Abstract
1,3-butadiene (BD) is a high volume industrial chemical commonly used in polymer and rubber production. It is also present in cigarette smoke, automobile exhaust, and urban air, leading to widespread exposure of human populations. Upon entering the body, BD is metabolized to electrophilic epoxides, 3,4-epoxy-1-butene (EB), diepoxybutane (DEB), and 3,4-epoxy-1,2-diol (EBD), which can alkylate DNA nucleobases. The most abundant BD epoxide, EBD, modifies the N7-guanine positions in DNA to form N7-(2, 3, 4-trihydroxybut-1-yl) guanine (N7-THBG) adducts, which can be useful as biomarkers of BD exposure and metabolic activation to DNA-reactive epoxides. In the present work, a capillary HPLC- high resolution ESI+-MS/MS (HPLC-ESI+-HRMS/MS) methodology was developed for accurate, sensitive, and reproducible quantification of N7-THBG in cell culture and in human white blood cells. In our approach, DNA is subjected to neutral thermal hydrolysis to release N7-guanine adducts from the DNA backbone, followed by ultrafiltration, solid phase extraction, and isotope dilution HPLC-ESI+-HRMS/MS analysis on an Orbitrap Velos mass spectrometer. Following method validation, N7-THBG was quantified in human fibrosarcoma (HT1080) cells treated with μmolar concentrations of DEB and in DNA isolated from blood of smokers, nonsmokers, individuals participating in a smoking cessation program, and occupationally exposed workers. N7-THBG concentrations increased linearly from 31.4μ± 4.84 to 966.55 ± 128.05 adducts per 109 nucleotides in HT1080 cells treated with 1 -100 μM DEB. N7-THBG amounts in leukocyte DNA of non-smokers, smokers, and occupationally exposed workers were 7.08 ± 5.29, 8.20 ± 5.12, and 9.72 ± 3.80 adducts per 109 nucleotides, respectively, suggesting the presence of an endogenous or environmental source for this adduct. The availability of sensitive HPLC-ESI+-HRMS/MS methodology for BD-induced DNA adducts in humans will enable future population studies of inter-individual and ethnic differences in BD bioactivation to DNA-reactive epoxides.
Introduction
1,3- Butadiene (BD) is an important industrial and environmental chemical classified as an animal1,2 and human carcinogen.3-6 Humans are commonly exposed to BD due to its widespread use in polymer industries,7 its presence in cigarette smoke,8 urban environment,9 automobile exhaust,10 and its formation upon wood burning and forest fires.11 Inhalation exposure to BD causes multi-site tumors in laboratory rats and mice1,2 and is associated with an increased incidence of leukemia in occupationally exposed workers.3-6 The adverse health effects of BD are mainly attributed to its cytochrome P450-mediated metabolism to electrophilic epoxides 3,4-epoxy-1-butene (EB) and 1,2-dihydroxy-3,4-epoxybutane (EBD), and 1,2,3,4-diepoxybutane (DEB).12,13 Among these, EBD is the most abundant in vivo.14-17 Multiple stereoisomers of each epoxide are produced in vivo (Scheme 1).18-20
Scheme 1.
BD bioactivation to epoxide metabolites and the formation of thermally labile N7-THBG adducts in DNA.
Epoxide metabolites of 1, 3-butadiene can alkylate DNA to form a range of nucleobase adducts.14,20-24 Specifically, EBD binds to the N7 position of guanine to give N7-(2, 3, 4 – trihydroxybut-1-yl) guanine (N7-THBG) adducts (Scheme 1).14 N7-THBG are also induced by DEB via a two-step process including hydrolysis of one of the two epoxide rings.25 Several stereoisomers of N7-THBG have been observed in vivo18-20. N7-THBG are the most abundant nucleobase adducts in DNA treated with EBD or DEB in vitro23 and in tissues of laboratory animals exposed to BD by inhalation.14,23,26 Because N7-guanine alkylation generates a positive charge on the modified nucleobase, it destabilizes the N-glycosidic bond, leading to spontaneous depurination of N7-THBG and the formation of abasic site (Scheme 1).26 The hydrolytic half-lives of N7-THBG adducts in vivo (mouse and rat liver DNA) are 3.6-5.5 days, depending on chirality.27 In the laboratory, N7-THBG nucleobases can be readily released from the DNA backbone by heating, facilitating their analysis by mass spectrometry. Although N7-THBG adducts may not be mutagenic because they retain normal Watson-Crick base pairing with C,28 they can be used as mechanism based biomarkers of BD metabolism to DNA-reactive epoxides.
In the present study, N7-THBG was selected as a DNA based biomarker of human exposure to BD due to its high prevalence in vivo as compared to other BD-DNA lesions.14,23 Previous studies have quantified N7-THBG adducts in tissues of laboratory mice and rats exposed to BD by inhalation using HPLC-ESI+-MS/MS.14 However, the sensitivity of existing methods is insufficient for studies in humans due to significantly lower amounts of DNA adducts observed in human tissues (< 1-2 adducts per 109 nucleotides in humans versus 1-10 adducts per 107 nucleotides in laboratory animals exposed to BD). In the present work, a highly sensitive and specific capillary HPLC - accurate mass ESI+ MS/MS methodology utilizing an Orbitrap Velos mass spectrometer was developed for quantitation of N7-THBG adducts in human white blood cells (WBCs). Under our conditions, all N7-THBG stereoisomers eluted as a single peak to maximize method sensitivity. The method was initially applied to human fibrosarcoma (HT1080) cells treated with sub-micromolar concentrations of DEB (10 nM – 100 μM). The validated method was subsequently used to determine the concentrations of N7-THBG adducts in DNA isolated from PBLs of smokers, nonsmokers, occupationally exposed workers, and individuals participating in a smoking cessation study.
Materials and methods
Note: DEB is a known human carcinogen and must be handled with adequate safety precautions. Phenol and chloroform are toxic chemicals that should be used only in a well ventilated fume hood with appropriate personal protective equipment.
N7-THBG and [15N5]-N7-THBG were synthesized in our laboratory as described elsewhere.23 Puregene DNA purification reagents were obtained from Qiagen (Valencia, CA). LC-MS grade water, methanol and acetonitrile were purchased from Fisher Scientific (Pittsburgh, PA). Isolute ENV+ 50 mg/1 mL SPE cartridges were provided by Biotage (Charlotte, NC). DNA oligodeoxynucleotides (5′-TCAGATTCGCGCCGGCTGCGATAAGCT-3′) were purchased from Integrated DNA Technologies (Coraville, IA) or synthesized in our laboratory using automated solid phase synthesis. All other chemicals and solvents were obtained from Sigma-Aldrich (Millwaukee, WI, St. Louis, MO). Human blood buffy coat samples were acquired from the Biorespository Facility, Masonic Cancer Center, University of Minnesota, Minneapolis, the NCI CRCHD repository, and the American Chemistry Council, Washington DC.
Cell culture
Human fibrosarcoma cells (HT1080) were grown in Dulbecco’s modified Eagle’s media supplemented with 9% fetal bovine serum (Atlanta Biologicals). Cells were cultured in a humidified atmosphere of 5% carbon dioxide, 95% air, at 37 °C.
N7-THBG formation in human cell culture
HT1080 cells were plated in 15 cm dishes using Dulbecco’s modified Eagle’s medium containing 9% FBS and permitted to adhere overnight. On the following morning, cells (in duplicate) were treated with increasing concentrations of DEB (0, 10 nM, 50 nM, 100 nM, 1 μM, 10 μM, 50 μM or 100 μM) for 3 h at 37 °C. Control and treated cells (N = 2) were harvested, washed with ice cold phosphate-buffered saline (PBS), and suspended in PBS for DNA extraction as described below.
Study subjects
The study was approved by the University of Minnesota Human Research Protection Programs Institutional Review Board. Blood samples from 13 smokers and 13 nonsmokers were obtained from the Tobacco Research Biorepository Facility, Masonic Cancer Center, University of Minnesota. All human subjects were age 18 years or older, not pregnant or breast feeding. They consumed less than 21 alcoholic drinks per week, and were in good physical and mental health. Smokers included in the study had been smoking for at least 5 years and consumed at least 7 cigarettes per day (CPD), with no change greater than 50% in CPD or brand within the last year, and had not used any other tobacco products in the last 6 months. Individuals with nonsmoking status were required to have smoked less than 100 cigarettes in their lifetime and were not using any tobacco products regularly. Individual smoking status was confirmed by expired carbon monoxide (CO) levels. CO levels were determined by having participants take a deep breath and hold it for 20 s before exhaling into a carbon monoxide monitor (Bedfont Scientific, Upchurch, UK). Smokers had CO levels of ≥10 ppm (range 7-29 ppm), while nonsmokers had CO levels ≤ 3 ppm (range 0-3 ppm). The average age of smokers was 41 ± 12 (range 19-61) and that of nonsmokers was 35 ± 12 years (range 20-61).
Human blood buffy coat samples from a smoking cessation study were obtained from National Cancer Institute. Subjects included were between the ages of 18 and 65, smoked at least 10 cigarettes daily for the past year, had carbon monoxide (CO) levels of >10, and were in good physical and mental health. Fasting blood was collected through venipuncture at various time points during the study. Blood buffy coat samples were obtained at 14 and 7 days prior to smoking cessation (baseline levels) and at two time points post smoking cessation (28 and 84 days). Smoking status was confirmed through CO measures and self-reported tobacco use diaries. To obtain sufficient sample size, buffy coat samples from 5 subjects were pooled for each time point (N = 2). DNA was isolated as described above. Samples were stored at − 80 °C until further analysis.
Human blood buffy coat samples from 10 workers with known occupational exposure to BD and 10 matched controls (administrative workers) were obtained from the American Chemistry Council, Washington DC. BD exposures were determined by personal monitoring tubes for 8-h work shifts on 10 separate occasions over a 4-month interval for each study subject. Ambient air samples were analyzed for BD concentrations. Co-exposures to toluene, styrene, and benzene were measured by personal monitoring in a subset of subjects on a single occasion and by ambient air sampling and other details of the study population are previously reported.29,30 The experimental protocol was approved by the Institutional Review Boards at Regional Institute of Hygiene of Central Bohemia, the University of Vermont. Samples were shipped to the University of Minnesota on dry ice and stored at − 80 °C until further analysis.
DNA isolation
DNA isolation from human blood samples was performed using the commercial protocol for DNA purification from buffy coat (Qiagen, Valencia, CA),31 with the following modifications. Human whole blood buffy coat fractions obtained from 10 mL of whole blood were lyzed in the presence of RBC lysis solution (3 mL). White blood cells were collected as a pellet via centrifugation at 2000 g for 10 min. RBC lysis solution (3 mL) was added to the pellet, and the samples were centrifugated at 2000g for 10 min. The WBC pellet was mixed with 2 mL of cell lysis solution and 8 μL of proteinase K solution (20 mg/mL) and incubated at room temperature overnight upon slow mixing. On the following day, a solution of RNase A (4 mg/mL) was added (8 μL), and the samples were incubated at room temperature for 2 h. Protein precipitation solution (700 μL) was added to the cell lysate, and the mixture is vortexed at a high speed for 20 seconds and centrifugated at 2000g for 15 min to remove proteins. DNA was precipitated with cold isopropyl alcohol (3 mL) and washed twice with 1 mL of 70% ethanol in water. DNA was dried under a stream of nitrogen and stored at − 20°C until further analysis. Typical DNA yields from 10 mL of whole blood were 0.1-0.15 mg.
DNA from control and DEB-treated human fibrosarcoma (HT1080) cells was isolated using standard phenol-chloroform extraction.32 DNA concentrations were estimated by UV spectrophotometry, and DNA purity was assessed from A260 /A280 absorbance ratios, which was typically between 1.6 and 1.7. DNA amounts were determined by dG quantitation in enzymatic hydrolysates as described below.
dG quantitation
DNA aliquots (~ 2 μg) were enzymatically digested with DNA Degradase Plus (Zymo Research, Irvine, CA) at 37 °C overnight. HPLC-UV analysis of dG was conducted by HPLC-UV using Eclipse XDB-C8 column (4.6 × 150 mm, 5 μm, from Agilent Technologies, Palo Alto, CA) eluted with a gradient of 150 mM ammonium acetate (A) and acetonitrile (B).32
Sample preparation and adduct enrichment
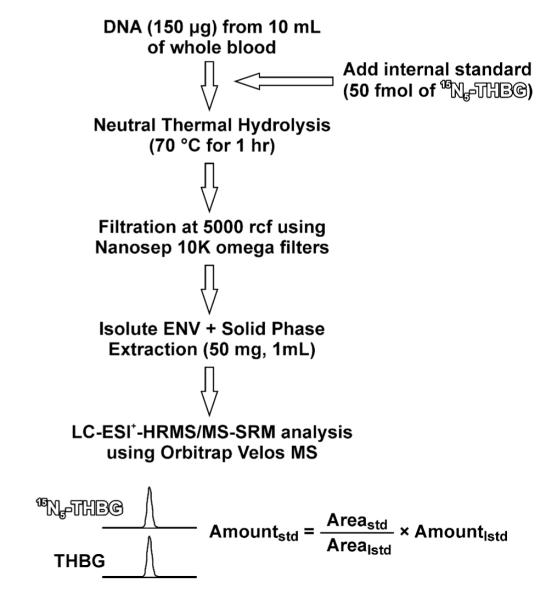
DNA samples (35-150 μg in water) were spiked with 50 fmol of 15N5-N7-THBG (99.9% purity, internal standard for mass spectrometry) and heated at 70 °C for 1 h to release N7-THBG adducts from the DNA backbone. Partially depurinated DNA was removed by ultrafiltration with Nanosep 10K filters (Pall Life Sciences, Ann Arbor, MI) at 5000 g for 10 min. The filtrates containing N7-THBG and its internal standard were subjected to solid phase extraction (SPE) on Isolute ENV+ 50 mg/1 mL cartridges from Biotage (Charlotte, NC). SPE cartridges were conditioned with 2 mL of CH3OH and 2 mL of H2O. Samples were loaded at neutral pH, washed with 1 mL of H2O and 1 mL of 0.1% formic acid in water, and eluted with 50% CH3CN in water. SPE fractions containing N7-THBG were collected, evaporated to dryness, and dissolved in water (24 μL) for HPLC-ESI+-HRMS/MS analysis. One third of each sample (8 μL, containing between 10 and 50 μg DNA) was injected on column for HPLC-ESI+-HRMS/MS analysis as described below.
HPLC-ESI+-HRMS/MS analysis of N7-THBG
A Nano2D-LC HPLC system (Eksigent, Dublin, CA) was interfaced to an LTQ Orbitrap Velos instrument (Thermo Scientific, Waltham, MA). HPLC solvents were LC-MS grade water containing 0.05% acetic acid (A) and LC-MS grade acetonitrile (B). Samples (8 μL) were injected on to Synergi Hydro RP 80R (250 × 0.5 mm, 4 μm) analytical column (Phenomenex, Torrance, CA). The column was eluted at a flow rate of 10 μL/min. Solvent composition was maintained at 1% B for 3 min and then linearly increased to 7% B in 12 min, further to 12% B in 1 min, to 20 % B in 2 min, to 50% B in 1 min, and kept at 50% B for 2 min. Solvent composition returned to 1% B for an 8 min equilibration period at a flow rate of 10 μL/min. Under these conditions, all stereoisomers of N7-THBG eluted as a single sharp peak at 17-18 min.
Samples were analyzed in the ESI+ mode using an LTQ Orbitrap Velos instrument (Thermo Scientific, Waltham, MA). The source temperature was set at 350°C, sheath gas was at 40.0, the ESI source voltage was at 4.0 kV, and S-Lens RF level was set at 80%. N7-THBG adducts were quantified in the SRM mode by monitoring the transitions m/z 256.1 [M + H]+ → m/z 152.05669 [Gua + H]+ and [15N5]-N7-THBG at m/z 261.1 → m/z 157.04187 for the analyte and its 15N5 labeled internal standard, respectively. CID was achieved using the HCD collision cell at the isolation width of 1 amu, collision energy of 50%, and mass resolution of 27,000. Accurate mass monitoring of the specified fragment ions was conducted at 5 ppm mass accuracy (152.05669 ± 0.0008 and 157.04187 ± 0.0008, respectively) with the Orbitrap Velos FTMS detector. A full scan event was also performed over m/z 50-270 to monitor for any co-eluting matrix components. HPLC-ESI+-HRMS/MS quantitation was conducted using the peak areas in extracted ion chromatograms corresponding to the analyte and the internal standard.
HPLC-ESI+-HRMS/MS standard curves were constructed by analyzing aqueous solutions containing fixed amount of 15N5-N7-THBG (50 fmol) and increasing amounts of N7-THBG (1.0, 5.0, 10.0, 15.0, 25.0, and 50.0 fmol) (in triplicate), followed by regression analysis of the actual and the observed amounts of N7-THBG (Figure S1 in the Supporting Information). Solvent blanks were periodically injected to monitor for potential analyte carry-over.
Method validation
DNA from untreated human fibrosarcoma (HT1080) cells (150 μg aliquots, in triplicate) was spiked with 0, 1, 5, 10, 15, 25 or 50 fmol of N7-THBG and 50 fmol of 15N5-N7-THBG internal standard. Following neutral thermal hydrolysis at 70 °C for 1 h, samples were filtered through Nanosep-10K ultra centrifugation devices to remove partially depurinated DNA. Samples were then enriched for N7-THBG and its internal standard by solid phase extraction on Isolute ENV+ and subjected to capillary HPLC-ESI+-HRMS/MS analysis as described above. N7-THBG amounts were expressed as adduct numbers per 109 normal nucleotides, and N7-THBG amounts endogenously present in human HT1080 cells (2.03 fmol/150 μg DNA) were subtracted from each value. The observed amounts of N7-THBG were plotted against the theoretical values, followed by regression analysis. DNA amounts were determined by HPLC-UV analysis of dG in DNA hydrolysates.26
Determination of LOD/LOQ, precession, and accuracy
The LOD values of the new HPLC-ESI+-HRMS/MS method were determined by spiking synthetic oligodeoxynucleotide (5′-TCAGATTCGCGCCGGCTGCGATAAGCT-3′,150 μg) with increasing amounts of N7-THBG (0, 0.3, 0.5, or 1 fmol) and a fixed amount of 15N5-N7-THBG (50 fmol, internal standard for mass spectrometry), followed by sample processing and HPLC-ESI+-HRMS/MS analysis by the standard methodology. Synthetic DNA was employed in these experiments because DNA from all biological sources examined contained endogenous N7-THBG (2 -3 fmol/150 μg DNA). The LOD value was determined as the analyte amount that consistently produced HPLC-ESI+-HRMS/MS signal-to-noise ratios above 3. The limit of quantitation was defined by identifying minimum analyte amounts that produced a coefficient of variation less than 15% and a signal-to-noise ratio (S/N) greater than 10.
To evaluate the inter-day and intra-day accuracy and precision of the new method, N7-THBG (1.0 fmol) and 15N5-N7-THBG (50.0 fmol) were spiked into synthetic oligodeoxynucleotide solution (150 μg). Samples were processed as described above and analyzed three times per day on three consecutive days. Method accuracy was calculated from the equation (Am/Aa × 100%), where Am is the measured amount of N7-THBG and Aa is the actual amount added.
SPE recovery was determined by adding [15N5]-N7-THBG (50 fmol) to synthetic oligodeoxynucleotide solution (150 μg) and processing the samples as described above. N7-THBG standard (75 fmol) was added immediately prior to HPLC-ESI+-HRMS/MS analysis, and the recovery was determined by comparing the theoretical and the observed [15N5]-N7-THBG:N7-THBG peak area ratios.
Results
Experimental approach
Human exposure to BD is common due to it ubiquitous presence in urban air,9-11 cigarette smoke,8,33 and its extensive use in plastic and rubber industries.4-7 Classified as a human carcinogen, BD induces tumors in laboratory animals and increases the risk for hematopoietic cancer in occupationally exposed individuals.3 The goal of the present study was to develop a non-invasive, mechanism-based biomarker of BD exposure and metabolism to DNA-reactive epoxides. N7-THBG adducts (Scheme 1) have been chosen because they are the most abundant BD-DNA lesions in vivo.14,23,26
Ultra-sensitive analytical methodology was required to enable the detection and accurate quantification of N7-THBG in human samples. In our approach, DNA isolated from human blood buffy coat (35-150 μg) is spiked with 15N5-N7-THBG internal standard, and the adducts of interest are released from the DNA backbone by thermal hydrolysis (Scheme 2).14,23,34 This is possible due to the intrinsic instability of the N-glycosidic bond on N7-alkylguanine lesions in DNA.28 Initial experiments have compared the efficiency of N7-THBG release following neutral thermal hydrolysis for 1 h at 70 °C versus heating at 80 °C for 1 h as reported previously.23 We found that thermal hydrolysis at a lower temperature (70 °C) was equally effective at releasing N7-THBG, but produced lower background of unmodified bases than hydrolysis at 80 °C (results not shown). Following thermal hydrolysis, partially depurinated DNA was removed by ultrafiltration, and N7-THBG and its internal standard were purified by solid phase extraction on Isolute ENV+ (50 mg/1 mL) cartridges (Scheme 2). SPE recovery (92.6 ± 1.8%) was determined by spiking synthetic oligodeoxynucleotide (150 μg) with known amounts of the analyte. We found that Isolute ENV+ cartridges (Biotage) provided the best recovery as compared to other SPE stationary phases tested.
Scheme 2.
Analytical procedure for isotope dilution HPLC-ESI+-HRMS/MS analysis of N7-THBG in human leukocyte DNA.
HPLC-ESI+-HRMS/MS method development for N7-THBG
N7-THBG is a highly polar molecule due to the presence of hydrophilic 2,3,4-trihydroxybut-1-yl substituent on the guanine base, leading to its poor retention on reversed phase HPLC columns. Our HPLC-ESI+-HRMS/MS method development efforts for N7-THBG have explored a wide range of stationary phases, including Synergi Polar RP, Synergi Max RP, and Synergi Hydro RP from Phenomenex (Torrance, CA), Hypercarb (Thermo Fisher Scientific, West Palm Beach, FL), and Zorbax SB C18 (Agilent Technologies, Palo Alto, CA). The best results in terms of analyte retention and HPLC peak shape were achieved with Synergi Hydro RP column (Phenomenex). Among HPLC mobile phases tested, a gradient of 0.05% acetic acid in water and acetonitrile has afforded the best chromatography and optimal ionization efficiency.
Our initial HPLC-ESI+-MS/MS method for N7-THBG was based on column switching, with Synergi Max RP 80R (150 × 0.5 mm, 4 μm) as a trap column (5 min at 10 μL/min of 0.05% acetic acid in water ) and Synergi Hydro RP (250 × 0.5 mm, 4 μm) as an analytical column. HPLC separation was achieved using gradient elution with acetonitrile and 0.05% acetic acid in water at 10 μL/min. A TSQ Vantage triple quadrupole mass spectrometer was operated in the SRM mode by monitoring the neutral loss of guanine from the analyte and the 15N5-lebeled internal standard, respectively (m/z 256.1[M +H]+ → 152.1 [Gua + H]+) and 15N5 -guanine (m/z 261.1 [15N5-M + H]+ → m/z 157.1 [15N5-Gua + H]+). These SRM transitions were selected based on ESI-MS/MS infusion results for authentic standards.14,23,34 However, the sensitivity of this method (LOQ, 5 fmol) was insufficient for human biomonitoring (Figure S2) due to poor signal to noise ratios and the high sample matrix background in human samples.
To enable N7-THBG quantification in human DNA, we have developed a new method that takes advantage of the high resolution capabilities of the Orbitrap Velos mass spectrometer (HPLC-ESI+-HRMS/MS-MS). In our approach, SPE-purified samples (8 μL out of the total 24 μL) are directly injected onto capillary HPLC column for ESI+-HRMS/MS analysis, avoiding the need for lengthy column switching. The same SRM transitions listed above are monitored, but the fragment ions are detected in the accurate mass mode: m/z 256.1[M +H]+ → 152.05669 [Gua + H]+ for N7-THBG and m/z 261.1 [15N5-M + H]+ → 157.04187 [15N5-Gua + H]+ (Figure 1). As shown in Figure 2, the use of high resolution MS dramatically improves the signal to noise ratios for N7-THBG (LOD, 100 amol), facilitating its detection in human DNA. An additional SRM transition (m/z 256.1[M +H]+ → 135.03014 [Gua –NH2 + H]+ and m/z 261.1 [15N5-M + H]+ → 139.01828 [15N5-Gua –NH2 + H]+ was employed for the confirmation purposes.
Figure 1.
HPLC-ESI+-HRMS/MS extracted ion chromatograms (A) and MS/MS spectra of synthetic N7-THBG (4 fmol) and [15N5]-N7-THBG (50 fmol) (B).
Figure 2.
HPLC-ESI+-HRMS/MS analysis of N7-THBG in leukocyte DNA of a smoker (A, 34 μg DNA hydrolysate on column) and an occupationally BD exposed worker (B, 25 μg DNA on column). DNA isolated from human blood buffy coat (80-100 μg total) was spiked with 15N5-N7-THBG internal standard and subjected to thermal hydrolysis, sample processing, and high resolution HPLC-ESI+-MS/MS analysis on an Obitrap Velos mass spectrometer.
Method validation
The new capillary HPLC-ESI+-HRMS/MS method was validated by analyzing control DNA from untreated human fibrosarcoma (HT1080) cells spiked with 1.0 - 50.0 fmol N7-THBG and 50.0 fmol 15N5-N7-THBG internal standard. The calculated N7-THBG amounts were corrected for the analyte endogenously present in HT1080 cells (2.03 fmol/150 μg DNA). An excellent correlation between the spiked and the observed amounts of the analyte was obtained, with an R2 value of 0.998 (Figure 3). Method accuracy and precision were determined by analyzing replicate samples of N7-THBG (1.0 fmol) spiked into 150 μg of synthetic oligonucleotide solution (Figure S3). Method accuracy was calculated as 92.3 ± 6.7% (n = 5), with the interday and intraday precision less than 13% RSD (Table 1).
Figure 3.

Correlation between the theoretical and the observed amounts of N7-THBG in spiked DNA. DNA isolated from untreated human fibrosarcoma cells (150 μg) was spiked with 0, 1, 5, 10, 15, 25, or 50 fmol of N7-THBG and 50 fmol of 15N5-N7-THBG internal standard, followed by sample processing and capillary HPLC-ESI+-HRMS/MS analysis as shown in Scheme 2 and described in text. N7-THBG amounts observed in control sample (20 adducts/109 nucleotides) were subtracted from each value. The calculated N7-THBG amounts were plotted against the theoretical values.
Table 1.
Accuracy and precision results for N7-THBG at 1 fmol spiked in 150μg of oligonucleotide solution.
| Day 1 | Mean | 0.99 |
| RSD (%) | 3.35 | |
| Accuracy(%) | 99.8 | |
| N | 3 | |
|
| ||
| Day 2 | Mean | 1.10 |
| RSD (%) | 12.3 | |
| Accuracy(%) | 110.2 | |
| N | 3 | |
|
| ||
| Day 3 | Mean | 1.01 |
| RSD (%) | 12.99 | |
| Accuracy(%) | 101.7 | |
| N | 3 | |
|
| ||
| Interday | Mean | 1.03 |
| RSD (%) | 10.3 | |
| Accuracy(%) | 103.9 | |
| N | 9 | |
In order to evaluate HPLC-ESI+-HRMS/MS method sensitivity, aliquots of synthetic DNA oligodeoxynucleotide (150 μg) were spiked with known amounts of N7-THBG standard (0.1, 0.3. 0.5, or 1.0 fmol), followed by hydrolysis and sample processing by standard methodology (Scheme 2). We chose synthetic DNA for these experiments because all DNA samples of biological origin, e.g. calf thymus DNA, salmon sperm DNA, human placental DNA, and DNA isolated from human fibrosarcoma (HT1080) cells, contained significant amounts of N7-THBG (20-40 N7-THBG adducts /109 nucleotides). The HPLC-ESI+-HRMS/MS limit of detection for N7-THBG spiked into 150 μg synthetic oligodeoxynucleotide solution was determined as 0.3 fmol (0.9 adducts/109 nucleotides), which gave the signal to noise ratio ≥ 3. No N7-THBG was detected in unfortified oligodeoxynucleotide samples, confirming that there was no analyte carryover and no artifactual N7-THBG production during the sample preparation and analysis.
Method limit of quantitation was defined as the lowest N7-THBG amounts that afforded the signal to noise ratios of > 10 and intra/inter day precision within 15 % CV. We found that the LOQ value of our capillary HPLC-ESI+-HRMS/MS method for N7-THBG was 1 fmol analyte in 150 μg of DNA (3 adducts per 109 nucleotides, Figure S3).
N7-THBG quantitation in HT1080 cell culture
To examine N7-THBG formation in a cell culture model, HT1080 cells were treated with increasing concentrations of DEB (10 nM – 100 μM). DNA was extracted and analyzed by HPLC-ESI+-HRMS/MS methods described above. Adduct concentrations were unchanged from the background values in cells treated with 0 - 100 nM DEB, but increased linearly from 31.48 ± 4.84 adducts /109 nucleotides in cells treated with 1 μM DEB to 966.55 ± 128.05 adducts/109 nucleotides in cells treated with 100 μM DEB (Figure 4).
Figure 4.

Concentration-dependent formation of N7-THBG adduct levels in HT1080 cells treated with increasing amounts of DEB (1-100 μM). N7-THBG amounts observed in control sample (20 adducts/109 nucleotides) were subtracted from each value.
N7-THBG quantitation in blood of confirmed smokers and non-smokers and the effect of smoking cessation
Having confirmed the effectiveness of the new method in a cell culture model (Figure 4), we proceeded to quantify N7-THBG adducts in leukocyte DNA of known smokers and the corresponding controls. Cigarette smoke contains high amounts of BD (~ 46 μg/cigarette in main-stream smoke and 283 μg/cigarette in side-stream smoke),35 leading to significant exposure of smokers to this carcinogen. DNA samples from smokers and nonsmokers (13 per group) were analyzed.
HPLC-ESI+-HRMS/MS analysis has revealed the presence of N7-THBG in DNA hydrolysates from human samples (Figure 2). N7-THBG amounts in DNA were expressed as the number of adducts per 109 nucleotides, with DNA amounts determined from HPLC analysis of dG in DNA hydrolysates.22 The concentrations of N7-THBG in leukocyte DNA of smokers were 8.20 ± 5.12 adducts /109 nucleotides (range 2.22 – 19.03), while the corresponding values for nonsmokers were 7.08 ± 5.29 adducts /109 nucleotides (range 0.77 – 15.73) (Table 2). Although smoker DNA contained higher numbers of adducts, the differences in N7-THBG concentrations in DNA of smokers and non-smokers were not statistically significant in this pilot study (p = 0.60, two-sample t- test).
Table 2.
Effect of smoking on N7-THBG concentration in human leukocyte DNA
| Smokers | Non Smokers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Sample | Gender | Age | Amt. of DNA analyzed (μg)b |
Cigarettes per day |
Adducts/109 nucleotides |
Sample | Gender | Age | Amt. of DNA analyzed (μg)b |
Adducts/ 109 nucleotides |
| 1 | M | 52 | 77.3 | 11 | 11.23 | 14 | F | 27 | 93.3 | 0.79* |
| 2 | F | 35 | 77.8 | 26 | < 0.9 | 15 | M | 24 | 87.2 | 3.01 |
| 3 | M | 57 | 82.6 | 7 | 2.22 | 16 | F | 33 | 92.7 | 5.92 |
| 4 | M | 48 | 38.3 | 9 | 5.73 | 17 | F | 20 | 103.0 | 1.17* |
| 5 | F | 19 | 79.6 | 23 | 2.71* | 18 | F | 34 | 92.9 | < 0.9 |
| 6 | F | 32 | 73.9 | 13 | 6.03 | 19 | M | 24 | 108.2 | 15.73 |
| 7 | F | 61 | 78.8 | 24 | 19.03 | 20 | F | 22 | 154.5 | 11.54 |
| 8 | M | 23 | 89.8 | 19 | 6.39 | 21 | M | 53 | 122.9 | 8.45 |
| 9 | F | 51 | 101.6 | 12 | 14.97 | 22 | F | 53 | 111.7 | 16.05 |
| 10 | F | 54 | 107.5 | 26 | 10.28 | 23a | F | 44 | 102.4 | 6.83 |
| 11 | M | 43 | 100.7 | 29 | 5.36 | 24a | M | 28 | 107.9 | 0.91* |
| 12a | F | 33 | 38.8 | 19 | 3.97 | 25a | M | 28 | 59.7 | 7.40 |
| 13a | F | 44 | 42.1 | 15 | 10.58 | 26a | M | 35 | 35.9 | 7.14 |
| Mean ± SD | 42.5 ± 13.1 | 144.2 ± 20.8 | 17.9 ± 7.3 | 8.20 ± 5.12 | Mean ± SD | 32.7 ± 11.0 | 97.9 ± 28.5 | 7.07 ± 5.29 | ||
Sample adduct amount less than LOQ but greater than LOD.
Samples analyzed using an N7-THBG internal standard contaminated with 0.72 fmol of N7-THBG analyte which is finally subtracted from the quantified amount
One third of the total DNA amount was injected on column for HPLC-accurate mass MS/MS analysis.
In order to directly evaluate the influence of smoking on N7-THBG adduct concentrations, N7-THBG was quantified in DNA of 10 individuals participating in a smoking cessation study. Two baseline samples were obtained before smoking cessation, and two additional samples were collected 28 and 84 days post smoking cessation. To afford sufficient DNA amounts (150 μg), samples from five smokers were pooled prior to DNA extraction, and combined samples were subjected to HPLC-ESI+-HRMS/MS analysis of N7-THBG as described above. N7-THBG amounts post cessation were normalized to the baseline values. As shown in Figure 5, smoking cessation did not influence N7-THBG concentrations in human leukocyte DNA, suggesting that N7-THBG adducts in humans are not associated with smoking.
Figure 5.

Percent reduction in N7-THBG adducts in leukocyte DNA of 10 individuals following smoking cessation. For each time point, DNA from 5 different subjects was pooled together to obtain sufficient sample size. Samples were collected 2 times before smoking cessation and 28, and 84 days following smoking cessation. The day when the smokers quit smoking is designated as day 0. Number of adducts per 109 nucleotides (mean ± SD) are shown below each point.
Influence of Occupational BD Exposure on N7-THBG Levels in Human DNA
To evaluate the effects of occupational exposure on adduct N7-THBG prevalence in human DNA, blood samples were obtained from ten individuals exposed to BD in a workplace (average BD concentration, 1.51 ± 1.12 ppm) and the corresponding controls which were administrative workers at the same plant (average BD concentration, 0.009 ± 0.012 ppm). We found that N7-THBG adduct concentrations were significantly elevated in leukocyte DNA of individuals occupationally exposed to BD (9.72 ± 3.80 adducts/ 109 nucleotides) as compared to matched controls (3.08 ± 2.15 adducts/ 109 nucleotides, p = 0.00014) (Table 3).
Table 3.
Effect of occupational BD exposure on of N7-THBG concentrations in human leukocyte DNA
| Controls | BD Exposed | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sample | BD Exposure (ppm) |
Amt. of DNA analyzed (μg)a |
Adducts/ 109 nucleotides |
Sample | BD Exposure (ppm) |
Amt. of DNA analyzed (μg)a |
Adducts/ 109 nucleotides |
| 1 | 0.005 | 91.7 | 1.02* | 11 | 2.2 | 83.3 | 6.55 |
| 2 | 0.003 | 94.6 | 0.55* | 12 | 3.1 | 103.9 | 9.93 |
| 3 | 0.004 | 94.8 | 6.64 | 13 | 1.2 | 86.6 | 6.65 |
| 4 | 0.005 | 98.7 | 1.56* | 14 | 3.3 | 69.1 | 7.29 |
| 5 | 0.006 | 81.9 | 3.81 | 15 | 2.1 | 71.3 | 10.11 |
| 6 | 0.040 | 76.84 | 2.64* | 16 | 0.92 | 82.98 | 8.68 |
| 7 | 0.005 | 78.80 | 5.97 | 17 | 0.21 | 76.84 | 15.7 |
| 8 | 0.007 | 45.94 | 3.07 | 18 | 0.24 | 78.80 | 4.37 |
| 9 | 0.010 | 84.65 | 4.61 | 19 | 0.99 | 45.94 | 12.8 |
| 10 | 0.007 | 75.84 | 0.90* | 20 | 0.83 | 84.65 | 15.1 |
| Mean ± SD | 0.009 ± 0.01 | 82.4 ± 15.2 | 3.08 ± 2.15 | Mean ± SD | 1.51 ± 1.11 | 78.3 ± 14.9 | 9.72 ± 3.79 |
Adduct amounts less than LOQ but greater than LOD.
One third of total DNA amount was injected on column for HPLC-accurate mass MS/MS analysis.
Discussion
A recognized human and animal carcinogen, BD is metabolically activated to DNA-reactive epoxides which produce a range of nucleobase adducts including nucleobase monoadducts N7-(2-hydroxy-3-buten-1-yl)guanine (HB-Gua I), N7-(1-hydroxy-3-buten-2-yl)guanine (HB-Gua II), N7-(2,3,4-trihydroxy-3-buten-2-yl)guanine (THB-G), 1,N6-(2-hydroxy-3-hydroxymethylpropan-1,3-diyl)-2′-deoxyadenosine (1,N6-γ HMHP-dA), 1,N6-(1-hydroxymethyl-2-hydroxypropan-1,3-diyl)-2′-deoxyadenosine (1,N6-α HMHP-dA), DNA-DNA cross-links such as 4-bis (guan-7-yl)-2,3-butanediol (bis-N7G-BD) and 1-(guan-7-yl)-4-(aden-1-yl)-2,3-butanediol (N7G-N1A-BD), and numerous DNA-protein cross-links.3,24,26,36-38 If not repaired, BD-induced lesions can lead to heritable mutations by inducing polymerase errors.39-41 N7-THBG are the most abundant DNA adducts generated upon exposure to BD,14,23,26 and thus have been selected for development of mechanism-based human biomarkers of BD exposure and activation to DNA-reactive epoxides in the present study.
Several reports have previously quantified N7-THBG adducts in tissues of laboratory rats and mice exposed to BD by inhalation. The first method for N7-THBG employed analytical flow HPLC-ESI+-MS/MS to detect these adducts in livers of rats and mice exposed to 1250 ppm BD for 14 days.25 Neutral thermal hydrolysis was used to release N7-THBG bases from the DNA backbone, followed by ultra-filtration, solid phase extraction, and HPLC-ESI+-MS/MS analysis.25 N7-THBG adducts were observed in liver DNA of mice (7.6 ± 1.5 adducts/106 nucleotides) and rats (4.1 ± 1.5 adducts/106 nucleotides).25 Oe et al. further optimized the HPLC-ESI+-MS/MS methodology to separately quantify SS,RR and meso forms of N7-THBG in DNA of rats and mice treated with 1250 ppm of BD.19 (±) N7-THBG concentrations in liver DNA increased from 0.1 per 106 nucleotides to 1.6 adducts per 106 nucleotides in rats and from 1.3 adducts per 106 nucleotides to 3.9 adducts per 106 adducts in mice between 1 and 10 days of inhalation exposure to BD. Meso N7-THBG levels in the liver increased from 0.1 per 106 nucleotides to 0.8 adducts per 106 per 106 nucleotides in rats and from 0.6 per 106 nucleotides to 2.2 adducts per 106 nucleotides in mice upon continuous exposure to 1250 ppm BD for 1 or 10 days, respectively. Koc et al conducted a molecular dosimetry study of N7-THBG in mice and rats exposed to 0-625 ppm BD.14 They observed a non-linear increase in N7-THBG adduct levels in liver, lung and kidney DNA of laboratory mice and rats exposed to BD. This was attributed to saturation of metabolic activation of BD starting at 62.5 ppm.14 However, to our knowledge, N7-THBG has not been previously detected in humans.
N7-THBG adducts are relatively stable in double stranded DNA, with in vitro hydrolytic half-life of about 50 h as determined by first order kinetics.23 In vivo half-lives of N7-THBG in tissues of laboratory mice and rats exposed to 1250 ppm of 1,3-butadiene are between 3.6 to 5.5 days, depending on adduct stereochemistry.27 N7 guanine adducts removal from DNA by spontaneous hydrolysis or via active repair generates abasic sites.42
Human exposure to BD includes environmental sources (1-10 ppb in urban air),43,44 automobile exhaust (20-60 ppb),44 smoking (on average 46 μg/ cigarette in main-stream smoke and 283 μg/cigarette in side-stream smoke)35 and workplace sources at butadiene monomer and polymer plants (< 2 ppm in the workplace).45 Since these levels of exposure are orders of magnitude lower than those employed in inhalation studies of laboratory animals, human studies of BD-DNA adducts demand significantly more sensitive analytical methodologies.
Our initial studies have revealed that traditional HPLC-ESI+-MS/MS analysis on a triple quadrupole mass spectrometer did not have adequate sensitivity and specificity for N7-THBG detection in DNA isolated from human leukocytes (Figure S2). This difficulty may be a result of low N7-THBG concentrations, the polar nature of the analyte, and its relatively low molecular weight (m/z 256.1), leading to increased background and poor HPLC-ESI+-MS/MS signal to noise ratios. To overcome this challenge, we have taken advantage of the high resolution capabilities of the Orbitrap Velos mass spectrometer. A highly sensitive isotope dilution HPLC-ESI+-HRMS/MS method has been developed that is characterized by greatly improved signal to noise ratios, high accuracy, and excellent precision as compared to traditional HPLC-ESI+-MS/MS (compare Figures S2 and 2). To our knowledge, this the first report of N7-THBG formation in human DNA. The LOD and LOQ values of the new method (0.3 fmol and 1 fmol, respectively, when spiked into 150 μg of DNA) are sufficiently low to enable sensitive and accurate N7-THBG quantitation in humans. Our HPLC-ESI+-HRMS/MS method does not separate stereoisomeric forms of N7-THBG, so the total adduct amounts were determined.
The new method was first employed to quantify N7-THBG in DNA of human fibrosarcoma (HT1080) cells treated with a range of DEB concentrations (0, 10 nM, 50 nM, 100 nM, 1 μM, 10 μM, 50 μM and 100 μM). Unexpectedly, we found that untreated cells contained measurable amounts of N7-THBG (~ 20 adducts per 109 nts). Careful method validation and multiple control experiments have confirmed that this was not a result of contamination or analyte carryover. These results suggest N7-THBG adduct can be formed endogenously. It has been hypothesized that 1,2-dihydroxy-3,4-epoxybutane (EBD) might be formed from dietary and/or endogenous sources linked to catabolism of carbohydrates.46,47 Consistent with this observation, structurally analogous N-(2,3,4-trihydroxy-butyl)valine (THB-Val) hemoglobin adducts have been detected in control subjects with no known exposure to BD.48 Similarly, DNA adducts such as N7-methylguanine,49 N7-ethylguanine,31 1,N2-etheno-2′-deoxyguanosine,50,51 1,N2-propanodeoxyguanosine,52 etheno adducts such as 1,N6-ethenodeoxyadenosine (εdA), heptanone-etheno-2′-deoxyguanosine,53 and 3,N4-ethenodeoxycytine (εdC) are also endogenously found in human DNA.54,55
To determine the influence of smoking on N7-THBG concentrations in human DNA, HPLC-ESI+-HRMS/MS analysis was conducted using DNA isolated from blood of 13 smokers and 13 non-smokers. N7-THBG concentrations were similar in DNA of smokers and non-smokers (8.21 ± 5.12 and 7.08 ± 5.29 adducts /109 nucleotides, respectively; P = 0.60, based on a two-sample t test) (Table 2). Furthermore, N7-THBG levels remained unchanged upon smoking cessation (Figure 5), suggesting that N7-THBG adduct levels in human leukocytes are not related to smoking. Environmental and/or endogenous sources may be responsible for this observation, as well as adduct repair may play a role. In contrast, N7-THBG levels were significantly increased in leukocyte DNA of occupationally exposed individuals (9.73 ± 3.80 adducts/ 109 nucleotides in workers exposed to 1.51 ± 1.12 ppm, N = 10) as compared to matched controls exposed to 0.009 ± 0.012 ppm (3.07 ± 2.15 adducts/ 109 nucleotides, N = 10) (Table 3). This is not unexpected because occupational exposures to BD (~ 1- 2 ppm in our study) are much higher than those associated with smoking. However, it should be noted that in addition to BD, smokers are exposed to ~ 60 of other known carcinogens, which can contribute to lung cancer development.8,33
Our quantitative results for N7-THBG in human leukocyte DNA are consistent with previously published results for other human BD-DNA adducts. For example, Hemminki et al. have employed 32P-post-labelling to quantify N1-(2, 3, 4-trihydroxybut-1-yl)adenine (N1-THB-Ade) adducts in occupationally exposed individuals.56 These authors have found statistically higher levels of N1-THB-Ade adducts in male workers occupationally exposed (4.5 ± 7.7 adducts/109 nucleotides) as compared to controls (0.8 ± 1.2 adducts/109 nucleotides).56 The differences in N1-THB-Ade adduct levels in smokers and nonsmokers were not statistically significant.56 Since N7-THBG concentrations in DNA of smokers and non-smokers determined in our study are similar (Table 2) and are not influenced by smoking cessation (Figure 5), this suggests the presence of an endogenous source of N7-THBG in humans. Indeed, background levels of N7-THBG were also found in DNA isolated from freshly cultured human fibrosarcoma (HT1080) cells. Future studies are needed to establish the origins of endogenous N7-THBG in humans. However, occupational exposure to BD does increase N7-THBG concentrations in human blood (Table 3), suggesting that it can be a useful biomarker useful in BD risk assessment.
In summary, we have developed and validated an isotope dilution HPLC-ESI+-HRMS/MS method for N7-THBG in human leukocyte DNA. The method has adequate accuracy and sensitivity for human biomonitoring. Future studies involving larger numbers of subjects need to be performed to further validate N7-THBG as a biomarker for BD exposure and metabolic activation to DNA-reactive metabolites.
Supplementary Material
Acknowledgements
All HPLC-MS/MS analyses were performed at the University of Minnesota Masonic Cancer Center Analytical Biochemistry facility. We thank the Biorepository Facility at the Masonic Cancer Center for providing human blood samples from smokers and non-smokers and the National Cancer Institute for providing samples from a smoking cessation study. The smoking cessation study activities were supported by National Cancer Institute contract HHSN261200644002 (Laboratory Assessment of Tobacco Use Behavior and Exposure to Toxins Among Users of New Tobacco Products Promoted to Reduce Harm; PI: Peter Shields, MD). We thank the American Chemistry Council for providing samples from occupationally exposed individuals. We are grateful to Bob Carlson for preparing figures for this manuscript.
Funding: This work is supported in part by Program Project Grant from the National Cancer Institute (1P01 CA138338). The shared resources of the Masonic Cancer Center are supported in part by Cancer Center Support Grant CA-77598. Orbitrap Velos mass spectrometer was purchased using NIH Shared Instrumentation Grant S10-RR-024618.
Abbreviations
- BD
1, 3-butadiene
- EB
3, 4-epoxy-1-butene
- EBD
1, 2-dihydroxy-3, 4-epoxybutane
- DEB
1, 2, 3, 4-diepoxybutane
- N7-THBG
N7-(2, 3, 4-trihydroxybut-1-yl) guanine
- HPLC-ESI+-MS/MS
High performance liquid chromatography electrospray ionization tandem mass spectrometry in positive mode
- SPE
Solid phase extraction
- HRMS
High resolution mass spectrometry
- LOD
Limit of detection
- LOQ
Limit of quantitation
Footnotes
Supporting information: Calibration curves for HPLC-ESI+-HRMS/MS analysis of N7-THBG using isotope dilution with the corresponding 15N labeled internal standard (15N5- N7-THBG) in water (N=3) (0-50.0 fmol) (Figure S1), representative HPLC-ESI+-HRMS/MS chromatogram of N7-THBG in human DNA using column switching TSQ vantage triple quadrupole SRM method (Figure S2), extracted ion chromatogram of 1 fmol of N7-THBG and 50 fmol [15N5]-N7-THBG LOQ sample spiked in oligonucleotide solution (150 μg) analyzed using capillary HPLC-ESI+-HRMS/ MS-SRM and results are shown with a 5 ppm mass tolerance (Figure S3). This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.Owen PE, Glaister JR, Gaunt IF, Pullinger DH. Inhalation toxicity studies with 1,3-butadiene. 3. Two year toxicity/carcinogenicity study in rats. Am. Ind. Hyg. Assoc. J. 1987;48:407–413. doi: 10.1080/15298668791384959. [DOI] [PubMed] [Google Scholar]
- 2.Melnick RL, Huff J, Chou BJ, Miller RA. Carcinogenicity of 1,3-butadiene in C57BL/6 × C3H F1 mice at low exposure concentrations. Cancer Res. 1990;50:6592–6599. [PubMed] [Google Scholar]
- 3.Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Toxicology and epidemiology of 1,3-butadiene. Crit. Rev. Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Sathiakumar N, Graff J, Matthews R, Delzell E. 1,3-Butadiene and leukemia among synthetic rubber industry workers: exposure-response relationships. Chem. Biol. Interact. 2007;166:15–24. doi: 10.1016/j.cbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Delzell E, Sathiakumar N, Hovinga M, Macaluso M, Julian J, Larson R, Cole P, Muir DC. A follow-up study of synthetic rubber workers. Toxicology. 1996;113:182–189. doi: 10.1016/0300-483x(96)03443-9. [DOI] [PubMed] [Google Scholar]
- 6.Sathiakumar N, Graff J, Macaluso M, Maldonado G, Matthews R, Delzell E. An updated study of mortality among North American synthetic rubber industry workers. Occup. Environ. Med. 2005;62:822–829. doi: 10.1136/oem.2004.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fajen JM, Roberts DR, Ungers LJ, Krishnan ER. Occupational exposure of workers to 1,3-butadiene. Environ. Health Perspect. 1990;86:11–18. doi: 10.1289/ehp.908611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 9.Grant RL, Leopold V, McCant D, Honeycutt M. Spatial and temporal trend evaluation of ambient concentrations of 1,3-butadiene and chloroprene in Texas. Chem. Biol. Interact. 2007;166:44–51. doi: 10.1016/j.cbi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Pelz N, Dempster NM, Shore PR. Analysis of low molecular weight hydrocarbons including 1,3-butadiene in engine exhaust gases using an aluminum oxide porous-layer open-tubular fused-silica column. J. Chromatogr. Sci. 1990;28:230–235. doi: 10.1093/chromsci/28.5.230. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services . Public Health Statement for 1,3-Butadiene. Agency for Toxic Substances and Disease Registry; [accessed July 26, 2013]. 2013. http://www.atsdr.cdc.gov/PHS/PHS.asp?id=457&tid=81. [Google Scholar]
- 12.Kirman CR, Albertini RA, Gargas ML. 1,3-Butadiene: III. Assessing carcinogenic modes of action. Crit. Rev. Toxicol. 2010;40(Suppl 1):74–92. doi: 10.3109/10408444.2010.507183. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: I. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis. 1994;15:713–717. doi: 10.1093/carcin/15.4.713. [DOI] [PubMed] [Google Scholar]
- 14.Koc H, Tretyakova NY, Walker VE, Henderson RF, Swenberg JA. Molecular dosimetry of N-7 guanine adduct formation in mice and rats exposed to 1,3-butadiene. Chem. Res. Toxicol. 1999;12:566–574. doi: 10.1021/tx980265f. [DOI] [PubMed] [Google Scholar]
- 15.Koivisto P, Kilpelainen I, Rasanen I, Adler ID, Pacchierotti F, Peltonen K. Butadiene diolepoxide- and diepoxybutane-derived DNA adducts at N7-guanine: a high occurrence of diolepoxide-derived adducts in mouse lung after 1,3-butadiene exposure. Carcinogenesis. 1999;20:1253–1259. doi: 10.1093/carcin/20.7.1253. [DOI] [PubMed] [Google Scholar]
- 16.Perez HL, Lahdetie J, Landin H, Kilpelainen I, Koivisto P, Peltonen K, Osterman-Golkar S. Haemoglobin adducts of epoxybutanediol from exposure to 1,3-butadiene or butadiene epoxides. Chem. Biol. Interact. 1997;105:181–198. doi: 10.1016/s0009-2797(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 17.Swenberg JA, Christova-Gueorguieva NI, Upton PB, Ranasinghe A, Scheller N, Wu KY, Yen TY, Hayes R. 1,3-butadiene: cancer, mutations, and adducts. Part V: Hemoglobin adducts as biomarkers of 1,3-butadiene exposure and metabolism. Res. Rep. Health Eff. Inst. 2000:191–210. [PubMed] [Google Scholar]
- 18.Krause RJ, Elfarra AA. Oxidation of butadiene monoxide to meso- and (+/−)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch. Biochem. Biophys. 1997;337:176–184. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- 19.Oe T, Kambouris SJ, Walker VE, Meng Q, Recio L, Wherli S, Chaudhary AK, Blair IA. Persistence of N7-(2,3,4-trihydroxybutyl)guanine adducts in the livers of mice and rats exposed to 1,3-butadiene. Chem. Res. Toxicol. 1999;12:247–257. doi: 10.1021/tx980193s. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Anderson C, Loeber R, Seetharaman M, Jones R, Tretyakova N. Interstrand and intrastrand DNA-DNA cross-linking by 1,2,3,4-diepoxybutane: role of stereochemistry. J. Am. Chem. Soc. 2005;127:14355–14365. doi: 10.1021/ja051979x. [DOI] [PubMed] [Google Scholar]
- 21.Goggin M, Seneviratne U, Swenberg JA, Walker VE, Tretyakova N. Column switching HPLC-ESI+-MS/MS methods for quantitative analysis of exocyclic dA adducts in the DNA of laboratory animals exposed to 1,3-butadiene. Chem. Res. Toxicol. 2010;23:808–812. doi: 10.1021/tx900439w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goggin M, Anderson C, Park S, Swenberg J, Walker V, Tretyakova N. Quantitative high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry analysis of the adenine-guanine cross-links of 1,2,3,4-diepoxybutane in tissues of butadiene-exposed B6C3F1 mice. Chem. Res. Toxicol. 2008;21:1163–1170. doi: 10.1021/tx800051y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Tretyakova N. Structural characterization of the major DNA-DNA cross-link of 1,2,3,4-diepoxybutane. Chem. Res. Toxicol. 2004;17:129–136. doi: 10.1021/tx0342058. [DOI] [PubMed] [Google Scholar]
- 24.Seneviratne U, Antsypovich S, Goggin M, Dorr DQ, Guza R, Moser A, Thompson C, York DM, Tretyakova N. Exocyclic deoxyadenosine adducts of 1,2,3,4-diepoxybutane: synthesis, structural elucidation, and mechanistic studies. Chem. Res. Toxicol. 2010;23:118–133. doi: 10.1021/tx900312e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tretyakova NY, Chiang SY, Walker VE, Swenberg JA. Quantitative analysis of 1,3-butadiene-induced DNA adducts in vivo and in vitro using liquid chromatography electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 1998;33:363–376. doi: 10.1002/(SICI)1096-9888(199804)33:4<363::AID-JMS643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Goggin M, Sangaraju D, Walker VE, Wickliffe J, Swenberg JA, Tretyakova N. Persistence and repair of bifunctional DNA adducts in tissues of laboratory animals exposed to 1,3-butadiene by inhalation. Chem. Res. Toxicol. 2011;24:809–817. doi: 10.1021/tx200009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair IA, Oe T, Kambouris S, Chaudhary AK. 1,3-butadiene: cancer, mutations, and adducts. Part IV: Molecular dosimetry of 1,3-butadiene. Res. Rep. Health Eff. Inst. 2000:151–190. [PubMed] [Google Scholar]
- 28.Gates KS, Nooner T, Dutta S. Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem. Res. Toxicol. 2004;17:839–856. doi: 10.1021/tx049965c. [DOI] [PubMed] [Google Scholar]
- 29.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Wright M, Nicklas JA, Boogaard PJ, Henderson RF, Swenberg JA, Tates AD, Ward JB., Jr Biomarkers for assessing occupational exposures to 1,3-butadiene. Chem. Biol. Interact. 2001;135-136:429–453. doi: 10.1016/s0009-2797(01)00181-8. [DOI] [PubMed] [Google Scholar]
- 30.Boysen G, Georgieva NI, Bordeerat NK, Sram RJ, Vacek P, Albertini RJ, Swenberg JA. Formation of 1,2:3,4-diepoxybutane-specific hemoglobin adducts in 1,3-butadiene exposed workers. Toxicol. Sci. 2012;125:30–40. doi: 10.1093/toxsci/kfr272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balbo S, Villalta PW, Hecht SS. Quantitation of 7-ethylguanine in leukocyte DNA from smokers and nonsmokers by liquid chromatography-nanoelectrospray-high resolution tandem mass spectrometry. Chem. Res. Toxicol. 2011;24:1729–1734. doi: 10.1021/tx200262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michaelson-Richie ED, Ming X, Codreanu SG, Loeber RL, Liebler DC, Campbell C, Tretyakova NY. Mechlorethamine-induced DNA--protein cross-linking in human fibrosarcoma (HT1080) cells. J. Proteome. Res. 2011;10:2785–2796. doi: 10.1021/pr200042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 34.Goggin M, Loeber R, Park S, Walker V, Wickliffe J, Tretyakova N. HPLC-ESI+-MS/MS analysis of N7-guanine-N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene. Chem. Res. Toxicol. 2007;20:839–847. doi: 10.1021/tx700020q. [DOI] [PubMed] [Google Scholar]
- 35.Brunnemann KD, Kagan MR, Cox JE, Hoffmann D. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis. 1990;11:1863–1868. doi: 10.1093/carcin/11.10.1863. [DOI] [PubMed] [Google Scholar]
- 36.Swenberg JA, Bordeerat NK, Boysen G, Carro S, Georgieva NI, Nakamura J, Troutman JM, Upton PB, Albertini RJ, Vacek PM, Walker VE, Sram RJ, Goggin M, Tretyakova N. 1,3-Butadiene: Biomarkers and application to risk assessment. Chem. Biol. Interact. 2011;192:150–154. doi: 10.1016/j.cbi.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tretyakova NY, Michaelson-Richie ED, Gherezghiher TB, Kurtz J, Ming X, Wickramaratne S, Campion M, Kanugula S, Pegg AE, Campbell C. DNA-reactive protein monoepoxides induce cell death and mutagenesis in mammalian cells. Biochemistry. 2013;52:3171–3181. doi: 10.1021/bi400273m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gherezghiher TB, Ming X, Villalta PW, Campbell C, Tretyakova NY. 1,2,3,4-Diepoxybutane-induced DNA-protein cross-linking in human fibrosarcoma (HT1080) cells. J. Proteome. Res. 2013;12:2151–2164. doi: 10.1021/pr3011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotapati S, Maddukuri L, Wickramaratne S, Seneviratne U, Goggin M, Pence MG, Villalta P, Guengerich FP, Marnett L, Tretyakova N. Translesion synthesis across 1,N6-(2-hydroxy-3-hydroxymethylpropan-1,3-diyl)-2′-deoxyadenosine (1,N6-gamma-HMHP-dA) adducts by human and archebacterial DNA polymerases. J. Biol. Chem. 2012;287:38800–38811. doi: 10.1074/jbc.M112.396788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez DA, Kowalczyk A, Ward JB, Jr., Harris CM, Harris TM, Lloyd RS. Point mutations induced by 1,2-epoxy-3-butene N1 deoxyinosine adducts. Environ. Mol. Mutagen. 2001;38:292–296. doi: 10.1002/em.10026. [DOI] [PubMed] [Google Scholar]
- 41.Carmical JR, Kowalczyk A, Zou Y, Van Houten B, Nechev LV, Harris CM, Harris TM, Lloyd RS. Butadiene-induced intrastrand DNA cross-links: a possible role in deletion mutagenesis. J. Biol. Chem. 2000;275:19482–19489. doi: 10.1074/jbc.M002037200. [DOI] [PubMed] [Google Scholar]
- 42.Boysen G, Pachkowski BF, Nakamura J, Swenberg JA. The formation and biological significance of N7-guanine adducts. Mutat. Res. 2009;678:76–94. doi: 10.1016/j.mrgentox.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cote IL, Bayard SP. Cancer risk assessment of 1,3-butadiene. Environ. Health Perspect. 1990;86:149–153. doi: 10.1289/ehp.9086149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neligan RE. Hydrocarbons in the Los Angeles atmosphere. A comparison between the hydrocarbons in automobile exhaust and those found in the Los Angeles atmosphere. Arch. Environ. Health. 1962;5:581–591. doi: 10.1080/00039896.1962.10663334. [DOI] [PubMed] [Google Scholar]
- 45.United States Department of Labor . Safety and Health Topics | 1,3-Butadiene. Occupational Safety & Health Administration; [accessed July 26, 2013]. 2013. https://www.osha.gov/SLTC/butadiene/index.html#standards. [Google Scholar]
- 46.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol. Sci. 2000;56:189–202. doi: 10.1093/toxsci/56.1.189. [DOI] [PubMed] [Google Scholar]
- 47.Fustinoni S, Soleo L, Warholm M, Begemann P, Rannug A, Neumann HG, Swenberg JA, Vimercati L, Colombi A. Influence of metabolic genotypes on biomarkers of exposure to 1,3-butadiene in humans. Cancer Epidemiol. Biomarkers Prev. 2002;11:1082–1090. [PubMed] [Google Scholar]
- 48.Vacek PM, Albertini RJ, Sram RJ, Upton P, Swenberg JA. Hemoglobin adducts in 1,3-butadiene exposed Czech workers: female-male comparisons. Chem. Biol. Interact. 2010;188:668–676. doi: 10.1016/j.cbi.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 49.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 50.Chen HJ, Lin GJ, Lin WP. Simultaneous quantification of three lipid peroxidation-derived etheno adducts in human DNA by stable isotope dilution nanoflow liquid chromatography nanospray ionization tandem mass spectrometry. Anal. Chem. 2010;82:4486–4493. doi: 10.1021/ac100391f. [DOI] [PubMed] [Google Scholar]
- 51.Chen HJ, Lin WP. Quantitative analysis of multiple exocyclic DNA adducts in human salivary DNA by stable isotope dilution nanoflow liquid chromatography nanospray ionization tandem mass spectrometry. Anal. Chem. 2011;83:85438–8551. doi: 10.1021/ac201874d. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Balbo S, Wang M, Hecht SS. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem. Res. Toxicol. 2011;24:119–124. doi: 10.1021/tx100321y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda T, Tao H, Goto M, Yamada H, Suzuki M, Wu Y, Xiao N, He Q, Guo W, Cai Z, Kurabe N, Ishino K, Matsushima Y, Shinmura K, Konno H, Maekawa M, Wang Y, Sugimura H. Lipid peroxidation-induced DNA adducts in human gastric mucosa. Carcinogenesis. 2013;34:121–127. doi: 10.1093/carcin/bgs327. [DOI] [PubMed] [Google Scholar]
- 54.Nair J, Carmichael PL, Fernando RC, Phillips DH, Strain AJ, Bartsch H. Lipid peroxidation-induced etheno-DNA adducts in the liver of patients with the genetic metal storage disorders Wilson’s disease and primary hemochromatosis. Cancer Epidemiol. Biomarkers Prev. 1998;7:435–440. [PubMed] [Google Scholar]
- 55.Bartsch H. DNA adducts in human carcinogenesis: etiological relevance and structure-activity relationship. Mutat. Res. 1996;340:67–79. doi: 10.1016/s0165-1110(96)90040-8. [DOI] [PubMed] [Google Scholar]
- 56.Zhao C, Vodicka P, Sram1 RJ, Hemminki K. Human DNA adducts of 1,3-butadiene, an important environmental carcinogen. Carcinogenesis. 2000;21:107–111. doi: 10.1093/carcin/21.1.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.