Abstract
BACKGROUND
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in premature neonates. The pathogenesis of NEC is characterized by an intestinal epithelial injury caused by perinatal insults, leading to the activation of the mucosal innate immune system and exacerbation of the epithelial barrier damage. Cytokines play an important role in mucosal immunity. Interleukin-10 (IL-10) is an anti-inflammatory cytokine that has been shown to play a role in epithelial integrity and modulation of the mucosal immune system. We hypothesized that IL-10 may protect against the development of experimental NEC by blunting the inflammatory response in the intestine.
METHODS
Wild-type and IL-10 −/− mice underwent a NEC-inducing regimen of formula feeding in combination with hypoxia and hypothermia (FF+HH). Integrity of the gut barrier was assessed through measurement of epithelial apoptosis, tight junction disruption, and inducible nitric oxide synthase. A total of 5 μg of exogenous IL-10 was administered intraperitoneally to IL-10−/−mouse pups before the initiation of FF+HH to test dependence of gene knockout phenotype on IL-10.
RESULTS
IL-10 −/− FF+HH showed more severe morphologic and histologic changes compared with controls as evidenced by increased epithelial apoptosis, decreased junctional adhesion molecule-1 localization, and increased intestinal inducible nitric oxide synthase expression. Administration of exogenous IL-10 alleviated the mucosal injury.
CONCLUSIONS
We conclude that IL-10 plays a protective role in the pathogenesis of NEC by attenuating the degree of intestinal inflammation.
Keywords: Necrotizing enterocolitis (NEC), Animal models of NEC, Apoptosis, IL-10, JAM-1, iNOS
Necrotizing enterocolitis (NEC) is the most devastating gastrointestinal disease affecting newborn infants. Although more than 90% of the infants affected by NEC are premature, some cases have been reported in full-term infants.1 The incidence of NEC continues to increase because the number of premature births has increased during the last decade and a half.2 Furthermore, recent advances in neonatal care have improved the survival of premature infants markedly. NEC is associated with a high mortality rate, which ranges from 10% to 50% according to different reports. However, in the group of infants with severe NEC, which is characterized by pan-intestinal involvement, the mortality rate approaches 100%.3,4 Although the pathogenesis of NEC remains elusive, the principal initiating events are believed to involve gut ischemia, formula feeding, and intestinal colonization with opportunistic pathogens. These perinatal insults compromise the integrity of the immature gut barrier, leading to bacterial translocation and activation of innate immune responses.5 NEC has been associated with increased levels of proinflammatory and anti-inflammatory cytokines in the inflamed intestine.6 Although proinflammatory cytokines tend to promote gut barrier failure, evidence suggests that anti-inflammatory cytokines such as interleukin-10 (IL-10) are likely to play a protective role by dampening the inflammatory responses. Indeed, IL-10–deficient mice develop intestinal inflammation as evidenced by the presence of colitis.7 By contrast, intraperitoneal injection of IL-10 in a mouse model of intestinal ischemia/reperfusion reduces local and systemic inflammation.8
In this study, we examined the role of IL-10 in the pathogenesis of experimental NEC. We show that levels of IL-10 increase in the serum of animals subjected to the NEC-inducing regimen of formula feeding, hypoxia, and hypothermia (FF+HH). IL-10 deficiency exacerbates the degree of intestinal inflammation in response to FF+HH. Administration of exogenous IL-10 before the initiation of FF+HH decreases the severity of NEC. These results show that IL-10 plays a protective role in the pathogenesis of NEC.
Methods
Animals
The Institutional Animal Care and Use Committee approved all animal experiments in this study. Time-dated, pregnant Sprague–Dawley rats were purchased from Charles River Laboratories (Hollister, CA) and delivered at term. Newborn rat pups were randomly separated and subjected to the NEC-inducing regimen of FF+HH as previously described, or left to breastfeed with their mother.9 Wild-type (WT) C57Bl/J and congenic IL-10−/− mice were purchased from Jackson Laboratories (Sacramento, CA) and bred at the Children's Hospital Los Angeles Animal Research Facility. Newborn mice were delivered by cesarean section of timed-pregnant mice at gestational day 18. NEC was induced using FF+HH as previously described; control animals were left to breastfeed with their mothers.6 The newborn mice in the experimental group were kept at 35°C and 70% relative humidity; they were gavage-fed every 3 hours with formula (2× Esbilac Milk Replacer for Puppies; PetAg, Hampshire, IL; 20 μL on day 1 and 30 μL thereafter) through a 24-gauge polyurethane catheter (Luther Medical Products, Tustin, CA) inserted in the esophagus. The mouse model of NEC differs from the rat model with regard to feeding frequency (once every 3 h in mice vs 3 times/d in rats); amount of formula per feeding (20–30 μL in mice vs 200 μL in rats); administration of hypoxia (1 min at 0% O2 in mice vs 10 min at 5% O2 in rats); and the addition of hypothermia (10 min at 8°C).6 Therefore, this latter regimen is referred to as formula feeding plus hypoxia and hypothermia (FF+HH). Animals displaying symptoms of NEC (abdominal distension, rectal bleeding, pneumatosis intestinalis) were promptly euthanized. All surviving animals were euthanized on day 3. NEC was diagnosed by microscopic examination of H&E–stained sections of terminal ileum. NEC grade (0–4, with increments of .5) was determined by a pathologist blinded to the groups, based on the extent of epithelial sloughing, submucosal edema, neutrophil infiltration, and derangement of intestinal villus architecture.10
Cytokine measurement
Blood samples from the pups were obtained via cardiac puncture and sent out to our collaborating laboratories at the University of Pittsburgh for analysis using the Luminex assay (Luminex, Austin, TX).
Immunofluorescence microscopy
Paraffin sections of small intestine were stained with anti–JAM-A antibody (Zymed, San Francisco, CA) or anti-inducible nitric oxide synthase (iNOS) antibody (Transduction Laboratories, San Diego, CA), followed by appropriate fluor-labeled secondary antibodies (Jackson Immunoresearch, West Grove, PA), as recommended by the manufacturers. Sections were stained for apoptosis using the ApopTag Red In Situ Apoptosis Detection Kit (Chemicon, Billerca, MA). Fluorescent images were obtained using the Olympus BX51 microscope equipped with a color camera and Picture Frame software (Olympus, Center Valley, PA). For comparisons, samples were processed in parallel on the same slide, and images were acquired and adjusted identically.
Results
Experimental NEC is associated with increased levels of IL-10
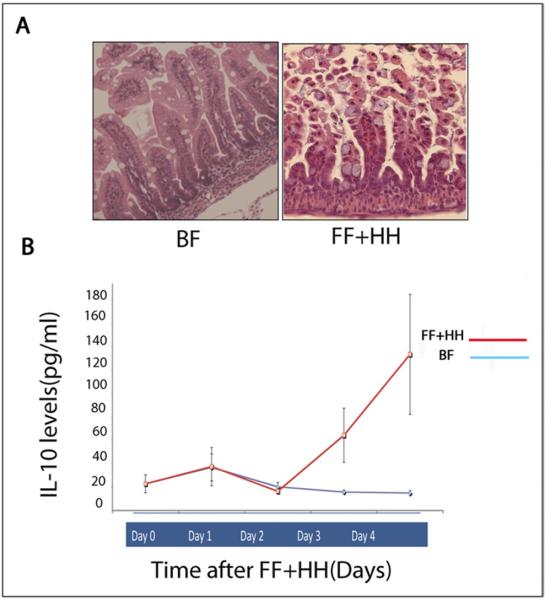
We previously showed that newborn rats subjected to formula feeding combined with hypoxia develop epithelial injury resembling NEC (Fig. 1A).10 To elucidate the pattern of inflammatory cytokine expression in experimental NEC, we performed a Luminex analysis of the serum from neonatal rats after breastfeeding (BF) or FF+HH. Levels of IL-10 increased significantly after the second day of FF+HH compared with BF controls (Fig. 1B). These results indicate that experimental NEC is associated with increased levels of IL-10.
Figure 1.
(A) Hematoxylin-eosin staining of rat intestine: BF and formula fed + hypoxia and hypothermia. (B) Levels of IL-10 (pg/mL) in the serum of the pups measured by Luminex Assay from day of life 1 to day of life 4 (N = minimum of 3 animals per group for each day).
IL-10 deficiency is associated with increased intestinal inflammation
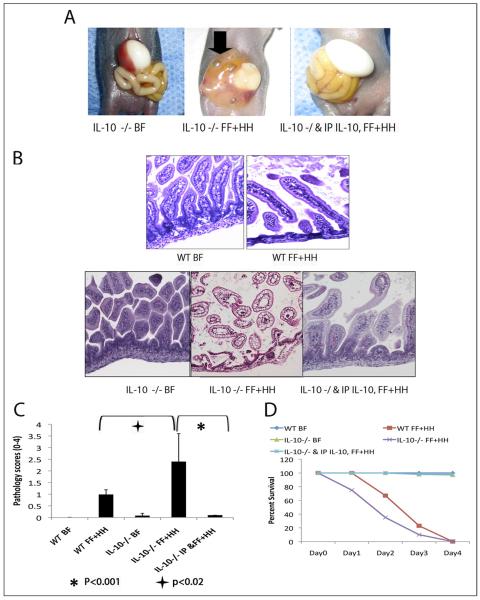
Increased levels of IL-10 in neonatal rats subjected to the FF/H regimen prompted us to examine the role of IL-10 in the pathogenesis of experimental NEC. To this end, we compared manifestations of NEC in IL-10–deficient mice and in congenic wild-type mice (C57Bl/6). Representative examples of gross pathology from WT and IL-10 −/− mice are shown in Fig. 2A. IL-10−/− mice had pronounced discoloration of the small intestine with pneumatosis intestinalis represented by the air bubbles, as well as areas of perforation or necrosis (Fig. 2A, arrowheads). IL-10−/− mice subjected to FF+HH had more derangement of the intestinal villus architecture with sloughing of villi compared with the WT controls (Fig. 2B). Consistent with these morphologic changes, IL-10–deficient mice had higher pathology scores and lower survival rates than their WT counterparts (Fig. 2C and D).
Figure 2.
(A) The intestinal morphology of IL-10 −/− mice that underwent BF (left), FF+HH (center) or intraperitoneal injection of 5 ug IL-10 before FF+HH (right). Arrow indicates dilated, perforated intestine. (B) Hematoxylin-eosin sections of experimental animals. BF IL-10 – deficient mice show normal histology. FF+HH IL-10 – deficient mice show extensive mucosal pathology (center). Exogenous IL-10 reverses the effects of FF+HH in IL-10 – deficient mice (right). (C) Pathology scores for BF versus FF+HH WT and IL-10 −/− mice (P < .02). Exogenous IL-10 significantly improves pathology scores compared with IL-10 −/− FF+HH mice (P < .001, analysis of variance). The error bars represent the standard error of the mean. The experiment was repeated 3 times and n = 30 in each group. (D) Survival curve from all animal experiments. N = minimum of 30 in each group.
IL-10 deficiency promotes epithelial apoptosis and derangement of tight junctions in the mouse model of NEC
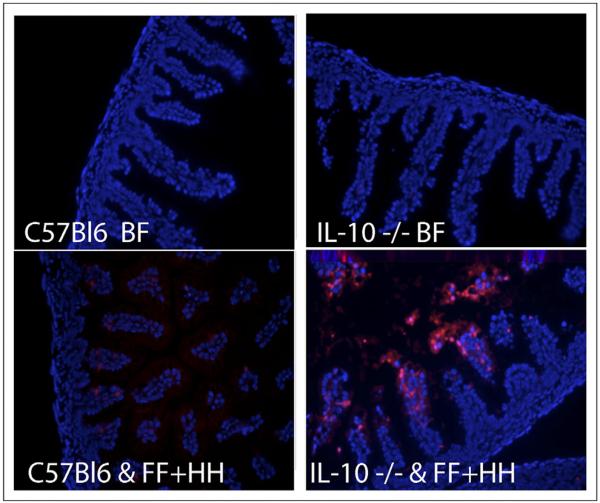
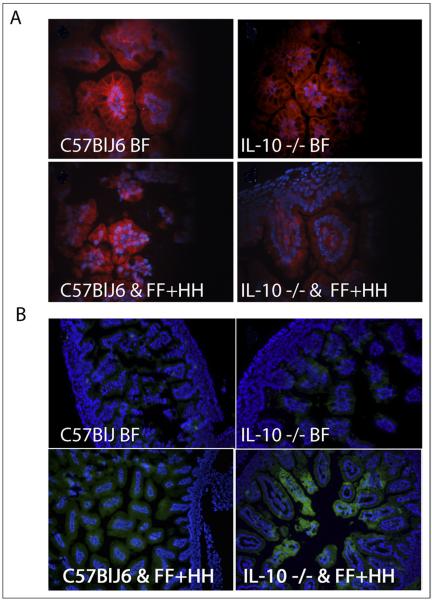
Because NEC is associated with abnormally increased epithelial apoptosis,5 we measured the number of apoptotic nuclei in WT and IL-10 −/− neonatal mice subjected to the FF+HH regimen. Small-intestine sections from both groups were stained for apoptotic cells using terminal transferase deoxyuridine nick-end labeling. IL-10–deficient animals showed dramatically increased levels of apoptosis compared with WT mice or with the BF control group (Fig. 3). Derangement of the intestinal barrier is another hallmark of NEC. To evaluate the effect of IL-10 deficiency on the integrity of the intestinal barrier during NEC, we examined the structure of intestinal tight junctions (TJs) in WT and IL-10−/−mice subjected to the FF+HH regimen, using immunostaining with anti–JAM-A antibody. JAM-A is a TJ protein that predominantly localizes to enterocyte borders under normal conditions. Dissociation of TJ is accompanied by internalization of JAM-A, resulting in a more diffuse distribution. As expected, JAM-A localized predominantly to enterocyte borders in BF animals, regardless of their genotype (Fig. 4A, top), consistent with a healthy gut barrier. In the majority of WT mice subjected to FF+HH, there was considerable intracellular localization of JAM-A in the epithelium (Fig. 4A, bottom left). The internalization of JAM-A was increased in IL-10−/− FF+HH mice. Typically, the cell border localization was completely lost, and only diffuse intracellular localization was observed (Fig. 4A, bottom right). These results indicate that IL-10 deficiency exacerbates gut barrier derangement in the mouse model of NEC.
Figure 3.
Fluorescence microscopy and immunohistochemistry of intestinal samples. 4',6-diamidino-2-phenylindole (DAPI)-stained nuclei are dark, phycoerythrin (PE)-stained apoptotic nuclei are lighter. Top left: WT BF mouse pups. Top right: IL-10 −/− BF pups. Bottom left: WT FF + HH mouse pups. Bottom right: IL-10 −/− FF+HH mouse pups.
Figure 4.
Fluorescence microscopy and immunohistochemistry of intestinal samples. (A) Tight junction staining, JAM-1 localization. Top left: WT BF mouse pups. Top right: IL-10 −/− BF pups. Bottom left: WT FF + HH mouse pups. Bottom right: IL-10 −/− FF+HH mouse pups. (B) iNOS localization fluorescein isothiocyanate (FITC). Top left: WT BF mouse pups. Top right: IL-10 −/− BF pups. Bottom left: WT FF + HH mouse pups. Bottom right: IL-10 −/− FF+HH mouse pups. Also shown are DAPI-stained nuclei.
IL-10 deficiency increases intestinal expression of inducible nitric oxide synthase during NEC
Our previous studies have shown that intestinal iNOS expression is increased in NEC both in vivo and in vitro.9 To test whether IL-10 deficiency leads to a further increase in intestinal iNOS levels during NEC, we performed immunofluorescence microscopy to evaluate iNOS expression in the small intestine of WT and IL-10 −/− mice subjected to the FF+HH regimen. Breastfed mice of both genotypes displayed low levels of iNOS in their intestines (Fig. 4B, top). By contrast, and consistent with our previous observations, WT mice subjected to the NEC-inducing regimen typically displayed increased iNOS signal in the epithelium (Fig. 4B, bottom left). Levels of iNOS were increased in IL-10 −/− mice subjected to the FF+HH regimen (Fig. 4B, bottom right). Thus, IL-10 deficiency exacerbates experimental NEC by augmenting iNOS expression.
Exogenous IL-10 attenuates intestinal inflammation in IL-10 −/− mice
To confirm that IL-10 deficiency, as opposed to other factors, is responsible for the increased susceptibility to NEC, we compared the severity of intestinal inflammation in IL-10 −/− mice injected with exogenous IL-10 or phosphate-buffered saline. A total of 10 μL of IL-10 (5 μg/pup) was administered via intraperitoneal injection to the newborn mice 1 hour before formula feeding. Administration of exogenous IL-10 increased survival of IL-10 −/− pups compared with those receiving phosphate-buffered saline (Fig. 2D). Administration of exogenous IL-10 before the beginning of FF+HH significantly improved intestinal villus architecture (Fig. 2B) and the corresponding pathology scores (Fig. 2C).
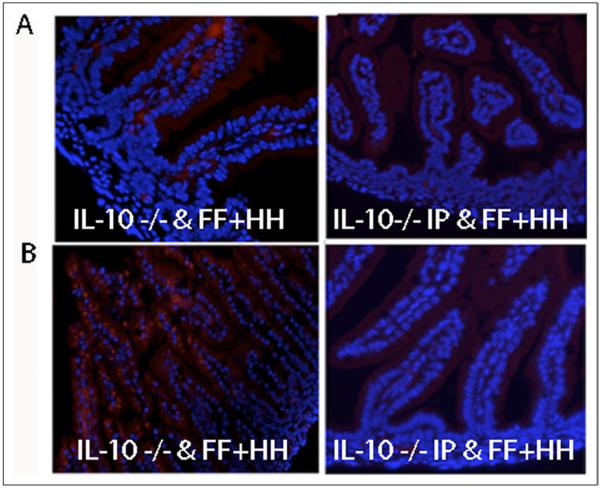
IL-10 administration reduces intestinal iNOS expression and apoptosis in the IL-10−/− animals
In the animal group that received exogenous IL-10, mucosal iNOS expression and apoptosis were measured as previously described. Exogenous IL-10 attenuated the degree of epithelial apoptosis (Fig. 5A) and suppressed iNOS expression in IL-10 – deficient pups subjected to FF+HH (Fig. 5B). Of note, tight junctions were intact in these animals despite FF+HH (data not shown).
Figure 5.
Fluorescence microscopy and immunohistochemistry of intestinal samples. (A) iNOS protein expression. Top left: IL-10 −/− FF+HH mouse pup. Top right: IL-10 −/− FF+HH mouse pups after receiving an intraperitoneal injection of 5 μg of IL-10. (B) Apoptosis by terminal transferase deoxyuridine nick-end labeling staining. Bottom left: IL-10 −/−FF + HH mouse pups. IL-10 −/− FF+HH mouse pup after receiving an intraperitoneal injection of 5 μg of IL-10. Also shown are DAPI-stained nuclei. Representative sections from 3 separate experiments are shown.
Comments
IL-10 was first described as a cytokine secretion inhibitory factor. The first report described this cytokine as a product of the T helper clone of CD4+ T cells, which could suppress the secretion of proinflammatory cytokines such as IL-2, tumor necrosis factor-α, or interferon-γ.11–13 Based on these preliminary reports it was concluded that IL-10 plays mostly an anti-inflammatory role in the immune system14 and its most important function is to limit the magnitude of the immune response. In cases of clinical sepsis and NEC, IL-10 levels are shown to increase in the serum after the acute phase of the disease is finished.6,10 In addition, animal studies have shown that mice lacking IL-10 develop spontaneous enterocolitis and symptoms similar to inflammatory bowel disease.7 In particular, IL-10–deficient mice show an exuberant immune response.15–17
Previous work has shown that proinflammatory cytokines such as tumor necrosis factor-α or IL-6 play an important role in the pathogenesis of NEC.18,19 The anti-inflammatory cytokine, IL-10, which is abundant in human breast milk,20 has been postulated to be one of the putative protective factors that confers protection against NEC.21,22 Interestingly, targeted deletion of the IL-10 gene in mice results in post-weaning enterocolitis, similar to NEC. Also, in mouse models of NEC, it has been shown that breast milk results in an increase in IL-10 production.23 Finally, it has been shown that IL-10 works to suppress the expression of iNOS, particularly from immunocytes such as macrophages, at the mucosal level.24
We already have established a link between FF+HH in both rat and mouse models of NEC.9 In this report, we examined the role of the anti-inflammatory cytokine IL-10 in the pathogenesis of NEC within our FF+HH model. In newborn rat pups we observed a significant increase in serum IL-10 after formula feeding and hypoxia. In the mouse model, IL-10 deficiency resulted in a significant increase in the severity of intestinal inflammation after FF+HH, as evidenced by an increase in mortality, more severe macroscopic and microscopic pathology scores, a marked increase in enterocyte apoptosis, increased derangement of TJ, and increased expression of iNOS. Moreover, exogenous administration of IL-10 reversed these morphologic changes in IL-10–deficient mice subjected to FF+HH. Taken together, these data point to the important role of IL-10 in the pathogenesis of experimental NEC. Consistent with these findings, BF IL-10–deficient mice do not develop intestinal inflammation and the intestinal morphology resembles that of BF WT mice. It is likely that the protection afforded by IL-10 is the result of down-regulation of iNOS expression.
Based on these results we conclude that IL-10 plays an important role in mucosal protection against injury caused by formula feeding and hypoxia. Our results show that the insult caused by FF+HH results in activation of the mucosal immune system. It is possible that IL-10 is up-regulated as a compensatory mechanism to attenuate the inflamma-tory response and therefore protect the mucosa from further injury. Based on our results, it is likely that the absence of IL-10 results in a more exuberant inflammatory response as evidenced by increased epithelial iNOS expression, which in turn leads to uncontrolled damage to the intestinal mucosa. Further investigation is necessary to elucidate the exact mechanisms by which IL-10 plays a protective role in modulating the mucosal proinflammatory immune response, thereby protecting against epithelial injury.
References
- 1.Wiswell TE, Robertson CF, Jones TA, et al. Necrotizing enterocolitis in full-term infants. A case-control study. Am J Dis Child. 1988;142:532–5. doi: 10.1001/archpedi.1988.02150050070034. [DOI] [PubMed] [Google Scholar]
- 2.Distribution of births by gestational age in the United States. Centers for disease control and prevention; [Accessed: November 11, 2010]. Available at: http://www.cdc.gov/datastatistics/2007/births. [Google Scholar]
- 3.Blakely ML, Lally KP, McDonald S, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD neonatal research network. Ann Surg. 2005;241:984–94. doi: 10.1097/01.sla.0000164181.67862.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccia D, Stolfi I, Lana S, et al. Nosocomial necrotising enterocolitis outbreaks: epidemiology and control measures. Eur J Pediatr. 2001;160:385–91. doi: 10.1007/s004310100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford HR. Mechanism of nitric oxide-mediated intestinal barrier failure: insight into the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2006;41:294–9. doi: 10.1016/j.jpedsurg.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–82. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 8.Lane JS, Todd KE, Lewis MP, et al. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery. 1997;122:288–94. doi: 10.1016/s0039-6060(97)90020-9. [DOI] [PubMed] [Google Scholar]
- 9.Hunter CJ, Singamsetty VK, Chokshi NK, et al. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. 2008;198:586–93. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadler EP, Dickinson E, Kniseley A, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–7. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 11.O'garra A, Stapleton G, Dhar V, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–32. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 12.Pestka S, Krause CD, Sarkar D, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 13.Moore KW, Vieira P, Fiorentino DF, et al. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;200:1230–4. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 14.Abbas A, Lichtman A, Pober J. Cellular and Molecular Immunology. 2nd ed W.B. Saunders Co; Philadelphia: 1994. [Google Scholar]
- 15.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–95. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez-Torres A, Jones-Carson J, Wagner RD, et al. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun. 1999;67:670–4. doi: 10.1128/iai.67.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 18.Morecroft JA, Spitz L, Hamilton PA, et al. Plasma interleukin-6 and tumour necrosis factor levels as predictors of disease severity and outcome in necrotizing enterocolitis. J Pediatr Surg. 1994;29:798–800. doi: 10.1016/0022-3468(94)90374-3. [DOI] [PubMed] [Google Scholar]
- 19.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro and counter inflammatory levels and severity of necrotizing enterocolitis. Pediatrics. 1999;1:766–71. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 20.Garofalo R, Chheda S, Mei F, et al. Interleukin-10 in human milk. Pediatr Res. 1995;37:444–9. doi: 10.1203/00006450-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 22.Schanler RJ, Lau C, Hurst NM. Randomized trial of donor human milk or preterm formula as supplements to mother's own milk for extremely premature infants. Pediatr Res. 2002;51:319A. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak B, Halpern MD, Holubec H, et al. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res. 2003;53:426–33. doi: 10.1203/01.PDR.0000050657.56817.E0. [DOI] [PubMed] [Google Scholar]
- 24.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–6. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]