Abstract
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective at controlling pain in children, especially in the treatment of fractures. Adult animal and adult clinical studies demonstrate conflicting evidence for the inhibitory relationship between NSAIDs and fracture healing. Published pediatric orthopaedic clinical studies do not demonstrate an inhibitory effect of ketorolac on bone healing. Little is known about the effects of any NSAID on bone formation in juvenile animals. This study investigates the effects of the NSAID ketorolac on fracture healing in a juvenile rat model.
Methods
Unilateral surgically induced and stabilized tibial shaft fractures were created in 45 juvenile (3 to 4wk old) male Sprague-Dawley rats. Either ketorolac (5 mg/kg; n=24) or saline (0.9% normal saline; n=21) was then administered to the rats 6 d/wk by intraperitoneal injections. Animals were then randomly assigned into time groups and euthanized at 7 days (n=8 ketorolac, n=7 saline), 14 days (n=8 ketorolac, n=7 saline), or 21 days (n=8 ketorolac, n=7 saline) postfracture. Biomechanical analysis was performed using a custom-designed 4-point bending loading apparatus. Statistics for tibial stiffness and strength data were performed using software package Systat 11. Specimens were also evaluated histologically using hematoxylin and eosin staining.
Results
Strength and stiffness of all fractured tibiae increased over time from day 7 to day 21 regardless of treatment type. No statistical difference was found between the fractured tibiae strength or stiffness in the ketorolac or control-treated specimens at the same time point. In addition, the quality of the fracture callus was similar in both groups at each of the time points.
Conclusions
In this study of a juvenile rat model with a stabilized tibia fracture, fracture callus strength, stiffness, and histologic characteristics were not affected by the administration of ketorolac during the first 21 days of fracture healing.
Clinical Relevance
The absence of inhibitory effects of ketorolac on early juvenile rat fracture healing supports the clinical practice of utilizing NSAIDs for analgesia in children with long bone fractures.
Keywords: nonsteroidal anti-inflammatory drugs, NSAIDs, fracture healing, pediatric, ketorolac, biomechanical analysis, histology, histologic analysis, rat
Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective at controlling pain in the pediatric population, especially in the treatment of postoperative pain and orthopaedic injuries.1–8 These analgesics also have fewer side effects than narcotics in the pediatric population. 1,7,9–11 Narcotics are known to have common side effects such as nausea, vomiting, respiratory depression, and constipation, which can be especially problematic in pediatric patients.1,6,10,12 Despite this, NSAID use in the treatment of pain associated with bony injury and healing in children is now considered controversial.
This controversy arises from the theory that NSAIDs delay bone healing, especially in adults. Several studies in various adult mammals including the rat, mouse, and rabbit have demonstrated an inhibition of bony repair with the use of various NSAIDs.13–28 However, there are also animal studies showing no effect of NSAIDs on bone formation or fracture healing.29–33
The published adult clinical studies are also conflicting. Some human adult studies investigating the effects of the NSAID ketorolac on spinal fusion have demonstrated an inhibition to bone healing34–36 but one larger clinical study did not.37 The effect of NSAIDs on bone healing in human adults after fracture or osteotomy is less clear, with some studies demonstrating increased rates of nonunion with NSAID use,38,39 whereas other studies finding no correlation.40,41
There is no evidence in the clinical pediatric orthopaedic literature suggesting NSAIDs delay bone healing in children. Clinical retrospective studies in the pediatric population do not demonstrate these inhibitory effects of the NSAID ketorolac on bone healing for posterior spinal fusion4,42,43 or operative fracture care44 or osteotomy healing.45 Furthermore, to our knowledge, there are no published studies investigating the effects of ketorolac in a juvenile animal model.
The present study was performed to evaluate the effect of ketorolac administration on the healing of surgically induced and stabilized tibia shaft fractures in a juvenile rat model. Ketorolac was chosen because it is practically utilized in orthopaedic surgery, has been studied in both adults34–37 and children,4,42–45 and there is evidence that it delays bone formation in adult rats13 and adult rabbits.14,22,27 We hypothesized that the NSAID ketorolac would not lead to delayed union or failure of bone formation in healing tibia fractures in these juvenile rats.
METHODS
All animal studies were approved by the Loyola University Institutional Animal Care and Use Committee. Forty-five juvenile male Sprague-Dawley rats were obtained at 20 to 21 days of age (Harlan, Indianapolis, IN) and acclimated to the laboratory environment for 3 to 4 days. On the basis of a power analysis of prior studies investigating inhibitory effects of alcohol in fracture repair in our laboratory, this number of subjects is sufficient to avoid a type II error.46 Animals were randomly assigned to receive intraperitoneal (IP) ketorolac (5 mg/kg) or isotonic saline control for 7 days (ketorolac n=8, saline n=7), 14 days (ketorolac n=8, saline n=7), or 21 days (ketorolac n=8, saline n=7) postoperatively. The animals were fed ad libitum during the study.
Surgical Preparation and NSAID Administration
The animals in both groups were given a preoperative injection of buprenorphine (0.1 mg/kg, SC) 30 minutes before surgery for analgesia, followed by antibiotic prophylaxis of gentamycin (5 mg/kg, SC) 5 minutes before surgery. For anesthesia rats were given ketamine (30 mg/kg, IP) and xylazine (3.2 mg/kg, IP). The level of anesthesia was maintained by isofluorane oxygen inhalation (2 mL/min; 1% isofluorane, 8% oxygen). Surgical procedures were performed using aseptic technique, and animals were kept on a heating pad during surgery to maintain core body temperature. An approximate 5-mm incision was made on the dorsolateral aspect of the thigh and extended over the knee joint. A longitudinal incision was made in the patellar tendon to expose the articular surface of the proximal tibia. A small hole was created just above the tibial tuberosity by inserting a 25-G 5/8-inch needle into the medullary canal. The needle was removed and a 0.35-mm-diameter stainless-steel insect pin was inserted into the medullary canal. For fracture creation, a second 5-mm incision was made at the midpoint of the tibia. Angled dissector scissors with stout blades to cut bone (Fine Science Tools #14082-09, Foster City, CA) were used to make a mid-diaphyseal cut in the tibia. The skin was closed with 6-0 prolene sutures. Animals were resuscitated with 1.0mL isotonic saline warmed to 37°C. After surgery, the animals were placed in warmed cages and monitored continuously until full recovery from anesthesia, followed by hourly observations for the first 8 hours postoperatively.
In both groups, postoperative pain was controlled by subcutaneous administration of buprenorphine (0.06 mg/kg, SC) administered every 8 hours for 24 hours postoperatively. The NSAID group was given ketorolac (5 mg/kg, IP) once daily starting the day after surgery until time of euthanasia at one of the randomly assigned time points (postoperative day 7, 14, or 21). In the same manner, the control group received saline (0.9% normal saline, IP) once daily until euthanasia.
The 5 mg/kg daily dose of ketorolac was based on previous publications studying the effects of ketorolac on bone healing in an adult rat model at a dose of 4 mg/kg given either orally or by IP injection.13,29,32 From these previous studies, we believe our dose would have the appropriate capability to prevent callus formation and subsequent fracture healing, if the effect exists in this population.
Human pediatric dosing of ketorolac ranges from 0.5 mg/kg IV to 1 mg/kg IM every 6 hours for up to 5 days.47,48 This correlates to a maximum of 2 to 4 mg/kg in a 24-hour time frame which is still less than the dosing of the juvenile rats in this study that received 5 mg/kg daily.
Tibiae Harvesting and Storage
In order to examine the role of NSAIDs in different stages of fracture healing in the juvenile rat, those in the control and NSAID groups were randomly assigned to be euthanized by carbon dioxide inhalation at 7, 14, or 21 days postfracture. Tibiae were harvested from each animal, the pin removed then stored at 20°C before biomechanical analysis or in 10% formalin solution before histologic analysis.
Biomechanical Testing
Four-point bending testing was performed on each harvested specimen to measure strength and stiffness of the fracture callus of the injured tibia and of the contralateral tibia using an Instron materials testing machine (Model 5544, Canton, MA). Custom-designed 4-point bending loading apparatuses were used to monitor load, and a precision sensor was used to measure the axial deformation of the specimen, as previously described.49 The test was performed with a constant rate of 0.5 mm/min, and the endpoint was set at a reduction rate of 30% of the peak compressive load. The load-deformation data were collected to calculate strength and stiffness, defined as maximum load sustained before failure (N) and property of the material (N/mm), respectively.
Histology
One fracture callus specimen for each time group was sent for histologic analysis. The specimens were decalcified, embedded, sectioned, and stained with hematoxylin and eosin. These were evaluated by a board certified pathologist under light microscopy for evidence of normal fracture soft callus formation by day 7, callus size at early and later stages of repair, and evidence of bridging or hard callus formed throughout the process of endochondral ossification of existing cartilage matrix. Any reduction in soft or hard callus size or decrease in endochondral ossification, which might be affected by the NSAID administration, was also noted.
Statistical Analysis
Statistics for tibia stiffness and strength data were performed using software package Systat 11 (Systat Software Inc., San Jose, CA). The treatment groups were compared at each time point (7, 14, 21 d) using 1-way analysis of variance and Tukey HSD multiple comparison procedure. Significance was noted at P<0.05.
RESULTS
Biomechanical Properties of Tibiae
Fractured Tibiae
When comparing the group that received ketorolac with the group that received saline for 7 days postfracture, there was no difference seen in strength (P=0.41) or stiffness (P=0.99). Similarly, the strength and stiffness were comparable in the 14-day fracture groups (strength, P=0.88; stiffness, P=0.98) and 21-day fracture groups (strength, P=0.55; stiffness, P=0.99; Figs. 1, 2).
FIGURE 1.
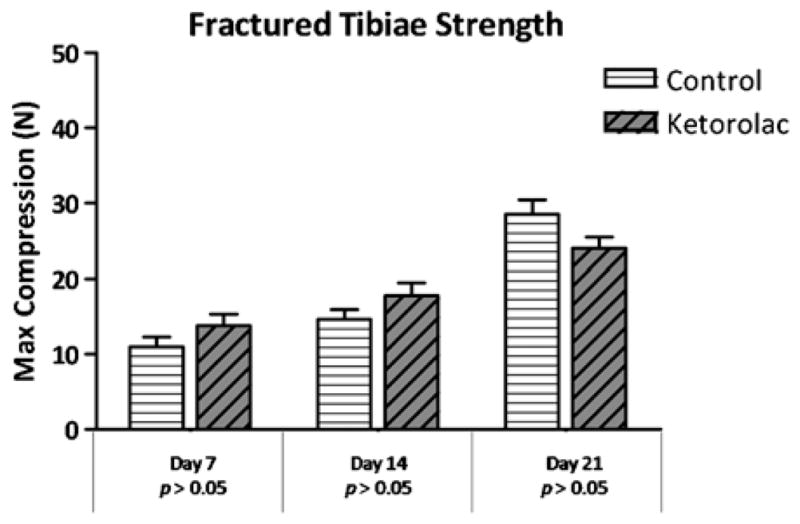
Fractured tibiae strength. Strength, measured by maximum compression in Newtons, of the fractured saline control groups and the fractured ketorolac treatment groups at each time point.
FIGURE 2.
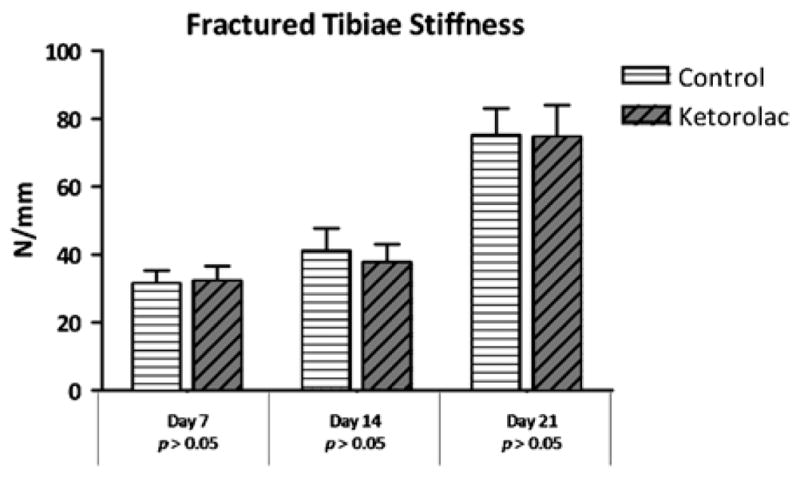
Fractured tibiae stiffness. Stiffness, measured in Newtons per millimeter, of the fractured saline control groups and ketorolac groups at each time point.
Contralateral Nonfractured Tibiae
The strength of the contralateral nonfractured tibiae were similar between the saline and ketorolac-treated groups at 7 days (P=0.45) as was the stiffness (P= 0.51). Likewise, the strength and stiffness were comparable between the 2 groups at 14 days (strength, P=0.99; stiffness, P=0.97) and 21 days (strength, P= 0.32; stiffness, P=0.42).
The strength and stiffness of both the saline and ketorolac-treated fracture groups increased over time (Figs. 1, 2). However, by day 21 postfracture, the strength of neither the saline (P=0.04) nor ketorolac (P<0.001)- treated fracture groups reached the strength of the contralateral intact tibiae (Fig. 3).
FIGURE 3.
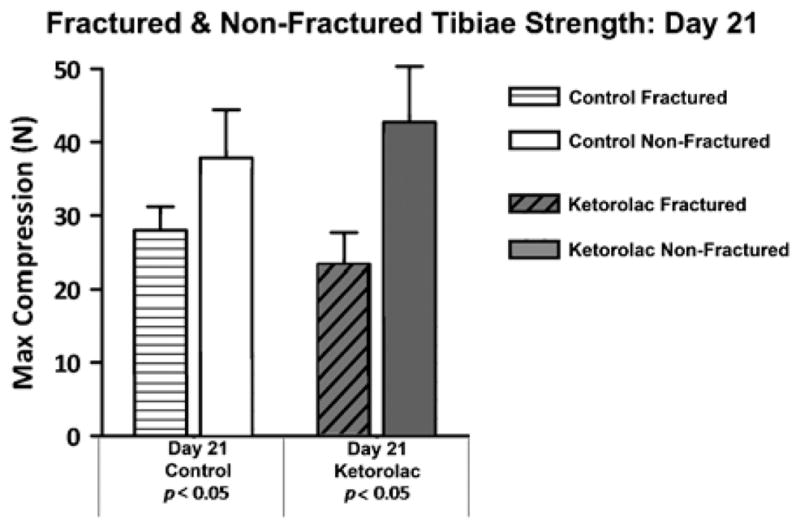
Fractured tibiae versus contralateral tibiae strength at day 21. Neither the saline control nor the ketorolac fractured tibiae reached full strength by day 21 postfracture compared with the contralateral nonfractured tibiae.
Histology
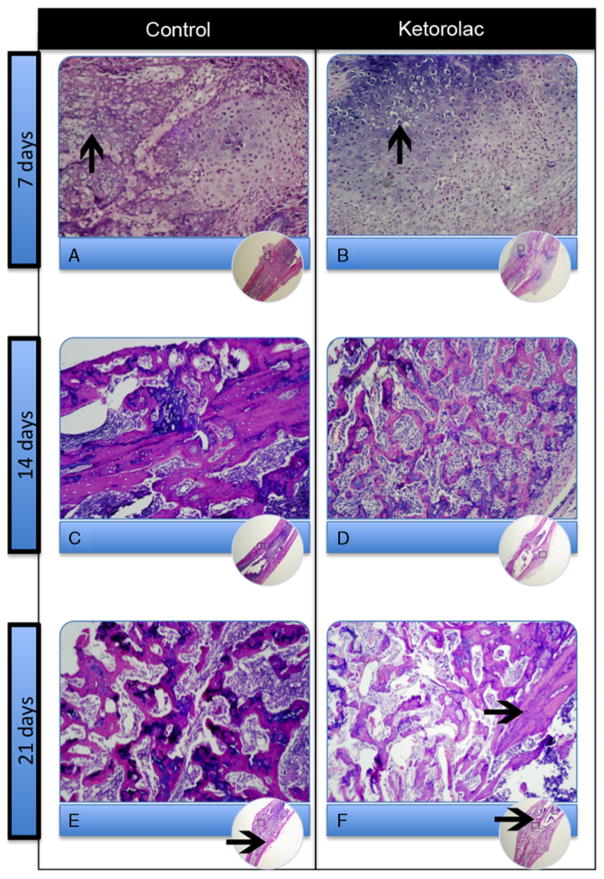
In both the ketorolac-treated fractured tibiae and the control fractured tibiae, at 7 days postinjury cartilaginous external and bony periosteal callus were present and hypertrophic chondrocytes were seen (Figs. 4A, B). At 14 days postinjury, hypertrophic chondrocytes were seen in the external callus with associated endochondral ossification activity and formation of woven bone (Figs. 4C, D). By 21 days postinjury, the cartilaginous component of the external callus was largely replaced by woven bone and there were signs of compact lamellar bone (Figs. 4E, and F). At each of the 3 time points, the ketorolac treatment groups seemed qualitatively similar to the saline control groups.
FIGURE 4.
Images of hematoxylin and eosin-stained specimens of the fractured tibiae at each time point comparing the saline control groups and ketorolac treatment groups. Hypertrophied chondrocytes (arrows in A and B) at day 7; woven bone at day 14 (C and D); further woven bone and early compact lamellar bone (arrows in E and F) at day 21.
DISCUSSION
NSAIDs have been shown to be effective for pain management in children with an acute fracture or musculoskeletal injury.1–3 These effective analgesics also have fewer side effects than narcotics in children.1,7,9–11 But concerns have been raised about the use of NSAIDs for pain associated with fracture or postoperative pain after fracture, osteotomy, or spinal fusion.50–52 Although much of the work investigating the role of NSAIDs in bone healing has been performed in adult humans or adult animal models, the use of NSAIDs has also been avoided in children with healing bone.
The biochemical effects of prostaglandins and the inhibitory effect of NSAIDs on prostaglandins in healing bone have been elucidated. Prostaglandins are important inflammatory mediators during the early phase of bone healing. NSAIDs block cyclooxygenase, an enzyme that catalyzes the creation of prostaglandins from arachadonic acid. It does follow that disruption of this pathway through NSAID use could lead to impairment of proper bone healing.15,50–53
A variety of NSAIDs and their effects on bone healing and formation have been studied—both clinically and in animal studies. Indomethacin seems to be well studied and most consistently shown to delay bone formation and healing in adult animal models.14,16–21,23–26 This is consistent with a clinical study of long bone fracture healing38 and its continued practical use in preventing heterotopic ossification after surgery for hip replacement54,55 and acetabular fracture.56–58
Ibuprofen has been less studied with some adult animal studies showing effects16,30 and another not.31 Little has been published on the clinical effects of ibuprofen during bone healing with some evidence showing a possible association with nonunion39 and other studies not specifying which NSAID was used post-operatively. 35,40,41
Ketorolac has been better studied both in the adult clinical and basic science realms; however, controversy still exists. In adult rat and rabbit studies, ketorolac administration has been shown to delay bone healing in some studies13,14,22,27 but not in others.29,32,33 In adult clinical studies, both Glassman et al34 and Park et al36 demonstrated increased nonunion rates in patients undergoing posterior spinal fusion who used ketorolac postoperatively. But Pradhan et al37 failed to demonstrate any difference.
But children are not small adults, and there has been consistency in the published clinical literature regarding the use of ketorolac postoperatively for pediatric orthopaedic patients. None of the published studies in children or adolescents have shown any deleterious effects of ketorolac on bone healing or bone formation after posterior spinal fusion for scoliosis,4,42,43 operative fracture care,44 or osteotomy.45
It does not seem that ketorolac has been studied in a juvenile animal model. To the best of our knowledge, there are only 2 studies to include young adult rats. Elves et al20 differentiated between “young” and “old” rats and showed no delay in bone formation in drilled vertebral bodies after indomethacin was administered to younger rats (2mo of age) compared with a consistent delay in bone formation in rats 6 to 9 months of age. Allen et al26 studied 45-day old rats with radius and ulna fractures and found histologic signs of delayed healing with exposure to indomethacin but no increase in pseudarthrosis rate. In fact, Vitale et al43 in their clinical study of the use ketorolac in children undergoing scoliosis surgery called for studies of young animals to determine the effects of ketorolac on either long bone fracture healing or spinal fusion—that leads us to the results of our current study.
Our results support our hypothesis that the NSAID ketorolac does not have an inhibitory effect on the early phases of bone healing in a juvenile rat with a stabilized tibia fracture. No statistical difference was seen in the biomechanical properties of the fractured juvenile rat tibiae or in the qualitative evaluation of fracture callus histology when rats were treated with administration of the NSAID ketorolac or saline. This corroborates the clinical data from the pediatric orthopaedic literature, which has yet to describe a delay in bone healing in the human pediatric population with ketorolac use.
The present study does have its limitations. First, we relied upon previous published studies in adult rats as historical experimental controls.13,29 This precludes our current study from being definitive, yet the results of this pilot study support the feasibility of our protocol. Previously published reports of the inhibitory effect of NSAID treatment on fracture healing in adult animal model systems13,14,27 have demonstrated that the NSAID exposure regimen used in the study is sufficient to impact fracture healing in a sensitive animal cohort. A follow-up study is warranted to definitively compare side-by-side the effects of ketorolac on juvenile versus adult rat tibiae fracture healing.
Second, the biomechanical data from our last time point suggest that complete fracture healing has not yet occurred at 21 days postfracture (Fig. 3). Thus, our study can only comment on the early phases of fracture healing: the inflammatory and repair stages. These early phases, the inflammatory stage in particular, are the phases one would expect NSAIDs to inhibit during fracture healing. In our study these stages seem to follow the normal progression even in the presence of NSAIDs in our juvenile rat model. Further investigation is warranted that follow the subjects through to complete healing.
As this was a pilot study and our numbers limited, only 1 specimen per group underwent histologic examination. The protocol for the follow-up study includes up to 3 specimens per group for histologic examination in order to better delineate reproducible results.
In addition, rats never reach full skeletal maturity. But distinct juvenile and adult phases do exist. During the first 3 to 4 months of life, rats grow rapidly in weight and length. Then the rat’s weight continues to slowly increase but its length does not. The most rapid period of growth in the male rat has been shown to occur at 6 weeks, which may correlate to the adolescent growth spurt in humans.59 Sprague-Dawley rats have been studied specifically, and it is accepted their adolescence occurs between 4 and 6 weeks of age and that 10 weeks of age is considered adult.60 Our juvenile rats were just 3 to 4 weeks of age at the commencement of our study. They were all euthanized by 7 to 8 weeks of age. The maximum weight reached by any rat in our study was <200 g. In published adult rat NSAID studies,13,17–19,30 the male rat weighed between 300 and 450 g and were all deemed “adult.” Therefore we do believe our younger rat population represents a juvenile or adolescent phase versus the adult phase that has been studied previously.
In conclusion, the data from this pilot study of a juvenile rat model with a stabilized tibia fracture demonstrates that fracture callus mechanical and histologic characteristics were not affected by the administration of ketorolac during the inflammatory and repair stages of fracture healing. These data, combined with prior adult rat studies, suggest that there is a differential effect of NSAIDs on juvenile and adult fracture healing in a rat model. The absence of inhibitory effects of ketorolac on early fracture healing in the juvenile rat specifically supports the use of ketorolac in postoperative pediatric orthopaedic patients and broadly supports the clinical use of NSAIDs for analgesia in children with musculoskeletal pain.
Acknowledgments
Supported by the Loyola Department of Orthopaedic Surgery Faculty Research Start-Up Fund and The Orthopaedic Research and Education Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Drendel AL, Gorelick MH, Weisman SJ, et al. A randomized clinical trial of ibuprofen versus acetaminophen with codeine for acute pediatric arm fracture pain. Ann Emerg Med. 2009;54:553–560. doi: 10.1016/j.annemergmed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Koller DM, Myers AB, Lorenz D, et al. Effectiveness of oxycodone, ibuprofen, or the combination in the initial management of orthopedic injury-related pain in children. Pediatr Emerg Care. 2007;23:627–633. doi: 10.1097/PEC.0b013e31814a6a39. [DOI] [PubMed] [Google Scholar]
- 3.Clark E, Plint AC, Correll R, et al. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics. 2007;119:460–467. doi: 10.1542/peds.2006-1347. [DOI] [PubMed] [Google Scholar]
- 4.Munro HM, Walton SR, Malviya S, et al. Low-dose ketorolac improves analgesia and reduces morphine requirements following posterior spinal fusion in adolescents. Can J Anaesth. 2002;49:461–466. doi: 10.1007/BF03017921. [DOI] [PubMed] [Google Scholar]
- 5.Sutters KA, Shaw BA, Gerardi JA, et al. Comparison of morphine patient-controlled analgesia with and without ketorolac for post-operative analgesia in pediatric orthopedic surgery. Am J Orthop. 1999;28:351–358. [PubMed] [Google Scholar]
- 6.Vetter TR, Heiner EJ. Intravenous ketorolac as an adjuvant to pediatric patient-controlled analgesia with morphine. J Clin Anesth. 1994;6:110–113. doi: 10.1016/0952-8180(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 7.DiMassa A, Scardigli M, Bruni L, et al. Ketorolac for paediatric postopertive pain: a review. Minerva Anestesiol. 2000;66:749–756. [PubMed] [Google Scholar]
- 8.Kokki H. Nonsteroidal anti-inflammatory drugs for postopertive pain: a focus on children. Pediatr Drugs. 2003;5:103–123. doi: 10.2165/00128072-200305020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Eberson CP, Pacicca DM, Ehrlich MG. The role of ketorolac in decreasing length of stay and narcotic complications in the postoperative pediatric orthopaedic patient. J Pediatr Orthop. 1999;19:688–692. [PubMed] [Google Scholar]
- 10.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347:1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 11.Fahey SM, Silver RM. Use of NSAIDs and COX-2 inhibitors in children with musculoskeletal disorders. J Pediatr Orthop. 2003;23:794–799. doi: 10.1097/00004694-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 12.McCann HL, Stanitski DF. Pediatric orthopaedic surgery pain management. J Pediatr Orthop. 2004;24:581–586. doi: 10.1097/00004694-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 14.Martin GJ, Jr, Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188–2193. doi: 10.1097/00007632-199911010-00003. discussion 2193–4. [DOI] [PubMed] [Google Scholar]
- 15.Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89:500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 16.Altman RD, Latta LL, Keer R, et al. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392–400. doi: 10.1097/00005131-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Brown KM, Saunders MM, Kirsch T, et al. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86-A:116–123. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Dimar JR, II, Ante WA, Zhang YP, et al. The effects of nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine. 1996;21:1870–1876. doi: 10.1097/00007632-199608150-00006. [DOI] [PubMed] [Google Scholar]
- 19.Hogevold HE, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing. Acta Orthop Scand. 1992;63:607–611. doi: 10.1080/17453679209169718. [DOI] [PubMed] [Google Scholar]
- 20.Elves MW, Bayley I, Roylance PJ. The effect of indomethacin upon experimental fractures in the rat. Acta Orthop Scand. 1982;53:35–41. doi: 10.3109/17453678208992176. [DOI] [PubMed] [Google Scholar]
- 21.Bergenstock M, Min W, Simon AM, et al. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–723. doi: 10.1097/01.bot.0000184144.98071.5d. [DOI] [PubMed] [Google Scholar]
- 22.Ho ML, Chang JK, Wang GJ. Antiinflammatory drug effects on bone repair and remodeling in rabbits. Clin Orthop Relat Res. 1995;313:270–278. [PubMed] [Google Scholar]
- 23.Dimmen S, Nordsletten L, Madsen JE. Parecoxib and indomethacin delay early fracture healing. Clin Orthop Relat Res. 2009;467:1992–1999. doi: 10.1007/s11999-009-0783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long J, Lewis S, Kuklo T, et al. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg Am. 2002;84-A:1763–1768. doi: 10.2106/00004623-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ro J, Sudmann E, Marton PF. Effect of indomethacin on fracture healing in rats. Acta Orthop Scand. 1976;47:588–599. doi: 10.3109/17453677608988744. [DOI] [PubMed] [Google Scholar]
- 26.Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51:595–600. doi: 10.3109/17453678008990848. [DOI] [PubMed] [Google Scholar]
- 27.Ho ML, Chang JK, Wang GJ. Effects of ketorolac on bone repair: a radiographic study in modeled demineralized bone matrix grafted rabbits. Pharmacology. 1998;57:148–159. doi: 10.1159/000028236. [DOI] [PubMed] [Google Scholar]
- 28.Spiro AS, Beil FT, Baranowsky A, et al. BMP-7-induced ectopic bone formation and fracture healing is impaired by systemic NSAID application in C57BL/6-mice. J Orthop Res. 2010;28:785–791. doi: 10.1002/jor.21044. [DOI] [PubMed] [Google Scholar]
- 29.Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114–125. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- 30.Lindholm TS, Törnkvist H. Inhibitory effect on bone formation and calcification exerted by the anti-inflammatory drug ibuprofen. An experimental study on adult rat with fracture. Scand J Rheumatol. 1981;10:38–42. [PubMed] [Google Scholar]
- 31.Huo MH, Troiano NW, Pelker RR, et al. The influence of ibuprofen on fracture repair: biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J Orthop Res. 1991;9:383–390. doi: 10.1002/jor.1100090310. [DOI] [PubMed] [Google Scholar]
- 32.Fracon RN, Teofilo JM, Moris IC, et al. Treatment with paracetamol, ketorolac or etoricoxib did not hinder alveolar bone healing: a histometric study in rats. J Appl Oral Sci. 2010;18:630–634. doi: 10.1590/S1678-77572010000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reikeraas O, Engebretsen L. Effects of ketorolac tromethamine and indomethacin on primary and secondary bone healing: an experimental study in rats. Arch Orthop Trauma Surg. 1998;118:50–52. doi: 10.1007/s004020050310. [DOI] [PubMed] [Google Scholar]
- 34.Glassman SD, Rose SM, Dimar JR, et al. The effect of post-operative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834–838. doi: 10.1097/00007632-199804010-00020. [DOI] [PubMed] [Google Scholar]
- 35.Deguchi M, Rapoff AJ, Zdeblick TA. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord. 1998;11:459–464. [PubMed] [Google Scholar]
- 36.Park SY, Moon SH, Park MS, et al. The effects of ketorolac injected via patient controlled analgesia postoperatively on spinal fusion. Yonsei Med J. 2005;46:245–251. doi: 10.3349/ymj.2005.46.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan BB, Tatsumi RL, Gallina J, et al. Ketorolac and spinal fusion. Spine. 2008;33:2079–2082. doi: 10.1097/BRS.0b013e31818396f4. [DOI] [PubMed] [Google Scholar]
- 38.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700–705. [PubMed] [Google Scholar]
- 39.Giannoudis PV, MacDonald DA, Matthews SJ, et al. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655–658. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharyya T, Levin R, Vrahas MS, et al. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364–367. doi: 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 41.Bhandari M, Tornetta P, III, Sprague S. Predictors of reoperation following operative management of fractures of the tibial shaft. J Orthop Trauma. 2003;17:353–361. doi: 10.1097/00005131-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Sucato DJ, Lovejoy JF, Agrawal S, et al. Postoperative ketorolac does not predispose to pseudoarthrosis following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine. 2008;33:1119–1124. doi: 10.1097/BRS.0b013e31816f6a2a. [DOI] [PubMed] [Google Scholar]
- 43.Vitale MG, Choe JC, Hwang MW, et al. Use of ketorolac tromethamine in children undergoing scoliosis surgery. An analysis of complications. Spine J. 2003;3:55–62. doi: 10.1016/s1529-9430(02)00446-1. [DOI] [PubMed] [Google Scholar]
- 44.Kay RM, Directo MP, Leathers M, et al. Complications of ketorolac use in children undergoing operative fracture care. J Pediatr Orthop. 2010;30:655–658. doi: 10.1097/BPO.0b013e3181efb8b4. [DOI] [PubMed] [Google Scholar]
- 45.Kay RM, Leathers M, Directo MP, et al. Perioperative ketorolac use in children undergoing lower extremity osteotomies. J Pediatr Orthop. 2011;31:783–786. doi: 10.1097/BPO.0b013e31822ed33a. [DOI] [PubMed] [Google Scholar]
- 46.Volkmer D, Lauing KL, Nauer RK, et al. Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma. 2011;25:516–521. doi: 10.1097/BOT.0b013e31821f65cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ketorolac Dosage. [Accessed October 29, 2012];Drugs. 2012 Available at: http://www.drugs.com/dosage/ketorolac.html.
- 48.Cote C, Lerman J, Todres ID. A Practice of Anesthesia for Infants and Children. 4. Philadelphia: Saunders Elsevier; 2009. [Google Scholar]
- 49.Callaci JJ, Juknelis D, Patwardhan A, et al. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcohol Clin Exp Res. 2004;28:182–191. doi: 10.1097/01.ALC.0000108661.41560.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdul-Hadi O, Parvizi J, Austin MS, et al. Nonsteroidal anti-inflammatory drugs in orthopaedics. J Bone Joint Surg Am. 2009;91:2020–2027. [PubMed] [Google Scholar]
- 51.Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg. 2004;12:139–143. doi: 10.5435/00124635-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Vuolteenaho K, Moilanen T, Moilanen E. Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin Pharmacol Toxicol. 2008;102:10–14. doi: 10.1111/j.1742-7843.2007.00149.x. [DOI] [PubMed] [Google Scholar]
- 53.Bergmann P, Schoutens A. Prostaglandins and bone. Bone. 1995;16:485–488. doi: 10.1016/8756-3282(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 54.Ritter MA, Gioe TJ. The effect of indomethacin on para-articular ectopic ossification following total hip arthroplasty. Clin Orthop Relat Res. 1982;167:113–117. [PubMed] [Google Scholar]
- 55.Schmidt SA, Kjaersgaard-Anderson P, Pedersen NW, et al. The use of indomethacin to prevent the formation of heterotopic bone after total hip replacement. J Bone Joint Surg Am. 1988;70:834–838. [PubMed] [Google Scholar]
- 56.Moed BR, Letournel E. Low-dose irradiation and indomethacin prevent heterotopic ossification after acetabular fracture surgery. J Bone Joint Surg Br. 1994;76:895–900. [PubMed] [Google Scholar]
- 57.McLaren AC. Prophylaxis with indomethacin for heterotopic bone. J Bone Joint Surg Am. 1990;72:245–247. [PubMed] [Google Scholar]
- 58.Moore KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures. J Bone Joint Surg Br. 1998;80:259–263. doi: 10.1302/0301-620x.80b2.8157. [DOI] [PubMed] [Google Scholar]
- 59.Pahl P. Growth curves for body weight of the laboratory rat. Aust J Biol Sci. 1969;22:1077–1080. doi: 10.1071/bi9691077. [DOI] [PubMed] [Google Scholar]
- 60.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]