Abstract
We review trapping mechanisms in the carnivorous flowering plant family Droseraceae (order Caryophyllales). Its members are generally known to attract, capture, retain and digest prey animals (mainly arthropods) with active snap-traps (Aldrovanda, Dionaea) or with active sticky flypaper traps (Drosera) and to absorb the resulting nutrients. Recent investigations revealed how the snap-traps of Aldrovanda vesiculosa (waterwheel plant) and Dionaea muscipula (Venus’ flytrap) work mechanically and how these apparently similar devices differ as to their functional morphology and shutting mechanics. The Sundews (Drosera spp.) are generally known to possess leaves covered with glue-tentacles that both can bend toward and around stuck prey. Recently, it was shown that there exists in this genus a higher diversity of different tentacle types and trap configurations than previously known which presumably reflect adaptations to different prey spectra. Based on these recent findings, we finally comment on possible ways for intrafamiliar trap evolution.
Keywords: Aldrovanda, carnivorous plant, catapult-flypaper-trap, Dionaea, Drosera, sticky flypaper trap, snap-trap
Introduction
The Droseraceae (~200 species) comprise the two monotypic genera Aldrovanda and Dionaea and the large genus Drosera.1,2 The aquatic Aldrovanda vesiculosa (waterwheel plant) and the terrestrial Dionaea muscipula (Venus’ flytrap) both feature active snap-traps, whereas the terrestrial Sundews (Drosera spp.) are generally known for possessing active flypaper traps.3-5 Molecular and morphological data suggest that Aldrovanda and Dionaea are sister and form a clade that is for its part sister to Drosera, with the South African species Drosera regia being basal in this clade.6-10 According to this scenario, snap-traps are likely to have evolved only once in angiosperms, with Aldrovanda and Dionaea sharing a common ancestor with Drosera. How snap-traps might have evolved from this ancestor is a challenging question since there is no fossil record of intermediate forms. A recent evolutionary model proposes that the ability for capturing larger prey was the main selective driving force for the evolution of snap-traps in Dionaea/Aldrovanda and for the evolution of elongated leaves and fast tentacles in the genus Drosera.11 Such traps are able to capture and retain single but larger and stronger prey in contrast to other trap types that are specialized to capture, e.g., small flying or crawling insects. This scenario would favor the evolution of several functional traits, such as rapid mechanisms for stimulus perception and conduction, fast mechanical responses and efficient mechanisms for enclosing and retaining prey.
It should be added that this model is likely to be limited to Dionaea and Drosera because Aldrovanda, as far as known, primarily catches small prey.12 The evolution of the underwater snap-traps of the waterwheel plant is likely to be a consequence of other driving forces which is discussed in section 4 of this article. In the following, we will summarize how Aldrovanda, Dionaea and Drosera traps work and highlight the many configurations of Drosera traps as well as structural and physiological pre-adaptations for snap-traps.
The Snap-Traps of Aldrovanda and Dionaea
Snap-traps can close within 100 ms at fastest and work in water and/or air, depending on the habitat of the respective plant.1,4,5,13,14 The fast shutting movement of the comparably large, up to 5 cm long aerial trap of Dionaea (Fig. 1A) relies on hydraulically actuated motion. The motion sequence comprises the initial bending and final closing of the trap lobes, and an intermediate elastic instability mechanism facilitated by the doubly-curved trap lobe surface, which enables the fast snap-buckling.13-16 The first, hydraulic part is generally believed to be due to an osmotically driven displacement of water between the cells of each lobe, but this was recently put into question.17 The trap midrib, which connects the two lobes, does not take part in the trapping motion and does not change its curvature during the snap-buckling process. Since Darwin’s first experiments, it was considered that Dionaea’s traps were adaptations to selectively catch and retain relatively larger prey animals than those typically caught by Drosera, although recent investigations have shown that Dionaea’s prey capture may be rather more opportunistic than selective.5,11,18,19
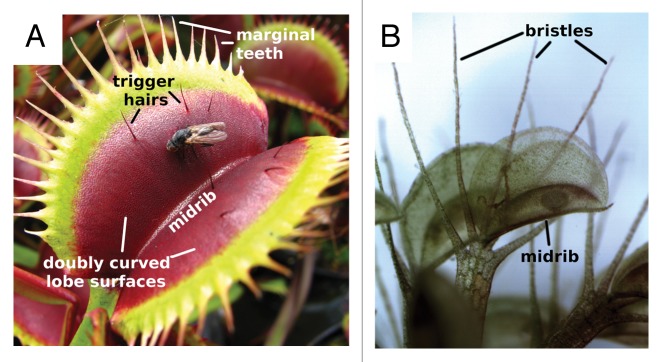
Figure 1. Snap-trapping in carnivorous plants. (A) The Venus’ flytrap (Dionaea muscipula) features doubly curved trap lobe surfaces that act as flexible flaps, a kinematically “inactive” midrib, and marginal teeth that interlock when the trap is shut. The trigger hairs are clearly visible. (B) The waterwheel plant (Aldrovanda vesiculosa) possesses a movable trap midrib that kinematically connects the two inflexible trap lobes. The leaf bristles extending beyond the lamina are clearly visible.
In contrast, the smaller, 2.5–6 mm long4 underwater trap of Aldrovanda (Fig. 1B) does not show elastic instability of the trap lobes. In this plant, hydraulically actuated bending of the trap midrib leads to a kinematically amplified opening/closing process of the inflexible trap lobes.14,20-22 It is hypothesized that this mechanism is a well-adapted way of snap-trapping underwater without excessive water displacement and potential loss of prey. Aldrovanda mainly traps small zooplankton.4,12,23
In summary, both snap-trap types differ from each other in functional morphology and consequential mechanics of snap-trapping: Dionaea traps consist of two independent kinematic elements (the trap lobes) that possess hydraulically actuated motion as well as snap-buckling, which are kinematically separated by the midrib. In contrast, in Aldrovanda the trap midrib forms a moveable element that kinematically connects the two (inflexible) trap lobes which show no individual deformation. The extent of morphological and concomitant mechanical adaptation to the respective surrounding medium and prey spectrum will be a fruitful topic for future investigations.
The Compound Traps of Drosera
The Sundews are generally described as possessing leaves of various shapes with a multitude of radially symmetric, glandular emergences (glue-tentacles) that consist of a stalk and a terminal head, the mucus-producing gland.1,3,18,24 After direct mechanical irritation or indirect irritation by touching of neighboring tentacles or chemical stimulation, these glue-tentacles reversibly bend toward stuck prey from any direction. In many species, as in e.g., D. regia, the leaf blades also wrap around the prey.25,26 Both of these thigmonastic and thigmotropic movements can last from several minutes up to hours and are considered to be based mainly on acid growth processes due to cell wall loosening.25,27-29 These “typical” glue-tentacles appear on all Drosera species and are termed “T0-tentacles” (Fig. 2).26,30,31
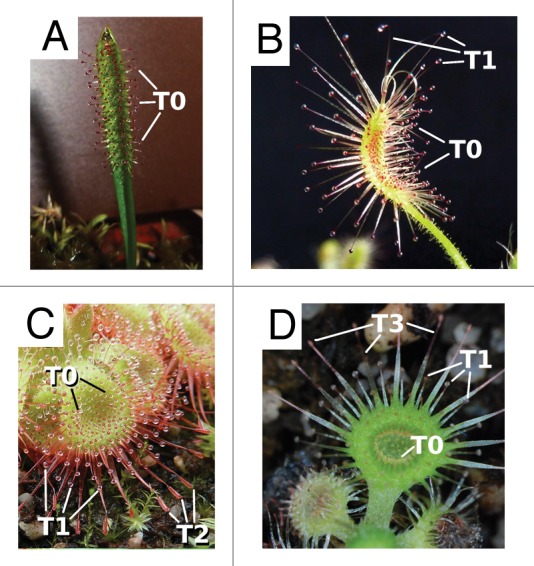
Figure 2. The compound traps of Drosera. (A) D. arcturi trap leaves feature only T0-tentacles. (B) The trap leaf of D. scorpioides features T0- and long-stalked, marginal T1-tentacles. This trap is very efficient in capturing flying arthropods as prey. (C) D. sessilifolia has T2-snap-tentacles for effective and fast retention of walking prey and several rows of T1- and T0-tentacles. (D) D. glanduligera catapults walking prey with outstretched T3-tentacles onto the sticky trap leaf which is covered with T0- and T1-tentacles.
Different in motion, structure and sometimes function are the various tentacle types produced on the margins of the trap leaves in many Drosera species. Most of them have a much longer stalk than the T0-tentacles, with a broad, flattened base that may account for the uniplanar, circular bending motion they are capable of (T0-tentacles, in contrast, can bend in more than one plane). Following the terminology proposed by Hartmeyer and Hartmeyer,30,31 one can distinguish between the following marginal tentacle types in the genus Drosera:
T1-tentacles (Fig. 2B–D): These are long-stalked, radially symmetric glue-tentacles which reversibly and comparably quickly bend toward prey within a time range of several seconds (~15 sec in D. macrantha) and up to some minutes (e.g., in D. villosa). T1-tentacles are commonly found on erect sundews, e.g., in species of the sections Bryastrum and Ergaleium, which predominantly capture flying arthropods as prey.11,26,32,33 With their comparably fast bending motion they most presumably help the plants effectively retain the prey animals. T1-tentacles also appear on traps that feature additionally T2- or T3-tentacles. Here they are smaller, more densely arranged in one or two rows (Fig. 2C and D) and help the trap to quickly draw caught prey toward the center of the sticky leaf.31,34
T2-tentacles (Fig. 2C): These are bilaterally symmetric, glue-free snap-tentacles that can reversibly bend toward the prey within five seconds at the fastest, as observed in Drosera nitidula ssp. omissa.30 The bending is triggered by touch on the sensitive, mucus-free tentacle head, or indirectly, with a delay of some seconds to several minutes, after triggering of the nearby T0- or T1-tentacles. The tentacle head is spoon-shaped and characterized by a distinct margin.24 T2-tentacles are able to effectively retain and fix even relatively large prey struggling on the trap, as demonstrated in D. burmannii (see also www.youtube.com/watch?v=XPRg7tHtPEE at 3:58 min).30 This might also be considered as a very effective protection against kleptoparasites such as ants and bugs. T2-tentacles occur mainly in seedlings and species that grow as basal rosettes, e.g., in many species of the section Bryastrum where the tentacles feature more or less spherical heads, or in species of the section Drosera, where the tentacle heads are mostly elongated.
T3-tentacles (Fig. 2D): These are bilaterally symmetric, glue-free snap tentacles that irreversibly bend within less than a tenth of a second.24,31,34 This tentacle type is only known from D. glanduligera (section Coelophylla) which grows as a basal rosette. Tentacle motion is hypothesized to be actuated hydraulically and is unique in the genus Drosera, not only in its morphology and speed but also in its irreversible “one-shot” character.31,34,35 T3-tentacles can be easily distinguished from T2-tentacles by their raised head and their unique, broadened hinge-zone near the tentacle base. At this distinct hinge zone, bending takes place after mechanically triggering the head. T3-tentacles cannot be triggered indirectly (e.g., by airborne prey landing on the row of nearby T1-tentacles), which apparently accounts for their unique function: instead of playing a role mainly in prey retention (like the T2-tentacles), they catapult walking prey onto the central regions of the sticky trap leaf. Hence, they play an active role in a unique capture process. Subsequently, after the prey has become catapulted, T1-tentacles lift it to the concave center of the leaf where T0-tentacles continue to pull it into the central cavity of the leaf. This two-step trapping mechanism has been termed a catapult-flypaper trap.34 It is not yet clear which processes are involved in the mechano-sensitive nature of the T2-/T3-tentacle heads and in the actuation of snap-tentacle bending.35
There exist even more tentacle types with divergent types of heads,24 but there are no reports available about their sensitivity or about the way in which they contribute to prey capture and/or retention. D. erythrogyne and a few Australian species of the section Ergaleium feature bilaterally symmetric tentacles with heads that lack the distinct margins typical of T2-tentacles. Moreover, the glandular tissue which normally is responsible for glue secretion occurs on the abaxial side of the tentacle heads. In particular, the climbing species of the section Ergaleium typically develop traps with very prominent and fast moving T1-tentacles. D. rosulata (section Erythrorhizae) features radially symmetric bristles without glandular tissue. These structures resemble rudimentary tentacles without heads, though their function is unclear. Another exception is D. prolifera, the only species of the section Prolifera (the Queensland Sundews) that develops bilaterally symmetric marginal tentacles (T2-tentacles?) in adult traps, which produce sticky mucus (personal observation).
Moreover, some Drosera species feature unique, modified tentacles and leaf trichomes, which are of taxonomic significance.9,24 Some of them are hypothesized to take part in prey attraction and most likely mirror a high degree of specialization as to prey.26,31 Most noteworthy, D. hartmeyerorum displays clusters of reflective lens-headed tentacles that are likely to visually attract prey insects. The closely related D. indica features several aberrant, different trichome types, ranging from mushroom-shaped to stellate structures. It is still up to future studies to examine the exact function of these conspicuous types of trichomes.
The above concise compilation proves that the genus Drosera is much more diverse in terms of trap characteristics than commonly acknowledged. One can at least distinguish between the following, general types of traps: (1) Trap leaves only with “normal” T0-tentacles (e.g., D. arcturi) (Fig. 2A); (2) Trap leaves with T0- and long T1-tentacles (e.g., D. scorpioides) for effectively catching and retaining flying prey (Fig. 2B ); (3) Trap leaves with T0-, T1- and T2-tentacles (e.g., D. sessilifolia) that help to capture and retain relatively large walking prey (Fig. 2C) and (4) Trap leaves with T0-, T1- and T3-tentacles (so far only known from D. glanduligera) (Fig. 2D), with T3-tentacles catapulting walking prey onto the trap leaf center.
There is no overview available showing which Drosera species feature leaf blade movement. Integrating this potentially functional important feature into the above classification would lead to even more different trap types. A classification of trap types in the genus Drosera is further complicated by the fact that some species switch the tentacle composition from their juvenile to their adult stage.30,31,35 D. glanduligera, for example, features T0- and T1-tentacles as a seedling, but T3-tentacles (instead of T1) as an adult. T3-snap-tentacles help in capturing relatively large walking prey, but as a seedling the trap leaves most presumably are too small to retain and digest prey of large size.11 D. scorpioides starts with T2-tentacles and “switches” to T1-tentacles later. D. cistiflora develops T2 snap-tentacles as long as it grows as a rosette, supposedly to successfully capture and retain large walking prey, but switches to T1-tentacles for flying prey after the erect growth continues until flowering. Also, D. binata seedlings develop T2-tentacles, but as soon as the long-stalked, dichotomous trap-leaves arise for the first time, T1-tentacles occur on the leaf margins. For a better understanding of the biological significance of these developmental stages, detailed prey spectra analyses are needed. For D. glanduligera, it was shown that this short-lived species is extremely “hungry” in cultivation and dies back unless fed regularly.30,35 Most presumably, each trap configuration and developmental stage is an adaptation to meet the needs with respect to prey type and nutrient demands.
Discussion
Although recent DNA analyses suppose Dionaea and Aldrovanda to be closely related and to share a common ancestor, it is not clear whether the ancestor was terrestrial or aquatic and which lineage evolved first.5 This is reflected by the fact that both snap-trap systems are very complex in their bauplan and functional integrity. Today’s Aldrovanda and Dionaea are thought to be relict members of an ancient snap-trap clade that once was much more diverse, involving several events of diversification and extinction.5 It is commonly assumed that Aldrovanda is a descendant of the Dionaea clade, although the transition from a land plant with functionally more complex large traps to an aquatic plant with reduced trap size is far from being proven and lacks satisfying structural and functional explanation.5,7,18 All evolutionary scenarios based on vague Aldrovanda fossil records are furthermore flawed by the fact that there exist no known fossils of Dionaea. It may be hypothesized that a loss of trap size and the development of a different (more simple?) closure mechanism was an evolutionary ‘response' of Aldrovanda to cope with the physically different constraints in water as a surrounding medium.5,14
At least as interesting as the above described relationship is how the Dionaea snap-trap evolved in general, which is hypothesized to be favored by selection to capture and retain large prey.11 The structurally and functionally most important adaptations for this purposes are: (1) Efficient enclosing of the prey, as found, for example, in the leaf movement present in basal Drosera regia; (2) Rapid mechanisms for prey stimulus perception and conduction, as present in the mechano-sensitive T3-tentacles of D. glanduligera; (3) Fast mechanical responses, as seen in T2- and T3-tentacles. A fusion of marginal, hydraulically actuated tentacles and reduction of a sticky lamina could have led to the formation of two lobes that are able to bend and close quickly.1,11 Adding a double surface curvature to implement snap-buckling as a speed boost for large traps would finally result in a hypothetical Dionaea-like trap. All necessary sensory and mechanical parts for such a snap-trap, including also the sessile glands, are present in Drosera tentacles. Especially the T3-tentacles of D. glanduligera appear as adequate precursor structures of snap-traps, due to their bending speed and sensitivity to touch. Nonetheless, owing to its derived position (in comparison to basal D. regia and D. arcturi) as a member of a clade within Drosera s.str,8,9 this species more likely possesses a further, unique trap adaptation apart from the snap-trap split-off. The same holds true for Drosera species which possess trap leaves that resemble Dionaea leaves (e.g., D. falconeri), which are also nested within the Drosera clade and therefore do not represent promising candidates as closest living relatives to the snap-trap clade that diverged much earlier.5 However, traditionally Drosera tentacles are being considered as homologous to Aldrovanda and Dionaea trigger hairs and/or snap-trap-teeth. Hence, from an evolutionary point of view, conceivable evolutionary transitions from a flypaper trap to a snap-trap include tentacle modifications into these structures.1,11,25 Leaf blade modifications required for snap-trap formation are a selective loss of tentacles and the ability to temporarily form a digestive cavity.1,11 Recently, it was shown for D. capensis that such leaf bending and formation of an “outer stomach” is triggered by an accumulation of endogenous jasmonates and is likely to represent a chemonastic response36 in contrast to the thigmonastic trap closure in Dionaea where these hormone signals apparently play no role.37 It is up to future studies to elucidate why the leaf bending feature does not exist in other Drosera species like D. glanduligera or long-leaved D. binata.
It is particularly interesting that such a high diversity of trap configurations and (probably) adaptations to different prey situations as described have evolved in Drosera. There are e.g., rosette species that predominantly capture walking prey, erect and climbing species with traps for catching flying prey, species with long leaves capable of bending motions for retaining large prey, species with peltate leaves that do not move, different tentacle types and many more. Trap configurations may even switch during the different developmental stages of a species. Future investigations on trap functioning, prey spectra, plant development and intrafamiliar phylogeny will hopefully shed more light on the trap diversity and evolution in the family Droseraceae.
Acknowledgments
We are grateful to Dr Frantisek Baluska for kindly inviting this review. We would like to thank the two anonymous reviewers for their constructive and helpful comments and Alex Chepstow-Lusty for his linguistic correction of the paper. S.P., T.M. and T.S. gratefully acknowledge the financial support by the funding directive BIONA of the German Federal Ministry of Education and Research (BMBF).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24685
References
- 1.Juniper BE, Robins RJ, Joel DM. The carnivorous plants: Academic; 1989. [Google Scholar]
- 2.Król E, Plachno BJ, Adamec L, Stolarz M, Dziubinska H, Trebacz K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann Bot (Lond) 2012;109:47–64. doi: 10.1093/aob/mcr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd FE. The carnivorous plants. Chron Bot. 1942 [Google Scholar]
- 4.Cross A. Aldrovanda The Waterwheel Plant. Redfern Natural History Productions; 2012. [Google Scholar]
- 5.Bailey T, McPherson S. Dionaea The Venus's Flytrap. Redfern Natural History Productions; 2012. [Google Scholar]
- 6.Williams SE, Albert VA, Case MW. Relationships of Droseraceae: a cladistic analysis of rbcL sequence and morphological data. Am J Bot. 1994;81:1027–37. doi: 10.2307/2445297. [DOI] [Google Scholar]
- 7.Cameron KM, Wurdack KJ, Jobson RW. Molecular evidence for the common origin of snap-traps among carnivorous plants. Am J Bot. 2002;89:1503–9. doi: 10.3732/ajb.89.9.1503. [DOI] [PubMed] [Google Scholar]
- 8.Rivadavia F, Kondo K, Kato M, Hasebe M. Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA Sequences. Am J Bot. 2003;90:123–30. doi: 10.3732/ajb.90.1.123. [DOI] [PubMed] [Google Scholar]
- 9.Rivadavia F, de Miranda VFO, Hoogenstrijd G, Pinheiro F, Heubl G, Fleischmann A. Is Drosera meristocaulis a pygmy sundew? Evidence of a long-distance dispersal between Western Australia and northern South America. Ann Bot. 2012;110:11–21. doi: 10.1093/aob/mcs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heubl G, Bringmann G, Meimberg H. Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales—revisited. Plant Biol (Stuttg) 2006;8:821–30. doi: 10.1055/s-2006-924460. [DOI] [PubMed] [Google Scholar]
- 11.Gibson TC, Waller DM. Evolving Darwin’s ‘most wonderful’ plant: ecological steps to a snap-trap. New Phytol. 2009;183:575–87. doi: 10.1111/j.1469-8137.2009.02935.x. [DOI] [PubMed] [Google Scholar]
- 12.Akeret B. Ein neuer Fundort von Aldrovanda vesiculosa L. in der Nordschweiz und einige Bemerkungen zu Stratiotes aloides L. Bot Helv. 1993;103:193–9. [Google Scholar]
- 13.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–5. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 14.Poppinga S, Joyeux M. Different mechanics of snap-trapping in the two closely related carnivorous plants Dionaea muscipula and Aldrovanda vesiculosa. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;84:041928. doi: 10.1103/PhysRevE.84.041928. [DOI] [PubMed] [Google Scholar]
- 15.Hodick D, Sievers A. On the mechanism of trap closure of Venus flytrap (Dionaea muscipula Ellis) Planta. 1989;179:32–42. doi: 10.1007/BF00395768. [DOI] [PubMed] [Google Scholar]
- 16.Fagerberg WR, Allain D. A quantitative study of tissue dynamics during closure in the traps of Venus's Flytrap Dionaea muscipula Ellis. Am J Bot. 1991;78:647–57. doi: 10.2307/2445086. [DOI] [Google Scholar]
- 17.Colombani M, Forterre Y. Biomechanics of rapid movements in plants: poroelastic measurements at the cell scale. Comput Method Biomec. 2011;14:115–7. doi: 10.1080/10255842.2011.593757. [DOI] [Google Scholar]
- 18.Darwin C. Insectivorous plants. John Murray; 1875. [Google Scholar]
- 19.Hutchens JJ, Luken JO. Prey capture in the Venus flytrap: collection or selection? Botany. 2009;87:1007–10. doi: 10.1139/B09-064. [DOI] [Google Scholar]
- 20.Ashida J. Studies on the leaf movement of Aldrovanda vesiculosa L. I. Process and mechanism of the movement. Mem Coll Sci Kyoto Imp Univ Ser. 1934;B9:141–244. [Google Scholar]
- 21.Skotheim JM, Mahadevan L. Physical limits and design principles for plant and fungal movements. Science. 2005;308:1308–10. doi: 10.1126/science.1107976. [DOI] [PubMed] [Google Scholar]
- 22.Schleicher S, Lienhard J, Poppinga S, Masselter T, Speck T, Knippers J. Bio-inspired kinematics of adaptive shading systems for free form facades. Proceedings of the IABSE-IASS Symposium,Taller Longer Lighter, London, UK 2011; 9 [Google Scholar]
- 23.Adamec L. The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquat Bot. 2008;89:66–70. doi: 10.1016/j.aquabot.2008.01.008. [DOI] [Google Scholar]
- 24.Seine R, Barthlott W. On the morphology of trichomes and tentacles of Droseraceae Salisb. Beitr Biol Pflanzen. 1993;67:345–66. [Google Scholar]
- 25.Williams SE. Comparative sensory physiology of the Droseraceae - The evolution of a plant sensory system. Proc Am Philos Soc. 1976;120:187–204. [Google Scholar]
- 26.McPherson S. Glistening carnivores. The sticky-leaved carnivorous plants. Redfern Natural History Productions; 2008. [Google Scholar]
- 27.Williams SE. Comparative physiology of the Droseraceae sensu stricto - How do tentacles bend and traps close? Proceedings of the 4th International Carnivorous Plant Conference, Tokyo, Japan 2002; 77-81. [Google Scholar]
- 28.Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165:373–89. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 29.Koller D. The restless plant: Harvard University Press; 2011. [Google Scholar]
- 30.Hartmeyer I, Hartmeyer SRH. Clandestine diversity: snap-tentacles of the genus Drosera. Carniflora Australis. 2006;7:4–18. [Google Scholar]
- 31.Hartmeyer I, Hartmeyer SRH. Snap-tentacles and runway lights. Carnivorous Plant Newsletter. 2010;39:101–13. [Google Scholar]
- 32.Watson AP, Matthiessen JN, Springett BP. Arthropod associates and macronutrient status of the red-ink sundew (Drosera erythrorhiza Lindl.) Aust J Ecol. 1982;7:13–22. doi: 10.1111/j.1442-9993.1982.tb01296.x. [DOI] [Google Scholar]
- 33.Verbeek NAM, Boasson R. Relationship between types of prey captured and growth form in Drosera in southwestern Australia. Aust J Ecol. 1993;18:203–7. doi: 10.1111/j.1442-9993.1993.tb00444.x. [DOI] [Google Scholar]
- 34.Poppinga S, Hartmeyer SRH, Seidel R, Masselter T, Hartmeyer I, Speck T. Catapulting tentacles in a sticky carnivorous plant. PLoS ONE. 2012;7:e45735. doi: 10.1371/journal.pone.0045735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartmeyer I, Hartmeyer SRH, Masselter T, Seidel R, Speck T, Poppinga S. Catapults into a deadly trap: The unique prey-capture mechanism of Drosera glanduligera. Carnivorous Plant Newsletter. 2013;42:4–14. [Google Scholar]
- 36.Nakamura Y, Reichelt M, Mayer VE, Mithöfer A. Jasmonates trigger prey-induced formation of ‘outer stomach’ in carnivorous sundew plants. Proc Biol Sci. 2013;280:20130228. doi: 10.1098/rspb.2013.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escalante-Pérez M, Krol E, Stange A, Geiger D, Al-Rasheid KA, Hause B, et al. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proc Natl Acad Sci USA. 2011;108:15492–7. doi: 10.1073/pnas.1112535108. [DOI] [PMC free article] [PubMed] [Google Scholar]


