Abstract
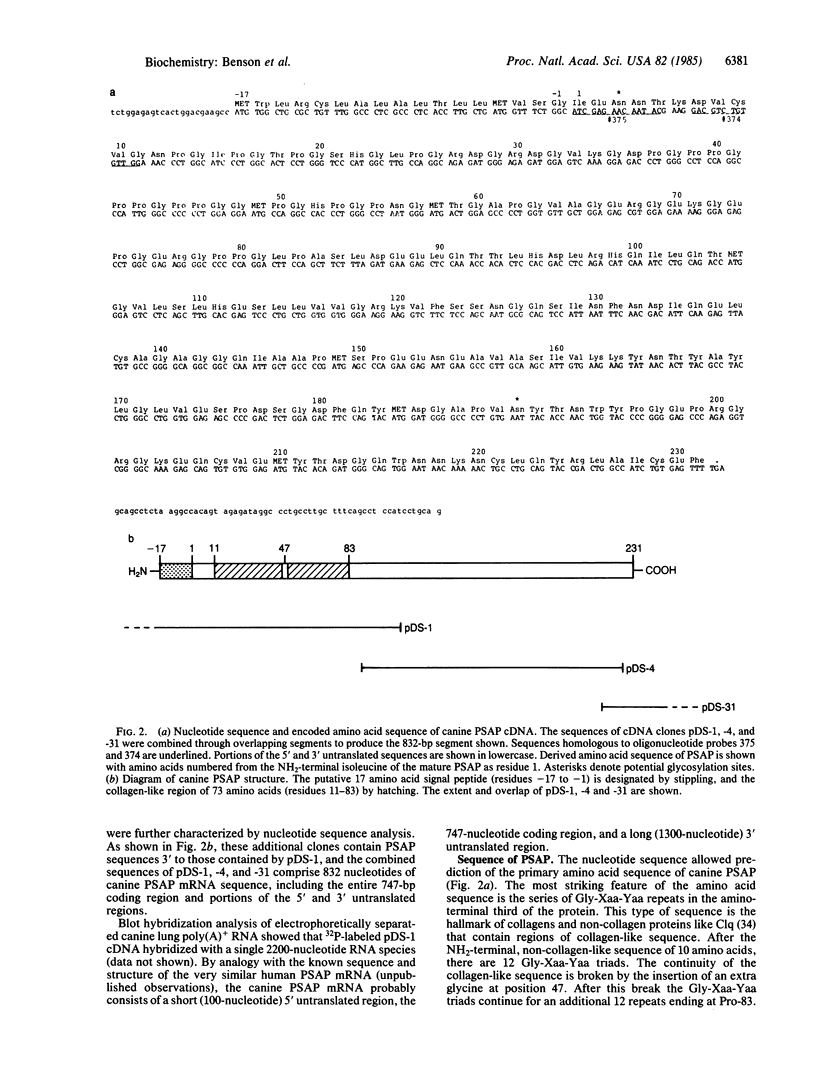
The apoproteins of pulmonary surfactant (PSAP) are thought to be critical for normal surfactant function. They bind to surfactant phospholipids and enhance their ability to form surface films in vitro. These acidic glycoproteins have monomeric molecular weights of 36,000, 32,000, and 28,000 (PSAP-36, -32, and -28). Each member of this family of proteins has a similar amino acid composition and their differences in electrophoretic mobility are due in part to glycosylation. We have derived the full amino acid sequence of PSAP-32 from the nucleotide sequence of PSAP cDNA. A cDNA library was prepared from canine lung poly(A)+ RNA and screened with oligonucleotide probes that were based on the NH2-terminal amino acids of PSAP-32 determined by Edman degradation. This protein has the striking feature of collagen-like and non-collagen-like sequences in the same polypeptide chain. There are 24 Gly-Xaa-Yaa triplets, where Yaa is often hydroxyproline. These repeats comprise one-third of PSAP near the NH2 terminus. The remaining two-thirds of PSAP is resistant to bacterial collagenase digestion and contains a possible N-glycosylation site near the carboxyl terminus. The NH2-terminal one-third of PSAP-32 probably contains the cysteine involved in interchain disulfide bonds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson B. J., Hawgood S., Williams M. C. Role of apoprotein and calcium ions in surfactant function. Exp Lung Res. 1984;6(3-4):223–236. doi: 10.3109/01902148409109250. [DOI] [PubMed] [Google Scholar]
- Benson B. J., Williams M. C., Sueishi K., Goerke J., Sargeant T. Role of calcium ions the structure and function of pulmonary surfactant. Biochim Biophys Acta. 1984 Mar 27;793(1):18–27. doi: 10.1016/0005-2760(84)90048-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Sahu S., Lynn W. S. Structural studies on a glycoprotein isolated from alveoli of patients with alveolar proteinosis. Biochim Biophys Acta. 1976 Mar 18;427(1):91–106. doi: 10.1016/0005-2795(76)90288-9. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Floros J., Phelps D. S., Taeusch H. W. Biosynthesis and in vitro translation of the major surfactant-associated protein from human lung. J Biol Chem. 1985 Jan 10;260(1):495–500. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Katyal S. L., Singh G. Analysis of pulmonary surfactant apoproteins by electrophoresis. Biochim Biophys Acta. 1981 Oct 28;670(3):323–331. doi: 10.1016/0005-2795(81)90104-5. [DOI] [PubMed] [Google Scholar]
- King R. J., Carmichael M. C., Horowitz P. M. Reassembly of lipid-protein complexes of pulmonary surfactant. Proposed mechanism of interaction. J Biol Chem. 1983 Sep 10;258(17):10672–10680. [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. I. Method of isolation. Am J Physiol. 1972 Sep;223(3):707–714. doi: 10.1152/ajplegacy.1972.223.3.707. [DOI] [PubMed] [Google Scholar]
- King R. J., Klass D. J., Gikas E. G., Clements J. A. Isolation of apoproteins from canine surface active material. Am J Physiol. 1973 Apr;224(4):788–795. doi: 10.1152/ajplegacy.1973.224.4.788. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Taylor P. Structural characterization of the asymmetric (17 + 13) S species of acetylcholinesterase from Torpedo. II. Component peptides obtained by selective proteolysis and disulfide bond reduction. J Biol Chem. 1982 Oct 25;257(20):12292–12301. [PubMed] [Google Scholar]
- Lynn W. S. Alveolyn--structure and source: a review. Exp Lung Res. 1984;6(3-4):191–196. doi: 10.3109/01902148409109247. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Ng V. L., Herndon V. L., Mendelson C. R., Snyder J. M. Characterization of rabbit surfactant-associated proteins. Biochim Biophys Acta. 1983 Nov 29;754(2):218–226. doi: 10.1016/0005-2760(83)90164-9. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Taeusch H. W., Jr, Benson B., Hawgood S. An electrophoretic and immunochemical characterization of human surfactant-associated proteins. Biochim Biophys Acta. 1984 Dec 7;791(2):226–238. doi: 10.1016/0167-4838(84)90013-x. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada H., Kashiwamata S. Sodium dodecyl sulfate-disc gel electrophoresis patterns of bovine lung surfactant. Biochim Biophys Acta. 1977 Jan 25;490(1):44–50. doi: 10.1016/0005-2795(77)90104-0. [DOI] [PubMed] [Google Scholar]
- Shelley S. A., Balis J. U., Paciga J. E., Espinoza C. G., Richman A. V. Biochemical composition of adult human lung surfactant. Lung. 1982;160(4):195–206. doi: 10.1007/BF02719293. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Benson B. J. Isolation of a major apolipoprotein of canine and murine pulmonary surfactant. Biochemical and immunochemical characteristics. Biochim Biophys Acta. 1981 Sep 24;665(3):442–453. doi: 10.1016/0005-2760(81)90257-5. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Wright J. R., Benson B. J., Williams M. C., Goerke J., Clements J. A. Protein composition of rabbit alveolar surfactant subfractions. Biochim Biophys Acta. 1984 Dec 21;791(3):320–332. doi: 10.1016/0167-4838(84)90343-1. [DOI] [PubMed] [Google Scholar]
- Yamanaka M., Greenberg B., Johnson L., Seilhamer J., Brewer M., Friedemann T., Miller J., Atlas S., Laragh J., Lewicki J. Cloning and sequence analysis of the cDNA for the rat atrial natriuretic factor precursor. Nature. 1984 Jun 21;309(5970):719–722. doi: 10.1038/309719a0. [DOI] [PubMed] [Google Scholar]