Abstract
Background
Standard therapy for older patients with AML has a poor outcome. We have designed a combination of clofarabine plus low-dose cytarabine followed by a prolonged consolidation alternating with decitabine.
Methods
Sixty patients with a median age of 70 years (range 60-81) with newly diagnosed AML were included. They received clofarabine 20mg/m2 intravenously daily × 5 days plus cytarabine 20mg subcutaneously twice daily × 10 days. Responding patients continued for up to 17 courses of consolidation therapy including decitabine.
Results
Forty of 59 evaluable patients responded (66%). Complete remission rate was 58%. Median relapse-free survival (RFS) was 14.1 (95% CI: 6.9-not estimable) and median overall survival (OS) 12.7 months (95% CI: 8.8-not estimable). Median OS of responding patients (CR/CRp) was 24.2 months (95% CI: 17-not estimable). Compared to a historical group of patients who received clofarabine plus low-dose cytarabine with a shorter consolidation, RFS was not statistically different. Induction mortality was low (7% at 8 weeks) and toxicities manageable.
Conclusions
Clofarabine plus low-dose cytarabine alternating with decitabine in consolidation is active in older patients with newly diagnosed AML. The benefits of a prolonged consolidation remain unproven.
Keywords: acute myeloid leukemia, clofarabine, decitabine, cytarabine, induction therapy
Introduction
Therapy for newly diagnosed patients ≥ 60 years with acute myeloid leukemia (AML) remains challenging with low response rates, short durability of responses, and a high risk of treatment-related toxicities following standard dose-intensive therapy.1,2 The recent years have therefore seen a heightened level of activity in the exploration of new drugs and lower-intensity approaches.
Clofarabine is a deoxyadenosine nucleoside analog with Food and Drug Administration (FDA) approval for children with relapsed acute lymphoblastic leukemia (ALL). The recommended phase 2 dose of clofarabine for adults with acute leukemias was 40 mg/m2 intravenously daily for 5 days.3 However, two large multicenter studies, from the United States and Europe, respectively, have since shown that lower doses of clofarabine can improve the toxicity profile while still demonstrating activity in the up-front treatment of newly diagnosed older patients with AML.4,5
We have shown in a randomized trial that the combination of lower dose clofarabine with low dose cytarabine produced higher response rates with a comparable safety profile compared to single agent clofarabine. 6 However, beyond achieving high remission rates, the ultimate goal is to improve survival. The current study was therefore designed with the following rationale: 1) to deliver lower doses of clofarabine than in the previous study; 2) to expand the duration of therapy; and 3) to provide multiple drugs with different mechanisms of action to prevent cross-resistance. As additional drug to be administered during consolidation we chose the DNA methyltransferase (DNMT) inhibitor decitabine. It can be delivered at low doses with acceptable toxicity and with activity in AML.7,8 We also compare survival and relapse-free survival between patients on the current study with a group of patients who received the combination therapy in a previous protocol where the number of consolidation cycles was shorter and DNMT inhibitors were not used.6
Patients and Methods
Patients
Sixty patients were enrolled between October 2008 and January 2010 of whom 59 are evaluable for response. Patients were eligible if they were ≥ 60 years of age with a diagnosis of previously untreated AML (based on World Health Organization [WHO] criteria) or high-risk myelodysplastic syndrome (MDS; ≥ 10% blasts or ≥ intermediate-2 by the International Prognostic Scoring System [IPSS]). Prior therapy with hydroxyurea, biological or targeted therapy was allowed. Nobody received prior clofarabine or decitabine although previous use of azacitidine was permissible. Additional requirements included an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2 and adequate organ function (serum total bilirubin ≤ 2 mg/dL, alanine aminotransferase [ALT] or aspartate aminotransferase [AST] ≤ 4 × of the upper limit of normal, serum creatinine ≤ 2 mg/dL, and cardiac ejection fraction [by either echocardiography or multigated acquisition {MUGA} scan] of > 40%). The study was approved by the Institutional Review Board (IRB) of The University of Texas MD Anderson Cancer Center (MDACC) and was conducted in the accordance with the basic principles of the Declaration of Helsinki. All patients provided written informed consent according to institutional guidelines.
Treatment design and monitoring
Induction therapy consisted of clofarabine 20 mg/m2 by intravenous infusion daily for five days on days 1 to 5 plus cytarabine 20 mg subcutaneously twice daily for 10 days on days 1 to 10. On days 1 through 5 clofarabine preceded the cytarabine injections by about 3 to 4 hours.6 Patients who did not achieve a complete remission could receive one re-induction cycle at the same dose and schedule but not before at least 28 days had passed after start of cycle 1. In the case of persistent disease following re-induction, patients could proceed with decitabine 20 mg/m2 as a one to two hour intravenous infusion daily for 5 days on days 1 to 5 as an alternative attempt to achieve a remission. Once in remission, patients would receive up to 17 cycles of consolidation therapy. Consolidation was administered in blocks of three cycles where clofarabine plus cytarabine at an abbreviated schedule alternated with decitabine (Figure 1). Consolidation cycles were repeated every 4 to 7 weeks depending on hematopoietic recovery (absolute neutrophil count [ANC] ≥ 1×109/L and platelet count ≥ 50 × 109/L) and resolution of toxicities (any non-hematologic toxicity had to return to at least grade 1).
Figure 1.
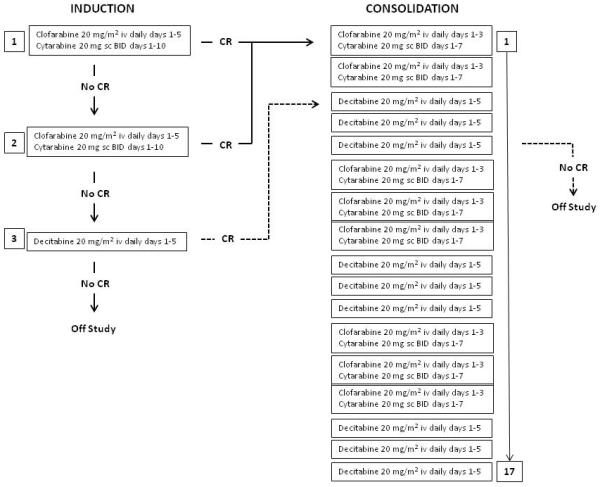
Treatment flow diagram
It was recommended that all patients receive cycle 1 of the induction in a laminar air flow room where they stayed hospitalized for the duration of the induction (on average 30 days). Patients received anti-infectious prophylaxis consisting of levofloxacin, valacyclovir, and voriconazole (or equivalent). To avoid hepatotoxicity, he latter was held on the first 5 days while clofarabine was administered. Hematopoietic growth factors (e.g. erythropoietin, filgrastim, pegfilgrastim, sagromostim) were used at the discretion of the treating physician. Anti-emetic therapy was routinely provided as appropriate. The patients’ fluid status, hepatic, and renal function were carefully monitored daily during the drug administration period.
Patients were monitored with complete blood count (CBC), differential and platelet count, and chemistry profile daily during induction and then at least weekly (CBC) or every 2 to 4 weeks (chemistry) as long as receiving drug therapy. Repeat marrow aspirates were performed starting on day 21 and then at least every 2 weeks until confirmation of remission or nonresponse. For most patients this occurred within 42 days.
Response criteria
Response was assessed based on criteria by the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia.12 Complete remission (CR) required an ANC of ≥ 1× 109/L, platelet count of ≥ 100×109/L, and marrow blasts ≤ 5%. CRp was defined as CR but with platelet counts < 100×109/L. Any other response was considered treatment failure.
Statistical Considerations
The primary objective of this phase 2 trial was to determine relapse-free (RFS) and overall survival (OS). Stopping boundaries were developed for monitoring efficacy and safety. Patient characteristics were summarized using frequency (percentage) for categorical variables and median (range) for continuous variables. Fisher’s exact test was used to assess the differences in categorical variables between patients accrued on the historical and current trials, respectively. Wilcoxon rank-sum test was used to compare continuous variables. OS was defined as the time interval between the date of treatment and the date of death due to any cause or last follow-up date, whichever occurred first. Among patients who achieved CR or CRp, RFS was defined as the time interval between the date of response (i.e., CR or CRp) and the date of relapse or date of death, whichever occurred first. CR or CRp patients who were alive and relapse-free were censored at the off-study date. OS and RFS were estimated using the method of Kaplan and Meier.9 Log-rank test was used to compare OS or RFS between patients treated in the two trials.10 Univariate and multivariable Cox proportional hazards models were fit to compare OS or RFS between patients treated in these two studies, after adjusting for other patient characteristics or clinical factors. All statistical analyses were performed using SAS and Splus.11
Results
Study Group
Characteristics for all patients (current and historical) are summarized in Table 1. Among the current group of patients, all had a diagnosis of AML (WHO).12 Patients with unfavorable pretreatment characteristics included those with age ≥ 75 years (29%), an ECOG performance status of 2 (18%), secondary AML with an antecedent hematologic disorder (AHD) (23%), and patients with complex cytogenetic abnormalities (33%). Of the 14 patients with AHD, 11 had a diagnosis of MDS. Four of these patients received prior azacitidine (one in combination with an investigational histone deacetylase inhibitor). Other treatments for MDS, which preceded enrollment into the study included hematopoietic growth factors (erythropoietin, darbepoietin, filgrastim), lenalidomide, cyclosporine and prednisone (in one patient with hypoplastic MDS), and the PR-1 vaccine. Two patients had a preceding diagnosis of chronic myelomonocytic leukemia (CMML) and one of a myeloproliferative neoplasm (MPN), not otherwise specified. The latter three patients received hydroxyurea at some point prior. The other 18 patients with non-hematologic preexisting malignancies received either chemotherapy, radiation, or both. Patients who were treated with surgery only were not included in this group.
Table 1.
Patient Characteristics
| Variable | Current | Historical |
|---|---|---|
| N | 60 | 79 |
| Age in yrs, median (range) | 69.5 (60-81) | 70 (60-82) |
| Age ≥ 75 yrs, N (%) | 17 (29) | 20 (25) |
| ECOG performance status 2, N (%) | 11 (18) | 11 (14) |
| AML diagnosis, N (%) | 60 (100) | 75 (95)* |
| Secondary AML, N (%) | 14 (23) | 18 (23) |
| Cytogenetics, N (%) | ||
| Diploid, −Y | 26 (43) | 40 (41) |
| −5, −7 | 19 (32) | 19 (24) |
| Others | 15 (25) | 20 (25) |
| FLT3/ITD positive | 7 (13) | 9 (12) |
| WBC 109/L, median (range) | 2.2 (0.4-61.2) | 2.8 (0.8-433) |
| Hemoglobin g/dL, median (range) | 8.8 (5-13.4) | 8.9 (4-12.9) |
| Platelets 109/L, median (range) | 47 (6-416) | 64 (8-300) |
| % PB blasts, median (range) | 7 (0-97) | 4 (0-93) |
| % BM blasts, median (range) | 40 (7-95) | 43 (9-94) |
Two patients with MDS/RAEB-1 and CMML, respectively
p values are non-significant for any variable
WBC, white blood cell count; PB, peripheral blood; BM, bone marrow
Outcome
Response
Fifty-nine patients are evaluable for response. One patient elected not to continue and was taken off study by day 6 before a response assessment was possible. Forty patients (66%) responded: 35 (58%) achieved CR and 5 (8%) CRp. Seven patients (18%; 5 with CR and 2 with CRp) required at least 2 cycles to respond. Median time to CR was 38 days (range 27 to 103). For patients with CRp, median time to establishing the response was 83 days (range 25 to 177).
Responding patients received a median of 4 consolidation cycles (range 0 to 17). Eight (20%) patients received at least 10 consolidation cycles and consolidation therapy is still ongoing in 14 (35%) of patients.
Responses by subgroup are summarized in Table 2. Responses (CR and overall response rate) were numerically lower in patients with an AHD and complex cytogenetic abnormalities. On the other hand, all of the 7 patients with a FLT3/ITD abnormality responded, which included CR in 6 (86%).
Table 2.
Response Rates (%) by Patient Subgroups
| Age | Performance status |
Primary vs AHD |
Karyotype | FLT3/ITD status |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 70 | ≥ 70 | < 2 | 2 | 1 ° | AHD | Dip | Com | Neg | Pos | |
| N | 29 | 31 | 49 | 11 | 30 | 14 | 30 | 20 | 53 | 7 |
| CR | 59 | 58 | 57 | 64 | 70 | 36 | 70 | 45 | 55 | 86 |
| CRp | 7 | 10 | 8 | 9 | 10 | 14 | 7 | 0 | 8 | 14 |
| OR | 66 | 68 | 65 | 73 | 80 | 50 | 77 | 45 | 63 | 100 |
AHD, antecedent hematologic disorder; Dip, diploid; Com, complex; neg, negative; pos, positive
Relapse-free and Overall Survival
The median follow up is 19.6 months. Among the 40 patients who have achieved CR/CRp, 24 patients (60%) relapsed or died later on and the median RFS was 14.1 months (95% CI: 6.9 – not estimable). Among the 60 patients, 34 (57%) died and the median OS was 12.7 months (95% CI: 8.8 – not estimable). Median OS of responding patients only (CR/CRp) was 24.2 months (95% CI: 17.0 – not estimable) (Figure 2).
Figure 2.
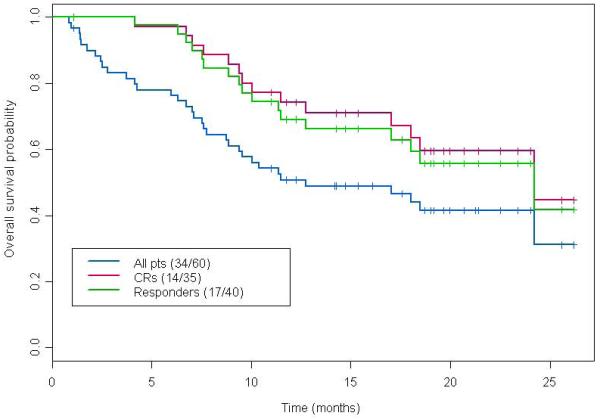
Kaplan-Meier estimates for OS by response status
We compared OS and RFS to a historical group of 79 patients. This group of patients was enrolled on a separate clinical study from August 2004 to June 2006 and received induction therapy with clofarabine 30 mg/m2 intravenously daily for 5 days plus cytarabine 20 mg/m2 subcutaneously daily for 14 days. Consolidation consisted of 3 days of clofarabine and 7 days of cytarabine without inclusion of a hypomethylating agent.6 All other supportive care and monitoring parameters were the same. There were no statistically significant differences in the characteristics between the two patient groups (Table 1). Response rates in the historical group were also statistically similar (CR 62%, CRp 5%, ORR 67%).
With a median follow up of 62.9 months, the median RFS of the historical group of patients was 9.3 months (95% CI: 6.2 – 12 months). There was no significant difference between the current and historical group of patients with respect to RFS (p-value = 0.24; log-rank test) (Figure 3). Table 3 shows the fitted univariate Cox proportional hazards regression models for RFS, which suggests that older age was significantly associated with an increased risk of relapse or death following achievement of CR/CRp. The fitted multivariate Cox model for RFS suggests that after adjusting for age, there was no significant difference between patients treated on the current protocol and the historical patient group.
Figure 3.
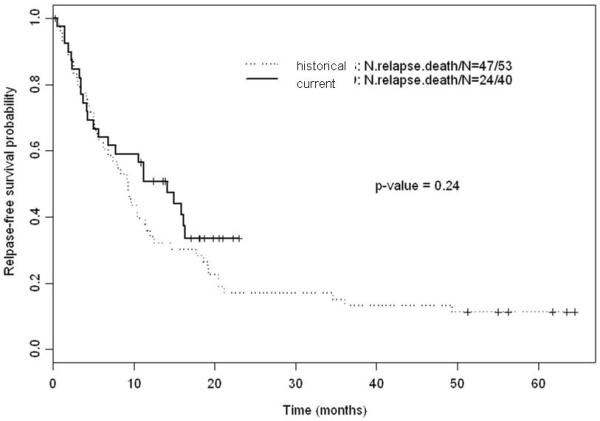
Kaplan-Meier estimates for RFS
Table 3.
Univariate Cox Proportional Hazards Model for RFS
| Variable | Estimate | SE | HR | P- value |
N | Relapse or death (N) |
|---|---|---|---|---|---|---|
| Age | 0.070 | 0.021 | 1.072 | 0.001 | 93 | 71 |
| log(WBC) | 0.143 | 0.078 | 1.154 | 0.068 | 93 | 71 |
| Hemoglobin | 0.039 | 0.070 | 1.039 | 0.580 | 93 | 71 |
| log(PLT) | 0.089 | 0.137 | 1.093 | 0.514 | 93 | 71 |
| log(PB blast) | 0.144 | 0.077 | 1.154 | 0.062 | 93 | 71 |
| BM blast | −0.002 | 0.005 | 0.997 | 0.632 | 93 | 71 |
| Male (vs. female) | 0.045 | 0.243 | 1.046 | 0.851 | 93 | 71 |
| AHD (vs. primary AML) | 0.314 | 0.333 | 1.369 | 0.346 | 93 | 71 |
| Cyto=Diploid (vs. others) | −0.128 | 0.238 | 0.879 | 0.591 | 93 | 71 |
| FLT3=pos (vs. neg) | 0.322 | 0.405 | 1.381 | 0.425 | 87 | 67 |
| PS=2 (vs. <2) | 0.508 | 0.319 | 1.662 | 0.111 | 93 | 71 |
| Current vs historical | −0.296 | 0.255 | 0.743 | 0.246 | 93 | 71 |
WBC, white blood cell count; PLT, platelet count; PB, peripheral blood; BM, bone marrow; AHD, antecedent hematologic disorder; PS, performance status.
The median OS of the 79 historical patients was 11.5 months (95% CI: 8.4 – 18.2 months) with no significant difference between the current and historical group of patients (p-value = 0.4; log-rank) (Figure 4). In a fitted univariate Cox proportional hazards regression model for OS (including age, white blood cell count, hemoglobin, platelet count, circulating blasts, gender, secondary AML, karyotype, FLT3 status, performance status and current versus historical group of patients) older age, higher white blood cell count, higher numbers of circulating blasts, secondary AML, karyotype other than diploid and a performance status of 2 were significantly associated with an increased risk of death. Only after adjusting in a fitted multivariable Cox model for older age, higher circulating blast numbers, secondary AML, and a performance status of 2, and after inclusion of baseline cytogenetics (although not statistically significant in the multivariate analysis) in an alternative multivariable Cox model, did the current group of patients demonstrate a better OS (p-value 0.02) (Table 4).
Figure 4.
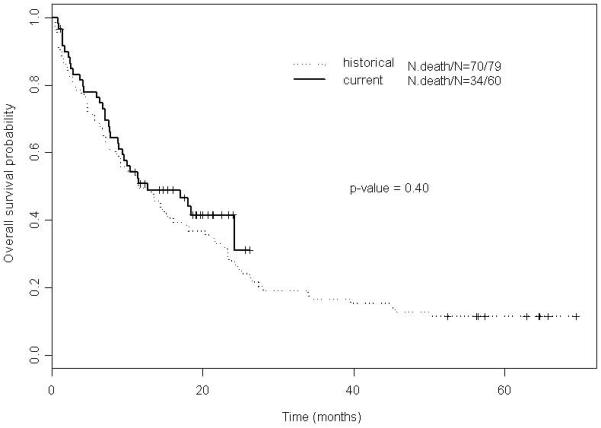
Kaplan-Meier estimates for OS
Table 4.
Alternative Multivariable Cox Proportional Hazards Model for OS (Cytogenetics Added)
| Variable | Coefficient | SE | HR | P-value |
|---|---|---|---|---|
| Age | 0.04 | 0.02 | 1.04 | 0.04 |
| Log (PB blast) | 0.22 | 0.06 | 1.24 | 0.001 |
| AHD (vs. primary AML) | 1.13 | 0.26 | 3.09 | <.0001 |
| PS=2 (vs. <2) | 0.96 | 0.28 | 2.62 | 0.001 |
| Cyto=good (vs. Others) | −0.27 | 0.21 | 0.76 | 0.20 |
| Current vs historical | −0.56 | 0.23 | 0.57 | 0.02 |
Adverse Events
Adverse events are summarized in Table 5 and grade and frequency. Most toxicities did not exceed grade 2. Gastro-intestinal-related adverse events including nausea and vomiting, increases of total bilirubin and transaminases, and diarrhea were observed most frequently, followed by skin rashes including palmoplantar dyserythesias in few patients.
Table 5.
Adverse Events (Frequency ≥ 10%)
| Adverse Event | Grade 1-2 (%) | Grade 3-4 (%) |
|---|---|---|
| Nausea | 62 | - |
| ALT increase | 57 | 10 |
| Skin rash | 58 | - |
| Bilirubin increase | 52 | 3 |
| Diarrhea | 30 | 2 |
| Vomiting | 20 | - |
| AST increase | 13 | 3 |
| Creatinine increase | 12 | 2 |
| Mucositis | 12 | - |
| Headache | 12 | - |
| Hand-foot-syndrome | 7 | 2 |
Seven patients died while on study. Four of these patients (7%) died within the first 8 weeks of study enrollment. No early deaths within the first 14 days occurred. Only one patient died while undergoing consolidation therapies and still in CR. Causes of deaths were infection-related secondary to myelosuppression in all patients.
Discussion
The combination of clofarabine with low-dose cytarabine followed by a prolonged consolidation with alternating decitabine achieved a response rate of 66% (58% CR), median RFS of 14.1 months, and median overall survival of 12.7 months. Median overall survival of responding patients (CR/CRp) was 24.2 months. Eight week mortality was 7% and most patients had manageable toxicities of grade ≤ 2.
The study design was based on our previous experience with the combination of clofarabine and low-dose cytarabine alone without the addition of decitabine. The rationale of this study was to attenuate induction doses to minimize early mortality, be able and deliver a more extended post-remission therapy, and include a third drug with activity in AML which is nn cross-resistant and might circumvent build-up of drug resistance to the two-drug combination. The first two goals have been largely achieved: induction mortality (measured at 8 weeks to incorporate effects of drug-related toxicity and of resistant disease) has been low and whereas a fifth of the patients received at least 10 consolidation cycles, consolidation is ongoing in another third of the patients. Hence, it is feasible to provide extended therapy with this regimen. As for the third goal, circumvention of drug resistance by adding additional drugs (in this case decitabine), it remains largely speculative. It will also remain difficult to answer in the absence of a direct randomized comparison. We therefore compared the long-term outcome of patients who received clofarabine and low-dose cytarabine in a previous study without decitabine with the current study.6 It needs to be emphasized however that there were other differences in the historical group: the induction dose of clofarabine was higher (30 mg/m2 daily × 5 days), the schedule of low-dose cytarabine was different (20 mg/m2 daily × 14 days), and the number of consolidation cycles administered was lower (median of 2). With these caveats in mind, response rates were identical although induction mortality appeared higher (19%) in the historical group. More importantly, RFS and overall survival were not different unless an alternative multivariable Cox model was applied that included information from baseline cytogenetics.
Comparisons with induction therapies that use single agent decitabine only are also limited. The two studies by Cashen et al. and Blum et al., respectively differed in the number of days of decitabine per cycle (5 versus 10).7,8 Whereas the overall response with the 5-day schedule has been only 25% (24% CR), the overall response rate with the 10-day schedule has been 64% (47% CR). The differences extended to median survival: 7.7 months with the 5-days schedule (14 months for responders only) versus 13 months (no information regarding responders only) with the 10-day schedule.
One of the major issues, which are debated with regard to induction therapy for older patients with AML is the value of “standard” or “intensive therapy”. The historical experience based on the “3+7” schedule is sobering. CR rates are typically below 50%, most patients relapse quickly, and 3-year survival expectations are < 10%.13,14 The value of intensive chemotherapy for older patients has been recently analyzed in a retrospective study by Kantarjian et al.1 Of 446 patients ≥ 70 years of age who have been treated with cytarabine-based therapy, CR rate was 45%, 8-week mortality 36%, median survival 4.6 months, and 1-year survival probability 28%. Juliusson from the Swedish AML Group argued against the conclusion that intensive therapy benefits only few older patients with AML. 15 Data from the Swedish Acute Leukemia Registry showed that 55% of 70 to 79 year olds received intensive therapy and half of those treated achieved CR. Outcomes were clearly better when compared with patients who opted for or received only palliative care. On the other hand, the registry experience does not provide any comparsions of different treatment approaches with each other and it remains therefore impossible to assess the value of established, intensive therapy vis-à-vis novel therapies.
Despite the conservative doses, this treatment causes myelosuppression with its accompanying risks of infectious complications and has a number of other adverse events typical for chemotherapy regimens (see Table 5). To treat patients with this combination requires meticulous observation and follow up. We admitted all our patients to a laminar airflow room during their first induction cycle and all received broad-spectrum antibiotic prophylaxis (antibacterials, antifungals, and antivirals). It does however provide a different approach based on attenuated induction and consolidation doses (validated by low mortality rates), changing drugs during consolidation, and a prolonged number of consolidation cycles. Both remission and survival rates confirm the activity of this regimen. Where it will be positioned among other approaches and in comparison to conventional therapy (such as “3+7”) cannot be answered from our study, but requires a more concerted effort of a larger randomized study. None of these regimens are mutually exclusive either. With better definitions of subsets of patients, there is likely to be a role for conventional therapy in some whereas investigational therapies are more appropriate for others. In this respect various prognostic models aid the decision making process and investigators should be encouraged to utilize them.16-18
Acknowledgments
SF designed the research, included patients, collected data, analyzed data, wrote the manuscript, proofread the protocol; FR included patients, collected data, proofread the protocol; XH and XW designed the research, analyzed data, proofread the protocol; EJ included patients, collected data; GGM included patients, collected data; TK included patients, collected data; AF included patients, collected data; MK included patients, collected data; GB included patients, collected data; JB included patients, collected data; JF collected data; proofread the protocol; HMK designed the research, included patients, collected data.
Footnotes
Financial disclosures SF received research funding from Genzyme and Eisai and has participated in advisory board meetings conducted by Genzyme; JB received research funding from Genzyme; HMK received research funding from Genzyme and has participated in advisory board meetings conducted by Genzyme;
References
- 1.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller GJ. When a gold standard is made of tin. Blood. 2010;116:4386–4387. doi: 10.1182/blood-2010-08-300566. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AK, Russell NH, Kell J, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 6.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodyspastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashen AF, Schiller GJ, O’Donnell, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 8.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 10.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;60:163–170. [PubMed] [Google Scholar]
- 11.Cox DR. Regression models and life tables (with discussion) J. R. Statistical Soc B. 1972;34:187–220. [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Estey EH. How I treat patients with AML. Blood. 2000;96:1670–1673. [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. Acute myeloid leukemia. 2008;V.1 http://www.nccn.org/professionals/phsyician_gls/PDF/aml.pdf. [Google Scholar]
- 15.Juliusson G. Most 70- to 79-year old patients with acute myeloid leukemia do benefit from intensive treatment. Blood. 2011;117:3473. doi: 10.1182/blood-2010-11-321737. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 17.Malfuson JV, Etienne A, Turlure P, et al. Haematologica. 2008;93:1806–1813. doi: 10.3324/haematol.13309. [DOI] [PubMed] [Google Scholar]
- 18.Krug U, Röllig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukemia: a web-based application for prediction of outcomes. Lancet. 2010;376:2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]


