Abstract
Diffusion in macrohomogeneous and microheterogeneous media can be described as effective free diffusion only at sufficiently long times. At intermediate times, the mean-square displacement of a diffusing object shows a transient behavior that can be misinterpreted as anomalous subdiffusion. We discuss how to discriminate between the two.
Free diffusion is a universal description of unbiased motion in a macrohomogeneous media, which is widely used in the analysis of various problems in physics, chemistry, and biology. This is a coarse-grained description that is applicable at sufficiently long times when a typical displacement of the diffusing object exceeds a characteristic length scale associated with the heterogeneity of the medium. For example, treatment of molecular motion in water in terms of self-diffusion is applicable at times larger than 100 ps when the mean displacement exceeds the mean distance between water molecules (1). Another example provides diffusion in crystalline solids, where the displacement should exceed the lattice period (2). It is well known that diffusion in biological systems frequently occurs in microheterogeneous environments (for instance, diffusion in the crowded cytoplasm). In such systems, description in terms of effective free diffusion may become applicable only for times that are comparable or even longer than the duration of the experiment. Therefore, it is not surprising that the data on the mean-square displacements, 〈Δr2(t)〉, are frequently described by the dependences 〈Δr2(t)〉 ∝ tα with α < 1 (see recent review articles (3,4) and references therein), whereas for free diffusion the exponent α is unity. Because the dependence 〈Δr2(t)〉 ∝ tα with α < 1 is a fingerprint of anomalous subdiffusion (5), the question naturally arises how to discriminate between anomalous subdiffusion and transient behavior to effective free diffusion regime. The present Letter focuses on this question.
The fundamental difference between anomalous subdiffusion and the transient behavior is that the exponent α is a constant in the former case, whereas it is a function of time in the case of transient behavior. Therefore, in the case of anomalous diffusion, α obtained from the experimental data is independent of the method used to determine the exponent. However, as we show below, different methods lead to different functions α(t) in the case of transient behavior.
Consider three different methods of determining the exponent α from experimental data:
-
1.
Fitting the time dependence of the mean-square displacement to the exponential function 〈Δr2(t)〉 ∝ tα, one can find the exponent that we denote by αfit.
-
2.Assuming that α is independent of time and integrating the dependence 〈Δr2(t)〉 ∝ tα over time, one can find that the area under the curve 〈Δr2(t)〉 is
We use this relation to define the exponent, which we denote by αint:(1) (2) -
3.Differentiating 〈Δr2(t)〉 ∝ tα with respect to time, assuming that α is a constant, we obtain
(3)
Using this, we determine the exponent denoted by αdif:
| (4) |
When diffusion is anomalous, and α is a constant, all three methods lead to the same value of the exponent: αfit(t) = αint(t) = αdif(t) = α. However, in the case of transient behavior where the exponent is not a constant, the three methods lead to the time-dependent αfit(t), αint(t), and αdif(t), which, importantly, are different functions of time.
As an illustration, consider diffusion in the presence of periodically spaced permeable membranes modeled as infinitely thin planar partitions (see the inset in Fig. 1 A). The effective diffusion coefficient Deff of a particle in the direction normal to the membranes is given by (6)
| (5) |
where D0 is the particle diffusion coefficient in the absence of the membranes, P is the membrane permeability, and l is the intermembrane distance. As follows from Eq. 5, the presence of low permeability membranes leads to a significant slowdown of the diffusion. This model of a microheterogeneous system allows for exact solutions for the mean-square displacement 〈Δx2(t)〉 in the direction normal to the membranes, and for the exponents αint(t) and αdif(t). To be more specific, one can find an exact analytical solution for the Laplace transform of 〈Δx2(t)〉 (7,8) and then to find 〈Δx2(t)〉 by numerically inverting the transform. Knowing the Laplace transform of 〈Δx2(t)〉, one can find the Laplace transforms of
Inverting these transforms one can obtain αint(t) and αdif(t) using the definitions of these exponents in Eqs. 2 and 4, respectively (see the Supporting Material for details). In Fig. 1, we show 〈Δx2(t)〉 for D0 = 1, P = 0.01, and l = 10. The slowdown of diffusion does not manifest itself at short times, where 〈Δx2(t)〉 = 2D0t and hence α = 1. As time increases, the rate of diffusion slows down, and the mean-square displacement approaches its long-time behavior, 〈Δx2(t)〉 = 2Defft, which describes effective free diffusion with Deff given in Eq. 5 and . Functions αfit(t), αint(t), and αdif(t) obtained by fitting the dependence 〈Δx2(t)〉, and using the definitions in Eqs. 2 and 4, respectively, are shown in Fig. 1 B.
Figure 1.
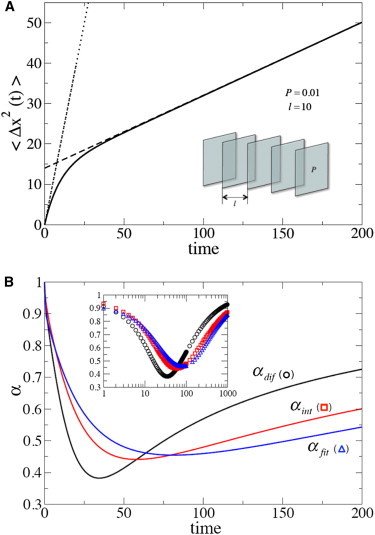
(A) The mean-square displacement 〈Δx2(t)〉 of a particle in the presence of periodically spaced permeable membranes in the direction normal to the membranes. (Inset) System geometry. The parameters in dimensionless units: membrane permeability is 0.01; intermembrane distance is 10; and particle diffusion coefficient in free space is 1. (Dotted and dashed straight lines) Short- and long-time asymptotic behaviors of 〈Δx2(t)〉, respectively. (B) The three exponents αfit(t), αint(t), and αdif(t) obtained by interpreting the transient behavior of 〈Δx2(t)〉 as anomalous subdiffusion. (Inset) The exponents as functions of log t. To see this figure in color, go online.
As another example, consider the tube with periodic dead ends shown in the inset in Fig. 2 A, which is a caricature of a spiny dendrite (9,10). Each dead end is formed by a spherical cavity of volume Vcav connected to the cylindrical part of the tube by a narrow bottleneck of radius a. Particles, from time to time, interrupt their motion along the tube by entering the dead ends that leads to the slowdown of their diffusion along the tube axis. The particle effective free diffusion coefficient, Deff, is related to its diffusion coefficient D0 in the absence of dead ends by the relation (11)
| (6) |
where Vtube is the tube volume per one dead end. As follows from Eq. 6, Deff is well below D0 when Vcav >> Vtube. For such a system, an analytical solution for the Laplace transform of the mean-square displacement 〈Δx2(t)〉 along the tube axis is also available (11). This Laplace transform was used to draw the dependence 〈Δx2(t)〉 shown in Fig. 2 A and the exponents αfit(t), αint(t), and αdif(t) shown in Fig. 2 B (see the Supporting Material for details).
Figure 2.
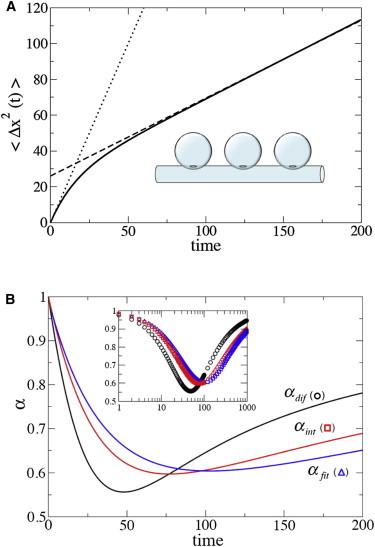
(A) The mean-square displacement 〈Δx2(t)〉 of a particle along the axis of a tube with periodic spherical dead ends (inset). The system parameters in dimensionless units: tube and cavity radii are 0.8 and 1.5, respectively; intercavity distance is 2; radius of the apertures connecting the tube with the cavities is 0.1; and particle diffusion coefficient in free space is 1. (Dotted and dashed straight lines) Short- and long-time asymptotic behaviors of 〈Δx2(t)〉, respectively. (B) The three exponents αfit(t), αint(t), and αdif(t) obtained by interpreting the transient behavior of 〈Δx2(t)〉 as anomalous subdiffusion. (Inset) The exponents as functions of log t. To see this figure in color, go online.
In both examples, the three exponents first decrease rapidly from unity at t = 0 to their minima and then increase slowly approaching unity at sufficiently long times. Although αdif(t) approaches unity faster than the two other exponents, differentiation of noisy experimental data on 〈Δx2(t)〉 may be difficult. In view of this circumstance, fitting the data on 〈Δx2(t)〉 and determining the area
seem more reliable procedures for analyzing data on the time dependence of the mean-square displacement. One can see that both αfit(t) and αint(t) approach unity rather slowly. It may happen that, on experimentally available times, both exponents are smaller than unity and almost do not change. As a result, this may lead to an incorrect interpretation of the transient behavior as anomalous subdiffusion. As shown in Figs. 1 B and 2 B, most of the time the two exponents are not equal to each other. Therefore, one can discriminate between anomalous subdiffusion and transient behavior by comparing αfit(t) and αint(t). In the case of anomalous subdiffusion, the two exponents must be identical and independent of time. If not, the observed deviation of the exponent α from unity can be related to the transient behavior to effective free diffusion regime.
Acknowledgments
We are grateful to Igor Sokolov for helpful discussions.
This study was supported by the Intramural Research Program of the National Institutes of Health, Center for Information Technology and Eunice Kennedy Shriver National Institute of Child Health and Human Development. L.D. thanks Consejo Nacional de Ciencia y Tecnología for partial support under grant No. 176452.
Supporting Material
References and Footnotes
- 1.Berezhkovskii A.M., Sutmann G. Time and length scales for diffusion in liquids. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002;65:060201. doi: 10.1103/PhysRevE.65.060201. [DOI] [PubMed] [Google Scholar]
- 2.Adda Y., Philbert J., Pontikis V. Validity of the diffusion equation at the atomic scale investigated via numerical simulations. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2012;85:144121. [Google Scholar]
- 3.Saxton M.J. Wanted: a positive control for anomalous subdiffusion. Biophys. J. 2012;103:2411–2422. doi: 10.1016/j.bpj.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkai E., Garini Y., Metzler R. Strange kinetics of single molecules in living cells. Phys. Today. 2012;65:29–35. [Google Scholar]
- 5.Sokolov I.M. Models of anomalous diffusion in crowded environments. Soft Matter. 2012;8:9043–9052. [Google Scholar]
- 6.Crick F. Diffusion in embryogenesis. Nature. 1970;225:420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- 7.Dudko O.K., Berezhkovskii A.M., Weiss G.H. Diffusion in the presence of periodically spaced permeable membranes. J. Chem. Phys. 2004;121:11283–11288. doi: 10.1063/1.1814055. [DOI] [PubMed] [Google Scholar]
- 8.Makhnovskii Yu. A., Berezhkovskii A.M., Zitserman V.Yu. Time-dependent diffusion in tubes with periodic partitions. J. Chem. Phys. 2009;131:104705. [Google Scholar]
- 9.Santamaria F., Wils S., Augustine G.J. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52:635–648. doi: 10.1016/j.neuron.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown S.A., Morgan F., Loew L.M. Analysis of phosphatidylinositol-4,5-bisphosphate signaling in cerebellar Purkinje spines. Biophys. J. 2008;95:1795–1812. doi: 10.1529/biophysj.108.130195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagdug L., Berezhkovskii A.M., Zitserman V.Yu. Transient diffusion in a tube with dead ends. J. Chem. Phys. 2007;127:224712. doi: 10.1063/1.2805068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


