Abstract
The mechanisms of circadian clock function in Arabidopsis rely on the complex relationships among core clock components. The current model of the Arabidopsis oscillator comprises a myriad of repressors but the mechanisms responsible for activation remain largely unknown. In our recent studies, we have demonstrated that the rhythms in H3 acetylation (H3ac) and H3K4 trimethylation (H3K4me3) are a key mechanism at the positive arm of the oscillator. H3K4me3 rhythmic accumulation is delayed compared to that of H3ac, which opens the possibility for separate roles for each mark. Indeed, the use of inhibitors that block H3K4me3 accumulation was concomitant with increased clock repressor binding, suggesting that H3K4me3 might control the timing from activation to repression. Plants mis-expressing the histone methyltransferase SET DOMAIN GROUP 2 (SDG2/ATXR3) displayed altered H3K4me3 accumulation, oscillator gene expression and clock repressor binding, suggesting that SDG2/ATXR3 is a key component contributing to proper circadian expression.
Keywords: circadian clock, chromatin, histone modifications, Arabidopsis thaliana
Circadian rhythms are daily oscillations in gene expression and biological activities that have been observed in almost all organisms, from cyanobacteria to mammals.1 The circadian clock is the internal mechanism that generates these rhythms allowing organisms to anticipate the environmental changes resulting from the day/night cycles. In Arabidopsis thaliana, the circadian clock regulates multiple biological functions, such as the photoperiodic-dependent flowering time, stem and hypocotyl elongation, leaf movement, stomata movement and gene expression of roughly one third of the genes.2 The fundamental architecture of the circadian clock comprises a complex network of feedback loops.3 The first transcriptional feedback loop identified in Arabidopsis4 included CCA1 (CIRCADIAN CLOCK ASSOCIATED 1) and LHY (LATE ELONGATED HYPOCOTYL),5,6 together with TOC1/PRR1 (TIMING OF CAB EXPRESSION 1/PSEUDO-RESPONSE REGULATOR 1), a CCT-domain containing transcription factor expressed in the evening.7,8 CCA1 and LHY are able to bind to the TOC1 promoter through the evening element (EE) and repress its expression.4 TOC1 initially was proposed to activate CCA1 and LHY expression4 but more recent reports have demonstrated that TOC1 also inhibits the expression of CCA1 and LHY.9,10 TOC1 not only represses CCA1 and LHY but also the components of the pseudo-response regulator (PRR) family (PRR5, PRR7, PRR9) and other evening-expressed genes.9 The current model thus contains a myriad of repressors, opening the question about positive factors that might function as activators at the core of the clock.
In eukaryotic cells, DNA wraps around a histone octamer to form nucleosomes, which are structured into higher-order structures to form a chromosome.11 Histones are subject to multiple post-translational modifications including acetylation, methylation, phosphorylation, ubiquitination and ADP-ribosylation. The complex combination of these modifications regulates gene transcription.12 Overall, histone acetyltransferases (HAT) acetylate histone lysine residues and favor transcription while histone deacetylases (HDAC) deacetylate histones and induce transcriptional repression.13-15 Histones can also be mono-, di- or trimethylated on lysines and mono-, symmetrically or asymmetrically dimethylated on arginines.16 Histone methylation acts as a signal for binding of chromatin remodeling factors, which can activate or repress transcriptional activity. The histone-modifying enzymes that catalyze the transfer of methyl groups are histone methyltransferases (HMT).16 The two major groups of HMT include lysine-specific (HKMT) and arginine-specific (PRMT) methyltransferases. In Arabidopsis, different methyltransferases have been described, including two ATX-RELATED (ATXR3/SDG2 and ATXR7/SDG25) and five ARABIDOPSIS TRITHORAX (ATX1–ATX5).17 Recently, SET DOMAIN GROUP 2 (ATXR3/SDG2) has been identified as the major histone methyltransferase responsible for H3K4me3 in Arabidopsis thaliana.18,19
Several studies have shown the connection between chromatin remodeling and the plant circadian clock. For instance, histone H3 acetylation at the TOC1 promoter was shown to closely correlate with TOC1 circadian expression.20 Moreover, recent reports have extended the analysis demonstrating that H3 acetylation and H3K4me3 associate with the rhythmic transcription of CCA1, LHY and TOC121,22 while H3K36me2 showed a negative correlation with their expression. Other studies have also implicated JUMONJI DOMAIN CONTAINING 5/30 (JMD5/JMD30), a putative histone demethylase, in circadian clock regulation.23,24 Taken together, these reports suggest that chromatin remodeling might be a key mechanism in the regulation of the plant circadian clock.
Our previous work revealed that circadian oscillations of H3ac at the TOC1 promoter regulate TOC1 rhythmic expression.20,25 In a recent report,26 we have extended these studies to other oscillator genes to demonstrate that the rhythms of H3K4me3, H3K9ac and H3K56ac are a regulatory mechanism common to the morning (CCA1, LHY, PRR9 and PRR7) and evening (TOC1 and LUX) expressed oscillator genes. Our studies show that H3K4me3 and H3K56ac mostly accumulated around the 5′ end of the oscillator genes, with a peak around the time of their maximal expression. Recent publications reporting H3K4me3 and H3ac location on TOC1, CCA1 and LHY loci support our results.21,22 The distribution of histone marks have been shown to be important for their effect on transcription. For instance, methylation of K36 by Set2 usually occurs within the ORF of actively transcribed genes. However, mis-accumulation of this mark within the promoter correlates with repression.27
The accumulation of H3K56ac and H3K4me3 around the peak of mRNA expression suggested that these marks might be associated with clock gene activation. Indeed, the rhythmic expression of the oscillator genes damped when acetylation and H3K4me3 were blocked with different inhibitors. Following the results of a previous study showing that nicotinamide (NAM) affected H3K4me3 and clock gene expression in mammals,28 we treated seedlings with NAM and found that oscillator gene expression was reduced by treatment with the inhibitor in a dose-dependent manner (Fig. 1). Treatment with NAM also associated with a significant reduction in H3K4me3. Although NAM was previously shown to inhibit histone deacetylase,29 our assay revealed that H3K56ac accumulation decreased when plants were treated with NAM. When we blocked histone acetylation by inhibition with MB-330 or with C64631 we also observed a dose-dependent reduction of oscillator gene expression (Fig. 1). Remarkably, the combined treatment with both NAM and C646 damped low the oscillation of clock gene expression suggesting that acetylation and H3K4me3 are key histone modifications for the activation of oscillator gene expression. Our conclusions suggesting a chromatin remodeling mechanism within the positive arm of the oscillator are consistent with the results demonstrating that the key clock component of the mammalian oscillator, CLOCK, has HAT activity that is crucial for the circadian clock.32 Therefore, it seems that the plant and mammal circadian systems share a common chromatin-dependent mechanism required for the activation of oscillator genes.
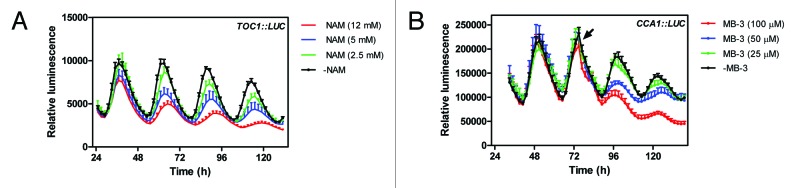
Figure 1. Effects of blocking histone acetylation and K4 trimethylation on circadian gene expression. (A) TOC1::LUC luminescence in WT plants entrained under LD cycles and subsequently released to constant light (LL) conditions. Luminescence was examined in the presence of 12 mM, 5 mM or 2.5 mM of NAM. As control, plants were treated only with the solvent. (B) CCA1::LUC luminescence in WT plants entrained under LD cycles and subsequently released to constant light (LL) conditions. Luminescence was examined in the presence of 100 μM, 50 μM or 25 μM of MB-3. As control, plants were treated only with the solvent. The arrow indicates the circadian time of inhibitor administration.
We also found that the oscillations in H3K4me3 were followed by rhythms in H3K4me2, which were antiphasic to H3ac and partially overlapping with H3K4me3. Detailed comparisons revealed clear coexistence of H3K4me2 and H3K4me3 around dusk for the oscillator genes expressed in the morning and around dawn for the genes expressed in the evening. Therefore, our results show the sequential enrichment H3ac→H3K4me3→H3K4me2 that can be temporally associated with the rhythmic expression of the oscillator genes, from peak to trough.
The detailed study of both H3ac and H3K4me3 accumulation in a time course experiment also revealed other interesting features. For instance, the peak of H3K4me3 was delayed in comparison to that of H3K56ac. A similar delay of H3K4me3 accumulation compared with H3K9ac was found at the promoter of the mouse albumin D element-binding protein (DBP).33 Acetylation seems to precisely coincide with the peak of maximal gene expression, suggesting a main role as an activating mark. The extended accumulation of H3K4me3 and its overlap with both acetylation and H3K4me2 suggest that H3K4me3 might somehow function as a transition mark at the boundaries between activation and repression. Notably, analysis of clock repressor binding showed that blocking H3K4me3 by pharmacological inhibition increases the binding and enhances gene repression. These results might be relevant for explaining the observed crosstalk between histone acetylation and methylation. We proposed that H3K4me3 might control the timing of repression by preventing an advanced repression phase, and thus ensuring the proper timing for activation by histone acetylation.
The last piece of the study focused on characterizing the histone methyltransferase SDG2/ATXR3 as the possible molecular component responsible for H3K4me3 accumulation at the promoters of the oscillator genes. Our analysis showed a decreased methylation and reduced gene expression in mutant lines while the opposite was observed in plants expressing additional copies of SDG2/ATXR3. Furthermore, clock repressor binding was also affected in these plants, which is fully consistent with the function of H3K4me3 as a transition mark controlling the timing of repression. The effects on repressor binding were reverted by treatment with NAM, suggesting that H3K4me3 accumulation is indeed able to directly modulate the clock repressor binding. Recent findings have shown that the methyltransferase MLL1 mediates H3K4 methylation to regulate CLOCK target genes.34 Further work would be required to check if the mechanism controlling clock repressor binding by H3K4me3 is conserved in other circadian systems.
Despite the advances in our understanding of the connections between chromatin remodeling and plant circadian function, many challenges are still in front of us. Discovering the chromatin related components and clock effectors that lie at the interface of pre- and post-transcriptional regulation is one of the plausible future objectives.
Acknowledgments
Work in PM lab is supported by grants from the Ramón Areces Foundation, the Spanish Ministry of Science and Innovation, the EMBO YIP program and from EUROHORCS (European Heads of Research Councils) and the European Science Foundation (ESF) through the EURYI Award.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24079
References
- 1.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–56. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet. 2010;44:419–44. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClung CR, Gutiérrez RA. Network news: prime time for systems biology of the plant circadian clock. Curr Opin Genet Dev. 2010;20:588–98. doi: 10.1016/j.gde.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293:880–3. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZY, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–17. doi: 10.1016/S0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 6.Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–29. doi: 10.1016/S0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- 7.Matsushika A, Makino S, Kojima M, Mizuno T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant Cell Physiol. 2000;41:1002–12. doi: 10.1093/pcp/pcd043. [DOI] [PubMed] [Google Scholar]
- 8.Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Mas P, Panda S, Kreps JA, Kay SA. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science. 2000;289:768–71. doi: 10.1126/science.289.5480.768. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Perez-Garcia P, Pokhilko A, Millar AJ, Antoshechkin I, Riechmann JL, et al. Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science. 2012;336:75–9. doi: 10.1126/science.1219075. [DOI] [PubMed] [Google Scholar]
- 10.Gendron JM, Pruneda-Paz JL, Doherty CJ, Gross AM, Kang SE, Kay SA. Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc Natl Acad Sci USA. 2012;109:3167–72. doi: 10.1073/pnas.1200355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–94. doi: 10.1016/S0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 12.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–52. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 14.Orphanides G, Reinberg D. RNA polymerase II elongation through chromatin. Nature. 2000;407:471–5. doi: 10.1038/35035000. [DOI] [PubMed] [Google Scholar]
- 15.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 16.Wood A, Shilatifard A. Posttranslational modifications of histones by methylation. Adv Protein Chem. 2004;67:201–22. doi: 10.1016/S0065-3233(04)67008-2. [DOI] [PubMed] [Google Scholar]
- 17.Ng DW, Wang T, Chandrasekharan MB, Aramayo R, Kertbundit S, Hall TC. Plant SET domain-containing proteins: structure, function and regulation. Biochim Biophys Acta. 2007;1769:316–29. doi: 10.1016/j.bbaexp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Yu Y, Law JA, Zhang X. SET DOMAIN GROUP2 is the major histone H3 lysine [corrected] 4 trimethyltransferase in Arabidopsis. Proc Natl Acad Sci USA. 2010;107:18557–62. doi: 10.1073/pnas.1010478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berr A, McCallum EJ, Ménard R, Meyer D, Fuchs J, Dong A, et al. Arabidopsis SET DOMAIN GROUP2 is required for H3K4 trimethylation and is crucial for both sporophyte and gametophyte development. Plant Cell. 2010;22:3232–48. doi: 10.1105/tpc.110.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perales M, Más P. A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell. 2007;19:2111–23. doi: 10.1105/tpc.107.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song HR, Noh YS. Rhythmic oscillation of histone acetylation and methylation at the Arabidopsis central clock loci. Mol Cells. 2012;34:279–87. doi: 10.1007/s10059-012-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmes H, Henriques R, Jang IC, Kim S, Chua NH. Circadian clock regulates dynamic chromatin modifications associated with Arabidopsis CCA1/LHY and TOC1 transcriptional rhythms. Plant Cell Physiol. 2012;53:2016–29. doi: 10.1093/pcp/pcs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones MA, Covington MF, DiTacchio L, Vollmers C, Panda S, Harmer SL. Jumonji domain protein JMJD5 functions in both the plant and human circadian systems. Proc Natl Acad Sci USA. 2010;107:21623–8. doi: 10.1073/pnas.1014204108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu SX, Knowles SM, Webb CJ, Celaya RB, Cha C, Siu JP, et al. The Jumonji C domain-containing protein JMJ30 regulates period length in the Arabidopsis circadian clock. Plant Physiol. 2011;155:906–15. doi: 10.1104/pp.110.167015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farinas B, Mas P. Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J. 2011;66:318–29. doi: 10.1111/j.1365-313X.2011.04484.x. [DOI] [PubMed] [Google Scholar]
- 26.Malapeira J, Khaitova LC, Mas P. Ordered changes in histone modifications at the core of the Arabidopsis circadian clock. Proc Natl Acad Sci USA. 2012;109:21540–5. doi: 10.1073/pnas.1217022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–19. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Xydous M, Sekeri-Pataryas KE, Prombona A, Sourlingas TG. Nicotinamide treatment reduces the levels of histone H3K4 trimethylation in the promoter of the mper1 circadian clock gene and blocks the ability of dexamethasone to induce the acute response. Biochim Biophys Acta. 2012;1819:877–84. doi: 10.1016/j.bbagrm.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Lawson M, Uciechowska U, Schemies J, Rumpf T, Jung M, Sippl W. Inhibitors to understand molecular mechanisms of NAD(+)-dependent deacetylases (sirtuins) Biochim Biophys Acta. 2010;1799:726–39. doi: 10.1016/j.bbagrm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Biel M, Kretsovali A, Karatzali E, Papamatheakis J, Giannis A. Design, synthesis, and biological evaluation of a small-molecule inhibitor of the histone acetyltransferase Gcn5. Angew Chem Int Ed Engl. 2004;43:3974–6. doi: 10.1002/anie.200453879. [DOI] [PubMed] [Google Scholar]
- 31.Bowers EM, Yan G, Mukherjee C, Orry A, Wang L, Holbert MA, et al. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol. 2010;17:471–82. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–74. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 34.Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17:1414–21. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]


