Abstract
Background
Vagally dependent gastric reflexes are mediated through vagal afferent fibers synapsing upon neurons of the nucleus tractus solitarius (NTS) which, in turn modulate the preganglionic parasympathetic dorsal motor nucleus of the vagus (DMV) neurons within the medullary dorsal vagal complex (DVC). The expression and transport of ghrelin receptors has been documented for the afferent vagus nerve, and functional studies have confirmed that vagal pathways are integral to ghrelin-induced stimulation of gastric motility. However, the central actions of ghrelin within the DVC have not been explored fully.
Methods
We assessed the responses to ghrelin in fasted rats using: 1) in vivo measurements of gastric tone and motility following IVth ventricle application or unilateral microinjection of ghrelin into the dorsal vagal complex (DVC); and, 2) whole cell recordings from gastric-projecting neurons of the dorsal motor nucleus of the vagus (DMV)
Results
1) IVth ventricle application or unilateral microinjection of ghrelin into the DVC elicited contractions of the gastric corpus via excitation of a vagal cholinergic efferent pathway; 2) Ghrelin facilitates excitatory, but not inhibitory, presynaptic transmission to DMV neurons.
Conclusions
Our data indicate that ghrelin acts centrally by activating excitatory synaptic inputs onto DMV neurons, resulting in increased cholinergic drive by way of vagal motor innervation to the stomach.
Keywords: vago-vagal reflexes, brainstem, gastrointestinal motility, gastrointestinal transit
Gastrointestinal peptide signaling occurs via neuronal or circulatory pathways, or through a combination of both pathways as we have proposed previously for cholecystokinin and GLP-1 1,2. The neuronal signaling pathway includes paracrine activation of luminal vagal afferent fibers or neurons that terminate in the nucleus tractus solitarius (NTS) within the brainstem dorsal vagal complex (DVC). The DVC is comprised of the NTS, the dorsal motor nucleus of the vagus (DMV) and the area postrema (AP) 3. The medullary integration of gastric sensory and motor activity occurs within brainstem vagal circuits and vagal augmentation of gastric motility and intramural pressure is mediated ultimately by a subset of vagal preganglionic motor efferent fibers in the DMV that activate cholinergic enteric neurons (reviewed in 3).
The neurohormone ghrelin, secreted from oxyntic cells within the gastric mucosa 4, is up-regulated during periods of negative energy balance, such as before meals, and is down-regulated after feeding 5. Ghrelin secretion exerts profound stimulatory effects upon gastric motility and acid secretion as well as food intake and energy metabolism 6–10. This stimulatory effect has been demonstrated in both fed and fasted states11,12. An inhibitory effect of ghrelin has been reported for fundic tone of fed animals 10. This particular experimental design, however, included a potentially confounding manipulation of the stomach in order to squeeze out gastric chyme prior to implantation of a luminal-dwelling balloon for detecting pressure.
Ghrelin is considered to exert its gastroexcitatory effect via a vagally-mediated pathway which involves activation of growth hormone secretagogue receptors that originate in the vagal afferent neurons within the nodose ganglion and are transported to afferent terminals 13. Furthermore, peripherally administered ghrelin diminishes vagal afferent activity while vagotomy, midbrain transection or perivagal capsaicin abolishes facilitation of feeding, growth hormone secretion, and activation of neuropeptide Y (NPY)- and growth hormone-releasing hormone (GHRH)-producing neurons 13,14. Ghrelin receptor expression has also been reported within the medullary brainstem and brainstem application of ghrelin exerts behavioral 15, gastric 10 and cardiovascular 16 responses.
Studies investigating both ghrelin receptor expression and functional outcomes of ghrelin receptor activation clearly indicate that while peripheral vagal pathways are integral to ghrelin-induced stimulation of gastric motility, central sites of action must also be considered. However, our understanding of the central actions of ghrelin within the DVC remains incomplete and controversial. The aim of the present work was to test the hypothesis that the promotility effects of ghrelin involve activation of NTS neurons which, in turn, modulate the DMV preganglionic parasympathetic neuronal outflow projecting to the stomach.
Materials and Methods
All experiments were conducted on male Long-Evans rats (n = 40; Harlan Labs, Fredrick MD). Rats were ≥8 weeks of age upon entrance into the experiment and were double housed in a room maintained at 21–24°C on a 12:12-hours light-dark cycle and food and water ad libitum. All procedures were performed according to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the Penn State University College of Medicine.
In vivo studies
Following an overnight fast (water ad libitum), animals were anesthetized deeply with thiobutabarbitol (Inactin®; 100–150 mg kg−1 ip) and administered dexamethasone (1 mg kg−1 sc) to prevent cerebral edema. The trachea was intubated and a jugular catheter was inserted for intravenous delivery of drugs. The stomach was isolated and placed upon a moisturized gauze pad, an encapsulated miniature strain gage (RB Products, Minneapolis, MN) was aligned with the circular smooth muscle fibers and sutured to the gastric corpus before closing the abdominal incision. Animals were placed in a stereotaxic frame and maintained at 37±1 °C using a feedback-controlled warming pad. The head of the animal was oriented in order to surgically expose the fourth ventricle and the vagal trigone. After a 1 hour recovery period, drugs were applied to the surface of the fourth ventricle at the level of the calamus scriptorius (2 μl; n = 12). In one control group of rats (n = 5), prior to attaching the gastric strain gage, the posterior sub-diaphragmatic vagus was sectioned 5 mm caudal to the hiatus. A silk suture ligature was also placed gently around the left cervical vagus approximately 5–10 mm caudal to the nodose ganglion. The ligature was exteriorized though a length of PE-240 tubing for later sectioning of the vagus nerve. After the first application of ghrelin, and a 30 min observation period, the ligature was withdrawn, thus interrupting the remaining vagal outflow to the stomach. In a second control group of rats (n = 3) three applications of a subthreshold dose of ghrelin (3pmol) were made to the fourth ventricle to test for additive effects of ghrelin. This sub-threshold dose was selected on the hypothesis that subtle additive effects of ghrelin would be more clearly detected by increases against a background of low motility.
Baseline gastric motility and tone were monitored continuously on a polygraph (model 79, Grass, Quincy, MA) and the effects of each treatment, beginning immediately following application and for 10 min thereafter, were compared to the average of the 10 min preceding the microinjection. Due to the prolonged responses to higher doses of ghrelin, analysis of gastric motility was limited to the first 10 min of response in order to ensure that motility scores of the lowest doses were not biased by unequal recovery periods. A minimum of 30 min return to baseline levels occurred prior to subsequent administration of any experimental treatment.
Separate groups of rats were prepared similarly for ghrelin microinjection into the left dorsal vagal complex (DVC) through a glass micropipette (30–40 μm tip diameter) directed by a micromanipulator at the following co-ordinates: 0.1–0.3 mm lateral from midline, 0.1–0.3 mm rostral to calamus scriptorius and 0.5–0.7 mm dorsoventral to the surface. Sixty nanoliters of phosphate buffer saline (PBS) or ghrelin (100 pmol dissolved in PBS) were microinjected using a picospritzer (Toohey, Pressure System IIe, Fairfield NJ). One group (n = 5) was prepared for left cervical vagotomy as described above. Microinjections of ghrelin were administered utilizing the same time course as fourth ventricle application. An additional group of rats (n = 5) was implanted with a jugular catheter for intravenous delivery of the cholinergic muscarinic antagonist atropine methyl nitrate (50 μg kg−1 bolus followed by continuous intravenous infusion 20 μg kg−1 hr−1 for 20 min). Atropine methyl nitrate infusion began 30 min after the first microinjection of ghrelin. A second microinjection of ghrelin into the DVC occurred within 10–15 min of the onset of atropine methyl nitrate infusion. A final group of rats (n = 4) were vagotomized during instrumentation at the right cervical vagus in order that microinjections of ghrelin (100 pmol) directed at the right caudal NTS would activate a subset of NTS neurons that project to contralateral DMV neurons.
The strain gage amplifiers were calibrated by setting peak-to-peak sensitivity of individual gages to equal a 1g static load. Gastric motility was calculated using the following formula17:
Based upon this formula, N equals the total number of peaks in a particular milligram range. Therefore, presuming that a 0 mg signal is indicative of no gastric motility, the grouping of peak-to-peak sinusoidal signals were calculated as 25–50 mg, 60–100 mg, 110–200 mg and signals greater than 210 mg for N1 through N4, respectively. Unlike area under the curve measurements, the motility index is independent of baseline tone fluctuations that naturally occur across several seconds or minutes.
Histological Processing
At the conclusion of the experiment, rats were perfused transcardially with a bolus of lidocaine and heparin followed by cold PBS then PBS with 4% paraformaldehyde. The region of the brainstem containing the injection site was removed and refrigerated overnight in PBS containing 20% sucrose and 4% paraformaldehyde. Alternate coronal slices (40μm thick) were mounted on gelatin-coated slides, dried, stained with cresyl violet and coverslipped. Images of the microinjection site were captured using a digital camera attached to a Zeiss Axioskop Microscope and imported into Adobe Photoshop CS5 for analysis.
Electrophysiology
The methods of preparing the brainstem slices and the identification of DMV neurons have been described previously 18,19. Briefly, Long-Evans rats (n=6) of either sex were anesthetized with isoflurane before being euthanized by a bilateral pneumothorax. After removal, 3–4 coronal slices (300μm-thick) of the brainstem were cut starting from the caudal area postrema and moving rostrally. The slices were incubated at 30±1°C in Krebs’ solution (in mM: 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, and 11 dextrose, maintained at pH 7.4 by bubbling with 95% O2-5% CO2) for 60–90 minutes before use. A single slice was then transferred to a perfusion chamber on the stage of a Nikon E600FN microscope, kept in place with a nylon mesh and maintained at 35±1°C by perfusion with warmed Krebs’ solution at a rate of 2.5–3.0 ml min−1. DMV neurons were identified by their soma size and location adjacent to the smaller, dorsal NTS neurons and dorsal to the larger, more heavily myelinated neurons of the hypoglossal nucleus. Recordings were made from DMV neurons at levels spanning from the caudal tip of the area postrema to approximately 0.5 mm rostral to its anterior portion with patch pipettes (2–4MΩ resistance) filled with a potassium gluconate solution (in mM: K gluconate 128, KCl 10, CaCl2 0.3, MgCl2 1, Hepes 10, EGTA 1, ATP 2, GTP 0.25 adjusted to pH 7.35 with KOH) by using an Axopatch 1D amplifier (Molecular Devices, Sunnydale, CA). Liquid junction potential was compensated at the beginning of the experiment. Recordings were accepted only if the series resistance was <20 MΩ. Whole cell recordings of spontaneous and miniature glutamatergic excitatory postsynaptic currents (EPSC) were conducted on DMV neurons, voltage clamped at −60 mV, in the presence of 50 μM picrotoxin to isolate glutamatergic currents pharmacologically and 1μM tetrodotoxin (TTX) to isolate action potential independent synaptic transmission. We have shown previously that the currents recorded under these conditions comprise glutamatergic ionotropic currents only 18. Whole cell recordings of spontaneous GABAergic inhibitory postsynaptic currents (IPSCs) were conducted on DMV neurons, voltage clamped at −50 mV, using patch pipettes (6–8MΩ) resistance filled with a potassium chloride solution (in mM:140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 EGTA, 2 ATP-Na, 0.25 GTP-Na, adjusted to pH 7.35 with HCl) in the presence of 1mM kynurenic acid to isolate GABAergic currents pharmacologically and 1 μM tetrodotoxin as above. We have shown previously that currents recorded under these conditions comprised GABAergic currents only 20.
Data were sampled at 10kHz and filtered at 2kHz, digitized via a Digidata 1320 interface, acquired with a PC utilizing pClamp9 software (Molecular Devices) and analyzed with Mini Analysis software (Jaejin Software, Leonia, NJ).
Drugs were applied to the bath via a series of manually operated valves. Cells were classified as responsive if perfusion with ghrelin (100nM) modified the frequency of s/mEPSC and mIPSC, respectively by a minimum of 50% from baseline (measured as the average frequency during 1min of recording in control conditions vs. 30s of recording centered on peak drug response, identified as the maximal ghrelin-induced effect). A shift in holding current greater than 20pA was considered a postsynaptic effect.
Drugs and chemicals
Inactin® and all other salts were purchased from Sigma (St. Louis, MO). Ghrelin was purchased from Tocris (Ellsville, MO). All drugs were dissolved in sterile isotonic phosphate buffered saline (PBS; in mM: 147.6 NaCl, 83.3 NaH2PO4, 12.9 KH2PO4).
Statistical Analysis
Results are expressed as means ± S.E.M. with significance defined as P < 0.05. Between group results from in vivo studies, between pre- and post-treatment values, were compared by one-way ANOVA and Tukey post hoc analysis or paired t-test as appropriate (SPSS Inc, Chicago, IL). Results from in vitro studies were compared before and after drug administration, with each neuron serving as its own control (Student’s paired t-test). Intergroup comparisons were conducted using the Student’s grouped t-test or the χ2 test.
Results
Fourth ventricle application of ghrelin increases gastric contractions
In fasted naïve rats (n = 12), application of PBS to the floor of the fourth ventricle did not induce any significant change in corpus tone or motility (P > 0.05; Fig. 1A&B). Fourth ventricle application of ghrelin (3–300 pmol) increased gastric corpus motility in a dose dependent manner (P < 0.05; Fig. 1B). Due to the variability of detectable responses following application of 3 pmol ghrelin, response duration was not calculated (Fig. 1C). For higher doses of ghrelin, the duration of the response was 17.1 ± 3.5 min for 10 pmol, 15.6 ± 2.1 min for 30 pmol, 20.1 ± 3.9 min for 100 pmol and 74.8 ± 16.9 min for 300 pmol. At the 300 pmol dose of ghrelin, 40% of animals (2/5) displayed a persistent increase in motility which did not return to baseline levels by the conclusion of the experiment. In a separate subset of animals, three sequential applications of 3 pmol ghrelin at intervals greater than 30 min induced comparable, non-significant, increases in corpus motility, suggesting that the effect of ghrelin was not additive in our preparation (n = 3; P > 0.05, 113±5%, 95±8% and 102±5%, respectively).
Figure 1. IVth ventricle application of ghrelin induces a vagally-mediated facilitation of corpus motility.
A: representative original polygraph traces from fasted animals after IVth ventricle application of PBS or ghrelin demonstrating the dose-dependent increase in corpus motility of fasted animals.
B: graphic summary of the increase in corpus motility after IVth ventricle application of PBS or ghrelin (n=12; expressed as percent increase from baseline; * P < 0.05 vs. baseline).
C: graphic summary of the duration of the ghrelin response after IVth ventricle application of PBS or ghrelin (duration defined as contraction amplitude greater than 10 percent of baseline. N/D = no detectable increase in contraction amplitude or duration; * P < 0.05 vs. 10, 30 or 100 pmol).
D: graphic summary of the increase in corpus motility after IVth ventricle application of PBS or ghrelin in the cohort of animals that received posterior subdiaphragmatic vagotomy (n=4). Following completion of vagotomy (Vgtx) IVth ventricle ghrelin (100 pmol) no longer elevated corpus motility (* P < 0.05 vs. baseline).
As with naïve rats, application of 100 pmol ghrelin to the fourth ventricle of rats which received a posterior subdiaphragmatic vagotomy (n = 4) induced a 233±35% increase in gastric corpus motility (P < 0.05; Fig. 1D) and the duration of the response was 16.3 ± 2.1 min. After 30 min stabilization and recovery, the bilateral vagotomy was completed by withdrawing the ligature around the left cervical vagus. This procedure reduced residual gastric contractions to 25±7% of pre-vagotomy levels (P < 0.05; data not shown). Following an additional 30-min stabilization and recovery, re-application of 100 pmol of ghrelin to the fourth ventricle no longer increased gastric corpus contractions (P > 0.05; Fig. 1D).
These data indicate that the increase in gastric motility following application of ghrelin to the fourth ventricle is mediated by activation of vagal neurocircuitry within the brainstem.
Microinjection of ghrelin into the DVC induced a vagally-mediated increase in corpus motility
To confirm that the effects of ghrelin were the result of localized activation of vagal neurocircuitry, dual-barrel microinjection pipettes were positioned within the left DVC of rats which received a posterior subdiaphragmatic vagotomy (n = 4) or rats with jugular intravenous catheters (n = 5). There was no significant difference in the baseline motility index of either group (P > 0.05). Microinjection of PBS in the left DVC of either group of these rats did not induce any significant change in gastric motility (P > 0.05).
Microinjection of ghrelin (100 pmol) in the left DVC significantly increased corpus motility in the cohort of prevagotomized rats (P < 0.05; Fig. 2). Upon recovery of corpus motility to baseline levels, the vagal ligature was withdrawn to complete the vagotomy. Following an additional 30 min post-vagotomy stabilization period, 100 pmol of ghrelin was microinjected into the DVC of completely vagotomized animals and no longer increased corpus motility above baseline levels (P > 0.05; Fig. 2).
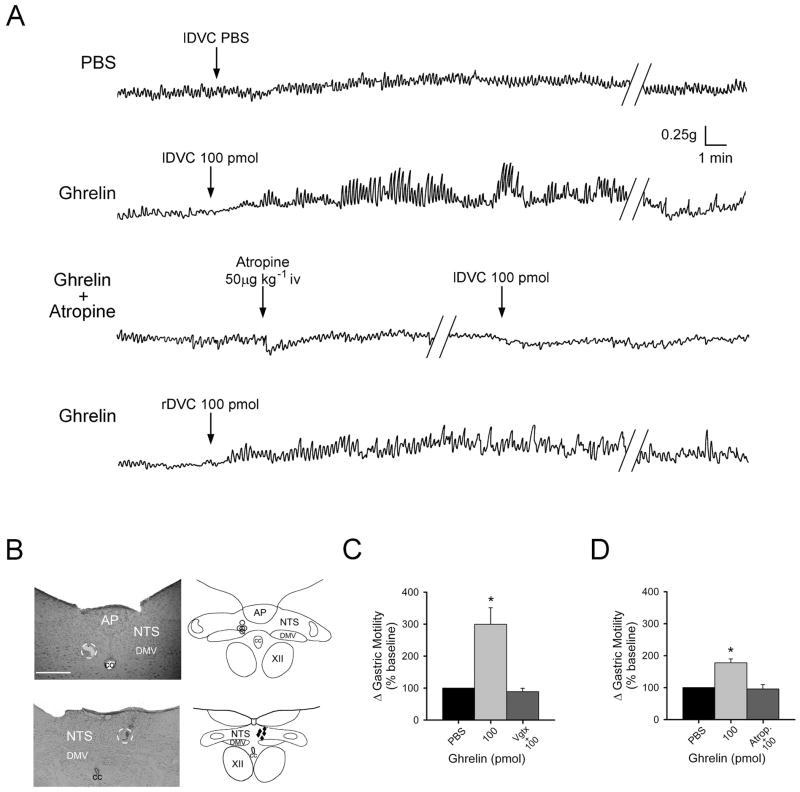
Figure 2. Microinjection of ghrelin in the left dorsal vagal complex (DVC) induces a vagally mediated increase in corpus motility.
A: representative original polygraph records of anterior corpus motility from fasted animals after unilateral microinjection of PBS into the left DVC (lDVC, upper trace), 100 pmol of ghrelin into the lDVC (second trace), intravenous administration of atropine (50 μg kg−1 bolus followed by 20 μg kg−1 hr−1) followed by a repeat lDVC injection of 100 pmol of ghrelin in the presence of systemic atropine (third trace), and unilateral microinjection of 100 pmol of ghrelin into the right DVC (rDVC, bottom trace) of an animal that had received a right cervical vagotomy prior to microinjection.
B: representative photomicrograph (top left) of left brain stem injection site with the injection region circled and schematic representation (top right) of effective injection areas (marked by ○). Representative injection site into the right brainstem (bottom left) with injection region circled and schematic representation (bottom right) of effective injection areas (marked by ◆). For clarity, only a few examples of the distribution of injection sites are depicted. AP, area postrema; NTS, nucleus tractus solitarius; DMV, dorsal motor nucleus of the vagus; CC, central canal; XII, hypoglossus. Calibration 250 μm.
C: graphic summary of the increase in corpus motility induced by microinjection of PBS or ghrelin (100 pmol, expressed as percent increase from baseline) in the left DVC of the cohort of animals prepared for vagotomy (Vgtx; n=12). Following completion of vagotomy, microinjection of ghrelin (100 pmol) no longer elevated corpus motility (* P < 0.05 vs. baseline).
D: graphic summary of the increase in corpus motility induced by microinjection of PBS or ghrelin (100pmol, expressed as percent increase from baseline) in the left DVC of the cohort of animals administered atropine (Atrop; n=5). In the presence of systemic atropine, microinjection of ghrelin (100 pmol) no longer elevated corpus motility (* P < 0.05 vs. baseline).
To verify that DVC microinjection of ghrelin increased corpus motility through the excitation of post-ganglionic cholinergic enteric neurons, peripheral muscarinic receptors were antagonized by the systemic administration of the cholinergic antagonist atropine methyl nitrate. Prior to administration of atropine, microinjection of 100 pmol of ghrelin in the DVC induced a 177±12% increase in corpus motility in the second cohort of rats (P < 0.05; Fig. 2). After 30 min recovery to return to baseline motility, atropine methyl nitrate administration (50 μg kg−1 bolus followed by continuous iv infusion of 25μg kg−1 hr−1) was initiated for 10–15 min prior to microinjection of ghrelin. Under these conditions, baseline motility was only reduced slightly in the presence of continuous systemic atropine (71±13% of baseline values pre-atropine; P > 0.05; data not shown). Following an additional 10 min of continuous atropine infusion, a second ghrelin microinjection into the DVC no longer increased corpus motility above baseline levels (P > 0.05; Fig. 2).
To demonstrate that the in vivo effects of ghrelin are through actions upon NTS neurons, microinjection of 100 pmol of ghrelin was directed at the ipsilateral DVC in rats that had the right cervical vagus transected. Microinjection of 100 pmol of ghrelin in the right DVC induced a 229±37 increase in gastric motility in this cohort of rats (P < 0.05).
Taken together, these data indicate that the ghrelin-mediated increase in corpus motility includes a central mechanism of action within the brainstem vagal pathways. In particular, the actions of ghrelin include a pro-motility effect likely mediated by NTS neurons. Furthermore, the increase in corpus motility includes vagal neurocircuitry that innervates the cholinergic enteric neurons of the stomach.
Ghrelin had no effect upon the membrane properties of DMV neurons
The action of ghrelin (100nM) to alter the membrane properties was assessed in 17 neurons under voltage clamp conditions. Of 11 neurons tested in control conditions, i.e. without TTX, ghrelin had no effect in 7 neurons (8.8±4.3pA; P>0.05) and induced an inward shift in holding current of −52.9±12.9pA in the remaining 4 neurons (P<0.05). In 6 further neurons, the effects of ghrelin were assessed in the presence of TTX (1μM) to block action potential dependent synaptic transmission; in these neurons ghrelin had no effect on holding current (−5.8±5.6pA; P > 0.05), suggesting the ghrelin-induced alterations in holding current observed in the absence of TTX were due to a synaptic-mediated action rather than a direct effect on the DMV neuronal membrane.
Ghrelin increased sEPSC frequency in DMV neurons
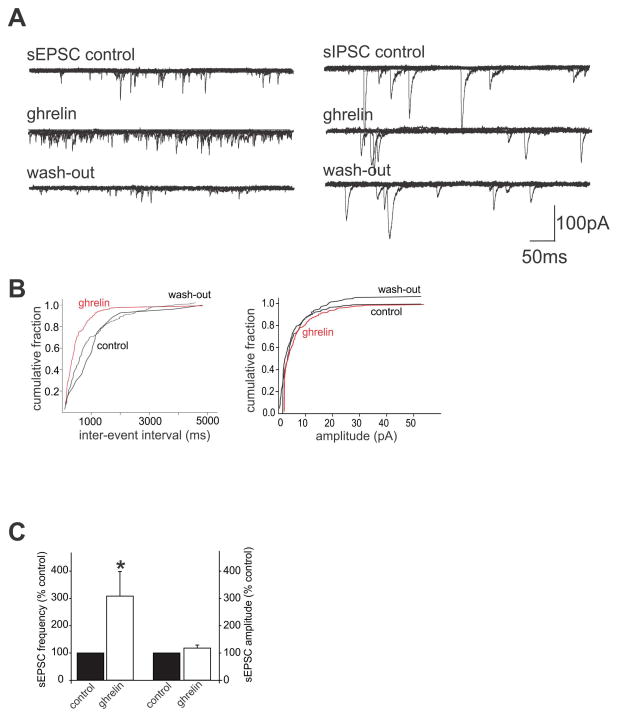
The effects of ghrelin to alter excitatory glutamatergic synaptic transmission was assessed in 6 DMV neurons. In 4 of these neurons, perfusion with ghrelin (100nM) increased the frequency of sEPSCs from 7.5±2.2 to 18.8±6.3 events s−1, i.e. ghrelin increased sEPSC frequency to 308±90% of control (P < 0.05, Fig. 3). The frequency of sEPSCs returned to baseline levels after 20 minutes of wash-out (9.5±4.3 events s−1). In these same neurons, ghrelin had no effect upon the amplitude of sEPSCs (35.6±8.5pA in control versus 44.9±16.6pA in the presence of ghrelin; P > 0.05).
Figure 3. Ghrelin increases the frequency of sEPSCs but not sIPSCs.
A: Six overlapping consecutive traces of sEPSCs (left) and sIPSCs (right) in DMV neurons voltage clamped at −60mV and −50mV, respectively. Ghrelin (100nM) was applied by superfusion for at least 3 minutes, or until the response reached plateau. Note that ghrelin increased the frequency of sEPSCs in a reversible manner, whereas ghrelin had no effect on sIPSC frequency.
B: computer generated graphics from the same neuron in A (left) showing the ghrelin-induced alteration in sEPSC frequency (left) but not amplitude (right).
C: Graphical summary of the effects of ghrelin to increase sEPSC frequency but not amplitude
Ghrelin has no effect upon mEPSC frequency in DMV neurons
Since ghrelin increased the frequency but not amplitude of sEPSCs, this suggested a presynaptic site of action. To confirm that ghrelin was acting at pre- rather than post-synaptic sites, a series of experiments were conducted in the presence of tetrodotoxin (TTX; 1μM) to block action potential-dependent synaptic transmission, i.e., we assessed the effects of ghrelin perfusion on mEPSC amplitude and frequency. In the presence of TTX, mEPSC frequency decreased from 4.3±2.2 to 2.3±1.0 events s−1 (P < 0.05) while mEPSC amplitude was unchanged (33.6±3.4pA in control versus 27.9±0.8pA in the presence of TTX; P > 0.05).
Perfusion with ghrelin had no effect on mEPSC frequency or amplitude in 4 of the 5 neurons tested (2.6±1.2 events s−1 and 29.5±2.1pA, respectively, i.e. 93±5% and 108±10% of mEPSC frequency and amplitude in the absence of ghrelin; P > 0.05). In the remaining neuron, ghrelin increased mEPSC frequency but had no effect on mEPSC amplitude (206% and 104%, respectively). This suggests that, in the majority of neurons, ghrelin increases mEPSC frequency via an increase in action potential dependent synaptic transmission.
Ghrelin has no effect upon sIPSC frequency in DMV neurons
The actions of ghrelin to alter inhibitory GABAergic synaptic transmission was assessed in 7 DMV neurons. Superfusion with ghrelin (100nM) had no effect upon either sIPSC frequency or amplitude in any of the neurons tested, i.e., sIPSC frequency was 3.3±1.1 in control versus 3.4±1.2 events s−1 in the presence of ghrelin while sIPSC amplitude was 60.3±10.5pA in control versus 59.7±9.9pA in the presence of ghrelin (P > 0.05 for both).
Taken together, our electrophysiological data indicate the action potential-dependent effect of ghrelin which is selective for presynaptic glutamate terminals terminating on DMV neurons.
Discussion
In the present study, our experimental data indicate that 1) similar to IVth ventricle application, ghrelin microinjected into the DVC elicits contractions of the gastric corpus; 2) ghrelin increases corpus contractions through the activation of a vagally-mediated cholinergic efferent pathway; 3) in vitro recordings suggest that ghrelin exerts its gastric prokinetic effects by facilitating excitatory synaptic transmission to DMV neurons; and 4) inhibitory synaptic transmission is not affected by ghrelin.
Previous studies report that intravenous or intracerebroventricular (ICV) administration of ghrelin facilitates contractility of the gastric corpus 6,9,12,21. These studies did not fully test the in vivo central effects of ghrelin specifically within the DVC neurocircuitry. For example, ghrelin administration into the lateral ventricle stimulates gastric motility through a neuropeptide Y (NPY) mediated pathway, since immunoneutralization of NPY blocked the effects of i.c.v. ghrelin 12. This specific action of ghrelin is probably mediated through one of many parallel descending systems originating in the hypothalamus and terminating in the DVC 19,22. Furthermore, central effects of NPY upon in vivo gastric motility and in vitro DVC firing appear to be mixed and receptor specific 23–25. The potential interactions between ghrelin and other peptidergic signaling pathways within the DVC are beyond the scope of our studies, but do highlight the complexity of brainstem-gastric neural circuits.
Previous studies have demonstrated that it is the acylated form of ghrelin that induces gastric motility in rats 26. While the half life of acylated ghrelin in response to a single bolus glucagon challenge is relatively short (ca. 10 min) 27, fasting levels of total ghrelin remain elevated during a 24 hour period of negative energy balance 28 and are likely to provide sufficient serum titers to affect DVC control of gastric motility. The presence of detectable ghrelin-immunoreactive nerve terminals in the CNS are controversial (reviewed in 29) and while it is often presumed that peptides do not cross the blood-brain barrier, there is evidence of blood-brain barrier permeability in regions of the NTS 30 and the presence of a saturable ghrelin transporter within the brainstem has been reported 31. Therefore, we propose that the central actions of ghrelin may include the transport of circulating peptide into the DMV.
As with the previous studies which employed ICV administration of ghrelin, our observed increase in corpus motility following application of ghrelin to the fourth ventricle could be attributed to diffusion of ghrelin into the systemic circulation. Peripheral administration of ghrelin to animals that have been vagotomized has been shown to induce antral contractions similar to the fasted state, presumably occurring through activation of ghrelin receptors located in the gastrointestinal tract 12. In the present study, the specificity of the effects of ghrelin on vagal brainstem neurocircuits were confirmed by the failure of repeated application of ghrelin, either to the fourth ventricle or microinjected into the DVC, to induce an increase in corpus motility following vagotomy. Furthermore, recent evidence supports a direct action of ghrelin on other neuronal systems 32 and reports of endogenous ghrelin receptor expression on the neurons of both the NTS and the DMV 16,33 supports a central site of action. We propose that in addition to peripheral or forebrain sites of action, ghrelin also acts directly within brainstem vagal neurocircuitry to increase gastric motility.
Our understanding of the gastric-directed motor output originating in the DMV reveals an increasingly complex level of organization 34. With regard to the stomach, the majority of vagal afferent signaling is directed toward second order neurons within the NTS through a glutamatergic synapse. The neurochemical phenotype of these NTS neurons comprises glutamatergic, GABAergic and/or noradrenergic synapses that ultimately terminate onto DMV neurons. Evidence following application of GABAergic antagonists to the DVC, suggests strongly that it is the NTS GABAergic inputs onto DMV neurons which regulate the basal motor outflow to the stomach tonically 35–39. Studies employing glutamatergic and noradrenergic antagonists within the DVC reveal little effect on basal response in the absence of ancillary reflex or pharmacological manipulation 35,40–42.
Ultimately, gastric motor reflexes are generated based upon the interplay between two separate postganglionic circuits. The parasympathetic preganglionic DMV neurons comprising the efferent vagal limb are cholinergic and activate postganglionic neurons via actions at a nicotinic receptor. Gastric projecting neurons within the DMV exhibit a basal rate of spontaneous action potentials 43–45 and it is this low frequency DMV firing which regulates an excitatory (cholinergic) circuit that is ultimately important to the antral milling of ingested solids and the delivery of reduced particles to the duodenum 46. Gastric relaxation can occur as a consequence of inhibiting this tonically active excitatory pathway 47–51. Conversely, activation of a non-adrenergic non-cholinergic (NANC) inhibitory vagal projection to the stomach potently elicits an inhibition of gastric motor functions. While this inhibition is often attributed to the release of nitric oxide (NO) 47,52–54, purinergic and vasoactive intestinal polypeptide mechanisms have also been identified (reviewed in 55).
Peripheral ghrelin increases gastric motility and emptying via activation of the aforementioned cholinergic efferent vagal pathway 9. In the present study, we confirmed the role of post-ganglionic cholinergic neurons in the centrally-mediated effect of ghrelin on corpus motility by demonstrating that peripheral blockade of muscarinic receptors by atropine blocked the stimulatory effect of ghrelin.
In order to further identify which neuronal pool within the DVC is responsible for our in vivo observations following microinjection of ghrelin, we performed a right cervical vagotomy, followed by microinjection of ghrelin directed at the right (ipsilateral) DVC. The rationale for this approach was based upon the bilateral projection to the DMV by NTS neurons of the caudal medulla and the subsequent ipsilateral efferent output of the DMV 56,57. Since the efferent outflow of ipsilateral DMV neurons was removed by the vagotomy, these injections targeted NTS neurons that also project contralaterally to intact DMV neural projections. Therefore, any preserved change in corpus motility would result from ghrelin acting upon NTS neurons that, in turn, would activate the intact contralateral DMV neurons in order for a change in corpus motility to occur.
Using an in vitro preparation in which a transgenic mouse model expressed enhanced green fluorescent protein under a tyrosine hydroxylase promoter (TH-EGFP), the effects of ghrelin upon identified neurons within the A2 region of the NTS has been investigated recently 58. This study demonstrated that ghrelin acted presynaptically to inhibit glutamatergic transmission, presumably from vagal afferent terminals, to TH-positive NTS neurons. In contrast, ghrelin inhibited glutamatergic transmission to only a minority of either non-TH-positive or pro-opiomelanocortin-positive (POMC+) NTS neurons, suggesting that ghrelin acts in a pathway-specific manner to modulate afferent neurotransmission within the brainstem. In the present study, however, ghrelin increased glutamatergic transmission to rat DMV neurons. While these effects appeared to be mediated presynaptically, since ghrelin modulated the frequency but not the amplitude of spontaneous glutamatergic currents, they were abolished by tetrodotoxin, implying that ghrelin increases glutamatergic synaptic transmission via modulation of action potential dependent neurotransmission. Within the coronal brainstem slice preparation utilized in the present study, the majority of glutamatergic input to DMV neurons arises from the adjacent NTS. It is likely, therefore, that ghrelin is acting to increase activity of NTS neurons, either directly or indirectly, that project to the DMV, although the possibility of effects at other brainstem neurons cannot be excluded. It also must be borne in mind, however, that in the present study, recordings are made from DMV neurons that receive polysynaptic inputs. It is conceivable, therefore, that the increase in excitatory inputs to DMV neurons induced by ghrelin is due to a disinhibition of presynaptic glutamatergic inputs. If ghrelin, for example, decreased the activity of tonically active inhibitory GABAergic or catecholaminergic neurons, as in the study by Appleyard’s group 58, the observed response in the recorded DMV neuron would be an increase in excitatory synaptic inputs. The precise site of action of ghrelin, and the phenotype of presynaptic neuron upon which it acts to regulate vagal efferent outflow, remains to be elucidated.
In conclusion, the present experiments describe the central actions of ghrelin upon gastric-projecting brainstem vagal neurocircuitry. Our observations demonstrate that ghrelin induces corpus motility through the indirect excitation of the preganglionic motor neurons within the DMV. These DMV neurons, in turn, activate the postganglionic cholinergic neurons which are responsible for gastric motility. Our data suggest that, in addition to peripheral activation of the afferent limb of vago-vagal reflex circuitry, circulating ghrelin may directly activate ghrelin receptors within the dorsal vagal complex. We have recently reported that alterations in the sensitivity of DVC neurons to central cholecystokinin in an animal model of spinal cord injury (SCI) 59. We speculate that similar alterations in the sensitivity of vagal neurocircuitry to ghrelin may contribute to gastric dysmotility observed in pathophysiological conditions including SCI.
Acknowledgments
The authors wish to acknowledge their gratitude to Melissa Tong and Emily Qualls-Creekmore who provided valuable technical assistance and generation of data on early portions of this study which were initiated at the Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA 70808
Funding
This work was supported by National Institutes of Health Grants NINDS 49177 (G.M. Holmes), NIDDK 55530 (R. A. Travagli) and NIDDK 78364 (K.N. Browning)
Footnotes
Disclosure
Competing Interests: the authors have no competing interests.
Author contribution
GMH designed the study and protocol, acquired and analyzed in vivo experimental data, and contributed to writing the manuscript. EMS acquired and analyzed in vivo experimental data, performed histological processing, and contributed to writing the manuscript. KNB contributed to the in vitro study design, data generation and analysis as well as the writing of the manuscript. AT contributed to all aspects of the study design and analysis and writing of the manuscript.
Reference List
- 1.Holmes GM, Tong M, Travagli RA. Effects of brainstem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am J Physiol Gastrointest Liver Physiol. 2009 Jan 8;296:G621–G631. doi: 10.1152/ajpgi.90567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. The Journal of Physiology. 2009 Oct 1;587:4749–59. doi: 10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a Novel Growth Hormone-Releasing Acylated Peptide, Is Synthesized in a Distinct Endocrine Cell Type in the Gastrointestinal Tracts of Rats and Humans. Endocrinology. 2000 Nov 1;141:4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 5.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001 Aug;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 6.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterology & Motility. 2007 Aug;19:675–80. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 7.Ariga H, Nakade Y, Tsukamoto K, Imai K, Chen C, Mantyh C, et al. Ghrelin accelerates gastric emptying via early manifestation of antro-pyloric coordination in conscious rats. Regulatory Peptides. 2008 Feb 7;146:112–6. doi: 10.1016/j.regpep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Wang WG, Chen X, Jiang H, Jiang ZY. Effects of ghrelin on glucose-sensing and gastric distension sensitive neurons in rat dorsal vagal complex. Regulatory Peptides. 2008 Feb 7;146:169–75. doi: 10.1016/j.regpep.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000 Oct 5;276:905–8. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 10.Kobashi M, Yanagihara M, Fujita M, Mitoh Y, Matsuo R. Fourth ventricular administration of ghrelin induces relaxation of the proximal stomach in the rat. Am J Physiol Regul Integr Comp Physiol. 2009 Feb 1;296:R217–R223. doi: 10.1152/ajpregu.00878.2007. [DOI] [PubMed] [Google Scholar]
- 11.Fujimiya M, Ataka K, Asakawa A, Chen CY, Kato I, Inui A. Ghrelin, des-acyl ghrelin and obestatin on the gastrointestinal motility. Peptides. 2011 Nov;32:2348–51. doi: 10.1016/j.peptides.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol (Lond) 2003 Jan 1;550:227–40. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002 Oct;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 14.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006 Oct;4:323–31. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic Effects of Brainstem Ghrelin Administration. Diabetes. 2003 Sep 1;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Matsumura K, Fukuhara M, Kagiyama S, Fujii K, Iida M. Ghrelin Acts at the Nucleus of the Solitary Tract to Decrease Arterial Pressure in Rats. Hypertension. 2004 May 1;43:977–82. doi: 10.1161/01.HYP.0000122803.91559.55. [DOI] [PubMed] [Google Scholar]
- 17.Ormsbee HS, Bass P. Gastroduodenal motor gradients in the dog after pyloroplasty. American Journal of Physiology. 1976;230:389–97. doi: 10.1152/ajplegacy.1976.230.2.389. [DOI] [PubMed] [Google Scholar]
- 18.Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Res. 2005;1052:139–46. doi: 10.1016/j.brainres.2005.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, et al. Presynaptic Melanocortin-4 Receptors on Vagal Afferent Fibers Modulate the Excitability of Rat Nucleus Tractus Solitarius Neurons. Journal of Neuroscience. 2008 May 7;28:4957–66. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Browning KN, Zheng Z, Gettys TW, Travagli RA. Vagal afferent control of opioidergic effects in rat brainstem circuits. J Physiol. 2006;575:761–76. doi: 10.1113/jphysiol.2006.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. European Journal of Pharmacology. 2005 May 16;515:160–8. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, et al. Role of Dorsomedial Hypothalamic Neuropeptide Y in Modulating Food Intake and Energy Balance. The Journal of Neuroscience. 2009 Jan 7;29:179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobashi M, Shimatani Y, Shirota K, Xuan SY, Mitoh Y, Matsuo R. Central neuropeptide Y induces proximal stomach relaxation via Y1 receptors in the dorsal vagal complex of the rat. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2006 Feb;290:R290–R297. doi: 10.1152/ajpregu.00423.2005. [DOI] [PubMed] [Google Scholar]
- 24.Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil. 2009 Dec;21:1309–e126. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997 Jun;9:109–16. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi H, Ariga H, Zheng J, Ludwig K, Takahashi T. Effects of ghrelin on interdigestive contractions of the rat gastrointestinal tract. World J Gastroenterol. 2008 Nov 7;14:6299–302. doi: 10.3748/wjg.14.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katayama T, Shimamoto S, Oda H, Nakahara K, Kangawa K, Murakami N. Glucagon receptor expression and glucagon stimulation of ghrelin secretion in rat stomach. Biochem Biophys Res Commun. 2007 Jun 15;357:865–70. doi: 10.1016/j.bbrc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, et al. Delayed Short-Term Secretory Regulation of Ghrelin in Obese Animals: Evidenced by a Specific RIA for the Active Form of Ghrelin. Endocrinology. 2002 Sep 1;143:3341–50. doi: 10.1210/en.2002-220225. [DOI] [PubMed] [Google Scholar]
- 29.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. 2012 May;9:271–85. doi: 10.1038/nrgastro.2012.57. [DOI] [PubMed] [Google Scholar]
- 30.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 1990 Dec 1;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- 31.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. Journal of Pharmacology and Experimental Therapeutics. 2002 Aug 1;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 32.Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, et al. Ghrelin Promotes and Protects Nigrostriatal Dopamine Function via a UCP2-Dependent Mitochondrial Mechanism. Journal of Neuroscience. 2009 Nov 11;29:14057–65. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006 Jan 20;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterology & Motility. 2010;22:1154–63. doi: 10.1111/j.1365-2982.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil. 1998 Aug;10:305–13. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 36.Browning KN, Travagli RA. Mechanism of action of baclofen in rat dorsal motor nucleus of the vagus. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2001 Jun 1;280:G1106–G1113. doi: 10.1152/ajpgi.2001.280.6.G1106. [DOI] [PubMed] [Google Scholar]
- 37.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2011 Jan 1;300:G21–G32. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman MA, Cruz MT, Sahibzada N, Verbalis J, Gillis RA. GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am J Physiol Gastrointest Liver Physiol. 2009 Jan 1;296:G101–G111. doi: 10.1152/ajpgi.90504.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman MA, Alayan A, Sahibzada N, Bayer B, Verbalis J, Dretchen KL, et al. μ-Opioid receptor stimulation in the medial subnucleus of the tractus solitarius inhibits gastric tone and motility by reducing local GABA activity. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2010 Aug 1;299:G494–G506. doi: 10.1152/ajpgi.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira M, Jr, Sahibzada N, Shi M, Panico W, Niedringhaus M, Wasserman A, et al. CNS Site of Action and Brainstem Circuitry Responsible for the Intravenous Effects of Nicotine on Gastric Tone. Journal of Neuroscience. 2002 Apr 1;22:2764–79. doi: 10.1523/JNEUROSCI.22-07-02764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira M, Jr, Sahibzada N, Shi M, Niedringhaus M, Wester MR, Jones AR, et al. Hindbrain chemical mediators of reflex-induced inhibition of gastric tone produced by esophageal distension and intravenous nicotine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1482–R1495. doi: 10.1152/ajpregu.00003.2005. [DOI] [PubMed] [Google Scholar]
- 43.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol. 1999 Jun 1;517(Pt 2):521–32. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991 Mar;260:G531–G536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- 45.Marks JD, Donnelly DF, Haddad GG. Adenosine-induced inhibition of vagal motoneuron excitability: receptor subtype and mechanisms. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1993 Feb 1;264:L124–L132. doi: 10.1152/ajplung.1993.264.2.L124. [DOI] [PubMed] [Google Scholar]
- 46.Malagelada J-R, Azpiroz F. Determinants of gastric emptying and transit in the small intestine. In: Wood J, editor. Handbook of physiology. 2. Bethesda: American Physiological Society; 1989. pp. 909–37. [Google Scholar]
- 47.Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973;390:1–38. [PubMed] [Google Scholar]
- 48.Abrahamsson H, Jansson G. Elicitation of reflex vagal relaxation of the stomach from pharynx and esophagus in the cat. Acta Physiol Scand. 1969 Sep;77:172–8. doi: 10.1111/j.1748-1716.1969.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 49.Gillis RA, Quest JA, Pagani FD, Norman WP. Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. Bethesda, MD: The American Physiological Society; 1989. Control centers in the central nervous system for regulating gastrointestinal motility; pp. 621–83. [Google Scholar]
- 50.McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol. 1992;453:401–11. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCann MJ, Rogers RC. Functional and chemical neuroanatomy of a gastric vago-vagal reflex. In: Tache Y, Wingate DL, Burks TF, editors. Innervation of the Gut: Pathophysiological Implications. Boca Raton: CRC Press; 1994. pp. 81–92. [Google Scholar]
- 52.Jansson G. Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiol Scand. 1969 Jan;75:245–52. doi: 10.1111/j.1748-1716.1969.tb04376.x. [DOI] [PubMed] [Google Scholar]
- 53.Krowicki ZK, Sivarao DV, Abrahams TP, Hornby PJ. Excitation of dorsal motor vagal neurons evokes non-nicotinic receptor-mediated gastric relaxation. J Auton Nerv Syst. 1999 Sep 24;77:83–9. [PubMed] [Google Scholar]
- 54.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997 Oct 15;504:479–88. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what’s new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- 56.Barnes KL, McQueeney AJ, Barrett WR, Knowles WD. Morphology and projections of neurobiotin-labeled nucleus tractus solitarii neurons recorded in vitro. Brain Research Bulletin. 1994;34:339–48. doi: 10.1016/0361-9230(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 57.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. The Journal of Comparative Neurology. 1982;211:248–64. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- 58.Cui RJ, Li X, Appleyard SM. Ghrelin Inhibits Visceral Afferent Activation of Catecholamine Neurons in the Solitary Tract Nucleus. The Journal of Neuroscience. 2011 Mar 2;31:3484–92. doi: 10.1523/JNEUROSCI.3187-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong M, Qualls-Creekmore E, Browning KN, Travagli RA, Holmes GM. Experimental spinal cord injury in rats diminishes vagally-mediated gastric responses to cholecystokinin-8s. Neurogastroenterology & Motility. 2011;23:e69–e79. doi: 10.1111/j.1365-2982.2010.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]