Abstract
Diisocyanates (dNCOs) are highly reactive low molecular weight chemicals commonly used in the manufacturing industry. Occupational exposures to dNCOs have been shown to elicit allergic sensitization and occupational asthma. Among the most commonly used dNCOs in industry are the aromatic dNCOs, toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI). This study aimed to develop enzyme linked immunosorbent assays (ELISA) utilizing aromatic dNCO-specific monoclonal antibodies (mAbs) for the detection of aromatic dNCO adducts. Two sandwich ELISAs were developed. The first sandwich ELISA utilized mAb 60G2 along with an anti-human serum albumin (HSA) polyclonal antibody. This assay detected MDI-, 2,4- and 2,6-TDI-HSA adducts with limits of detection (LOD) of 2.67, <0.10, and 1.70 ng/mL, respectively. When spiked into human serum, the LOD of this ELISA increased to 34.37, 7.64 and 24.06 ng/mL, respectively. The second ELISA utilized mAbs 62G5 and 60G2 for capture and detection. This assay was capable of detecting 2,4- and 2,6-TDI-HSA adducts with LODs of <4.90 and 26.92 ng/mL, respectively, and when spiked in human serum, <4.90 and 95.93 ng/mL, respectively. This 62G5-60G2 sandwich assay was also able to detect dNCO adducted transferrin, hemoglobin, keratin and actin, but with less sensitivity than dNCO-HSA. The results of this study demonstrate potential application of these ELISAs in the identification and characterization of aromatic dNCO adducts as well as in biomonitoring occupational and environmental dNCO exposures.
Keywords: Diisocyanate, Monoclonal antibody, Immunoassay, Occupational asthma
1. Introduction
Diisocyantes (dNCOs) are highly reactive, low molecular weight chemicals used in the manufacturing sector to produce polyurethane products, paints and glues. The aromatic dNCOs, methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI), are among the most common used in manufacturing (Allport et al., 2003). Workers handling these products without appropriate personal protective equipment may be at increased risk of developing occupational allergy and asthma. The Occupational Safety and Health Administration has recently initiated a National Emphasis Program to help protect workers from these adverse health effects associated with occupational exposures to isocyanates.
Current dNCO biological monitoring methods include the measurement of dNCO-specific antibodies in the serum and some dNCO-derived biomarkers in the blood and urine. Among these biomarkers, dNCO-derived diamines from hydrolyzed plasma and urine are commonly screened in biomonitoring studies (Gledhill et al., 2005; Budnik et al., 2011). However, detection of dNCO hydrolysis products may be limited by several confounding variables, including the lack of a standardized method for hydrolysis and the requirement for specialized instrumentation. This method also lacks specificity in that it does not distinguish between isocyanate exposure and direct exposure to diamines. Consequently, there is a need for alternative methods for the detection of dNCO exposures in the occupational environment.
dNCO haptenation to a variety human proteins following exposure has been hypothesized as a critical step in the development of dNCO sensitization and asthma. Efforts toward developing ELISA-based methods to detect dNCO-haptened proteins have remained limited due to the availability of monoclonal and polyclonal antibodies. Lemus and colleagues developed a sandwich ELISA capable of detecting as low as 3 ng of 1,6-hexamethylene diisocyanate (HDI) adducted human serum albumin (HSA) (Lemus et al., 2001). To our knowledge, no other ELISAs have been developed to assess proteins adducted by either MDI or TDI. This study aimed to develop sandwich ELISAs utilizing a set of recently produced TDI-specific monoclonal antibodies (mAbs) (Ruwona et al., 2011) for application in the biological monitoring of dNCO adducts.
2. Materials and methods
2.1. Conjugation of dNCOs to proteins
All chemicals and proteins used were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise noted. dNCOs, including 4,4&-MDI (CAS 101-68-8), 2,4-TDI (CAS 584-84-9), 2,6-TDI (CAS 91-06-7), and 1,6-HDI (CAS 822-06-0) were conjugated to 0.5 mg/mL HSA (CAS 70024-90-7), human transferrin (CAS 11096-37-0), human hemoglobin (CAS 9008-02-0), keratin from human epidermis (CAS 68238-35-7) and actin from human platelet (Cytoskeleton, Inc, Denver, CO) in 0.01 M phosphate buffered saline (PBS; pH 7.4). dNCO-protein adducts were prepared by adding 10 μL of each freshly prepared dNCO/acetone solution per 1 mL of 0.5 mg/mL protein solution drop wise while vortexing to obtain final molar ratios ranging from 5:1 to 40:1 dNCO:protein. The conjugates were then incubated while vortexing for 1 h at room temperature (RT). After incubation, conjugates were dialyzed using 12-14,000 MWCO dialysis membrane (Spectrum® Laboratories, Inc., Rancho Dominguez, CA) to remove residual hydrolyzed and polymerized dNCO. Samples were stored at 4°C until use.
2.2. dNCO-HSA-specific Sandwich ELISA
A sandwich ELISA specific for aromatic dNCO-HSA was developed using the aromatic dNCO-specific mAb 60G2 (IgG1). Briefly, Corning high protein binding 96-well plates (Corning, NY) were coated with 4 μg/mL AffiniPure goat anti-mouse IgG Fc, subclass 1 specific antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) in 0.1 M sodium carbonate buffer, pH 9.6 overnight at 4°C. Following overnight incubation, the wells were washed three times with PBS containing 0.05% Tween 20 (PBST) and incubated on a shaker for 1 h at RT with 2 μg/mL mAb 60G2. The wells were blocked with 200 μL/well 3% non-fat dry milk powder in PBST (SMPBST) for 1 h at 37°C. Duplicate wells containing 1 μg/mL dNCO-HSA conjugates at each molar ratio, 5:1 to 40:1, were 2 fold serially diluted in SMPBST and incubated for 1 h at 37°C. Wells were washed and incubated for 1 h at 37°C with biotin-conjugated affinity purified rabbit anti-HSA antibody (Rockland, Gilbertsville, PA) diluted 1/5000 (v/v) in SMPBST. Following incubation, the wells were washed and incubated for 1 h at 37°C with alkaline phosphatase (AP)-conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc.) diluted 1/5000 (v/v) in SMPBST. Binding of dNCO-HSA adducts was quantified using 0.5 mg/mL p-nitrophenyl phosphate in AP substrate. The optical density was determined spectrophotometrically at 405 nm using a SpectraMax M4 microplate reader (Molecular Devices, Sunnyvale, CA). The limit of detection (LOD) and limit of quantification (LOQ) for each dNCO-HSA adduct were defined as 3 and 10 times the standard deviation of 40 replicate HSA (0.5 μg/mL) control wells. This assay was additionally used to detect dNCO adducted HSA in human serum by diluting the dNCO-HSA in a 1/20 dilution of pooled human serum (Sigma Aldrich) in SMPBST.
2.3. 62G5-60G2 Sandwich ELISA
A secondary sandwich assay was developed utilizing aromatic dNCO-specific mAb 60G2 (IgG1) and TDI-specific mAb 62G5 (IgG2a) (Ruwona et al., 2011). Briefly, Corning high protein binding 96-well plates (Corning, NY) were coated with 4 μg/mL AffiniPure goat anti-mouse IgG Fc, subclass 2a specific antibody (Jackson ImmunoResearch Laboratories Inc.) in 0.1 M sodium carbonate buffer, pH 9.6, overnight at 4°C. Following incubation, the wells were washed three times with PBST and incubated on a shaker for 1 h at RT with 2 μg/mL mAb 62G5. The wells were blocked with 200 μL/well SMPBST for 1 h at 37°C. Duplicate wells containing 5 μg/mL dNCO-HSA conjugates at each molar ratio were 2 fold serially diluted in SMPBST and incubated for 1 h at 37°C. Other dNCO-protein adducts, including transferrin, hemoglobin, keratin, and actin, were tested starting at a concentration of 25 μg/mL. Wells were washed and incubated for 1 h at 37°C with 2 μg/mL mAb 60G2. Wells were washed and incubated for 1 h at 37°C with biotin-SP-conjugated AffiniPure goat anti-mouse IgG Fc, subclass 1 specific antibody (Jackson ImmunoResearch Laboratories Inc.) diluted 1/5000 (v/v) in SMPBST. Following incubation, the wells were washed and incubated for 1 h at 37°C with AP-conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc.) diluted 1/5000 (v/v) in SMPBST. Binding to each dNCO-HSA adduct was quantified using 0.5 mg/mL p-nitrophenyl phosphate in AP substrate. The optical density was determined spectrophotometrically as previously described. The LOD and LOQ for each dNCO were defined as 3 and 10 times the standard deviation of 40 replicate HSA or 16 replicate transferrin, hemoglobin, keratin or actin (0.5 μg/mL) control wells. This assay was additionally used to detect dNCO adducted HSA in human serum by diluting the dNCO-HSA in a 1/20 dilution of pooled human serum (Sigma Aldrich) in SMPBST.
3. Results and Discussion
3.1. Development of a Sandwich ELISA to detect dNCO adducted proteins
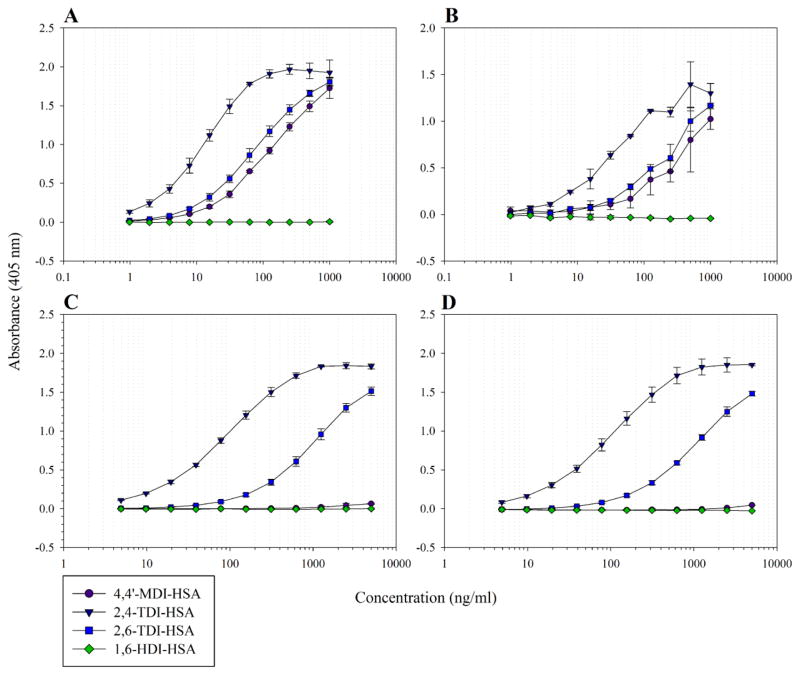
Two different sandwich ELISAs were developed to detect aromatic dNCO adducts. The first ELISA utilized the IgG1 mAb 60G2 along with an HSA-specific polyclonal antibody. This assay was capable of detecting aromatic MDI, 2,4-TDI and 2,6-TDI adducted HSA in a concentration dependent manner (Fig. 1A). Aliphatic HDI was not detected at any concentration (Fig. 1A). At a 40:1 dNCO to HSA molar ratio, the LOD for MDI, 2,4-TDI and 2,6-TDI adducted HSA was 2.67, <0.10, and 1.70 ng/mL, respectively, while the LOQ was 12.44, <0.10, and 5.34 ng/mL, respectively (Table 1). As the molar ratio of dNCO:HSA decreased, the respective LODs and LOQs increased. Multiple factors may contribute to the lower LODs observed with increasing dNCO:protein ratios. dNCOs can bind to multiple sites of a protein through intra- and intermolecular protein cross-linking. A recent study has shown that as the molar dNCO:protein ratio is increased, the percent and number of dNCO-protein adducts and the amount of cross-linking all increase (Mhike et al., 2013).
Figure 1.
Sandwich ELISA detection of aromatic dNCO adducts. (A) Detection of dNCO-HSA adducts using the 60G2 dNCO-HSA-specific sandwich ELISA, (B) detection of dNCO-HSA supplemented in pooled human serum using the 60G2 dNCO-HSA-specific sandwich ELISA, (C) detection of dNCO-HSA adducts using the 62G5-60G2 sandwich ELISA, (D) detection of dNCO-HSA supplemented in pooled human serum using the 62G5-60G2 sandwich ELISA. The results represent the mean OD405 values of each 40:1 hapten-protein combination corrected for background ± the standard deviation of replicate assays containing 2 ELISA well repeats. Background controls using 0.5 μg/mL each human protein were examined in parallel.
Table 1.
Comparison of two dNCO-specific sandwich ELISAs for the detection of aromatic dNCO-HSA (1 μg/mL).a
| dNCO adduct | 60G2 dNCO-HSA-Specific ELISA | 62G5-60G2 Sandwich ELISA | ||||
|---|---|---|---|---|---|---|
| OD (405 nm) | LOD (ng/ml) | LOQ (ng/ml) | OD (405 nm) | LOD (ng/ml) | LOQ (ng/ml) | |
| 4,4′-MDI-HSA | ||||||
| 40:1 | 0.82 ± 0.102 | 2.67 | 12.44 | 0.01 ± 0.008 | > 5000 | > 5000 |
| 20:1 | 0.41 ± 0.031 | 10.87 | 37.52 | ND | ND | ND |
| 10:1 | 0.11 ± 0.007 | 64.05 | 210.59 | ND | ND | ND |
| 5:1 | 0.03 ± 0.003 | 276.20 | 862.34 | ND | ND | ND |
|
| ||||||
| 2,4-TDI-HSA | ||||||
| 40:1 | 1.70 ± 0.004 | < 0.10 | < 0.10 | 1.56 ± 0.027 | < 4.90 | 7.02 |
| 20:1 | 1.10 ± 0.135 | 0.99 | 3.59 | 0.41 ± 0.012 | 33.77 | 117.22 |
| 10:1 | 0.50 ± 0.043 | 5.27 | 20.69 | 0.05 ± 0.003 | 313.25 | 939.16 |
| 5:1 | 0.20 ± 0.027 | 20.32 | 104.05 | ND | 2208.33 | > 5000 |
|
| ||||||
| 2,6-TDI-HSA | ||||||
| 40:1 | 1.20 ± 0.049 | 1.70 | 5.34 | 0.52 ± 0.048 | 26.92 | 96.46 |
| 20:1 | 0.34 ± 0.019 | 20.01 | 59.09 | 0.04 ± 0.006 | 334.25 | 960.16 |
| 10:1 | 0.03 ± 0.002 | 193.10 | 486.17 | 0.01 ± 0.006 | 750.62 | 2315.41 |
| 5:1 | 0.01 ± 0.005 | 730.51 | > 5000 | ND | ND | ND |
The results represent the mean OD405 values of each dNCO-HSA (1 μg/mL) corrected for background ± the standard deviation of 2 ELISA well repeats. Background controls using 0.5 μg/mL HSA were examined in parallel. Limits of detection (LOD) and quantification (LOQ) were determined by calculating the concentration of dNCO-HSA that corresponded to 3x and 10x the standard deviation of background control absorbance values, respectively. ND: not detected.
The second assay utilized two mAbs in a sandwich ELISA format and included IgG2a mAb 62G5 and the IgG1 mAb 60G2. In this ELISA, 2,4-TDI and 2,6-TDI, but not MDI or HDI, adducted HSA were detected in a concentration dependent manner (Fig. 1C). Loss of MDI adduct detection is due to the lack of cross-reactivity of the TDI-specific 62G5 mAb with MDI adducts. At a 40:1 dNCO-adduct molar ratio, the LODs for 2,4- and 2,6-TDI-HSA were <4.90 and 26.92 ng/mL, respectively, and the LOQs were 7.02 and 94.46 ng/mL, respectively (Table 1). As observed in the previously described sandwich ELISA, these limits increased as the molar ratio of dNCO to HSA decreased. While the LOD of this assay was higher than that of the first ELISA, this assay allows for the detection of other dNCO haptenated proteins; however, at least 2 bound dNCOs are theoretically required for the adduct to be detected.
Occupational exposures to dNCOs have resulted in haptenation of an array of host dermal and bronchial proteins. Along with the commonly investigated human proteins albumin and hemoglobin, other proteins, including; keratin 18, the 78-kD glucose-regulated protein, trans-1,2-dihyrobenzene-1,2-diol dehydrogenase, actin, and tubulin, have been identified as possible adducts (Lange et al., 1999; Wisnewski et al., 2000). In order to test the applicability of this assay for detecting human protein adducts, the 62G5-60G2 sandwich ELISA was utilized to test dNCO adducted transferrin, hemoglobin, keratin and actin. 2,4-TDI-transferrin, -keratin, and -actin were detected with an LOD of less than 49 ng/mL while 2,4-TDI-hemoglobin was additionally detected but with a higher LOD of 199.25 ng/mL (Table 2). 2,6-TDI-transferrin, hemoglobin, keratin and actin were also detected but had LODs that were generally higher than those reported for the 2,4-TDI adducts (<49, 408.65, 301.14, and 736.25, respectively) (Table 2). This result is not unexpected as the 2,6-TDI isomer is less reactive and may produce fewer and less complex adducts at molar equivalent binding ratios to 2,4-TDI.
Table 2.
Detection of different dNCO adducted human proteins.b
| dNCO adduct | 62G5-60G2 Sandwich ELISA | ||
|---|---|---|---|
| OD (405 nm) | LOD (ng/ml) | LOQ (ng/ml) | |
| 2,4-TDI- | |||
| Transferrin | 1.47 ± 0.053 | < 49.0 | < 49.0 |
| Hemoglobin | 0.11 ± 0.001 | 199.25 | 713.43 |
| Keratin | 0.77 ± 0.048 | < 49.0 | 114.15 |
| Actin | 1.65 ± 0.039 | < 49.0 | < 49.0 |
|
| |||
| 2,6-TDI- | |||
| Transferrin | 0.66 ± 0.010 | < 49.0 | 155.49 |
| Hemoglobin | 0.12 ± 0.007 | 408.65 | 1334.18 |
| Keratin | 0.29 ± 0.002 | 301.14 | 1078.47 |
| Actin | 0.08 ± 0.002 | 736.25 | 2073.06 |
The results represent the mean OD405 values of each 40:1 hapten-protein (3 μg/mL) corrected for background ± the standard deviation of replicate assays containing 2 ELISA well repeats. Background controls using 0.5 μg/mL each protein were examined in parallel. LOD and LOQ were determined by calculating the concentration of dNCO-protein that corresponded to 3x and 10x the standard deviation of background control absorbance values, respectively.
3.2. Detection of dNCO adducted HSA in human serum
The most commonly used biomonitoring methods utilize dNCO diamine hydrolysis products as biomarkers of occupational exposure in both plasma and urine. Levels of MDI-derived diamines have been shown to range from <0.1 to 45 ng/mL in hydrolyzed plasma (Sepai et al., 1995; Dalene et al., 1997). The diamines derived from 2,4 and 2-6-TDI have also been reported in hydrolyzed plasma within the range of <0.1 to 5.5 and <0.1 to 2.3 ng/mL, respectively (Dalene et al., 1997). However, dNCO diamine methods are time-consuming, labor intensive, lack a standardized hydrolysis method, are dependent on specialized analytical instrumentation, and are not specific to isocyanate exposure. To determine if sandwich ELISAs could provide an alternative biomonitoring approach, the ability to detect dNCO-HSA in human serum was additionally investigated. Pooled human serum obtained from Sigma Aldrich was diluted 1/20 in SMPBST, as this dilution was identified in previous studies to be optimal for reducing assay interference of serum proteins. The serum was then spiked with 40:1 dNCO-HSA and was assessed using both sandwich ELISA formats. The 62G5-60G2 sandwich assay detected both 2,4- and 2,6-TDI-HSA in a concentration-dependent manner similar to the original assay (Fig. 1D) with limits of detection of <4.90 and 95.93 ng/mL, respectively. The dNCO-HSA-specific assay utilizing 60G2 and anti-HSA polyclonal antibody was able to detect MDI, 2,4-TDI and 2,6-TDI in a concentration dependent manner (Fig. 1B) with limits of detection of 34.37, 7.64 and 24.06 ng/ml, respectively. These data demonstrate the potential utility of these mAbs in immunoassays for the biomonitoring of occupational and environmental dNCO exposures.
4. Conclusions
Two sandwich ELISAs utilizing aromatic dNCO-specific mAbs were developed to detect dNCO adducted human proteins. The sandwich ELISA utilizing both mAbs 62G5 and 60G2 could detect TDI adducted HSA as well as other adducted human proteins. To our knowledge, this is the first report of immunoassays that have the capability of detecting aromatic dNCO adducted human proteins. Due to the specificity and sensitivity of these ELISAs, they may have potential future application in the identification and characterization of aromatic dNCO adducts. The sandwich ELISAs reported in this study may also have potential utility in screening human samples, such as serum, for identifying aromatic dNCO adducts formed following occupational and environmental dNCO exposures.
Highlights.
Two sandwich ELISAs were developed to detect aromatic diisocyanate (dNCO) adducted human proteins using recently developed toluene diisocyanate (TDI)-specific monoclonal antibodies (mAb).
The mAb 60G2 and polyclonal anti-HSA sandwich ELISA could detect aromatic dNCO-HSA adducts with high specificity and sensitivity.
The sandwich ELISA utilizing mAbs 62G5 and 60G2 could detect TDI adducted HSA as well as other adducted human proteins.
Sandwich ELISAs were developed to detect aromatic dNCO adducted proteins.
dNCO-HSA adducts could be detected with high specificity and sensitivity.
TDI adducted HSA as well as other adducted human proteins could also be detected.
These ELISAs could be used to identify and characterize aromatic dNCO adducts.
The ELISAs may have application in biomonitoring occupational dNCO exposures.
Acknowledgments
The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. The authors declare no conflict of interest. This study was supported in part by an interagency agreement with NIEHS IAA# AES 12007-00100000.
Abbreviations
- dNCO
diisocyanate
- TDI
toluene diisocyanate
- MDI
methylene diphenyl diisocyanate
- HDI
hexamethylene diisocyanate
- ELISA
enzyme linked immunosorbent assay
- mAb
monoclonal antibody
- HSA
human serum albumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela R. Lemons, Email: wrw0@cdc.gov.
Toni A. Bledsoe, Email: tab4@cdc.gov.
Paul D. Siegel, Email: pds3@cdc.gov.
Donald H. Beezhold, Email: zec1@cdc.gov.
Brett J. Green, Email: dox6@cdc.gov.
References
- Allport DC, Gilbert DS, Outterside SM. A Source Book and Practical Guide. John Wiley & Sons, Ltd; West Sussex, England: 2003. MDI and TDI: Safety, Health and the Environment. [Google Scholar]
- Budnik LT, Nowak D, Merget R, Lemiere C, Baur X. Elimination kinetics of diisocyanates after specific inhalative challenges in humans: mass spectrometry analysis, as a basis for biomonitoring strategies. J Occup Med Toxicol. 2011;6:9. doi: 10.1186/1745-6673-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalene M, Skarping G, Lind P. Workers exposed to thermal degradation products of TDI- and MDI-based polyurethane: biomonitoring of 2,4-TDA, 2,6-TDA, and 4,4′-MDA in hydrolyzed urine and plasma. Am Ind Hyg Assoc J. 1997;58:587–91. doi: 10.1080/15428119791012522. [DOI] [PubMed] [Google Scholar]
- Gledhill A, Wake A, Hext P, Leibold E, Shiotsuka R. Absorption, distribution, metabolism and excretion of an inhalation dose of [14C] 4,4′-methylenediphenyl diisocyanate in the male rat. Xenobiotica. 2005;35:273–92. doi: 10.1080/00498250500057591. [DOI] [PubMed] [Google Scholar]
- Lange RW, Lantz RC, Stolz DB, Watkins SC, Sundareshan P, Lemus R, Karol MH. Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol Sci. 1999;50:64–71. doi: 10.1093/toxsci/50.1.64. [DOI] [PubMed] [Google Scholar]
- Lemus R, Lukinskeine L, Bier ME, Wisnewski AV, Redlich CA, Karol MH. Development of immunoassays for biomonitoring of hexamethylene diisocyanate exposure. Environ Health Perspect. 2001;109:1103–8. doi: 10.1289/ehp.011091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhike M, Chipinda I, Hettick JM, Simoyi RH, Lemons A, Green BJ, Siegel PD. Characterization of methylene diphenyl diisocyanate haptenated human serum albumin and hemoglobin. Anal Biochem. 2013;440:197–204. doi: 10.1016/j.ab.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwona TB, Johnson VJ, Hettick JM, Schmechel D, Beezhold D, Wang W, Simoyi RH, Siegel PD. Production, characterization and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J Immunol Methods. 2011;373:127–35. doi: 10.1016/j.jim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Sepai O, Henschler D, Sabbioni G. Albumin adducts, hemoglobin adducts and urinary metabolites in workers exposed to 4,4′-methylenediphenyl diisocyanate. Carcinogenesis. 1995;16:2583–7. doi: 10.1093/carcin/16.10.2583. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000;162:2330–6. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]