Abstract
Vitamin A is essential for diverse aspects of life ranging from embryogenesis to the proper functioning of most adult organs. Its derivatives (retinoid) have potent biological activities such as regulating cell growth and differentiation. Plasma retinol binding protein (RBP) is the specific vitamin A carrier protein in the blood that binds to vitamin A with high affinity and delivers it to target organs. A large amount of evidence has accumulated over the past decades supporting the existence of a cell surface receptor for RBP that mediates cellular vitamin A uptake. Using an unbiased strategy, this specific cell-surface RBP receptor has been identified as STRA6, a multitransmembrane domain protein with previously unknown function. STRA6 is not homologous to any membrane receptors, channels and transporters of known function and represents a new type of cell-surface receptor. Consistent with the diverse functions of vitamin A, STRA6 is widely expressed in embryonic development and in adult organ systems. Mutations in human STRA6 are associated with severe pathological phenotypes in many organs such as the eye, brain, heart, and lung. STRA6 binds to RBP with high affinity and mediates vitamin A uptake into cells. This review summarizes the history the RBP receptor research, its expression in the context of known functions of vitamin A in distinct human organs, structure/function analysis of this new type of membrane receptor, pertinent questions regarding its very existence, and its potential implication in treating human diseases.
Keywords: Vitamin A, Retinoid, RBP, STRA6, Membrane Receptor, Retinol, Anophthalmia, Mental Retardation
1. Introduction
The molecular mechanism for vitamin A's physiological function was first elucidated for vision (Wald, G., 1968). Vitamin A's multitasking ability kept on surprising researchers starting almost a century ago. Today, biological functions of vitamin A have been discovered in almost every vertebrate organ system. In addition to vision, known biological functions of vitamin A include its roles in embryonic growth and development, immune competence, reproduction, maintenance of epithelial surfaces, and proper functioning of the adult brain (Drager, U. C., 2006; Duester, G., 2008; Mangelsdorf, D. J. et al., 1993; Napoli, J. L., 1999; Ross, A. C., and Gardner, E. M., 1994). Since vitamin A derivatives have profound effects on cellular growth and differentiation, vitamin A also plays positive or negative roles in a wide-range of pathological conditions, such as visual disorders(Travis, G. H. et al., 2006), cancer (Love, J. M., and Gudas, L. J., 1994; Niles, R. M., 2004; Verma, A. K., 2003), infectious diseases (Stephensen, C. B., 2001), diabetes (Basu, T. K., and Basualdo, C., 1997; Yang, Q. et al., 2005), teratogenicity (Nau, H. et al., 1994), and skin diseases (Chivot, M., 2005; Orfanos, C. E. et al., 1997; Zouboulis, C. C., 2001). Except for vision, which depends on the aldehyde form of vitamin A, most of these physiological or pathological functions can be ascribed to retinoic acid's effects on nuclear hormone receptors (Chambon, P., 1996; Evans, R. M., 1994). New biological functions are still being discovered for vitamin A derivatives. For example, it was recently discovered that retinal inhibits adipogenesis (Ziouzenkova, O. et al., 2007).
Plasma retinol binding protein (RBP), a high-affinity vitamin A binding protein, is the principal means of vitamin A transport in the blood and is responsible for a well-regulated transport system that helps vertebrates adapt to fluctuations in vitamin A levels (Blomhoff, R. et al., 1990). RBP specifically binds to vitamin A, effectively solubilizes it in aqueous solution, and protects it from enzymatic and oxidative damage (Goodman, D. S., 1984). In addition, RBP was recently discovered to play a role in insulin resistance (Yang, Q. et al., 2005). Using an unbiased strategy combining specific photo-crosslinking, high-affinity purification and mass spectrometry, the high-affinity cell-surface RBP receptor has been identified as STRA6, a protein with a multi-transmembrane domain architecture typical of channels and transporters, but not homologous to any protein of known function. STRA6 binds to RBP with high affinity and mediates cellular uptake of vitamin A from the vitamin A/RBP complex (holo-RBP). Consistent with the diverse functions of vitamin A, human STRA6 mutations cause severe pathological phenotypes including the absence of eyes (anophthalmia), mental retardation, congenital heart defects, lung hyperplasia, and intrauterine growth retardation (Golzio, C. et al., 2007; Pasutto, F. et al., 2007).
In this review, we provide a summary of current knowledge of vitamin A and RBP, describing in detail our current knowledge of the RBP receptor including its identification, the unique features of its function both as a membrane receptor and a membrane transporter, and the relationships between its tissue distribution and the known organ specific functions of vitamin A. In addition, we provide answers to some pertinent questions related to the RBP receptor and its potential relationships with human diseases.
2. Diverse Physiological Functions of Vitamin A, a Molecule Essential for Vertebrate Survival
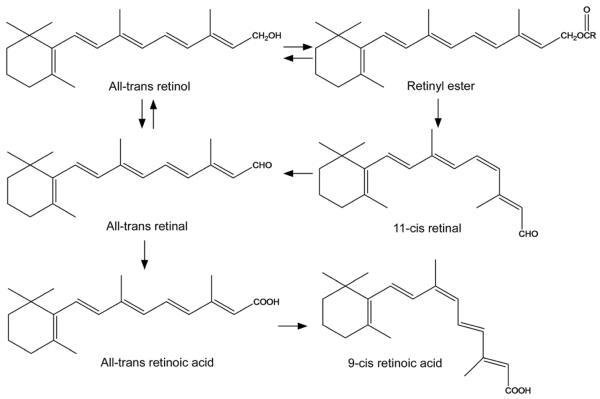
Vitamin A has alcohol, aldehyde, acid and ester forms (Figure 1). Some forms have direct biological activities and other forms serve as important reaction intermediates or as the storage form of vitamin A. Known functions of vitamin A and its derivatives and pathological effects of vitamin A deficiency are discussed below.
Figure 1.
Vitamin A and its major derivatives that have biological activities or serve as important intermediates.
2.1. The alcohol form of vitamin A
A main function of the alcohol form of vitamin A is to serve as the substrate for RBP for delivery in the blood. The fact that retinol evolved to be the major transport form of vitamin A is likely due to fact it is less toxic than retinal and retinoic acid. Free retinal has been shown to be highly toxic (Maeda, A. et al., 2009; Maeda, A. et al., 2008). Retinoic acid is the most biologically active form of vitamin A and is also most toxic form. In addition, retinol can be converted to retinal and retinoic acid.
Retinol also serves as an intermediate in the visual cycle for chromophore regeneration (Chen, C., and Koutalos, Y., 2010; Chen, C. et al., 2005). In addition, the alcohol derivatives of vitamin A have biological activities distinct from retinoic acid. For example, they control the growth of B lymphocytes (Buck, J. et al., 1991; Buck, J. et al., 1990) and function as a survival factor in serum for fibroblasts (Chen, Y. et al., 1997). Anhydroretinol, another physiological metabolite of vitamin A, can induce cell death (Chen, Y. et al., 1999). Another known function of vitamin A is in the testis. Degeneration of germinal epithelium in testis caused by vitamin A deficiency can be reversed by vitamin A but not by retinoic acid (Griswold, M. D. et al., 1989; Howell, J. M. et al., 1963). One likely explanation for the distinction between retinol and retinoic acid is that retinol can be efficiently transported via RBP/retinol in vivo but retinoic acid cannot. In addition, retinol can also have biochemical functions distinct from the precursor to retinoic acid (Chen, L., and Khillan, J. S., 2010; Hoyos, B. et al., 2005). It was recently discovered that retinol, but not retinoic acid, regulates BMP4 expression in male germ line cells (Baleato, R. M. et al., 2005). In addition, retinol, but not retinoic acid, prevents the differentiation and promotes the feeder-independent culture of embryonic stem cells (Chen, L., and Khillan, J. S., 2008, 2010; Chen, L. et al., 2007).
2.2. The aldehyde forms of vitamin A
Photoreceptor cells in the retina use 11-cis retinal as the chromophore (Dowling, J. E., 1966). In addition, the aldehyde form of vitamin A serves as an intermediate in the synthesis of retinoic acid, the vitamin A derivative with the most diverse biological functions. It was also recently discovered to inhibit adipogenesis (Ziouzenkova, O. et al., 2007).
2.3. The acid forms of vitamin A
Retinoic acid (vitamin A acid) was initially known as a morphogen in development (Marshall, H. et al., 1996; Reijntjes, S. et al., 2005). Retinoic acid is essential in organogenesis (Maden, M., 1994). The nuclear receptors for retinoic acid were discovered in 1987 (Giguere, V. et al., 1987; Petkovich, M. et al., 1987). Nuclear retinoic acid receptors regulate the transcriptions of a large number of genes (Chambon, P., 1996; Evans, R. M., 1994). Retinoic acid can both stimulate or suppress mitogenesis depending on the cellular context (Chen, S., and Gardner, D. G., 1998). In addition to gene transcription, retinoic acid may also mediate its effect by retinoylation of proteins (Takahashi, N., and Breitman, T. R., 1994). Recently, retinoic acid was discovered to acutely regulate protein translation in neurons independent of its roles in regulating gene transcription (Aoto, J. et al., 2008; Chen, N. et al., 2008). In addition to its developmental roles, retinoic acid is also important in the function of many adult organs such as the nervous system, the immune system, the reproductive system, the respiratory system, and the skin. These functions will be discussed in detail in the context of the RBP receptor.
2.4. The ester forms of vitamin A
Retinyl ester is the major storage form of vitamin A inside cells (Batten, M. L. et al., 2004; Liu, L., and Gudas, L. J., 2005; O'Byrne, S. M. et al., 2005; Ruiz, A. et al., 2007) and is an alternative form for vitamin A delivery. However, vitamin A delivery through retinyl ester is associated with toxicity (Goodman, D. S., 1984; Smith, F. R., and Goodman, D. S., 1976).
2.5. Pathological effects of vitamin A deficiency
Vitamin A deficiency affects a wide-range of organ systems in vertebrates (West, K. P., Jr., 1994; Wolbach, S. R., and Howe, P. R., 1925). For humans, the most well known effects of vitamin A deficiency are night blindness (Dowling, J. E., 1966) and childhood mortality and morbidity (Sommer, A., 1997a). Even mild vitamin A deficiency (presymptomatic for ocular phenotypes) has a large impact on childhood mortality (Sommer, A., 1997a). This is due to vitamin A's role in boosting immunity (Semba, R. D., 1999; Stephensen, C. B., 2001). It was estimated that 140 million children and more than 7 million pregnant women suffer from vitamin A deficiency every year worldwide and 1.2–3 million children die each year due to vitamin A deficiency alone (Sommer, A., and Davidson, F. R., 2002). Maternal vitamin A deficiency is associated with maternal mortality and congenital defects in multiple organs for newborn children. In adults, vitamin A deficiency can affect brain function. Experiments in animal models show that vitamin A deficiency can lead to profound impairment of hippocampal long-term potentiation and a virtual abolishment of long-term depression (Misner, D. L. et al., 2001). Consistently, vitamin A deficiency leads to impairment in special learning and memory (Cocco, S. et al., 2002). In addition, vitamin A deficiency can lead to abnormal functions of the lung (Biesalski, H. K., 2003), the skin (Vahlquist, A., 1994), the thyroid (Morley, J. E. et al., 1978) and the male and female reproductive systems (Livera, G. et al., 2002; Wolbach, S. R., and Howe, P. R., 1925).
3. Retinol Binding Protein, the Specific Carrier of Vitamin A in the Blood
Since mammals cannot synthesize vitamin A, the only sources of vitamin A are from the diet and maternal vitamin A (which is also ultimately from the diet). The majority of dietary vitamin A is stored in the liver. Vitamin A is insoluble in aqueous media, is chemically unstable, and is toxic to cells at low levels. Vitamin A transport to different cell types needs to be precisely regulated because too little or too much vitamin A can be detrimental both to cellular survival and function (Goodman, D. S., 1984). Plasma retinol binding protein (RBP) is a member of lipocalin superfamily (Newcomer, M. E., and Ong, D. E., 2000). RBP is the principal means of transporting vitamin A in the blood and is responsible for a well-regulated transport system that helps vertebrates to adapt to fluctuations in vitamin A level (e.g. during seasonal changes in natural environments) (Blomhoff, R. et al., 1990). RBP specifically binds to vitamin A and effectively solubilizes vitamin A in aqueous solution and allows for a plasma vitamin A concentration 1000-fold higher than would occur for free vitamin A. RBP also protects vitamin A from enzymatic and oxidative damage (Goodman, D. S., 1984). RBP helps to maintain a physiological range of vitamin A concentration so that vitamin A concentration is stable despite variable intake from food (Redondo, C. et al., 2006). Another function of the RBP system is to decrease the toxicity associated with unregulated distribution of vitamin A (Dingle, J. T. et al., 1972; Goodman, D. S., 1984). Experiments in rats (Mallia, A. K. et al., 1975) and a study of human patients with hypervitaminosis A (Smith, F. R., and Goodman, D. S., 1976) both suggested that more toxicity is associated with vitamin A delivery independent of RBP. An excessive dose of vitamin A is toxic in vivo only when the level of vitamin A in the circulation is presented to cells in a form other than bound to RBP, such as in retinyl esters (Goodman, D. S., 1984).
RBP in complex with vitamin A (holo-RBP) is mainly produced in the liver, but is also produced in many other organs. For example, RBP is highly expressed in adipose tissue (Makover, A. et al., 1989). However, extrahepatic RBP cannot mobilize the vitamin A stores in liver (Quadro, L. et al., 2004). The exact roles of RBP secreted by tissues other than liver are not clear. One exception is that RBP secreted by adipocytes has recently been found to be an adipokine for insulin resistance (Tamori, Y. et al., 2006; Yang, Q. et al., 2005). This is a function of RBP other than vitamin A delivery. Holo-RBP in the blood is in complex with the thyroxine binding protein transthyretin (TTR), which is also called prealbumin. This complex increases the molecular weight of holo-RBP and reduces its loss through glomerular filtration in the kidney. The crystal structure of holo-RBP in complex with TTR has been determined (Figure 2) (Monaco, H. L. et al., 1995; Naylor, H. M., and Newcomer, M. E., 1999; Zanotti, G., and Berni, R., 2004).
Figure 2.
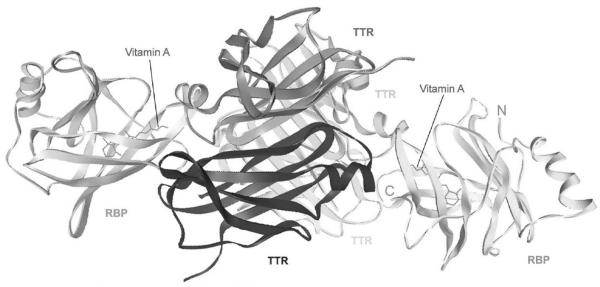
Crystal structure of the holo-RBP and TTR complex.
The main conclusion from studies of RBP knockout mice is that loss of RBP makes mice extremely sensitive to vitamin A deficiency. RBP knockout mice cannot mobilize the hepatic vitamin A store (Quadro, L. et al., 1999). Even with a nutritionally complete diet, knockout mice have a dramatically lower serum vitamin A level, similar to the level in the later stages of vitamin A deficiency in humans. Given the role of vitamin A in immune regulation and the susceptibility of vitamin A deficient children to infection before visual symptoms (Semba, R. D., 1998; Sommer, A., 1997a; Stephensen, C. B., 2001), it is likely that the immune system is also sensitive to RBP defect under vitamin A sufficient conditions. Indeed, the circulating immunoglobulin level in RBP knockout mice is half of that in the wild-type mice even under vitamin A sufficiency (Quadro, L. et al., 2000). It will be interesting to systematically study the effects of loss of RBP functions on mouse susceptibility to infection, which is well correlated with vitamin A status in humans (Semba, R. D., 1999; Sommer, A., 1997a; Stephensen, C. B., 2001). As demonstrated by a LacZ reporter system for retinoic acid level, there is a dramatic decrease in retinoic acid level in the developing brain of RBP knockout mice even under vitamin A-sufficient conditions (Quadro, L. et al., 2005). Therefore, it will be interesting to test whether these mice have any cognitive defects in adulthood. In addition, RBP knockout mice have abnormal heart development (Wendler, C. C. et al., 2003) and impaired vision (Quadro, L. et al., 1999; Quadro, L. et al., 2003). Systematic study of several organ functions in RBP knockout mice demonstrated that RBP is essential for survival under vitamin A deficient conditions (Ghyselinck, N. B. et al., 2006; Quadro, L. et al., 1999; Quadro, L. et al., 2005). Vitamin A deficient conditions are common for most, if not all, animals living in natural environments. Studies of the RBP knockout mice subjected to different lengths of time of vitamin A deficiency revealed that RBP is the primary vitamin A source for fetal development (Quadro, L. et al., 2005). Depending on the extent of dietary vitamin A deficiency, malformations in RBP knockout mice range from mild symptoms to complete fetal resorption. Under conditions of vitamin A deficiency, in which wild-type mice behave normally, RBP knockout mice have rapid vision loss in adults after merely a week of vitamin A deficiency (Quadro, L. et al., 1999). In addition, these mice rapidly develop testicular defects (Ghyselinck, N. B. et al., 2006).
4. Diverse Evidence for the Existence of an RBP Receptor that Mediates Vitamin A Uptake
Diverse experimental evidence accumulated since the 1970s from independent research groups supports the existence of a specific cell surface RBP receptor that mediates vitamin A uptake (Table 1). It was first shown in the 1970s that there exists a specific cell surface receptor for retinol binding protein on the retinal pigment epithelium (RPE) cells and mucosal epithelial cells (Bok, D., and Heller, J., 1976; Chen, C. C., and Heller, J., 1977; Heller, J., 1975; Heller, M., and Bok, D., 1976; Maraini, G., and Gozzoli, F., 1975; Rask, L., and Peterson, P. A., 1976). During the past 30 years, there has been strong evidence for the existence of RBP receptors not only on RPE cells but also on other tissues including placenta (Sivaprasadarao, A. et al., 1994; Sivaprasadarao, A., and Findlay, J. B., 1988a; Smeland, S. et al., 1995), choroid plexus (MacDonald, P. N. et al., 1990; Smeland, S. et al., 1995), Sertoli cells of the testis (Bhat, M. K., and Cama, H. R., 1979; Bishop, P. D., and Griswold, M. D., 1987; Shingleton, J. L. et al., 1989; Smeland, S. et al., 1995) and macrophages (Hagen, E. et al., 1999). Evidence for a specific RBP receptor include saturable binding of 125I-RBP to cell membrane (Bhat, M. K., and Cama, H. R., 1979; Heller, J., 1975; Pfeffer, B. A. et al., 1986). Binding can be inhibited by an excess of unlabeled RBP (Bhat, M. K., and Cama, H. R., 1979; Heller, J., 1975; Heller, M., and Bok, D., 1976; Sivaprasadarao, A., and Findlay, J. B., 1988a; Torma, H., and Vahlquist, A., 1986), an antibody to RBP (Melhus, H. et al., 1995; Rask, L., and Peterson, P. A., 1976) or by a cysteine modification compound (Sivaprasadarao, A., and Findlay, J. B., 1988a). When 125I-RBP was injected into rat, specific labeling was observed on the basolateral membrane of the RPE (Bok, D., and Heller, J., 1976) and in the choroid plexus (MacDonald, P. N. et al., 1990). In a systematic comparison of RBP binding between different tissues and cell types, the highest RBP binding activities were found in membranes from the RPE, the placenta, the bone marrow, the choroid plexus and undifferentiated keratinocytes (Smeland, S. et al., 1995). The observed tissue distribution of the putative RBP receptor agrees well with what we know about vitamin A function and metabolism. For example, in order for vitamin A to exert its effect on adult brain, it must cross the choroid plexus, the blood-brain barrier, and therefore a high level of RBP receptor should be expressed in this tissue.
Table 1.
In vitro and in vivo experimental evidence (in chronological order) for the existence of an RBP receptor on RPE cells and several other cell types and a mechanism for receptor-mediated vitamin A uptake from holo-RBP.
| References | Experimental Systems | Experimental Evidence |
|---|---|---|
| Heller, 1975 | Bovine RPE cells | Binding of retinol-125I-RBP to RPE cells is saturable and can be inhibited by an excess of unlabeled holo-RBP. Apo-RBP is less effective in displacing bound retinol-125I-RBP. |
| Bok and Heller, 1976 | Live rats | Injection of 125I-RBP into rat leads to specific labeling of the basal and lateral membrane of the RPE. |
| Chen and Heller, 1976 | Bovine RPE cells | RPE cells but not red blood cells or white blood cells efficiently take up 3H-retinol from 3H-retinol/RBP. RPE cells cannot take up 3H-retinol bound to BSA. |
| Heller and Bok, 1976 | Bovine RPE cells | Autoradiography of retinol-125I-RBP bound to RPE cells showed specific labeling of the choroidal surface of the cells but not the retinal surface. The labeling can be abolished by an excess of unlabeled holo-RBP. |
| Rask and Peterson, 1976 | Monkey mucosal epithelial cells of small intestine | Uptake of 3H-retinol from 3H-retinol/RBP can be inhibited by unlabeled holo-RBP, apo-RBP or antibody against RBP but not by the metabolite form of RBP. |
| Bhat and Cama, 1979 | Chicken testis membrane | Binding of 125I-RBP is saturable and can be inhibited by an excess of unlabeled RBP. |
| Rask et al., 1980 | Bovine cornea | Uptake of 3H-retinol from 3H-retinol/RBP is saturable and can be inhibited by unlabeled holo-RBP. |
| Torma and Vahlquist, 1984 | Human skin | Uptake of 3H-retinol from 3H-retinol/RBP is saturable by substrate and can be inhibited by an excess of unlabeled holo-RBP. |
| Pfeffer et al., 1986 | Human RPE culture | Binding of retinol-25I-RBP to RPE cells is saturable. Uptake of 3H-retinol from 3H-retinol/RBP can be inhibited by unlabeled holo-RBP. |
| Torma and Vahlquist, 1986 | Human placenta | Specific binding of 125I-RBP and uptake of 3H-retinol from 3H-retinol/RBP (both are inhibited by an excess of cold RBP) |
| Eriksson et al., 1986 | Embryonal carcinoma cell line F9 | Differentiated, but not undifferentiated, F9 cells specifically bind to 125I-RBP and take up 3H-retinol from 3H-retinol/RBP (both are inhibited by an excess of unlabeled RBP) |
| Ottonello et al., 1987 | Bovine RPE membrane | Isolated RPE membrane can specifically take up 3H-retinol from 3H-retinol/RBP. This uptake can be inhibited by an excess of unlabeled holo-RBP. |
| Sivaprasadarao and Findlay, 1988 | Human placenta microvilli | Binding of 125I-RBP can be inhibited by an excess of unlabeled RBP or TTR. PCMBS treatment of membrane can abolish 125I-RBP binding. |
| Sivaprasadarao and Findlay, 1988 | Human placenta membrane vesicles | Uptake of 3H-retinol from 3H-retinol/RBP can be inhibited by an excess of unlabeled holo-RBP or apo-RBP, but not by serum albumin. PCMBS treatment of membrane can abolish uptake. |
| Shingleton et al, 1989 | Sertoli cells of rat testis | Uptake of 3H-retinol from 3H-retinol/RBP is saturable and can be inhibited by an excess of unlabeled holo-RBP. |
| Sivaprasadarao and Findlay, 1994 | Human placenta membrane | Specific mutations on the open end of the β-barrel of RBP can block RBP's interaction with its receptor. |
| Melhus et al., 1995 | Bovine RPE membrane | A monoclonal antibody recognizing an entrance loop of RBP can specifically block its interaction with its receptor on RPE membrane. |
| Smeland et al., 1995 | Membrane prepared from many organs | High binding activity of125I-RBP was found in placenta, RPE, bone marrow and kidney and undifferentiated, but not differentiated, keratinocytes. |
| Sundaram et al., 1998 | Human placenta membrane | Specific transfer of 3H-retinol from 3H-retinol/RBP to CRBP is dependent on the RBP receptor on placenta membrane. Serum albumin and β-lactoglobulin cannot substitute for RBP in this process. |
| Vogel et al., 2002 | RBP knockout mouse model | The rate of retinol uptake from holo-RBP by the eye markedly exceeds all other tissues except for the kidney. This suggests that the RPE can specifically recognize and efficiently absorb retinol when bound to RBP. |
| Liden and Eriksson, 2005 | A new retinol uptake assay in cell culture | A monoclonal antibody to RBP can block retinol uptake from holo-RBP by cells. |
The receptor on RPE membrane can not only specifically bind to RBP, but also can mediate vitamin A uptake from vitamin A-loaded RBP (holo-RBP) (Chen, C. C., and Heller, J., 1977; Maraini, G., and Gozzoli, F., 1975; Ottonello, S. et al., 1987; Pfeffer, B. A. et al., 1986). The uptake mechanism is highly specific because red blood cells don't take up vitamin A from RBP. In addition, the efficiency of vitamin A uptake from vitamin A bound to BSA is much less efficient than from vitamin A bound to RBP. Specific vitamin A uptake has also been demonstrated for mucosal epithelial cells (Rask, L., and Peterson, P. A., 1976), human placenta (Sivaprasadarao, A., and Findlay, J. B., 1988b; Sundaram, M. et al., 1998; Torma, H., and Vahlquist, A., 1986), Sertoli cells of the testis (Bishop, P. D., and Griswold, M. D., 1987; Shingleton, J. L. et al., 1989), human skin (Torma, H., and Vahlquist, A., 1984) and macrophages (Hagen, E. et al., 1999). Previous studies also showed that specific mutations in RBP or a monoclonal antibody against a specific region of RBP can abolish its interaction with the RBP receptor (Liden, M., and Eriksson, U., 2005; Melhus, H. et al., 1995; Sivaprasadarao, A., and Findlay, J. B., 1994). Another strong piece of evidence that vitamin A uptake is mediated by a protein is that the uptake in human placenta membrane can be inhibited by a cysteine modification reagent (Sivaprasadarao, A., and Findlay, J. B., 1988b). Not listed in Table 1 are indirect pieces of evidence for the existence of an RBP receptor. For example, in an unbiased search for a serum factor that stimulates the growth of B cells, it was found that holo-RBP is this factor (Buck, J. et al., 1990). For lymphoblastoid cell lines Mou and BH, holo-RBP is much more potent than retinol itself in growth stimulation. These experiments also suggest the existence of a cell surface receptor for holo-RBP on these cell types.
5. Identification of the RBP Receptor
5.1. Identification of the high-affinity RBP receptor as STRA6
Despite the large amount of evidence, the RBP receptor turned out to be very difficult to identify. Potential obstacles to purifying the RBP receptor include the fragility of the receptor protein and the transient nature of the binding of RBP to its receptor. These challenges likely prevented the purification of the receptor using traditional biochemical approaches. To overcome these two challenges, a strategy was designed that can stabilize the RBP/receptor interaction and permit high-affinity purification of the RBP/receptor complex without requiring the receptor to remain active during the purification (Kawaguchi, R. et al., 2007). Another advantage of this strategy is that it permits stringent washing with high salt and urea, which can dissociate non-specifically bound protein without causing membrane protein aggregation. Using this strategy, the RBP receptor was identified as STRA6, a multi-transmembrane protein of previously unknown function (Kawaguchi, R. et al., 2007). STRA6 binds to RBP with high affinity and specificity and facilitates the release of vitamin A from holo-RBP and the transport of vitamin A into the cell (Kawaguchi, R. et al., 2007).
STRA6 was first characterized as a retinoic acid induced gene in P19 embryonic carcinoma cells (Bouillet, P. et al., 1995). STRA6 stands for “stimulated by retinoic acid 6”. It was also identified as a gene induced by Wnt-1 and retinoic acid in mouse mammary epithelial cells (Szeto, W. et al., 2001). The induction by Wnt-1 and retinoic acid is synergistic. Strikingly, STRA6 was found to be overexpressed up to 172 fold in 14 out of 14 human colorectal tumors relative to the normal tissue (Szeto, W. et al., 2001). STRA6 is widely expressed during embryonic development and in adult organ systems. In development, STRA6 is widely expressed, consistent with the diverse roles of vitamin A in development. For example, its expression during mouse limb development suggested that STRA6 may participate in early dorsovental limb patterning and in controlling endochondral ossification (Chazaud, C. et al., 1996). In developing eye, it is expressed in the inner nuclear layer of the developing retina, and in developing RPE (Bouillet, P. et al., 1997). Information on the distribution of STRA6 mRNA in adult tissues is available from the NCBI's comprehensive tissue EST profiler (Table 2) and from two tissue distribution studies of STRA6 (Bouillet, P. et al., 1997; Chazaud, C. et al., 1996). It was suggested that a high level of RBP receptors should be expressed in cells comprising blood-tissue barriers (MacDonald, P. N. et al., 1990). Indeed, in adult tissues, STRA6 expression is enriched in blood-organ barriers such as the RPE (blood-retina barrier), the placenta (maternal-fetal barrier), the choroid plexus (blood-brain barrier) and the Sertoli cells of testis (blood-testis barrier), although STRA6's expression is not limited to blood-organ barriers. Correlations between STRA6 expression and known functions of vitamin A in adult organs are discussed below.
Table 2.
Correlation between STRA6's tissue expression and known functions of vitamin A, its derivatives, and RBP. VAD, vitamin A deficiency. The color intensity of the ovals indicates the abundance of STRA6 message. Tissue EST counts are suggested by the current NCBI's EST ProfileViewer for human STRA6. The relative abundance of STRA6 in different organs is similar but not identical between human and mouse. Expression from rare cell types, such as the RPE, may be underrepresented in this analysis. The sensitivity of this analysis is also affected by the total number of ESTs available per tissue.
| Tissues | Transcripts per million | STRA6 EST/Total EST | Known functions of vitamin A, its derivatives and RBP | References |
|---|---|---|---|---|
| brain | 51 
|
48/933463 | Maintenance of synaptic plasticity and cortical synchronization during sleep | Misner et al., 2001;Maret et al., 2005; Drager, 2006 |
| connective tissue | 27 
|
3/107437 | Vitamin A promotes collagen synthesis | Anstead, 1998 |
| parathyroid | 0 | 0/20837 | ||
| thyroid | 72 
|
4/54920 | VAD leads to hyperthyroidism and higher basal metabolic rate | Morley et al., 1978 |
| pituitary gland | 0 | 0/17477 | ||
| placenta | 195 
|
58/296769 | Vitamin A is essential for embryonic development. | Maden, 1994; Quadro et al., 2005 |
| eye | 28 
|
6/207123 | Vitamin A is essential for light perception in vision and modulates neuronal signaling | Wald, 1968; Weiler et al., 2001 |
| embryonic tissue | 60 
|
12/199222 | Vitamin A is essential in almost all steps in organogenesis. | Maden, 1994; Reijntjes et al., 2005 |
| abdominal cavity | 0 | 0/40397 | ||
| cervix | 20 
|
1/48034 | ||
| ovary | 28 
|
3/106265 | Vitamin A promotes ovarian follicular growth and ooctye maturation | Ikeda et al., 2005 |
| uterus | 41 
|
10/238763 | VAD leads to replacement of uterus mucosa by stratified, keratinizing epithelium | Wolbach and Howe, 1925 |
| prostate | 6 
|
1/154822 | ||
| testis | 94 
|
33/348176 | Vitamin A is essential for spermatogenesis | Livera et al., 2002; Chung and Wolgemuth, 2004 |
| bladder | 0 | 0/30298 | ||
| kidney | 42 
|
9/212609 | Vitamin A has antifibrotic and cytoprotective effects on various renal cell types | Xu et al., 2004a |
| tongue | 75 
|
5/66626 | Maintenance of normal taste bud function | Biesalski etal., 1985 |
| larynx | 32 
|
1/30370 | VAD leads to replacement of respiratory mucosa by keratinizing epithelium | Wolbach and Howe, 1925 |
| pharynx | 67 
|
1/14868 | VAD leads to replacement of respiratory mucosa by keratinizing epithelium | Wolbach and Howe, 1925 |
| salivary gland | 0 | 0/20411 | ||
| heart | 11 
|
1/89584 | Vitamin A has anti-growth activity in fully differentiated cardiac cells | Gardner and Chen, 1999 |
| lymph node | 10 
|
1/95317 | Regulation of hematopoiesis and maintenance of immune competence | Blomhoff and Smeland, 1994;Stephensen, 2001 |
| lymph | 0 | 0/44599 | ||
| tonsil | 0 | 0/17168 | ||
| spleen | 18 
|
1/52804 | Regulation of hematopoiesis and maintenance of immune competence | Blomhoff and Smeland, 1994;Stephensen, 2001 |
| thymus | 40 
|
3/73960 | Regulation of hematopoiesis and maintenance of immune competence | Blomhoff and Smeland, 1994;Stephensen, 2001 |
| mammary gland | 17 
|
3/170913 | Lactation transfers vitamin A from mother to infant for its growth and development | Ross and Gardner, 1994 |
| muscle | 34 
|
4/114677 | RBP is an adipokine that induces insulin resistance in muscle | Yang et al., 2005a |
| lung | 43 
|
15/347374 | Vitamin A is involved in the maintenance of normal lung function. | Baybutt et al., 2000;Biesalski, 2003 |
| trachea | 20 
|
1/48470 | VAD leads to replacement of respiratory mucosa by keratinizing epithelium | Wolbach and Howe, 1925 |
| skin | 15 
|
3/187916 | Vitamin A is essential for the maintenance of normal skin. | Vahlquist, 1994; Varani et al., 2000 |
5.2. STRA6 in the eye
In the eye, STRA6 is abundantly expressed in the RPE. In contrast to its absence in the endothelial cells of the choriocapillaris, STRA6 is expressed in retinal blood vessels, although the signal is much weaker than that in the RPE (Kawaguchi, R. et al., 2007). The retinal blood vessels are another location that constitutes a blood-retina barrier. As holo-RBP in choriocapillaris blood is the source of vitamin A for the RPE, holo-RBP from retinal blood vessels is a potential source of vitamin A for Müller cells in the retina (Mata, N. L. et al., 2002). In the RPE, STRA6 is largely localized to the basolateral membrane of the RPE cells. This localization is exactly what is expected for an RBP receptor, which should be localized to the basolateral membrane of the RPE facing the choroidal circulation (Heller, M., and Bok, D., 1976; Pfeffer, B. A. et al., 1986). STRA6, localized to the lateral membrane of the RPE, is in close proximity to the retinosome, a recently discovered RPE structure that stores retinyl esters (Imanishi, Y. et al., 2004). This suggests that vitamin A absorbed by STRA6 from holo-RBP is in close proximity to the cellular structures that store vitamin A. Interestingly, there are also STRA6 signals on distinct intracellular vesicles in the RPE. These vesicles may play a role in targeting STRA6 to the basolateral membrane or in recycling. Another major location in the eye that expresses STRA6 is the cornea (unpublished results). Consistently, it was known that RBP supplies vitamin A to the cornea (Rask, L. et al., 1980).
5.3. STRA6 in the reproductive systems
Consistent with the essential role of vitamin A in both male and female reproductive functions, EST analysis show that both male and female reproductive systems express STRA6 at high levels (Table 2). Vitamin A plays an essential role in spermatogenesis (Chung, S. S., and Wolgemuth, D. J., 2004; Livera, G. et al., 2002). In testis, STRA6 is expressed in the Sertoli cells (Bouillet, P. et al., 1997). STRA6 protein has been localized to the plasma membrane of Sertoli cells (Bouillet, P. et al., 1997), a localization consistent with Sertoli cells' RBP binding activity (Smeland, S. et al., 1995) and their ability to take up vitamin A from holo-RBP (Bishop, P. D., and Griswold, M. D., 1987; Shingleton, J. L. et al., 1989).
Consistent with the diverse roles of vitamin A in female reproductive functions (Clagett-Dame, M., and DeLuca, H. F., 2002), STRA6 is highly expressed in several female reproductive organs such as placenta, uterus, ovary and mammary gland (Table 2). Placenta has the highest level of STRA6 expression of all organs according to EST analysis (Table 2). This is consistent with the facts that RBP is the most important source of vitamin A for embryos (Quadro, L. et al., 2005) and placental membrane has one of the highest RBP binding activities of any cell or tissue tested (Smeland, S. et al., 1995). Human placental membrane has been used in the past as a model system to study RBP binding to the RBP receptor and vitamin A uptake (Sivaprasadarao, A., and Findlay, J. B., 1988a, b; Sundaram, M. et al., 1998).
5.4. STRA6 in the nervous system
Retinoic acid is a modulator of the nervous system (Drager, U. C., 2006; Lane, M. A., and Bailey, S. J., 2005; Weiler, R. et al., 2001). For example, retinoic acid plays an important role in maintaining synaptic plasticity in hippocampus (Misner, D. L. et al., 2001) and cortical synchrony during sleep (Maret, S. et al., 2005). Independent of its roles in regulating gene transcription, it also regulates protein translation in neurons (Aoto, J. et al., 2008; Chen, N. et al., 2008). It also plays an important role in regenerative processes in the adult central nervous system (Maden, M., 2007; Vergara, M. N. et al., 2005). Although retinoic acid is not preferentially transported from the blood to the brain, the brain has a high concentration of retinoic acid (Werner, E. A., and Deluca, H. F., 2002). Highly abundant expression of STRA6 in the brain is consistent with the local absorption and conversion of vitamin A to retinoic acid. Consistent with a previous study (Bouillet, P. et al., 1997), we observed strong STRA6 signals in the choroid plexus and meninges of the brain and weaker signals in a large subset of brain endothelial cells (unpublished results). Interestingly, these three sites constitute the blood-brain barriers (Abbott, N. J. et al., 2006). Consistent with the strong expression of STRA6 in meninges, retinoic acid from the meninges has been shown to regulate cortical neuron generation (Siegenthaler, J. A. et al., 2009). We also found that blood vessels negative for STRA6 were surrounded by astrocyte perivascular endfeet (Abbott, N. J. et al., 2006) that are positive for STRA6 (Kawaguchi, R. et al., 2007).
5.5. STRA6 in the lymphoid organs
Vitamin A plays important roles in hematopoiesis and in maintaining immunocompetence (Blomhoff, H. K., and Smeland, E. B., 1994; Oren, T. et al., 2003; Stephensen, C. B., 2001). For example, retinoic acid attenuates B cell proliferation to promote maturation and antibody production (Chen, Q., and Ross, A. C., 2005). In an unbiased search for a serum factor that stimulates the growth of B cells, holo-RBP was identified as this factor (Buck, J. et al., 1990). Circulating immunoglobulin level in RBP knockout mice is half that in wild-type mice, even under vitamin A sufficiency (Quadro, L. et al., 2000). Consistently, STRA6 is highly expressed in lymphoid organs thymus, spleen, and lymph nodes (Table 2). There are two possible mechanisms by which vitamin A is absorbed for immune regulation. One possibility is that specialized cell types in lymphoid organs other than leukocytes and their precursors absorb vitamin A from holo-RBP and generate retinoic acid locally for immune cells, similar to the role of the RPE cells in absorbing vitamin A and generating 11-cis retinal for photoreceptor cells. It was discovered as early as 1925 that vitamin A deficiency leads to thymus and spleen atrophy in rat (Wolbach, S. R., and Howe, P. R., 1925). Another possible mechanism is that leukocytes and their precursors directly absorb vitamin A from holo-RBP and produce retinoic acid themselves. A combination of both mechanisms is also possible.
5.6. STRA6 in the skin
Vitamin A plays essential roles in maintaining normal skin (Vahlquist, A., 1994). Vitamin A treatment reduces matrix metalloproteinase expression and stimulates collagen synthesis in both naturally aged, sun-protected skin and photoaged skin (Varani, J. et al., 2000). Because of vitamin A's role in epithelial and bone formation, cellular differentiation, and immune regulation, vitamin A deficiency can impede wound healing (Anstead, G. M., 1998; MacKay, D., and Miller, A. L., 2003). Human skin can specifically and efficiently take up vitamin A from holo-RBP (Torma, H., and Vahlquist, A., 1984). Epidermis has much higher activity than dermis. Undifferentiated human skin keratinocytes were found to have the highest RBP binding activity of any cell or tissue type tested, even higher than placenta and RPE cells (Smeland, S. et al., 1995). Consistent with the functions of vitamin A in skin and the ability of cells in the skin to take up vitamin A from holo-RBP, STRA6 is expressed relatively abundantly in the skin (Table 2).
5.7. STRA6 in the lung
Vitamin A plays important roles in maintaining the normal function of the lung (Biesalski, H. K., 2003). Vitamin A deficiency produces morphologic changes in the lung, impairs pneumocyte function (Baybutt, R. C. et al., 2000), and potentiates hyperoxic lung injury (Veness-Meehan, K. A., 1997). Vitamin A also has a protective effect on respiratory status in patients with cystic fibrosis (Aird, F. K. et al., 2006). STRA6 was originally identified as a retinoic acid stimulated gene (Bouillet, P. et al., 1995). Consistent with the ability of retinoic acid to stimulate STRA6 expression, Vitamin A combined with retinoic acid increases retinol uptake in the lung in a synergistic manner (Ross, A. C. et al., 2006).
5.8. STRA6 in the kidney
Both EST analysis and Northern blot analysis have shown that kidney expresses STRA6 at a fairly high level (Table 3). The detailed localization of STRA6 in kidney is unknown. STRA6 may function in vitamin A absorption for the kidney itself or in the recycling of vitamin A. Vitamin A is known to have antifibrotic effect and a cytoprotective effect on various renal cell types (Xu, Q. et al., 2004).
5.9. STRA6 in the heart
STRA6 expression in the heart is seen in both EST analysis and Northern blot analysis (Bouillet, P. et al., 1997). Retinoids have anti-growth activity in fully differentiated cardiac cells. Retinoids may be useful in the management of hypertrophic/hyperproliferative disorders of the heart and vascular wall (Gardner, D. G., and Chen, S., 1999). In addition, vitamin A deficiency causes a significant decrease in contractile responsiveness of aortic smooth muscle as a result of a down-regulation in the expression of contractile-related proteins (Wright, G. L. et al., 2002). Retinoids have also been shown to regulate cardiac mitochondrial membrane potential (Korichneva, I. et al., 2003).
6. Structure and Function Analysis of the RBP Receptor's Interaction with RBP
6.1. Transmembrane topology of STRA6
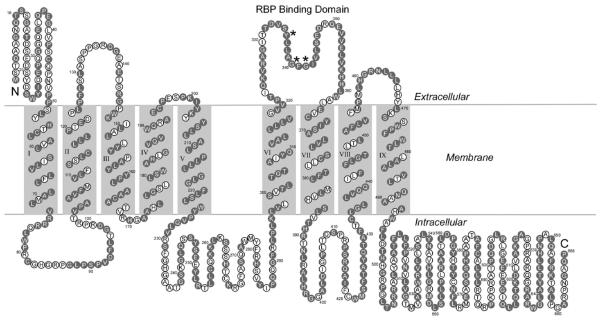
At the amino acid sequence level, STRA6 has no homology to any proteins or protein domains to indicate its function. The transmembrane topology of STRA6 has been determined experimentally (Kawaguchi, R. et al., 2008c). The topology model suggests that STRA6 has 19 distinct domains including 5 extracellular domains, 9 transmembrane domains, and 5 intracellular domains (Figure 3). Many membrane transporters have 8–12 transmembrane domains (Hediger, M. A. et al., 2004). Although STRA6 represents a new membrane transport protein, its number of transmembrane domains lies within this range. Most proteins with more than 7 transmembrane domains function as membrane transporters or channels. The large number of transmembrane domains potentially makes it more feasible to form a specific transmembrane pore, through which the ligand can pass through.
Figure 3.
Transmembrane topology of STRA6. Bovine sequence is shown. Resides conserved between human, mouse and bovine STRA6 are labeled in red. Resides essential for RBP binding are marked with asterisks.
6.2. The RBP binding domain in STRA6
Because STRA6 represents a new type of cell-surface receptor that is not homologous to any membrane receptor, transporter or channel and has no obvious functional domains, we decided to use an unbiased strategy to study its structure and function. By creating and analyzing more than 900 random mutants of STRA6, an essential RBP binding domain has been identified (Kawaguchi, R. et al., 2008a). The locations of three essential residues involved in RBP binding are indicated in the transmembrane topology model of STRA6 (Figure 3). Mutations in any of three essential residues in this domain can abolish the binding of STRA6 to RBP and its vitamin A uptake activity without affecting its cell surface expression. The advantage of an unbiased screening approach is evident because one of the transmembrane domains predicted by computer softwares turned out to be the RBP binding domain, which is an extracellular domain. Although this extracellular domain is most essential for RBP binding, other extracellular domains also contribute to RBP binding (Kawaguchi, R. et al., 2008c).
6.3. STRA6 mutants associated with human disease
Human genetic studies found that mutations in STRA6 are associated with severe pathological phenotypes such as mental retardation, anophthalmia, congenital heart defects, lung hyperplasia, duodenal stenosis, pancreatic malformations, and intrauterine growth retardation(Golzio, C. et al., 2007; Pasutto, F. et al., 2007). More human genetic studies have further confirmed the role of STRA6 mutations in malformations in humans (Chassaing, N. et al., 2009; Segel, R. et al., 2009; West, B. et al., 2009; White, T. et al., 2008). One of the most prominent features of loss of STRA6 function in human is the absence of the eye. Consistently, STRA6 knockdown also causes developmental defects in zebrafish (Isken, A. et al., 2008). The loss of retinoid uptake in the eye due to loss of STRA6 has also been demonstrated in the zebrafish model (Isken, A. et al., 2008).
Functional assays showed that the pathogenic missense mutations identified in the human genetic study abolish the vitamin A uptake activity of STRA6 (Kawaguchi, R. et al., 2008b), consistent with the severe clinical phenotypes. The locations of these mutations are depicted in Figure 3. As mentioned in a previous review (Niederreither, K., and Dolle, P., 2008), this is the first example of a retinoid signaling pathway mutation causing developmental abnormalities in humans.
6.4. RBP's interaction with its receptor
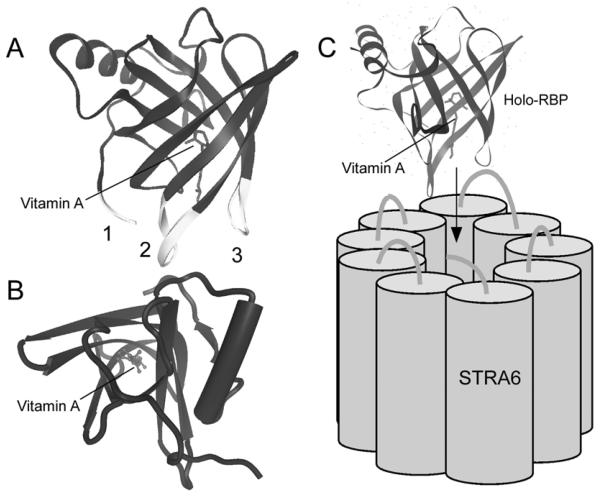
Three regions of RBP are potentially involved in binding to the RBP receptor (Figure 6A and 6B). The first region (C-tail) was implicated in a study of urine RBP. It was found that there are two main forms of RBP in human urine (Rask, L. et al., 1971). One form has vitamin A bound and can bind to the RBP receptor. The second form has no vitamin A bound and cannot bind the RBP receptor (Rask, L., and Peterson, P. A., 1976). The second form is missing a lysine residue at the C-terminus compared with the first form. Since the extreme C-terminal residue in full-length human RBP protein is not lysine, both products may represent RBP losing C-terminal residues with the second form having lost more. Given the fact that full-length apo-RBP can bind the RBP receptor, these studies suggest a role of the RBP C-terminus in interacting with the RBP receptor. In addition, alkaline phosphatase (AP) tagged at the N-terminus, but not the C-terminus, of RBP binds to the RBP receptor (Kawaguchi, R. et al., 2007). The second region, a loop near the vitamin A exit site of RBP, was implicated by two independent studies. The first showed the effect of mutating this region (Sivaprasadarao, A., and Findlay, J. B., 1994) and the second study found that a monoclonal antibody recognizing this region can block the binding of RBP with its receptor (Melhus, H. et al., 1995). The third region, another loop near the vitamin A exit site of RBP, was implicated by a deletion experiment (Sivaprasadarao, A., and Findlay, J. B., 1994). Consistent with the role of the RBP receptor in vitamin A release from holo-RBP, all three regions in RBP implicated in RBP receptor binding are located around the vitamin A exit end of the β-barrel (Figure 4A and 4B). A hypothetical model based on the crystal structure of RBP is proposed that illustrates its interaction with its receptor (Figure 4C).
Figure 4.
A. Structure of holo-RBP. The three regions implicated in RPB receptor binding are indicated as 1, 2, and 3, respectively. These three regions (labeled in yellow) are all located on the vitamin A exit end of RBP. B. Structure of holo-RBP showing the vitamin A exit end facing up. C. A hypothetical model for RBP interacting with STRA6 via the vitamin A exit end.
7. Pertinent Questions Related to the RBP Receptor
7.1. Retinol has the ability to diffuse through membranes. Why is it necessary to have a multitransmembrane domain protein (the RBP receptor) to assist its transport?
First, although free retinol has the ability to diffuse through membranes, retinol seldom exists in its free form under physiological conditions. During its transport in the blood, virtually all retinol in the blood is bound to the RBP/TTR complex, which makes retinol membrane impermeable. The RBP receptor serves both as a “homing” device of holo-RBP and mediates cellular uptake of retinol from holo-RBP.
Second, even for molecules that can diffuse through membrane, membrane transporters are known to facilitate their transport. For example, although urea and water can diffuse through membrane, urea transporters (Sachs, G. et al., 2006; You, G. et al., 1993) and water channels (Agre, P., 2004) greatly facilitate their transport. Even for free retinoids, there exist membrane transport systems that facilitate their transport across membrane. For example, biochemical evidence suggests that retinol uptake from the small intestine is mediated by a membrane transporter (Dew, S. E., and Ong, D. E., 1994). There is also strong evidence for the existence of a specific mechanism to transport 11-cis retinal in the retinal pigment epithelium (RPE), and this mechanism depends on interphotoreceptor retinoid-binding protein (IRBP). Apo-IRBP is much more effective in promoting the release of 11-cis retinal from the RPE than the apo-forms of other retinoid binding proteins (Carlson, A., and Bok, D., 1992). In addition, apo-IRBP is only effective when it is present on the apical, but not basal, side of the RPE (Carlson, A., and Bok, D., 1999). Another finding that challenges the assumptions about random diffusion is the identification of an ATP-dependent transporter (ABCR or ABCA4) that transports all-trans retinal released from bleached rhodopsin across membranes (Ahn, J. et al., 2000; Sun, H. et al., 1999; Weng, J. et al., 1999). Prior to the surprising discovery of ABCR's role in retinoid transport, there was no biochemical or physiological evidence for the existence of such a transporter.
Third, free retinol diffusion is not efficient enough for tissues that demand a large quantity of vitamin A. Both our study and an earlier study (Maraini, G., and Gozzoli, F., 1975) demonstrated that the RBP receptor-mediated vitamin A uptake is much more efficient than vitamin A uptake depending on the association of vitamin A with cellular membranes due to its hydrophobicity. In addition, a non-specific diffusion mechanism would depend on free retinol's interaction with cell membrane, but free retinol is toxic to cell membranes. Specific delivery mediated by RBP would prevent cellular damage by free retinol (Goodman, D. S., 1984). The fact that retinol but not retinoic acid can prevent testicular degeneration (Howell et al. 1963) may be due to the efficient delivery of retinol by RBP and its receptor. Random retinoic acid diffusion may be insufficient to supply the amount of retinoid needed by the testis.
7.2. Why does RBP need a high-affinity receptor for vitamin A uptake?
Under normal physiological conditions of vitamin A sufficiency, the blood concentration of RBP is in the micromolar range (Mills, J. P. et al., 2008). In contrast, STRA6 with high affinity (Kd=59 nM) (Kawaguchi, R. et al., 2007). Why does RBP need a high-affinity receptor? The first likely reason is to compete with TTR to bind to RBP in the blood. Holo-RBP is complexed with TTR in the blood. However, both TTR and the RBP receptor bind to the vitamin A exit end of RBP, as discussed above. Therefore, the RBP receptor needs to bind to RBP with higher affinity than TTR in order to absorb vitamin A from the blood despite the high concentration of RBP in the blood. Indeed, RBP's affinity for STRA6 (Kawaguchi, R. et al., 2007) is higher than its affinity for TTR (Malpeli, G. et al., 1996). Consistently, cells and tissues naturally absorb vitamin A from the holo-RBP/TTR complex in the blood, and STRA6 not only mediates vitamin A uptake from purified holo-RBP but also from human serum (Kawaguchi, R. et al., 2007). Although TTR blocks the vitamin A exit end of RBP (Monaco, H. L. et al., 1995), a study using Sertoli cells showed that it only partially inhibits RBP receptor-mediated vitamin A uptake (Shingleton, J. L. et al., 1989). A second likely function of the high-affinity interaction between RBP and its receptor is to help vertebrates survive vitamin A deficient conditions, which can lower serum RBP level. Vitamin A deficient conditions are common for most, if not all, vertebrates living in natural environments.
7.3. Why is the interaction between RBP and its receptor transient?
Transient interaction between RBP and its receptor is crucial for a vitamin A uptake mechanism that does not depend on endocytosis because each RBP protein only carries one retinol molecule and stable interaction will prevent further retinol delivery by other holo-RBP complexes to the same RBP receptor. In a sense, the removal of apo-RBP from the RBP receptor is as important as the binding of holo-RBP to the receptor for vitamin A delivery to a cell. As discussed above, the transient nature of the interaction between RBP and its receptor was one of the major technical hurdles in the identification of the RBP receptor.
7.4. Why are the phenotypes of patients with RBP mutations different from patients with RBP receptor mutations?
Phenotypes associated with known human RBP mutations are different from phenotypes associated with known human STRA6 mutations. Two natural RBP mutations have been identified in humans, and they cause vision defects such as dystrophy of the RPE (Seeliger, M. W. et al., 1999). This phenotype is consistent with the high expression levels of STRA6 in RPE cells, which need to absorb a large amount of vitamin A for proper visual functions. In contrast, known human STRA6 mutations cause severe and systemic phenotypes (Golzio, C. et al., 2007; Pasutto, F. et al., 2007). There are several likely reasons that can explain these differences. First, biochemical analysis showed that the only two natural mutations found in human RBP cause only a partial loss of RBP function (Folli, C. et al., 2005). This is in sharp contrast to the complete or near-complete loss of STRA6 function caused by STRA6 mutations (Kawaguchi, R. et al., 2008b). The human RBP mutants can still bind retinol (Folli, C. et al., 2005). In addition, holo-RBP formed by the mutant RBP can still bind TTR like the wild-type RBP. The major defect identified is the relatively faster release of retinol in the presence of lipid membranes. The vision defect associated with human RBP mutations suggests that vision is most sensitive to the partial loss of RBP function. RBP is highly conserved in evolution. The fact that human RBP mutations are so rarely identified (only two so far) is consistent with its essential function. Moreover, the detection limit of serum retinol/RBP in patients with RBP mutations in the previous study is 200 nM (Seeliger, M. W. et al., 1999), which is still much higher than the Kd of the RBP/STRA6 interaction (Kawaguchi, R. et al., 2007).
Further, even for complete nulls, the loss of RBP and RBP receptor function doesn't necessarily generate the same phenotypes in the same species. For example, placental delivery of vitamin A from maternal RBP to the human embryo is very different between RBP null embryos and RBP receptor null embryos. A human embryo without the RBP receptor can be directly impacted by its loss of function in the placental absorption of vitamin A from maternal RBP. In contrast, a human embryo without RBP still has its functional RBP receptor and functional maternal RBP to ensure sufficient vitamin A delivery to the embryo through the placenta. Interestingly, RBP knockout mice have retinoic acid deficiency in the brain even under vitamin A sufficient condition (Quadro, L. et al., 2005) and may have cognitive defects. Consistently, STRA6 mutations in human are associated with mental retardation (Pasutto, F. et al., 2007). Even human pathological phenotypes caused by STRA6 mutations are variable (Chassaing, N. et al., 2009). The variability is likely caused by the variable degrees in the loss of STRA6 function and the variability in vitamin A intake of the affected individuals. If the RBP/STRA6 system of vitamin A delivery is lost, random diffusion of retinoid would become the primary route of vitamin A transport. When comparing human and mouse phenotypes, another source of variability is species differences in retinoid metabolism and transport. For example, the doses of retinoic acid needed to produce teratogenic effects have drastic species variation (Nau, H., 2001). Mouse and rat are about 100 times less sensitive to isotretinoin's teratogenic effect than human (Nau, H., 2001).
7.5. Retinoid has the ability to diffuse systemically. Why did such a complicated mechanism (RBP/STRA6) to deliver vitamin A to cells evolve?
As demonstrated by retinoid related drugs, retinoids can diffuse to most human organs without a special delivery system. What are the reasons for the existence of the special vitamin A delivery system mediated by RBP and STRA6? Compared with random diffusion, there are many advantages of specific vitamin A delivery.
First, random retinoid distribution is associated with mild to severe toxic side effects. This is not surprising given the fact that vitamin A derivatives (retinoids) have profound effects on the growth and differentiation of diverse cell types by controlling the activities of their nuclear hormone receptors (Chambon, P., 1996; Evans, R. M., 1994). Retinoids can both enhance and suppress gene expression. The potent biological effects of vitamin A are best illustrated during embryonic development. Both insufficient and excessive vitamin A can cause severe birth defects (Collins, M. D., and Mao, G. E., 1999). The aldehyde form of vitamin A (retinal) has also been demonstrated to be highly toxic in adults (Maeda, A. et al., 2009; Maeda, A. et al., 2008).
Retinoids, especially retinoic acid, have been used in the past to treat human diseases, especially in dermatology and oncology. However, retinoid therapy is often associated with undesirable side effects similar to the systemic toxicity found in hypervitaminosis A. The most well known side effect of treatment by retinoic acid (e.g., Accutane) is terotogenicity(Adams, J., 1993; Nau, H., 2001; Nau, H. et al., 1994). Excessive retinoic acid is more toxic than retinol (Adams, J., 1993). In addition, retinoid therapies in adults are generally associated with diverse side effects on mucocutaneous tissues, such as cheilitis, xerosis, desquamation, dryness of mucous membranes, ocular effects, hair loss, hypergranulation of tissue, bone toxicity, and serum lipid alterations(Shalita, A. R., 1987). Animal model studies found that chronic exposure to clinical doses of 13-cis retinoic acid suppresses hippocampal neurogenesis and disrupts hippocampal-dependent memory(Crandall, J. et al., 2004). In addition, 13-cis retinoic acid causes night blindness (Sieving, P. A. et al., 2001). The birth defects and widespread side effects on adult organs caused by retinoid drugs also demonstrate the danger of relying on random diffusion to distribute retinoid.
The RBP/STRA6 system is clearly designed to prevent random retinol diffusion. The specificity of this system in vitamin A delivery is achieved through three mechanisms. The first mechanism is the high affinity and low off rate in RBP's binding to retinol. The absence of vitamin A from abundant erythrocytes and serum albumin, which can bind vitamin A, argue against random release of retinol from the holo-RBP/TTR complex in the blood. The second mechanism is the specific binding of RBP to its high-affinity receptor STRA6, which acts as a “homing device” to target holo-RBP to specific cells.
Second, random retinoid distribution requires constant retinoid intake to supply target tissues. Although constant intake is routine for laboratory animals, it is impossible for most, if not all, animals living in natural environments and most people living in developing countries. Delivery of vitamin A to cells or tissues is analogous to delivery of water to a house. Although random mechanisms (e.g., rain or flooding) may achieve delivery, they cannot guarantee the appropriate quantity and are associated with undesired side effects (as described above). Random mechanisms also cannot provide a stable supply during times of insufficiency. RBP serves as a buffer to maintain stable vitamin A concentration in the blood. The buffering function is important given the adverse effect of both low and high retinoid on the growth and function of diverse organs. Without this buffering function, blood retinoid level would fluctuate dramatically depending on dietary intake.
Third, random retinoid distribution may not satisfy tissues that need a large amount of retinoid for proper function such as the eye and developing embryos. RBP solubilizes vitamin A, protects it against oxidative damage, and makes it possible to mobilize retinoid stored in the liver when there is low retinoid intake. As a special carrier for vitamin A, RBP efficiently and specifically delivers vitamin A to organs distant from the liver such as the eye, the brain, the lung, the testis, and the placenta. The efficient vitamin A uptake activity of the RBP receptor may be especially important for tissues or cell types demanding a large amount of vitamin A. As mentioned earlier, the efficient delivery of retinol by RBP is likely responsible for the ability of retinol, but not retinoic acid (which is not a natural ligand of RBP), to prevent testicular degeneration (Howell et al. 1963).
7.6. If the RBP/STRA6 system functions to specifically deliver vitamin A to target organs, why does excessive vitamin A uptake cause toxicity?
Like many essential things in life (e.g., water), too much vitamin A is as detrimental as too little (Penniston, K. L., and Tanumihardjo, S. A., 2006). In normal physiological conditions, RBP mobilizes vitamin A stored in the liver and delivers vitamin A to target organs by specifically binding to STRA6 on target cells. Using water delivery as an analogy, excessive vitamin A intake would overwhelm this system, analogous to excessive water overflowing riverbanks. Experiments in rats (Mallia, A. K. et al., 1975) and a study of human patients with hypervitaminosis A (Smith, F. R., and Goodman, D. S., 1976) both suggested that more toxicity is associated with vitamin A delivery independent of RBP. An excessive dose of vitamin A is toxic in vivo only when the level of vitamin A in the circulation is presented to cells in a form other than bound to RBP, such as in retinyl esters (Goodman, D. S., 1984). An increase of 10% in retinyl ester is regarded as a sign of vitamin A overload.
7.7. What is the role of megalin in vitamin A uptake?
Megalin, a 600-kD scavenger receptor in the renal proximal tubes, is a low-affinity nonspecific receptor for RBP that mediates the endocytosis and transcytosis of the RBP protein in the kidney for its recycling (Christensen, E. I. et al., 1999; Marino, M. et al., 2001). Megalin binds to a wide-range of extracellular proteins including the highly abundant serum proteins albumin and hemoglobin (Christensen, E. I., and Birn, H., 2002). For this reason, it is unlikely for a cell exposed to the blood to use megalin as a receptor to take up vitamin A from holo-RBP because megalin nonspecifically binds to most, if not all, serum proteins. Extremely abundant serum proteins like albumin will saturate its binding sites. Consistently, no study has shown that megalin can mediate vitamin A uptake from holo-RBP in the serum. Megalin's extremely large extracellular domain may be responsible for the promiscuity in its ligand binding. This promiscuity is clearly important in the recycling of proteins in the kidney to prevent their loss in the urine. In contrast, STRA6 is a highly specific membrane receptor for RBP and can even distinguish RBP from other retinol binding proteins (Kawaguchi, R. et al., 2007). This selectivity is essential for recognizing RBP in the complex mixture of proteins in the serum during vitamin A uptake. Consistently, STRA6 can mediate vitamin A uptake not only from purified holo-RBP but also from holo-RBP in the serum (Kawaguchi, R. et al., 2007). A large number of studies have shown that the RBP receptor-mediated vitamin A uptake does not depend on endocytosis (Bok, D., and Heller, J., 1976; Chen, C. C., and Heller, J., 1977; Heller, J., 1975; Ottonello, S. et al., 1987; Quadro, L. et al., 2002; Rask, L., and Peterson, P. A., 1976; Shingleton, J. L. et al., 1989; Sivaprasadarao, A., and Findlay, J. B., 1988b; Sundaram, M. et al., 1998). These studies distinguished megalin from the specific RBP receptor even before its identification.
8. The RBP Receptor as a Potential Target in Treating Human Diseases
Knowledge of RBP receptor may be used to design specific methods to increase or decrease tissue retinoid levels to treat human diseases and alleviate disease symptoms. Currently, the most common therapeutic uses of retinoids are in dermatology and oncology. Retinoids have been used to treat various types of cancer (Simoni, D., and Tolomeo, M., 2001; Verma, A. K., 2003) and various skin diseases such as psoriasis and other hyperkeratotic and parakeratotic skin disorders, keratotic genodermatoses, severe acne and acne-related dermatoses (Orfanos, C. E. et al., 1997). Given the potent biological effects of retinoids, current retinoid treatment is associated with diverse toxic effects such as teratogenicity, bone toxicity and increases in serum lipids.
The RBP receptor-mediated vitamin A uptake is a natural physiological mechanism for cellular absorption of vitamin A from the blood. Increasing retinoid level by stimulating RBP receptor activity using pharmacology or molecular biology methods can potentially avoid the toxic effects of systemic administration of retinoids. Upregulating RBP receptor activity will only increase uptake through a physiological system. An analogy of systemic administration of retinoid is to flood a city in drought with water in an uncontrolled manner. Upregulating RBP receptor activity in this analogy is to deliver more water to a city in drought through its natural water delivery system. When decreasing the retinoid level is desired in treating certain diseases, cell-specific or tissue-specific suppression of the RBP receptor activity is better than systemic lowering of vitamin A/RBP level in the blood.
Membrane transporters have been one of the most successful drug targets. For example, serotonin reuptake inhibitors including Prozac inhibit monoamine transporters and are widely used as anti-depressant. Given STRA6's function as a membrane transport protein for vitamin A uptake, it is possible to modulate tissue vitamin A level by specifically targeting this natural vitamin A uptake mechanism. This technique can avoid the use of retinoids, which are associated with a wide variety of toxic effects. Since STRA6 is not homologous to any membrane receptor, channels and transporters, its uniqueness can also be an advantage in pharmacological targeting of this receptor to create specific drugs. Modulating tissue retinoid level by targeting this natural vitamin A uptake mechanism has the potential to treat or alleviate the symptoms of the following diseases:
8.1. Visual disorders
Vitamin A is essential for vision because it is the precursor to photoreceptor chromophore (Crouch, R. K. et al., 1996; Wald, G., 1968). A variety of visual disorders has been associated with abnormal vitamin A metabolism in the eye (Thompson, D. A., and Gal, A., 2003; Travis, G. H. et al., 2006). Modulating vitamin A uptake is a known method to alleviate symptoms for some diseases such as Stargardt macular dystrophy (Radu, R. A. et al., 2005; Radu, R. A. et al., 2004). Therefore, STRA6 is a novel molecular target for treating visual disorders.
8.2. Cancer
STRA6 was originally identified as a cancer cell surface marker (Bouillet, P. et al., 1997; Szeto, W. et al., 2001). STRA6 was overexpressed up to 172 fold in 14 out of 14 human colorectal tumors relative to normal colon tissue (Szeto, W. et al., 2001). Since vitamin A is known to be required for cell proliferation in many contexts, these cancer cells likely use the enhanced retinoid level for proliferation. Thus, the identification of STRA6 as the RBP receptor makes it possible to inhibit cancer growth by inhibiting the RBP receptor. Retinoids have been used to treat many types of cancer (Verma, A. K., 2003), and targeting STRA6 is an alternative to systemic retinoid treatment.
8.3. Skin diseases
Vitamin A plays essential roles in maintaining normal skin (Vahlquist, A., 1994). Retinoid can reverse aging of the skin (Varani, J. et al., 1998). At the molecular level, retinoid treatment reduces matrix metalloproteinase expression and stimulates collagen synthesis in both naturally aged skin and photoaged skin (Varani, J. et al., 2000). Retinoids have been used for treatment of various skin diseases such as psoriasis and other hyperkeratotic and parakeratotic skin disorders, keratotic genodermatoses, severe acne and acne-related dermatoses (Orfanos, C. E. et al., 1997). Given the role of STRA6 as the natural mechanism of vitamin A uptake, modulating STRA6 activity in the skin is an alternative and potentially less toxic method to regulating skin retinoid level.
8.4. Lung diseases
Vitamin A plays important roles in maintaining normal development and function of the lung (Biesalski, H. K., 2003). Consistently, STRA6 mutations cause lung hypoplasia (Golzio, C. et al., 2007; Pasutto, F. et al., 2007). Vitamin A deficiency produces morphologic changes in the lung, impairs pneumocyte function (Baybutt, R. C. et al., 2000), and potentiates hyperoxic lung injury (Veness-Meehan, K. A., 1997). Vitamin A also has a protective effect on respiratory status in patients with cystic fibrosis (Aird, F. K. et al., 2006). Therefore, modulating STRA6 activity can potentially improve lung function under pathological conditions.
8.5. Immune disorders
Vitamin A plays important roles in hematopoiesis and in maintaining immunocompetence (Blomhoff, H. K., and Smeland, E. B., 1994; Oren, T. et al., 2003; Sommer, A., 1997a, b; Stephensen, C. B., 2001). Since STRA6 is highly expressed in lymphoid organs such as thymus, spleen, and lymph nodes, modulating STRA6 activity has the potential to improve immune function.
8.6. Neurological disorders
Vitamin A is required for cognition, learning, and memory of adult brain because retinoic acid is a modulator of the nervous system (Drager, U. C., 2006; Lane, M. A., and Bailey, S. J., 2005; Maden, M., 2007; Weiler, R. et al., 2001). For example, retinoic acid plays an important role in maintaining synaptic plasticity in hippocampus (Misner, D. L. et al., 2001) and cortical synchrony during sleep (Maret, S. et al., 2005). It also plays an important role in regenerative processes in the adult central nervous system (Vergara, M. N. et al., 2005). Highly abundant expression of STRA6 in the brain is consistent with vitamin A's function in the brain. Given the potential roles of retinoid signaling in depression, Parkinson disease, Huntington disease, neuronal regeneration, and Alzheimer disease (Goodman, A. B., 2006; Mey, J., and McCaffery, P., 2004; Vergara, M. N. et al., 2005), STRA6 is a potential target for treating or alleviating symptoms of neurological disorders.
8.7. Diabetes
RBP was recently discovered as a signal secreted by adipoctyes for insulin resistance (Yang, Q. et al., 2005). STRA6 is the only known high-affinity receptor for RBP and is potentially involved in pathological events in insulin resistance.
9. Concluding Remarks
It is a surprise that evolution came up with a completely new type of cell surface receptor to mediate cellular vitamin A uptake. This receptor is unlike any known membrane receptors, transporters or channels, although functionally it is both as a receptor for RBP and a membrane transport protein that mediates vitamin A entry into the cell. How this multitransmembrane domain protein performs these functions are just beginning to be understood. Future studies of this receptor will shed light on the molecular mechanism of how cells take up vitamin A under physiological conditions and how this uptake process is regulated to maintain tissue retinoid homeostasis. This knowledge will help to develop new treatment for human diseases caused by insufficient or excessive retinoid levels.
Acknowledgement
Supported by NIH/NEI grant 1R01EY018144 (H.S.).
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adams J. Structure-activity and dose-response relationships in the neural and behavioral teratogenesis of retinoids. Neurotoxicol Teratol. 1993;15:193–202. doi: 10.1016/0892-0362(93)90015-g. [DOI] [PubMed] [Google Scholar]
- Agre P. Nobel Lecture. Aquaporin water channels. Biosci Rep. 2004;24:127–163. doi: 10.1007/s10540-005-2577-2. [DOI] [PubMed] [Google Scholar]
- Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- Aird FK, Greene SA, Ogston SA, Macdonald TM, Mukhopadhyay S. Vitamin A and lung function in CF. J Cyst Fibros. 2006;5:129–131. doi: 10.1016/j.jcf.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998;11:277–285. [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleato RM, Aitken RJ, Roman SD. Vitamin A regulation of BMP4 expression in the male germ line. Dev Biol. 2005;286:78–90. doi: 10.1016/j.ydbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Basu TK, Basualdo C. Vitamin A homeostasis and diabetes mellitus. Nutrition. 1997;13:804–806. doi: 10.1016/s0899-9007(97)00192-5. [DOI] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt RC, Hu L, Molteni A. Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J Nutr. 2000;130:1159–1165. doi: 10.1093/jn/130.5.1159. [DOI] [PubMed] [Google Scholar]
- Bhat MK, Cama HR. Gonadal cell surface receptor for plasma retinol-binding protein. A method for its radioassay and studies on its level during spermatogenesis. Biochim Biophys Acta. 1979;587:273–281. doi: 10.1016/0304-4165(79)90360-x. [DOI] [PubMed] [Google Scholar]
- Biesalski HK. The significance of vitamin A for the development and function of the lung. Forum Nutr. 2003;56:37–40. [PubMed] [Google Scholar]
- Biesalski HK, Wellner U, Stofft E, Bassler KH. Vitamin A deficiency and sensory function. Acta Vitaminol Enzymol. 1985;7(Suppl):45–54. [PubMed] [Google Scholar]
- Bishop PD, Griswold MD. Uptake and metabolism of retinol in cultured Sertoli cells: evidence for a kinetic model. Biochemistry. 1987;26:7511–7518. doi: 10.1021/bi00397a046. [DOI] [PubMed] [Google Scholar]
- Blomhoff HK, Smeland EB. Role of Retinoids in Normal Hemtopoiesis and the Immune System. In: Blomhoff R, editor. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. pp. 451–484. [Google Scholar]
- Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- Bok D, Heller J. Transport of retinol from the blood to the retina: an autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein. Exp Eye Res. 1976;22:395–402. doi: 10.1016/0014-4835(76)90177-9. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier JM, Schuhbaur B, Dolle P, Chambon P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2) Dev Biol. 1995;170:420–433. doi: 10.1006/dbio.1995.1226. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991;254:1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- Buck J, Ritter G, Dannecker L, Katta V, Cohen SL, Chait BT, Hammerling U. Retinol is essential for growth of activated human B cells. J Exp Med. 1990;171:1613–1624. doi: 10.1084/jem.171.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A, Bok D. Promotion of the release of 11-cis-retinal from cultured retinal pigment epithelium by interphotoreceptor retinoid-binding protein. Biochemistry. 1992;31:9056–9062. doi: 10.1021/bi00152a049. [DOI] [PubMed] [Google Scholar]
- Carlson A, Bok D. Polarity of 11-cis retinal release from cultured retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:533–537. [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- Chassaing N, Golzio C, Odent S, Lequeux L, Vigouroux A, Martinovic-Bouriel J, Tiziano FD, Masini L, Piro F, Maragliano G, Delezoide AL, Attie-Bitach T, Manouvrier-Hanu S, Etchevers HC, Calvas P. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Hum Mutat. 2009;30:E673–681. doi: 10.1002/humu.21023. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Bouillet P, Oulad-Abdelghani M, Dolle P. Restricted expression of a novel retinoic acid responsive gene during limb bud dorsoventral patterning and endochondral ossification. Dev Genet. 1996;19:66–73. doi: 10.1002/(SICI)1520-6408(1996)19:1<66::AID-DVG7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Chen C, Koutalos Y. Rapid formation of all-trans retinol after bleaching in frog and mouse rod photoreceptor outer segments. Photochem Photobiol Sci. 2010;9:1475–1479. doi: 10.1039/c0pp00124d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Tsina E, Cornwall MC, Crouch RK, Vijayaraghavan S, Koutalos Y. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys J. 2005;88:2278–2287. doi: 10.1529/biophysj.104.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Heller J. Uptake of retinol and retinoic acid from serum retinol-binding protein by retinal pigment epithelial cells. J Biol Chem. 1977;252:5216–5221. [PubMed] [Google Scholar]
- Chen L, Khillan JS. Promotion of feeder-independent self-renewal of embryonic stem cells by retinol (vitamin A) Stem Cells. 2008;26:1858–1864. doi: 10.1634/stemcells.2008-0050. [DOI] [PubMed] [Google Scholar]
- Chen L, Khillan JS. A novel signaling by vitamin A/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells. 2010;28:57–63. doi: 10.1002/stem.251. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang M, Dawes J, Khillan JS. Suppression of ES cell differentiation by retinol (vitamin A) via the overexpression of Nanog. Differentiation. 2007;75:682–693. doi: 10.1111/j.1432-0436.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ross AC. Inaugural Article: Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci U S A. 2005;102:14142–14149. doi: 10.1073/pnas.0505018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Gardner DG. Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. J Clin Invest. 1998;102:653–662. doi: 10.1172/JCI3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Buck J, Derguini F. Anhydroretinol induces oxidative stress and cell death. Cancer Res. 1999;59:3985–3990. [PubMed] [Google Scholar]
- Chen Y, Derguini F, Buck J. Vitamin A in serum is a survival factor for fibroblasts. Proc Natl Acad Sci U S A. 1997;94:10205–10208. doi: 10.1073/pnas.94.19.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivot M. Retinoid therapy for acne. A comparative review. Am J Clin Dermatol. 2005;6:13–19. doi: 10.2165/00128071-200506010-00002. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK. Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol. 1999;10:685–695. doi: 10.1681/ASN.V104685. [DOI] [PubMed] [Google Scholar]
- Chung SS, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res. 2004;105:189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- Cocco S, Diaz G, Stancampiano R, Diana A, Carta M, Curreli R, Sarais L, Fadda F. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience. 2002;115:475–482. doi: 10.1016/s0306-4522(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharmacol Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- Crandall J, Sakai Y, Zhang J, Koul O, Mineur Y, Crusio WE, McCaffery P. 13-cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci U S A. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch RK, Chader GJ, Wiggert B, Pepperberg DR. Retinoids and the visual process. Photochem Photobiol. 1996;64:613–621. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Dew SE, Ong DE. Specificity of the retinol transporter of the rat small intestine brush border. Biochemistry. 1994;33:12340–12345. doi: 10.1021/bi00206a042. [DOI] [PubMed] [Google Scholar]
- Dingle JT, Fell HB, Goodman DS. The effect of retinol and of retinol-binding protein on embryonic skeletal tissue in organ culture. J Cell Sci. 1972;11:393–402. doi: 10.1242/jcs.11.2.393. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Night blindness. Sci Am. 1966;215:78–84. doi: 10.1038/scientificamerican1066-78. [DOI] [PubMed] [Google Scholar]
- Drager UC. Retinoic acid signaling in the functioning brain. Sci STKE. 2006;2006:e10. doi: 10.1126/stke.3242006pe10. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Hansson E, Nilsson M, Jonsson KH, Sundelin J, Peterson PA. Increased levels of several retinoid binding proteins resulting from retinoic acid-induced differentiation of F9 cells. Cancer Res. 1986;46:717–722. [PubMed] [Google Scholar]
- Evans RM. The molecular basis of signaling by vitamin A and its metabolites. Harvey Lect. 1994;90:105–117. [PubMed] [Google Scholar]
- Folli C, Viglione S, Busconi M, Berni R. Biochemical basis for retinol deficiency induced by the I41N and G75D mutations in human plasma retinol-binding protein. Biochem Biophys Res Commun. 2005;336:1017–1022. doi: 10.1016/j.bbrc.2005.08.227. [DOI] [PubMed] [Google Scholar]
- Gardner DG, Chen S. Retinoids and cell growth in the cardiovascular system. Life Sci. 1999;65:1607–1613. doi: 10.1016/s0024-3205(99)00255-6. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC. Matthew-Wood Syndrome Is Caused by Truncating Mutations in the Retinol-Binding Protein Receptor Gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AB. Retinoid receptors, transporters, and metabolizers as therapeutic targets in late onset Alzheimer disease. J Cell Physiol. 2006;209:598–603. doi: 10.1002/jcp.20784. [DOI] [PubMed] [Google Scholar]
- Goodman DS. Plasma Retinol-Binding Protein. In: Sporn MB, Boberts AB, Goodman DS, editors. The Retinoids. Vol. 2. Academic Press, Inc.; 1984. pp. 41–88. [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–172. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- Hagen E, Myhre AM, Smeland S, Halvorsen B, Norum KR, Blomhoff R. Uptake of vitamin A in macrophages from physiologic transport proteins: role of retinol-binding protein and chylomicron remnants. J Nutr Biochem. 1999;10:345–352. doi: 10.1016/s0955-2863(99)00013-3. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Heller J. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J Biol Chem. 1975;250:3613–3619. [PubMed] [Google Scholar]
- Heller M, Bok D. A specific receptor for retinol binding protein as detected by the binding of human and bovine retinol binding protein to pigment epithelial cells. Am J Ophthalmol. 1976;81:93–97. doi: 10.1016/0002-9394(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Howell JM, Thompson JN, Pitt GA. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. I. The male rat. J Reprod Fertil. 1963;5:159–167. doi: 10.1530/jrf.0.0050159. [DOI] [PubMed] [Google Scholar]
- Hoyos B, Jiang S, Hammerling U. Location and functional significance of retinol-binding sites on the serine/threonine kinase, c-Raf. J Biol Chem. 2005;280:6872–6878. doi: 10.1074/jbc.M412695200. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kitagawa M, Imai H, Yamada M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J Reprod Dev. 2005;51:23–35. doi: 10.1262/jrd.51.23. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 Disrupts Vitamin A Uptake Homeostasis in a STRA6-Deficient Animal Model for Matthew-Wood Syndrome. Cell Metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Wiita P, Honda J, Sun H. An essential ligand-binding domain in the membrane receptor for retinol-binding protein revealed by large-scale mutagenesis and a human polymorphism. J Biol Chem. 2008a;283:15160–15168. doi: 10.1074/jbc.M801060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Wiita P, Honda J, Sun H. An essential ligand binding domain in the membrane receptor for retinol binding protein revealed by large-scale mutagenesis and a human polymorphism. J Biol Chem. 2008b doi: 10.1074/jbc.M801060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Wiita P, Ter-Stepanian M, Sun H. Mapping the membrane topology and extracellular ligand binding domains of the retinol binding protein receptor. Biochemistry. 2008c;47:5387–5395. doi: 10.1021/bi8002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korichneva I, Waka J, Hammerling U. Regulation of the cardiac mitochondrial membrane potential by retinoids. J Pharmacol Exp Ther. 2003;305:426–433. doi: 10.1124/jpet.103.048900. [DOI] [PubMed] [Google Scholar]
- Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Prog Neurobiol. 2005;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Liden M, Eriksson U. Development of a versatile reporter assay for studies of retinol uptake and metabolism in vivo. Exp Cell Res. 2005;310:401–408. doi: 10.1016/j.yexcr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Pairault C, Levacher C, Habert R. Regulation and perturbation of testicular functions by vitamin A. Reproduction. 2002;124:173–180. [PubMed] [Google Scholar]
- Love JM, Gudas LJ. Vitamin A, differentiation and cancer. Curr Opin Cell Biol. 1994;6:825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- MacDonald PN, Bok D, Ong DE. Localization of cellular retinol-binding protein and retinol-binding protein in cells comprising the blood-brain barrier of rat and human. Proc Natl Acad Sci U S A. 1990;87:4265–4269. doi: 10.1073/pnas.87.11.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D, Miller AL. Nutritional support for wound healing. Altern Med Rev. 2003;8:359–377. [PubMed] [Google Scholar]
- Maden M. Role of Retinoids in Embryonic Development. In: Blomhoff R, editor. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. pp. 289–322. [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makover A, Soprano DR, Wyatt ML, Goodman DS. Localization of retinol-binding protein messenger RNA in the rat kidney and in perinephric fat tissue. J Lipid Res. 1989;30:171–180. [PubMed] [Google Scholar]
- Mallia AK, Smith JE, Goodman DW. Metabolism of retinol-binding protein and vitamin A during hypervitaminosis A in the rat. J Lipid Res. 1975;16:180–188. [PubMed] [Google Scholar]
- Malpeli G, Folli C, Berni R. Retinoid binding to retinol-binding protein and the interference with the interaction with transthyretin. Biochim Biophys Acta. 1996;1294:48–54. doi: 10.1016/0167-4838(95)00264-2. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Kliewer SA, Kakizuka A, Umesono K, Evans RM. Retinoid receptors. Recent Prog Horm Res. 1993;48:99–121. doi: 10.1016/b978-0-12-571148-7.50008-7. [DOI] [PubMed] [Google Scholar]
- Maraini G, Gozzoli F. Binding of retinol to isolated retinal pigment epithelium in the presence and absence of retinol-binding protein. Invest Ophthalmol. 1975;14:785–787. [PubMed] [Google Scholar]
- Maret S, Franken P, Dauvilliers Y, Ghyselinck NB, Chambon P, Tafti M. Retinoic acid signaling affects cortical synchrony during sleep. Science. 2005;310:111–113. doi: 10.1126/science.1117623. [DOI] [PubMed] [Google Scholar]
- Marino M, Andrews D, Brown D, McCluskey RT. Transcytosis of retinol-binding protein across renal proximal tubule cells after megalin (gp 330)-mediated endocytosis. J Am Soc Nephrol. 2001;12:637–648. doi: 10.1681/ASN.V124637. [DOI] [PubMed] [Google Scholar]
- Marshall H, Morrison A, Studer M, Popperl H, Krumlauf R. Retinoids and Hox genes. Faseb J. 1996;10:969–978. [PubMed] [Google Scholar]
- Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhus H, Bavik CO, Rask L, Peterson PA, Eriksson U. Epitope mapping of a monoclonal antibody that blocks the binding of retinol-binding protein to its receptor. Biochem Biophys Res Commun. 1995;210:105–112. doi: 10.1006/bbrc.1995.1633. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10:409–421. doi: 10.1177/1073858404263520. [DOI] [PubMed] [Google Scholar]
- Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood) 2008;233:1255–1261. doi: 10.3181/0803-RM-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misner DL, Jacobs S, Shimizu Y, de Urquiza AM, Solomin L, Perlmann T, De Luca LM, Stevens CF, Evans RM. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268:1039–1041. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- Morley JE, Damassa DA, Gordon J, Pekary AE, Hershman JM. Thyroid function and vitamin A deficiency. Life Sci. 1978;22:1901–1905. doi: 10.1016/0024-3205(78)90477-0. [DOI] [PubMed] [Google Scholar]
- Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Nau H. Teratogenicity of isotretinoin revisited: species variation and the role of all trans-retinoic acid. J Am Acad Dermatol. 2001;45:S183–187. doi: 10.1067/mjd.2001.113720. [DOI] [PubMed] [Google Scholar]
- Nau H, Chahoud I, Dencker L, Lammer EJ, Scott WJ. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. Teratogenicity of Vitamin A and Retinoids; pp. 615–664. [Google Scholar]
- Naylor HM, Newcomer ME. The structure of human retinol-binding protein (RBP) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of RBP. Biochemistry. 1999;38:2647–2653. doi: 10.1021/bi982291i. [DOI] [PubMed] [Google Scholar]
- Newcomer ME, Ong DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482:57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Niles RM. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat Res. 2004;555:81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren T, Sher JA, Evans T. Hematopoiesis and retinoids: development and disease. Leuk Lymphoma. 2003;44:1881–1891. doi: 10.1080/1042819031000116661. [DOI] [PubMed] [Google Scholar]
- Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53:358–388. doi: 10.2165/00003495-199753030-00003. [DOI] [PubMed] [Google Scholar]
- Ottonello S, Petrucco S, Maraini G. Vitamin A uptake from retinol-binding protein in a cell-free system from pigment epithelial cells of bovine retina. Retinol transfer from plasma retinol-binding protein to cytoplasmic retinol-binding protein with retinyl-ester formation as the intermediate step. J Biol Chem. 1987;262:3975–3981. [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 Cause a Broad Spectrum of Malformations Including Anophthalmia, Congenital Heart Defects, Diaphragmatic Hernia, Alveolar Capillary Dysplasia, Lung Hypoplasia, and Mental Retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Pfeffer BA, Clark VM, Flannery JG, Bok D. Membrane receptors for retinol-binding protein in cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1986;27:1031–1040. [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Hamberger L, Novikoff PM, Vogel S, Piantedosi R, Gottesman ME, Colantuoni V. The role of extrahepatic retinol-binding protein (RBP) in the mobilization of retinoid stores. J Lipid Res. 2004 doi: 10.1194/jlr.M400137-JLR200. [DOI] [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Hamberger L, Van Gelder RN, Vogel S, Piantedosi R, Gouras P, Colantuoni V, Gottesman ME. Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J Biol Chem. 2002;277:30191–30197. doi: 10.1074/jbc.M205046200. [DOI] [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadro L, Gamble MV, Vogel S, Lima AA, Piantedosi R, Moore SR, Colantuoni V, Gottesman ME, Guerrant RL, Blaner WS. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J Infect Dis. 2000;182(Suppl 1):S97–S102. doi: 10.1086/315920. [DOI] [PubMed] [Google Scholar]
- Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003;24:421–430. doi: 10.1016/s0098-2997(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- Radu RA, Mata NL, Nusinowitz S, Liu X, Travis GH. Isotretinoin treatment inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Novartis Found Symp. 2004;255:51–63. discussion 63–57, 177–178. [PubMed] [Google Scholar]
- Rask L, Geijer C, Bill A, Peterson PA. Vitamin A supply of the cornea. Exp Eye Res. 1980;31:201–211. doi: 10.1016/0014-4835(80)90078-0. [DOI] [PubMed] [Google Scholar]
- Rask L, Peterson PA. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey's small intestine. J Biol Chem. 1976;251:6360–6366. [PubMed] [Google Scholar]
- Rask L, Vahlquist A, Peterson PA. Studies on two physiological forms of the human retinol-binding protein differing in vitamin A and arginine content. J Biol Chem. 1971;246:6638–6646. [PubMed] [Google Scholar]
- Redondo C, Burke BJ, Findlay JB. The Retinol-binding Protein System: A Potential Paradigm for Steroid-binding Globulins? Horm Metab Res. 2006;38:269–278. doi: 10.1055/s-2006-925349. [DOI] [PubMed] [Google Scholar]
- Reijntjes S, Blentic A, Gale E, Maden M. The control of morphogen signalling: regulation of the synthesis and catabolism of retinoic acid in the developing embryo. Dev Biol. 2005;285:224–237. doi: 10.1016/j.ydbio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Ross AC, Ambalavanan N, Zolfaghari R, Li NQ. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in a synergistic manner in neonatal rats. J Lipid Res. 2006;47:1844–1851. doi: 10.1194/jlr.M600061-JLR200. [DOI] [PubMed] [Google Scholar]
- Ross AC, Gardner EM. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv Exp Med Biol. 1994;352:187–200. doi: 10.1007/978-1-4899-2575-6_15. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Ghyselinck NB, Mata N, Nusinowitz S, Lloyd M, Dennefeld C, Chambon P, Bok D. Somatic ablation of the lrat gene in the mouse retinal pigment epithelium drastically reduces its retinoid storage. Invest Ophthalmol Vis Sci. 2007;48:5377–5387. doi: 10.1167/iovs.07-0673. [DOI] [PubMed] [Google Scholar]
- Sachs G, Kraut JA, Wen Y, Feng J, Scott DR. Urea transport in bacteria: acid acclimation by gastric Helicobacter spp. J Membr Biol. 2006;212:71–82. doi: 10.1007/s00232-006-0867-7. [DOI] [PubMed] [Google Scholar]
- Seeliger MW, Biesalski HK, Wissinger B, Gollnick H, Gielen S, Frank J, Beck S, Zrenner E. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci. 1999;40:3–11. [PubMed] [Google Scholar]
- Segel R, Levy-Lahad E, Pasutto F, Picard E, Rauch A, Alterescu G, Schimmel MS. Pulmonary hypoplasia-diaphragmatic hernia-anophthalmia-cardiac defect (PDAC) syndrome due to STRA6 mutations--what are the minimal criteria? Am J Med Genet A. 2009;149A:2457–2463. doi: 10.1002/ajmg.a.33038. [DOI] [PubMed] [Google Scholar]
- Semba RD. The role of vitamin A and related retinoids in immune function. Nutr Rev. 1998;56:S38–48. doi: 10.1111/j.1753-4887.1998.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- Shalita AR. Mucocutaneous and systemic toxicity of retinoids: monitoring and management. Dermatologica. 1987;175(Suppl 1):151–157. doi: 10.1159/000248878. [DOI] [PubMed] [Google Scholar]
- Shingleton JL, Skinner MK, Ong DE. Characteristics of retinol accumulation from serum retinol-binding protein by cultured Sertoli cells. Biochemistry. 1989;28:9641–9647. doi: 10.1021/bi00451a015. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA, Chaudhry P, Kondo M, Provenzano M, Wu D, Carlson TJ, Bush RA, Thompson DA. Inhibition of the visual cycle in vivo by 13-cis retinoic acid protects from light damage and provides a mechanism for night blindness in isotretinoin therapy. Proc Natl Acad Sci U S A. 2001;98:1835–1840. doi: 10.1073/pnas.041606498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni D, Tolomeo M. Retinoids, apoptosis and cancer. Curr Pharm Des. 2001;7:1823–1837. doi: 10.2174/1381612013397168. [DOI] [PubMed] [Google Scholar]
- Sivaprasadarao A, Boudjelal M, Findlay JB. Solubilization and purification of the retinol-binding protein receptor from human placental membranes. Biochem J. 1994;302(Pt 1):245–251. doi: 10.1042/bj3020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasadarao A, Findlay JB. The interaction of retinol-binding protein with its plasma-membrane receptor. Biochem J. 1988a;255:561–569. [PMC free article] [PubMed] [Google Scholar]
- Sivaprasadarao A, Findlay JB. The mechanism of uptake of retinol by plasma-membrane vesicles. Biochem J. 1988b;255:571–579. [PMC free article] [PubMed] [Google Scholar]
- Sivaprasadarao A, Findlay JB. Structure-function studies on human retinol-binding protein using site-directed mutagenesis. Biochem J. 1994;300(Pt 2):437–442. doi: 10.1042/bj3000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeland S, Bjerknes T, Malaba L, Eskild W, Norum KR, Blomhoff R. Tissue distribution of the receptor for plasma retinol-binding protein. Biochem J. 1995;305(Pt 2):419–424. doi: 10.1042/bj3050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med. 1976;294:805–808. doi: 10.1056/NEJM197604082941503. [DOI] [PubMed] [Google Scholar]
- Sommer A. 1997 Albert Lasker Award for Clinical Research. Clinical research and the human condition: moving from observation to practice. Nat Med. 1997a;3:1061–1063. doi: 10.1038/nm1097-1061. [DOI] [PubMed] [Google Scholar]
- Sommer A. Vitamin A deficiency, child health, and survival. Nutrition. 1997b;13:484–485. doi: 10.1016/s0899-9007(97)00013-0. [DOI] [PubMed] [Google Scholar]
- Sommer A, Davidson FR. Assessment and control of vitamin A deficiency: the Annecy Accords. J Nutr. 2002;132:2845S–2850S. doi: 10.1093/jn/132.9.2845S. [DOI] [PubMed] [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- Sundaram M, Sivaprasadarao A, DeSousa MM, Findlay JB. The transfer of retinol from serum retinol-binding protein to cellular retinol-binding protein is mediated by a membrane receptor. J Biol Chem. 1998;273:3336–3342. doi: 10.1074/jbc.273.6.3336. [DOI] [PubMed] [Google Scholar]
- Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–4205. [PubMed] [Google Scholar]
- Takahashi N, Breitman TR. Retinoylation of Proteins in Mammalian Cells. In: Blomhoff R, editor. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. pp. 257–273. [Google Scholar]
- Tamori Y, Sakaue H, Kasuga M. RBP4, an unexpected adipokine. Nat Med. 2006;12:30–31. doi: 10.1038/nm0106-30. discussion 31. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Gal A. Genetic defects in vitamin A metabolism of the retinal pigment epithelium. Dev Ophthalmol. 2003;37:141–154. doi: 10.1159/000072044. [DOI] [PubMed] [Google Scholar]
- Torma H, Vahlquist A. Vitamin A uptake by human skin in vitro. Arch Dermatol Res. 1984;276:390–395. doi: 10.1007/BF00413360. [DOI] [PubMed] [Google Scholar]
- Torma H, Vahlquist A. Uptake of vitamin A and retinol-binding protein by human placenta in vitro. Placenta. 1986;7:295–305. doi: 10.1016/s0143-4004(86)80147-3. [DOI] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases Caused by Defects in the Visual Cycle: Retinoids as Potential Therapeutic Agents. Annu Rev Pharmacol Toxicol. 2006 doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlquist A. Role of Retinoids in Normal and Diseased Skin. In: Blomhoff R, editor. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. pp. 365–424. [Google Scholar]
- Varani J, Fisher GJ, Kang S, Voorhees JJ. Molecular mechanisms of intrinsic skin aging and retinoid-induced repair and reversal. J Investig Dermatol Symp Proc. 1998;3:57–60. [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Veness-Meehan KA. Effects of retinol deficiency and hyperoxia on collagen gene expression in rat lung. Exp Lung Res. 1997;23:569–581. doi: 10.3109/01902149709039244. [DOI] [PubMed] [Google Scholar]
- Vergara MN, Arsenijevic Y, Del Rio-Tsonis K. CNS regeneration: a morphogen's tale. J Neurobiol. 2005;64:491–507. doi: 10.1002/neu.20158. [DOI] [PubMed] [Google Scholar]
- Verma AK. Retinoids in chemoprevention of cancer. J Biol Regul Homeost Agents. 2003;17:92–97. [PubMed] [Google Scholar]
- Vogel S, Piantedosi R, O'Byrne SM, Kako Y, Quadro L, Gottesman ME, Goldberg IJ, Blaner WS. Retinol-binding protein-deficient mice: biochemical basis for impaired vision. Biochemistry. 2002;41:15360–15368. doi: 10.1021/bi0268551. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Weiler R, Pottek M, Schultz K, Janssen-Bienhold U. Retinoic acid, a neuromodulator in the retina. Prog Brain Res. 2001;131:309–318. doi: 10.1016/s0079-6123(01)31025-7. [DOI] [PubMed] [Google Scholar]
- Wendler CC, Schmoldt A, Flentke GR, Case LC, Quadro L, Blaner WS, Lough J, Smith SM. Increased fibronectin deposition in embryonic hearts of retinol-binding protein-null mice. Circ Res. 2003;92:920–928. doi: 10.1161/01.RES.0000069030.30886.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Werner EA, Deluca HF. Retinoic acid is detected at relatively high levels in the CNS of adult rats. Am J Physiol Endocrinol Metab. 2002;282:E672–678. doi: 10.1152/ajpendo.00280.2001. [DOI] [PubMed] [Google Scholar]
- West B, Bove KE, Slavotinek AM. Two novel STRA6 mutations in a patient with anophthalmia and diaphragmatic eventration. Am J Med Genet A. 2009;149A:539–542. doi: 10.1002/ajmg.a.32682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KP., Jr . Vitamin A Deficiency: Its Epidemiology and Relation to Child Mortality and Morbidity. In: Blomhoff R, editor. Vitamin A in Health and Disease. Marcel Dekker, Inc.; 1994. [Google Scholar]
- White T, Lu T, Metlapally R, Katowitz J, Kherani F, Wang TY, Tran-Viet KN, Young TL. Identification of STRA6 and SKI sequence variants in patients with anophthalmia/microphthalmia. Mol Vis. 2008;14:2458–2465. [PMC free article] [PubMed] [Google Scholar]
- Wolbach SR, Howe PR. Tissue change following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GL, Wang S, Fultz ME, Arif I, Matthews K, Chertow BS. Effect of vitamin A deficiency on cardiovascular function in the rat. Can J Physiol Pharmacol. 2002;80:1–7. doi: 10.1139/y01-093. [DOI] [PubMed] [Google Scholar]
- Xu Q, Lucio-Cazana J, Kitamura M, Ruan X, Fine LG, Norman JT. Retinoids in nephrology: promises and pitfalls. Kidney Int. 2004;66:2119–2131. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. Cloning and characterization of the vasopressin-regulated urea transporter. Nature. 1993;365:844–847. doi: 10.1038/365844a0. [DOI] [PubMed] [Google Scholar]
- Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
- Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis CC. Retinoids--which dermatological indications will benefit in the near future? Skin Pharmacol Appl Skin Physiol. 2001;14:303–315. doi: 10.1159/000056361. [DOI] [PubMed] [Google Scholar]