Abstract
Horizontal gene transfer (HGT) has been well documented in prokaryotes and unicellular eukaryotes, but its role in plants and animals remains elusive. In a recent study, we showed that at least 57 families of nuclear genes in the moss Physcomitrella patens were acquired from prokaryotes, fungi or viruses and that HGT played a critical role in plant colonization of land. In this paper, we categorize all acquired genes based on their putative functions and biological processes, and further address the importance of HGT in plant innovation and evolution.
Keywords: gene acquisition, adaptation, land plants, plant evolution
Horizontal gene transfer (HGT) is the process of genetic transmission between species and has long been recognized as a driving force for the evolution of prokaryotes.1 In bacteria, individual organisms in changing environments often sample from a large global gene pool through constant gene gain and loss. The acquisition of novel genes may allow the recipient organism to explore new niches or resources. Some prominent examples of HGT in bacteria involve the spread of antibiotic resistance and virulence genes.2 Evolutionary novelties introduced through HGT events have also been frequently reported in various unicellular eukaryotes, including many photosynthetic organisms.3 In multicellular eukaryotes, because acquired genes will have to pass through reproductive tissues in order to be transmitted to offspring, HGT is often considered to be rare and insignificant. However, recent studies show that HGT does occur in animals and plants. In particular, HGT is frequently reported in insects and nematodes.4 Reports of HGT events in land plants are rare except for mitochondrial genes.5 In our recent genome analyses of the moss Physcomitrella patens, 128 nuclear genes of 57 families were found to be acquired from prokaryotes, fungi or viruses.6 These genes are involved in some essential or plant-specific activities such as xylem formation, plant defense, nitrogen recycling as well as the biosynthesis of starch, polyamines, hormones and glutathione. In this addendum, we categorize all 128 acquired genes identified in our earlier analyses based on their putative functions and biological processes. We further discuss the adaptive significance of additional genes that were not addressed in the paper.
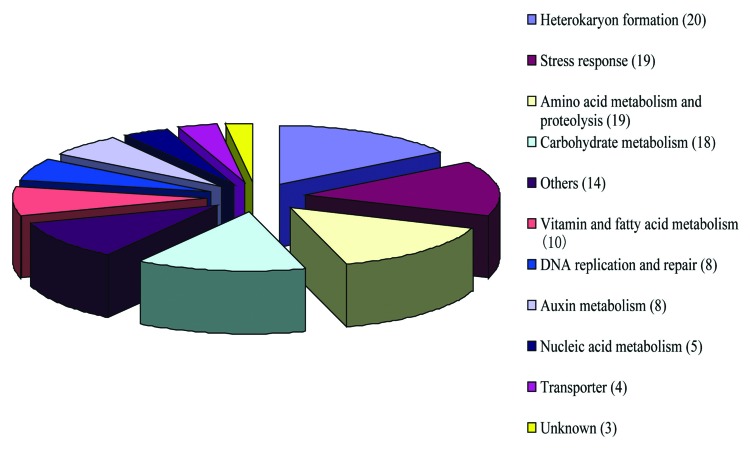
Of all 128 acquired genes identified in P. patens, 111 can be assigned to nine categories based on their more specific biological functions, an additional 14 genes have diverse functions and three other genes were annotated as unknown functions (Fig. 1). The category containing most genes is related to heterokaryon formation in filamentous fungi. These heterokaryon incompatibility (HET) gene homologs are only found in P. patens and filamentous fungi. Because heterokaryosis is not present in plants, the biochemical functions of these genes and involved biological processes in P. patens remains to be further investigated. Most genes assigned to other categories generally are broadly distributed in extant land plants. The acquisition of these genes has affected the evolution of the entire land plant lineage.
Figure 1. Functional categories of horizontally acquired genes identified in Physcomitrella patens, based on information of Table 1 in the original paper.6
HGT may confer recipient organisms novel metabolic capabilities. The gene encoding methionine gamma-lyase (MGL) was likely acquired from bacteria. MGL is involved in amino acid metabolism and proteolysis. In plants, MGL catalyzes the conversion of methionine to methanethiol and 2-ketobutyrate, an intermediate for isoleucine biosynthesis. It is believed that MGL plays a major role in the biosynthesis of isoleucine under osmotic stress and possibly salt and drought.7 Another gene acquired from bacteria encodes the nucleotide-binding domain of a novel class of mitochondrial ABC transporters that are involved in c-type cytochrome maturation in Arabidopsis.8 Photorespiration influences a wide range of processes from bioenergetics, carbon metabolism, to nitrogen assimilation. Plant photorespiratory metabolism was previously thought to be confined to chloroplasts, peroxisomes and mitochondria, but recent studies suggest that hydroxypyruvate reductase 2 (HPR2) is involved in a cytosolic bypass to the photorespiratory core cycle.9 The gene encoding HPR2 was also acquired from bacteria. Some other identified gene families are related to plant morphogenesis and development. In addition to the large and versatile subtilase gene family that is involved in the structural development of multiple organs, the vein patterning 1 (VEP1) gene family is related to vascular development.10 Our result is consistent with an earlier study that this gene family in land plants was acquired from bacteria and might have been instrumental to the transition of plants from water to land.11 These data provide additional evidence that acquired genes have contributed to the metabolic and structural complexity of land plants.
There are 19 acquired genes in P. patens that are either directly or indirectly related to stress response. In addition to genes involved in DNA repair, nitrogen fixation and biosynthesis of glutathione and polyamines,6 four acquired acid phosphatase genes, which encode vegetative storage protein (AtVSP) in Arabidopsis, might play a role in defense against herbivorous insects.12,13 Two other acquired genes encode N-acetyl-gamma-glutamyl-phosphate reductase (argC) and a HAD-superfamily hydrolase that are related to the tolerance of cadmium stress and cold stress respectively.14,15 These stress-related genes might have facilitated the adaptation of land plants to hostile environments.
It has long been speculated that HGT played an important role in the evolution of flowering plants.16 Although cases of HGT have frequently been reported in plants, most of them involve mitochondrial genes.5 Given the residential nature of mitochondria, these mitochondrial genes usually do not confer novel functions to the recipient. Similarly, recent HGT events of nuclear genes between grasses17 and plants of parasitic relationship18,19 have also been reported occasionally. These recent HGT events are important for understanding the mechanisms of HGT in plants, but usually do not affect the evolution of major plant lineages. Genes acquired during early stages of land plant evolution and retained in descendants might theoretically have the most significant impact on plant evolution.20 Several nuclear genes acquired during early land plant evolution have been reported by previous studies21-23 and some other genes acquired by more remote ancestors of plants were also identified.24,25 Clearly, some of these anciently acquired genes had long-term impact on plant development and adaptation. For example, the γ-glutamylcysteine ligase gene acquired from α-proteobacteria is involved in glutathione biosynthesis,21 and the phenylalanine ammonia lyase (PAL) gene acquired from soil bacterial encodes an critical precursor for both flavonoid and lignin monomer biosynthesis. The data generated from the analyses of P. patens represent the largest data set of acquired nuclear genes in land plants. However, because the study was based on analyses of a single genome using strict phylogenomic approach, it is reasonable to conclude that many other acquired genes likely exist in land plants. It merits further detailed genome analyses and experimental investigations to fully understand the importance of HGT in land plant evolution.
Acknowledgments
This work is supported in part by a NSF Assembling the Tree of Life (ATOL) grant (DEB 0830024) and the CAS/SAFEA International Partnership Program for Creative Research Teams.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24130
References
- 1.Gogarten JP, Doolittle WF, Lawrence JG. Prokaryotic evolution in light of gene transfer. Mol Biol Evol. 2002;19:2226–38. doi: 10.1093/oxfordjournals.molbev.a004046. [DOI] [PubMed] [Google Scholar]
- 2.Keen EC. Paradigms of pathogenesis: targeting the mobile genetic elements of disease. Front Cell Infect Microbiol. 2012;2:161. doi: 10.3389/fcimb.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J, Yue J. Horizontal gene transfer in the evolution of photosynthetic eukaryotes. J Syst Evol. 2013;51:13–29. doi: 10.1111/j.1759-6831.2012.00237.x. [DOI] [Google Scholar]
- 4.Dunning Hotopp JC. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011;27:157–63. doi: 10.1016/j.tig.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson AO, Palmer JD. Horizontal gene transfer in plants. J Exp Bot. 2007;58:1–9. doi: 10.1093/jxb/erl148. [DOI] [PubMed] [Google Scholar]
- 6.Yue J, Hu X, Sun H, Yang Y, Huang J. Widespread impact of horizontal gene transfer on plant colonization of land. Nat Commun. 2012;3:1152. doi: 10.1038/ncomms2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi V, Jander G. Arabidopsis methionine gamma-lyase is regulated according to isoleucine biosynthesis needs but plays a subordinate role to threonine deaminase. Plant Physiol. 2009;151:367–78. doi: 10.1104/pp.109.138651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayapuram N, Hagenmuller J, Grienenberger JM, Giegé P, Bonnard G. AtCCMA interacts with AtCcmB to form a novel mitochondrial ABC transporter involved in cytochrome c maturation in Arabidopsis. J Biol Chem. 2007;282:21015–23. doi: 10.1074/jbc.M704091200. [DOI] [PubMed] [Google Scholar]
- 9.Timm S, Nunes-Nesi A, Pärnik T, Morgenthal K, Wienkoop S, Keerberg O, et al. A cytosolic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in Arabidopsis. Plant Cell. 2008;20:2848–59. doi: 10.1105/tpc.108.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jun JH, Ha CM, Nam HG. Involvement of the VEP1 gene in vascular strand development in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:323–30. doi: 10.1093/pcp/pcf042. [DOI] [PubMed] [Google Scholar]
- 11.Tarrío R, Ayala FJ, Rodríguez-Trelles F. The Vein Patterning 1 (VEP1) gene family laterally spread through an ecological network. PLoS ONE. 2011;6:e22279. doi: 10.1371/journal.pone.0022279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Ahn JE, Datta S, Salzman RA, Moon J, Huyghues-Despointes B, et al. Arabidopsis vegetative storage protein is an anti-insect acid phosphatase. Plant Physiol. 2005;139:1545–56. doi: 10.1104/pp.105.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi YH, Jing X, Lei J, Ahn JE, Koo YD, Yun DJ, et al. Stability of AtVSP in the insect digestive canal determines its defensive capability. J Insect Physiol. 2011;57:391–9. doi: 10.1016/j.jinsphys.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Sarry JE, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V, et al. The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics. 2006;6:2180–98. doi: 10.1002/pmic.200500543. [DOI] [PubMed] [Google Scholar]
- 15.Yan C, Shen H, Li Q, He Z. A novel ABA-hypersensitive mutant in Arabidopsis defines a genetic locus that confers tolerance to xerothermic stress. Planta. 2006;224:889–99. doi: 10.1007/s00425-006-0272-6. [DOI] [PubMed] [Google Scholar]
- 16.Syvanen M. Horizontal gene transfer: evidence and possible consequences. Annu Rev Genet. 1994;28:237–61. doi: 10.1146/annurev.ge.28.120194.001321. [DOI] [PubMed] [Google Scholar]
- 17.Christin PA, Edwards EJ, Besnard G, Boxall SF, Gregory R, Kellogg EA, et al. Adaptive evolution of C(4) photosynthesis through recurrent lateral gene transfer. Curr Biol. 2012;22:445–9. doi: 10.1016/j.cub.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida S, Maruyama S, Nozaki H, Shirasu K. Horizontal gene transfer by the parasitic plant Striga hermonthica. Science. 2010;328:1128. doi: 10.1126/science.1187145. [DOI] [PubMed] [Google Scholar]
- 19.Xi Z, Bradley RK, Wurdack KJ, Wong K, Sugumaran M, Bomblies K, et al. Horizontal transfer of expressed genes in a parasitic flowering plant. BMC Genomics. 2012;13:227. doi: 10.1186/1471-2164-13-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Gogarten JP. Ancient gene transfer as a tool in phylogenetic reconstruction. Methods Mol Biol. 2009;532:127–39. doi: 10.1007/978-1-60327-853-9_7. [DOI] [PubMed] [Google Scholar]
- 21.Copley SD, Dhillon JK. Lateral gene transfer and parallel evolution in the history of glutathione biosynthesis genes. Genome biology 2002; 3:research0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emiliani G, Fondi M, Fani R, Gribaldo S. A horizontal gene transfer at the origin of phenylpropanoid metabolism: a key adaptation of plants to land. Biol Direct. 2009;4:7. doi: 10.1186/1745-6150-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards TA, Soanes DM, Foster PG, Leonard G, Thornton CR, Talbot NJ. Phylogenomic analysis demonstrates a pattern of rare and ancient horizontal gene transfer between plants and fungi. Plant Cell. 2009;21:1897–911. doi: 10.1105/tpc.109.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Gogarten JP. Concerted gene recruitment in early plant evolution. Genome Biol. 2008;9:R109. doi: 10.1186/gb-2008-9-7-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price DC, Chan CX, Yoon HS, Yang EC, Qiu H, Weber AP, et al. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science. 2012;335:843–7. doi: 10.1126/science.1213561. [DOI] [PubMed] [Google Scholar]