Thereafter, cells were incubated for 30 min with FGF2 (10 ng/mL), either alone or in combination with either heparin (5 ng/mL), CSA (5 ng/mL) or PAGE-purified CS/DS extracted from brittlestars (5 ng/mL) with or without previous CSase ABC digestion. FGF2 and GAGs were mixed just before application to the cells. Cell stimulation was stopped after 30 min and cells extracted as described earlier (Jastrebova et al. 2010). Protein concentrations of the lysates were determined by the bicinchoninic acid method (Wiechelman et al. 1988) and equivalent amounts (10 μg total protein) of different samples were separated by 8% sodium dodecyl sulfate (SDS)–PAGE (Laemmli 1970). After separation, proteins were transferred to Immobilon-FL membranes (Millipore, Solna, Sweden) as described (Jastrebova et al. 2010). Membranes were blocked at RT for 90 min using Odyssey blocking buffer (diluted 1:1 in PBS) and were then incubated at 4°C overnight with primary antibodies, rabbit polyclonal antibody (Cell Signaling, Danvers, MA) against phosphorylated ERK1/2 (Thr202/Thr204) (pERK) and monoclonal mouse antibody (Cell Signaling) against total ERK, both diluted 1:1000 in Odyssey blocking buffer including 0.1% Tween 20. The membranes were washed with TBS containing Tween (TBST: 50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 0.1% Tween 20) followed by incubation in the dark for 1 h with secondary IRDye®-labeled goat anti-rabbit immunoglobulin G (IgG) (excitation—683 nm, emission—710 nm) and goat anti-mouse IgG (excitation—778 nm, emission—795 nm) (1:10,000 dilution) antibodies (Li-Cor Biosciences, Cambridge, UK), respectively, in Odyssey blocking buffer containing 0.1% Tween 20 and 0.02% SDS. The membranes were washed with 2 changes of TBST and 2 changes of TBS and then scanned using an Odyssey-infrared scanner (Li-Cor Biosciences) at 700 and 800 nm. Band intensities were quantified using the Image J software. Ratios of phosphorylated ERK/total ERK were calculated and plotted, the signal observed after incubation with FGF alone for 30 min being set an arbitrary value of 1 to relate different experiments to one another. Student's “t-test” was used to calculate statistical significance with P < 0.05 using the Graphpad prism software.
Supplementary data
Supplementary data for this article are available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported by grants from the Swedish Cancer Foundation and the Foundation for Proteoglycan Research at Uppsala University to D.S.; S.T.D. is funded by the Linnaeus Centre for Marine Evolutionary Biology at the University of Gothenburg (http://www.cemeb.science.gu.se/) and supported by a Linnaeus-grant from the Swedish Research Councils VR and Formas. X.S. and J.Z. are funded by National Institutes of Health (grant numbers P41GM104603 and R01HL098950).
Conflict of interest
None declared.
Abbreviations
AT, antithrombin; BSA, bovine serum albumin; CHO, Chinese hamster ovary; CS, chondroitin sulfate; CSase, chondroitinase; DEAE, diethylaminoethyl; DS, dermatan sulfate; EDTA, ethylenediaminetetraacetic acid; ERK, extracellular signal-regulated kinase; FGF2, fibroblast growth factor 2; GAGs, glycosaminoglycans; GalNAc, N-acetyl galactosamine; GlcA, glucuronate; HexA, hexuronate; HPLC, high pressure (or high performance) liquid chromatography; HS, heparan sulfate; IdoA, iduronate; IgG, immunoglobulin G; MS, mass spectrometry; PAGE, polyacrylamide gel electrophoresis; pERK, phosphoERK; PF4, platelet factor 4; PF4, platelet factor 4; RPIP, reverse-phase ion-pairing; SDS, sodium dodecyl sulfate; SEC, size exclusion column; TBE, Tris-borate-EDTA; TBS, Tris-buffered saline; TBST, TBS containing Tween; VEGF, vascular endothelial growth factor.
Supplementary Material
Acknowledgements
We are grateful to Odd Lindahl for the help with collection of the mixed brittlestar population. We thank J. Esko for the CHO745 cells and Kurt Ballmer-Hofer for VEGFA.
References
- Bannister R, Mcgonnell IM, Graham A, Thorndyke MC, Beesley PW. Coelomic expression of a novel bone morphogenetic protein in regenerating arms of the brittle star Amphiura filiformis. Dev Genes Evol. 2008;218:33–38. doi: 10.1007/s00427-007-0193-9. [DOI] [PubMed] [Google Scholar]
- Bao X, Nishimura S, Mikami T, Yamada S, Itoh N, Sugahara K. Chondroitin sulfate/dermatan sulfate hybrid chains from embryonic pig brain, which contain a higher proportion of L-iduronic acid than those from adult pig brain, exhibit neuritogenic and growth factor binding activities. J Biol Chem. 2004;279:9765–9776. doi: 10.1074/jbc.M310877200. [DOI] [PubMed] [Google Scholar]
- Bergeron K-F, Xu X, Brandhorst BP. Oral-aboral patterning and gastrulation of sea urchin embryos depend on sulfated glycosaminoglycans. Mech Dev. 2011;128:71–89. doi: 10.1016/j.mod.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Burns G, Ortega-Martinez O, Thorndyke MC, Peck LS, Dupont S, Clark MS. Dynamic gene expression profiles during arm regneration in the brittle star Amphiura filiformis. J Exp Mar Biol Ecol. 2011;407:315–322. [Google Scholar]
- Cassaro CM, Dietrich CP. Distribution of sulfated mucopolysaccharides in invertebrates. J Biol Chem. 1977;252:2254–2261. [PubMed] [Google Scholar]
- Casu B. Structural features and binding properties of chondroitin sulfates, dermatan sulfate, and heparan sulfate. Semin Thromb Hemost. 1991;17:9–14. [PubMed] [Google Scholar]
- Casu B, Lindahl U. Structure and biological interactions of heparin and heparan sulfate. Adv Carbohyd Chem Biochem. 2001;57:159–206. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]
- Cébe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman MK, Slahetka MF, Hittner DM, Kim J, Forino M, Gadelrab G. Polyacrylamide-gel electrophoresis and Alcian blue staining of sulphated glycosaminoglycan oligosaccharides. Biochem J. 1984;221:707–716. doi: 10.1042/bj2210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa SS, Umehara Y, Higashiyama S, Itoh N, Sugahara K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors. Implications as a physiological binding partner in the brain and other tissues. J Biol Chem. 2002;277:43707–43716. doi: 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- Dische Z, Shettles LB. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J Biol Chem. 1948;175:595–603. [PubMed] [Google Scholar]
- Dupont S, Thorndyke MC. Bridging the regeneration gap: Insights from echinoderm models. Nat Rev Genetics. 2007;8:1923. [Google Scholar]
- Erlinger R, Welsch U, Scott JE. Ultrastructural and biochemical observations on proteoglycans and collagen in the mutable connective tissue of the feather star Antedon bifida (Echinodermata, Crinoidea) J Anat. 1993;183:1–11. [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi O, Sugiura K, Kawai N. Glucose branches in chondroitin sulfates from squid cartilage. J Biol Chem. 1977;252:4570–4576. [PubMed] [Google Scholar]
- Hampson IN, Gallagher JT. Separation of radiolabelled glycosamionoglycan oligosaccharides by polyacrylamide-gel electrophoresis. Biochem J. 1984;221:697–705. doi: 10.1042/bj2210697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama K, Okada S. Crystallization and some properties of chondroitinase from Arthrobacter aurescens. J Biol Chem. 1975;250:1824–1828. [PubMed] [Google Scholar]
- Jastrebova N, Vanwildemeersch M, Lindahl U, Spillmann D. Heparan sulfate domain organization and sulfation modulate FGF2 induced cell signaling. J Biol Chem. 2010;285:26842–26851. doi: 10.1074/jbc.M109.093542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Yamada S, Haslam S, Morris H, Dell A, Sugahara K. Novel tetrasaccharides isolated from squid cartilage chondroitin sulfate E contain unusual sulfated disaccharide units GlcA(3-O-sulfate)beta1–3GalNAc(6-O-sulfate) or GlcA(3-O-sulfate)beta1–3GalNAc. J Biol Chem. 1997;272:19656–19665. doi: 10.1074/jbc.272.32.19656. [DOI] [PubMed] [Google Scholar]
- Kinoshita-Toyoda A, Yamada S, Haslam SM, Khoo KH, Sugiura K, Morris HR, Dell A, Sugahara K. Structural determination of five novel tetrasaccharides containing 3-O-sulfated d-glucuronic acid and two rare oligosaccharides containing a beta-d-glucose branch isolated from squid cartilage chondroitin sulfate E. Biochemistry. 2004;43:11063–11074. doi: 10.1021/bi049622d. [DOI] [PubMed] [Google Scholar]
- Kozlowski EO, Gomes A, Sobral Silva C, Sa Pareira M, De Vilela Silva ACES, Pavao MSG. Structure and biological activities of glycosaminoglycan analogs from marine invertebrates: New therapeutic agents? In: Pavao MSG, editor. Glycans in Diseases and Therapeutics, Biology of Extracellular Matrix. Berlin,Heidelberg: Springer; 2011. pp. 159–184. [Google Scholar]
- Kreuger J, Jemth P, Sanders-Lindberg E, Eliahu L, Ron D, Basilico C, Salmivirta M, Lindahl U. Fibroblast growth factors share binding sites in heparan sulfate. Biochem J. 2005;389:145–150. doi: 10.1042/BJ20042129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledin J, Staatz W, Li JP, Götte M, Selleck S, Kjellén L, Spillmann D. Heparan sulfate structure in mice with genetically modified heparan sulfate production. J Biol Chem. 2004;279:42732–42741. doi: 10.1074/jbc.M405382200. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Cifonelli JA, Lindahl B, Rodén L. The role of serine in the linkage of heparin to protein. J Biol Chem. 1965;240:2817–2820. [PubMed] [Google Scholar]
- Lindahl U, Li J-P. Interactions between heparan sulfate and proteins-design and functional implications. Int Rev Cell Mol Biol. 2009;276:105–159. doi: 10.1016/S1937-6448(09)76003-4. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ, Avci FY, Toida T, Kim YS, Cygler M. CS-lyases: Structure, activity, and applications in analysis and treatment of diseases. Adv Pharmacol. 2006;53:187–215. doi: 10.1016/S1054-3589(05)53009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovtrup-Rein H, Lovtrup S. Sulfated polysaccharides and cell differentiation in the sea urchin embryo. Exp Cell Biol. 1984;52:383–388. doi: 10.1159/000163285. [DOI] [PubMed] [Google Scholar]
- Lyon M, Gallagher JT. A general method for the detection and mapping of submicrogram quantities of glycosaminoglycan oligosaccharides on polyacrylamide gels by sequential staining with azure A and ammoniacal silver. Anal Biochem. 1990;185:63–70. doi: 10.1016/0003-2697(90)90255-8. [DOI] [PubMed] [Google Scholar]
- Malavaki C, Mizumoto S, Karamanos NK, Sugahara K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect Tissue Res. 2008;49:133–139. doi: 10.1080/03008200802148546. [DOI] [PubMed] [Google Scholar]
- Malmström A, Bartolini B, Thelin MA, Pacheco B, Maccarana M. Iduronic acid in chondroitin/dermatan sulfate: Biosynthesis and biological function. J Histochem Cytochem. 2012;60:916–925. doi: 10.1369/0022155412459857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros GF, Mendes A, Castro RAB, Ba EC, Nader HB, Dietrich CP. Distribution of sulfated glycosaminoglycans in the animal kingdom: Widespread occurrence of heparin-like compounds in invertebrates. Biochim Biophys Acta. 2000;1475:287–294. doi: 10.1016/s0304-4165(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Michel G, Pojasek K, Li Y, Sulea T, Linhardt RJ, Raman R, Prabhakar V, Sasisekharan R, Cygler M. The structure of chondroitin B lyase complexed with glycosaminoglycan oligosaccharides unravels a calcium-dependent catalytic machinery. J Biol Chem. 2004;279:32882–32896. doi: 10.1074/jbc.M403421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misevic GN. Immunoblotting and immunobinding of acidic polysaccharides separated by gel electrophoresis. Methods Enzymol. 1989;179:95–104. doi: 10.1016/0076-6879(89)79117-5. [DOI] [PubMed] [Google Scholar]
- Mourão PAS, Pereira MS, Pavão MSG, Mulloy B, Tollefsen DM, Mowinckel M-C, Abildgaard U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from Echinoderm. Sulfated fucose branches on polysaccharide account for its high anticoagulant action. J Biol Chem. 1996;271:23973–23984. doi: 10.1074/jbc.271.39.23973. [DOI] [PubMed] [Google Scholar]
- Mulloy B, Mourão PAS, Gray E. Structure/function studies of anticoagulant sulphated polysaccharides using NMR. J Biotechnol. 2000;77:123–135. doi: 10.1016/s0168-1656(99)00211-4. [DOI] [PubMed] [Google Scholar]
- Nader HB, Ferreira TM, Paiva JF, Medeiros MG, Jeronimo SM, Paiva VM, Dietrich CP. Isolation and structural studies of heparan sulfates and chondroitin sulfates from three species of molluscs. J Biol Chem. 1984;259:1431–1435. [PubMed] [Google Scholar]
- Nandini CD, Itoh N, Sugahara K. Novel 70-kDa chondroitin sulfate/dermatan sulfate hybrid chains with a unique heterogeneous sulfation pattern from shark skin, which exhibit neuritogenic activity and binding activities for growth factors and neurotrophic factors. J Biol Chem. 2005;280:4058–4069. doi: 10.1074/jbc.M412074200. [DOI] [PubMed] [Google Scholar]
- Nandini CD, Mikami T, Ohta M, Itoh N, Akiyama-Nambu F, Sugahara K. Structural and functional characterization of oversulfated chondroitin sulfate/dermatan sulfate hybrid chains from the notochord of hagfish. Neuritogenic and binding activities for growth factors and neurotrophic factors. J Biol Chem. 2004;279:50799–50809. doi: 10.1074/jbc.M404746200. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGFS, heparan sulfate and FGFRs: Complex interactions essential for development. Bioessays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pavão MSG, Aiello KRM, Werneck CC, Silva LCF, Valente A-P, Mulloy B, Colwell NS, Tollefsen DM, Mourão PAS. High sulfated dermatan sulfate from ascidians. Structure versus anticoagulant activity of these glycosaminoglycans. J Biol Chem. 1998;273:27848–27857. doi: 10.1074/jbc.273.43.27848. [DOI] [PubMed] [Google Scholar]
- Pelkonen S, Häyrinen J, Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bact. 1988;170:2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem. 1998;273:28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- Petersen F, Brandt E, Lindahl U, Spillmann D. Characterization of a neutrophil cell surface glycosaminoglycan that mediates binding of platelet factor 4. J Biol Chem. 1999;274:12376–12382. doi: 10.1074/jbc.274.18.12376. [DOI] [PubMed] [Google Scholar]
- Pye DA, Vives RR, Turnbull JE, Hyde P, Gallagher JT. Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J Biol Chem. 1998;273:22936–22942. doi: 10.1074/jbc.273.36.22936. [DOI] [PubMed] [Google Scholar]
- Reiland J, Rapraeger AC. Heparan sulfate proteoglycan and FGF receptor target basic FGF to different intracellular destinations. J Cell Sci. 1993;105:1085–1093. doi: 10.1242/jcs.105.4.1085. [DOI] [PubMed] [Google Scholar]
- Sampaio LO, Nader HB. Emergence of structural characteristics of chondroitin sulfates in the animal kingdom. Adv Pharmacol. 2006;53:233–251. doi: 10.1016/S1054-3589(05)53011-4. [DOI] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zaia J. Organ-specific heparan sulfate structural phenotypes. J Biol Chem. 2009;284:11806–11814. doi: 10.1074/jbc.M809637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively JE, Conrad HE. Formation of anhydrosugars in chemical depolymerization of heparin. Biochemistry. 1976;15:3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Sperinde GV, Nugent MA. Heparan sulfate proteoglycans control intracellular processing of bFGF in vascular smooth muscle cells. Biochemistry. 1998;37:13153–13164. doi: 10.1021/bi980600z. [DOI] [PubMed] [Google Scholar]
- Spillmann D, Witt D, Lindahl U. Defining the interleukin-8-binding domain of heparan sulfate. J Biol Chem. 1998;273:15487–15493. doi: 10.1074/jbc.273.25.15487. [DOI] [PubMed] [Google Scholar]
- Staples GO, Shi Z, Zaia J. Extended N-sulfated domains reside at the nonreducing end of heparan sulfate chains. J Biol Chem. 2010;285:18336–18343. doi: 10.1074/jbc.M110.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K, Tanaka Y, Yamada S. Preparation of a series of sulfated tetrasaccharides from shark cartilage chondroitin sulfate D using testicular hyaluronidase and structure determination by 500 MHz 1H NMR spectroscopy. Glycoconj J. 1996;13:609–619. doi: 10.1007/BF00731449. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Yamada S. Structure and function of oversulfated chrondroitin sulfate variants: Unique sulfation patterns and neuroregulatory activities. Trends Glycosci Glycotechnol. 2000;12:321–349. [Google Scholar]
- Tapon-Bretaudière J, Chabut D, Zierer M, Matou S, Helley D, Bros A, Mourão PAS, Fischer AM. A fucosylated chondroitin sulfate from echinoderm modulates in vitro fibroblast growth factor 2-dependent angiogenesis. Mol Cancer Res. 2002;1:96–102. [PubMed] [Google Scholar]
- Thorndyke MC, Candia Carnevali MD. Regeneration neurohormones and growth factors in echinoderms. Can J Zool. 2001;79:1171–1208. [Google Scholar]
- Trowbridge JM, Gallo RL. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology. 2002;12:117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- Vieira RP, Mourão PAS. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J Biol Chem. 1988;263:18176–18183. [PubMed] [Google Scholar]
- Volpi N, Maccari F. Structural characterization and antithrombin activity of dermatan sulfate purified from marine clam Scapharca inaequivalvis. Glycobiology. 2009;19:356–367. doi: 10.1093/glycob/cwn140. [DOI] [PubMed] [Google Scholar]
- Wiechelman KJ, Braun RD, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal Biochem. 1988;175:231–237. doi: 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- Wilkie IC. Autotomy as a prelude to regeneration in echinoderms. Microsc Res Tech. 2001;55:369–396. doi: 10.1002/jemt.1185. [DOI] [PubMed] [Google Scholar]
- Yamada S, Sugahara K, Özbek S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun Integr Biol. 2011;4:150–158. doi: 10.4161/cib.4.2.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Van Die I, Van Den Eijnden DH, Yokota A, Kitagawa H, Sugahara K. Demonstration of glycosaminoglycans in Caenorhabditis elegans. FEBS Lett. 1999;459:327–331. doi: 10.1016/s0014-5793(99)01286-7. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968;243:1523–1535. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.