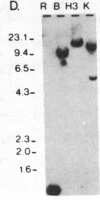
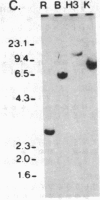
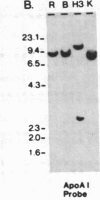
Abstract
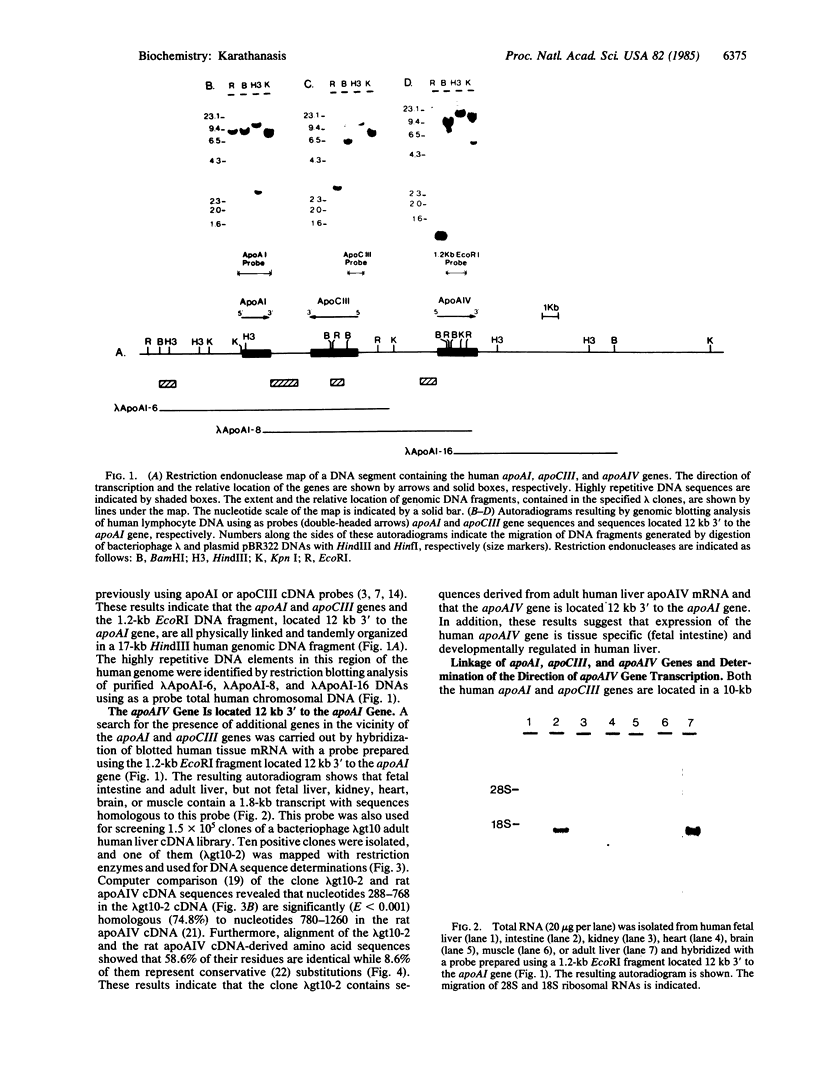
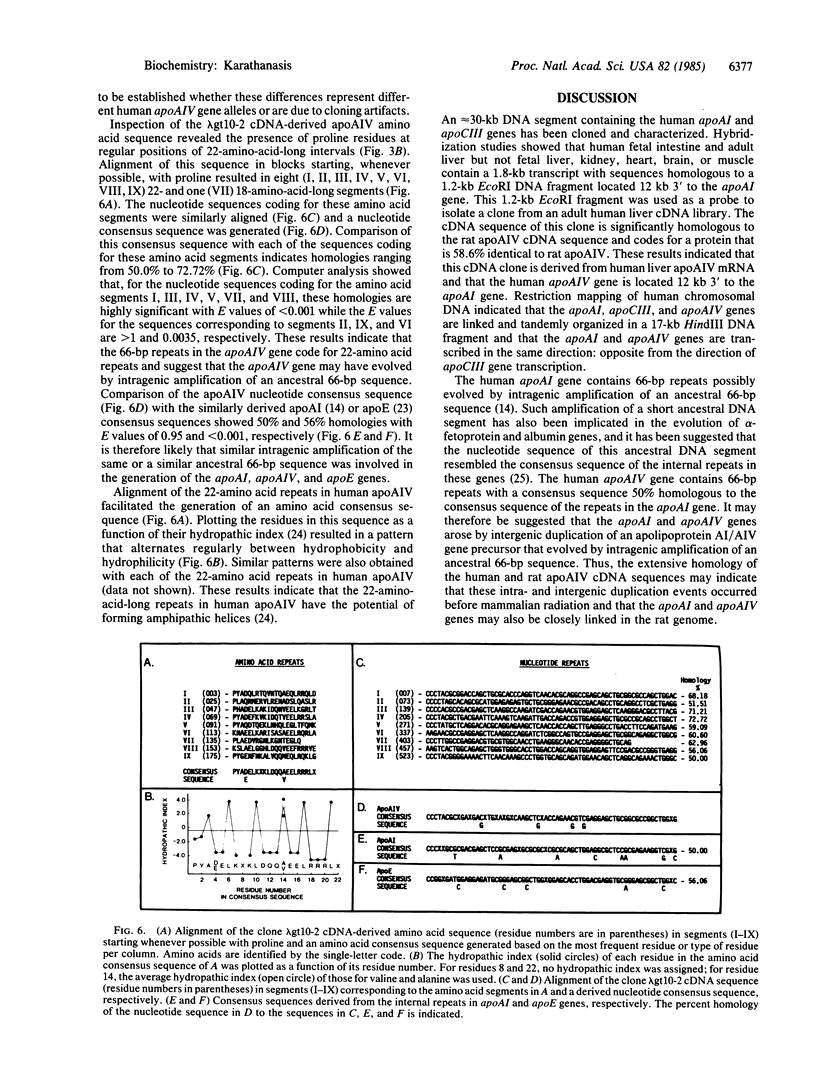
The genes for two of the proteins of the plasma lipid transport system, apolipoprotein AI (apoAI) and CIII (apoCIII) are closely linked in the human genome. An approximately equal to 30-kilobase (kb) DNA segment containing these genes and their flanking sequences has been cloned and extensively characterized. Hybridization studies revealed that a DNA fragment located 12 kb 3' to the apoAI gene contains sequences homologous to a 1.8-kb mRNA transcript in human fetal intestine and adult liver but not in fetal liver, kidney, heart, brain, or muscle. This DNA fragment was used as a probe to isolate a clone from an adult human liver cDNA library. The nucleotide sequence of this clone is 74.8% homologous to the cDNA sequence of rat apolipoprotein AIV (apoAIV), another protein of the lipid transport system, and codes for a protein that is 58.6% identical to rat apoAIV. These results indicate that apoAI, apoCIII, and apoAIV genes are closely linked in the human genome and suggest that all three of them are derived from a common ancestral precursor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander F., Young P. R., Tilghman S. M. Evolution of the albumin: alpha-fetoprotein ancestral gene from the amplification of a 27 nucleotide sequence. J Mol Biol. 1984 Feb 25;173(2):159–173. doi: 10.1016/0022-2836(84)90187-6. [DOI] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Evolution of lipoproteins deduced from protein sequence data. Comp Biochem Physiol B. 1977;57(4):309–315. doi: 10.1016/0305-0491(77)90060-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Rat apolipoprotein A-IV contains 13 tandem repetitions of a 22-amino acid segment with amphipathic helical potential. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5021–5025. doi: 10.1073/pnas.81.16.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D. Gene expression in eukaryotes. Science. 1981 Feb 13;211(4483):667–674. doi: 10.1126/science.6256857. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Karathanasis S. K., Breslow J. L. Human apolipoprotein A-I--C-III gene complex is located on chromosome 11. Arteriosclerosis. 1984 Mar-Apr;4(2):97–102. doi: 10.1161/01.atv.4.2.97. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L., Clayton J., Friedland P., Kedes L. H. SEQ: a nucleotide sequence analysis and recombination system. Nucleic Acids Res. 1982 Jan 11;10(1):279–294. doi: 10.1093/nar/10.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberg A. M., Kinniburgh A. J. Induction of liver apolipoprotein A-IV mRNA in porphyric mice. Nucleic Acids Res. 1985 Mar 25;13(6):1953–1963. doi: 10.1093/nar/13.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Das H. K., McPherson J., Bruns G. A., Karathanasis S. K., Breslow J. L. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J Biol Chem. 1985 May 25;260(10):6240–6247. [PubMed] [Google Scholar]
- Green P. H., Glickman R. M., Riley J. W., Quinet E. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J Clin Invest. 1980 Apr;65(4):911–919. doi: 10.1172/JCI109745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries S. E., Berg K., Gill L., Cumming A. M., Robertson F. W., Stalenhoef A. F., Williamson R., Børresen A. L. The gene for apolipoprotein C-II is closely linked to the gene for apolipo-protein E on chromosome 19. Clin Genet. 1984 Nov;26(5):389–396. [PubMed] [Google Scholar]
- Jackson C. L., Bruns G. A., Breslow J. L. Isolation and sequence of a human apolipoprotein CII cDNA clone and its use to isolate and map to human chromosome 19 the gene for apolipoprotein CII. Proc Natl Acad Sci U S A. 1984 May;81(10):2945–2949. doi: 10.1073/pnas.81.10.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanasis S. K., McPherson J., Zannis V. I., Breslow J. L. Linkage of human apolipoproteins A-I and C-III genes. 1983 Jul 28-Aug 3Nature. 304(5924):371–373. doi: 10.1038/304371a0. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., Norum R. A., Zannis V. I., Breslow J. L. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 1983 Feb 24;301(5902):718–720. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., Zannis V. I., Breslow J. L. A DNA insertion in the apolipoprotein A-I gene of patients with premature atherosclerosis. 1983 Oct 27-Nov 2Nature. 305(5937):823–825. doi: 10.1038/305823a0. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., Zannis V. I., Breslow J. L. Isolation and characterization of cDNA clones corresponding to two different human apoC-III alleles. J Lipid Res. 1985 Apr;26(4):451–456. [PubMed] [Google Scholar]
- Karathanasis S. K., Zannis V. I., Breslow J. L. Isolation and characterization of the human apolipoprotein A-I gene. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6147–6151. doi: 10.1073/pnas.80.20.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Law S. W., Gray G., Brewer H. B., Jr, Sakaguchi A. Y., Naylor S. L. Human apolipoprotein A-I and C-III genes reside in the p11----q13 region of chromosome 11. Biochem Biophys Res Commun. 1984 Feb 14;118(3):934–942. doi: 10.1016/0006-291x(84)91485-2. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipid-protein interactions in the plasma lipoproteins. Biochim Biophys Acta. 1977 Aug 9;472(2):93–133. doi: 10.1016/0304-4157(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Myklebost O., Rogne S., Olaisen B., Gedde-Dahl T., Jr, Prydz H. The locus for apolipoprotein CII is closely linked to the apolipoprotein E locus on chromosome 19 in man. Hum Genet. 1984;67(3):309–312. doi: 10.1007/BF00291359. [DOI] [PubMed] [Google Scholar]
- Protter A. A., Levy-Wilson B., Miller J., Bencen G., White T., Seilhamer J. J. Isolation and sequence analysis of the human apolipoprotein CIII gene and the intergenic region between the apo AI and apo CIII genes. DNA. 1984 Dec;3(6):449–456. doi: 10.1089/dna.1.1984.3.449. [DOI] [PubMed] [Google Scholar]
- Rees A., Shoulders C. C., Stocks J., Galton D. J., Baralle F. E. DNA polymorphism adjacent to human apoprotein A-1 gene: relation to hypertriglyceridaemia. Lancet. 1983 Feb 26;1(8322):444–446. doi: 10.1016/s0140-6736(83)91440-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz A., Utermann G. Activation of lecithin: cholesterol acyltransferase by human apolipoprotein A-IV. J Biol Chem. 1985 Feb 25;260(4):2258–2264. [PubMed] [Google Scholar]
- Tata F., Henry I., Markham A. F., Wallis S. C., Weil D., Grzeschik K. H., Junien C., Williamson R., Humphries S. E. Isolation and characterisation of a cDNA clone for human apolipoprotein CI and assignment of the gene to chromosome 19. Hum Genet. 1985;69(4):345–349. doi: 10.1007/BF00291654. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]