Abstract
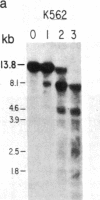
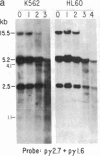
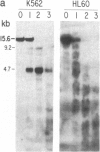
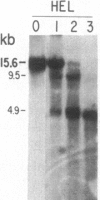
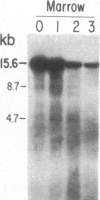
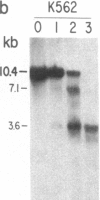
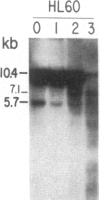
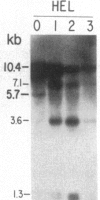
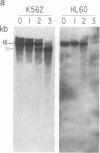
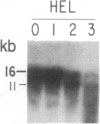
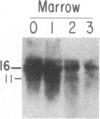
We have mapped the distribution of the major and minor DNase I-hypersensitive sites in the human "beta-like-globin" gene domain. The minor DNase I-hypersensitive sites map close to the 5' end of each of the beta-like-globin genes. Their presence is specifically associated with the transcription of the immediate downstream beta-like-globin genes. The major DNase I-hypersensitive sites map in what appear to be the 5' and 3' boundary areas of the human beta-like-globin gene domain, a region estimated to span at least 90 kilobases of DNA. These major sites are present in various erythroid cells, which express predominantly either the embryonic, the fetal, or the adult beta-like-globin genes, and seem to be involved in defining the active beta-like-globin genes domain in cells of erythroid lineage. The four major DNase I-hypersensitive sites in the 5' boundary area, when correlated with sequencing data, are shown to be located in DNA regions containing enhancer core-like sequences and alternating purine and pyrimidine bases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. W., Kaufman R. E., Kretschmer P. J., Harrison M., Nienhuis A. W. A family of long reiterated DNA sequences, one copy of which is next to the human beta globin gene. Nucleic Acids Res. 1980 Dec 20;8(24):6113–6128. doi: 10.1093/nar/8.24.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Chung S. Y., Folsom V., Wooley J. DNase I-hypersensitive sites in the chromatin of immunoglobulin kappa light chain genes. Proc Natl Acad Sci U S A. 1983 May;80(9):2427–2431. doi: 10.1073/pnas.80.9.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Dean A., Ley T. J., Humphries R. K., Fordis M., Schechter A. N. Inducible transcription of five globin genes in K562 human leukemia cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5515–5519. doi: 10.1073/pnas.80.18.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Mears J. G. DNase I hypersensitivity in the gamma globin gene locus of K562 cells. Nucleic Acids Res. 1983 Sep 10;11(17):6065–6077. doi: 10.1093/nar/11.17.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G. M., Knoll B. J., March C. J., Woo S. L., Tsai M. J., O'Malley B. W. Definition of 5' and 3' structural boundaries of the chromatin domain containing the ovalbumin multigene family. J Biol Chem. 1982 Feb 10;257(3):1501–1507. [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Mills F. C., Fisher L. M., Kuroda R., Ford A. M., Gould H. J. DNase I hypersensitive sites in the chromatin of human mu immunoglobulin heavy-chain genes. Nature. 1983 Dec 22;306(5945):809–812. doi: 10.1038/306809a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Rutherford T., Clegg J. B., Higgs D. R., Jones R. W., Thompson J., Weatherall D. J. Embryonic erythroid differentiation in the human leukemic cell line K562. Proc Natl Acad Sci U S A. 1981 Jan;78(1):348–352. doi: 10.1073/pnas.78.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Brown F. L., Maio J. J., Adams J. W. KpnI families of long, interspersed repetitive DNAs associated with the human beta-globin gene cluster. Gene. 1982 Dec;20(3):397–407. doi: 10.1016/0378-1119(82)90208-6. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., London I. M. Mapping of DNase I-hypersensitive sites in the upstream DNA of human embryonic epsilon-globin gene in K562 leukemia cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2718–2722. doi: 10.1073/pnas.81.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]