Abstract
Purpose
The gain in quantification precision that can be expected in human brain 1H MRS at very high field remains a matter of debate. Here we investigate this issue using Monte-Carlo simulations.
Methods
Simulated human brain-like 1H spectra were fitted repeatedly with different noise realizations using LCModel at B0 ranging from 1.5T to 11.7T, assuming a linear increase in SNR with B0 in the time domain, and assuming a linear increase in linewidth with B0 based on experimental measurements. Average quantification precision (Cramér-Rao Lower Bound, CRLB) was then determined for each metabolite as a function of B0.
Results
For singlets, CRLB improved (decreased) by a factor ~ as B0 increased, as predicted by theory. For most J-coupled metabolites, CRLBs decreased by a factor ranging from to B0 as B0 increased, reflecting additional gains in quantification precision compared to singlets due to simplification of spectral pattern and reduced overlap.
Conclusion
Quantification precision of 1H MRS in human brain continues to improve with B0 up to 11.7T, even though peak SNR in the frequency domain levels off above 3T. In most cases, the gain in quantification precision is higher for J-coupled metabolites than for singlets.
Keywords: 1H magnetic resonance spectroscopy, Human brain, Monte-Carlo simulations, Quantification precision, Cramér-Rao Lower Bounds
INTRODUCTION
Proton magnetic resonance spectroscopy (1H MRS) allows non-invasive measurement of the concentration of multiple metabolites in the brain in vivo. In principle, high magnetic fields are beneficial for 1H MRS due to increased signal-to-noise ratio (SNR), increased spectral dispersion and simplification of J-coupled spectral patterns. However, these benefits are mitigated by other factors, such as increased RF power and B0 shimming requirements. In addition, even with perfect B0 shimming, the spectral linewidth gets broader at high fields due to shorter T2 relaxation times and increased B0 microsusceptibility effects (1,2). As a result, even with a very short echo-time (TE) sequence (so that signal loss from T2 relaxation is minimized), the actual gain in sensitivity and quantification precision of 1H MRS in human brain at ultra high-field (7T and beyond) is still a matter of debate.
Multiple studies have documented experimental gains in SNR in human brain 1H MRS with high fields. However the reported gains vary significantly from study to study. Peak SNR in the frequency domain (SNRfreq, defined as peak height divided by RMS noise) was shown to increase 23% to 46% at 3T relative to 1.5T (3–5) while another study reported no improvement (6). Another study found a ~80% increase in SNRfreq at 4T compared to 1.5T (7). Furthermore a 1:1 linear increase in SNRfreq of singlet resonances with B0 was reported from 1.5T to 7T (8). More recently, a 1.7-fold increase in SNRfreq from 3T to 7T (9), and a 1.91-fold increase from 4T to 7T (10) were also reported. Therefore, there is significant variability in the actual sensitivity gains observed at high field. Such variability can be explained in part by the difficulty to match experimental conditions (design and construction of RF coils, B1 spatial distribution, etc) between different systems at different B0 field strengths.
Another possible approach to assess potential gains in quantification precision at high field is using simulations. Previous simulations in rodent brain suggested that SNRfreq is expected to flatten out as B0 increases, with little increase (< 10%) in SNRfreq of singlet resonances (NAA, Cr) above 10T (11). However, further gains in quantification can reasonably be expected for J-coupled metabolites due to simplification of spectral patterns and reduced overlap at high field (11). Therefore, the improvement in quantification precision for every individual metabolite as B0 increases remains an open question.
In this context, the goal of the present study was to determine the relationship between quantification precision (estimated by Cramér-Rao Lower Bounds or CRLBs) and B0 for every brain metabolite present in in vivo 1H MR spectra. To achieve this goal, we performed Monte-Carlo simulations together with LCModel and simulated ‘brain-like’ spectra at multiple B0 field strengths.
MATERIAL AND METHODS
Simulations were performed with two main assumptions: (a) the in vivo linewidth increases linearly with B0 above 1.5T as measured experimentally (1) and (b) the SNR in the time domain (SNRtime) increases linearly with B0 (12,13). ‘Brain-like’ 1H NMR spectra were simulated in Matlab (14), assuming no J-evolution (pulse-acquire sequence analogous to an ultrashort TE sequence). Nineteen metabolites were included with concentrations similar to those found in human occipital cortex: 0.5 mM alanine (Ala), 1 mM ascorbate (Asc), 2 mM aspartate (Asp), 4 mM creatine (Cr), 1 mM γ-aminobutyric acid (GABA), 1 mM glucose (Glc), 10 mM glutamate (Glu), 2.5 mM glutamine (Gln), 0.5 mM glycerophosphorylcholine (GPC), 1 mM glutathione (GSH), 0.5 mM lactate (Lac), 6 mM myo-inositol (Ins), 12 mM N-acetylaspartate (NAA), 1 mM N-acetylaspartylglutamate (NAAG), 4 mM phosphocreatine (PCr), 0.5 mM phosphorylcholine (PCho), 1.5 mM phosphorylethanolamine (PE), 0.25 mM scyllo-inositol (sIns) and 1.5 mM taurine (Tau). Macromolecules were also simulated and added to the spectrum (see Supplemental Methods for details).
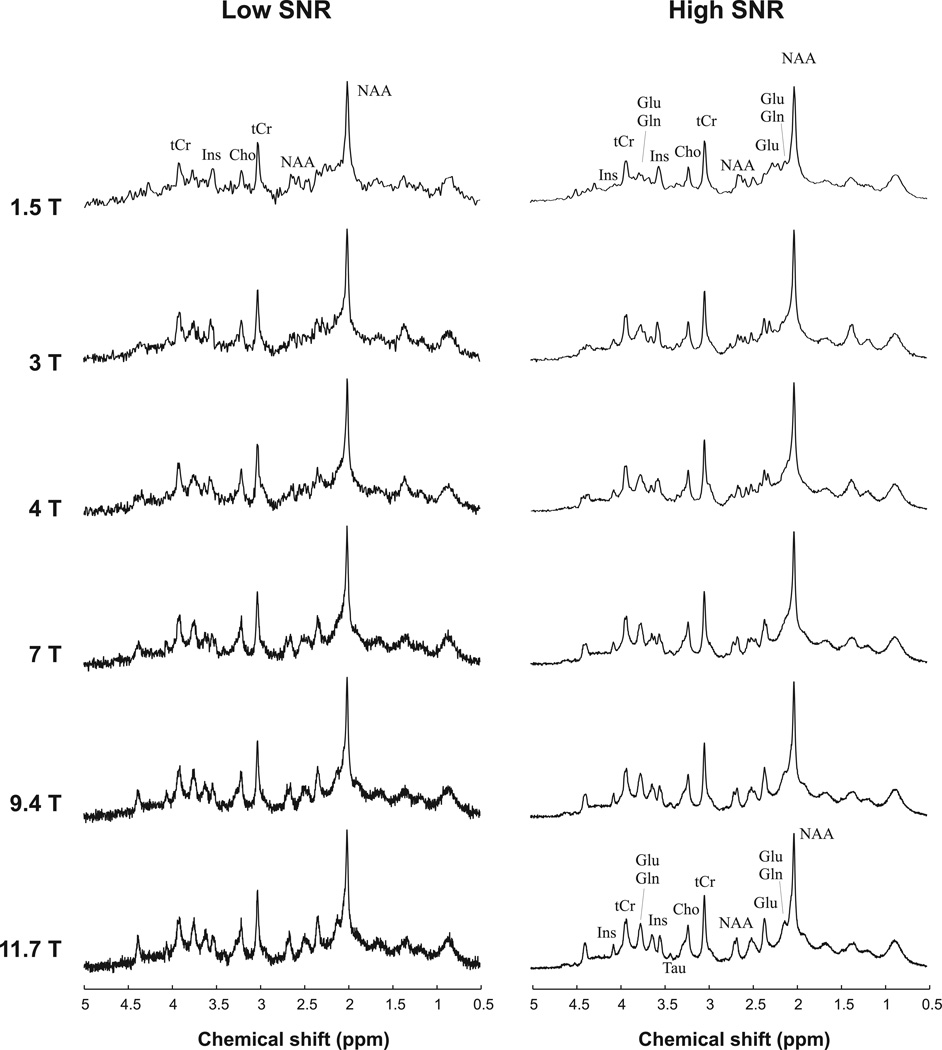
Brain-like spectra were then generated at 6 different magnetic field strengths: 1.5, 3, 4, 7, 9.4 and 11.7T with a spectral linewidth of 2.1, 3.7, 4.9, 8.5, 11.2 and 14.0 Hz respectively. These values were obtained by measuring the linewidth of the tCr peak at 3.03 ppm at 4T, 7T and 9.4T, and subtracting the Cr-PCr chemical shift difference (0.0033 ppm or 0.14 Hz/T). Values at 1.5T, 3T and 11.7T were obtained by linear extrapolation of the fit from 4T to 9.4T. The slope was 1.31 Hz/T and 1.17 Hz/T before and after subtraction of the Cr-PCr chemical shift difference respectively. All metabolites were simulated with the same linewidth, neglecting small differences in linewidth due to different T2 relaxation times. The resulting simulated spectra (Figure 1) were very similar in appearance to those obtained experimentally in the human brain (1,3,8–10,15).
Figure 1.
Examples of ‘brain-like’ simulated 1H NMR spectra with low SNR (left) and high SNR (right) from 1.5T (top) to 11.7T (bottom) using the linewidth values and the concentrations of metabolites given in the text. Fore each condition (low SNR or high SNR), spectra are scaled so that the RMS noise is identical at each magnetic field.
For Monte-Carlo simulations, Gaussian noise was added to the simulated 1H NMR spectra, with a noise level inversely proportional to B0. The resulting spectra were fitted with LCModel version 6.1-4A (16). This procedure was repeated 50 times with 50 different random noise realizations (sufficient for proper convergence), yielding 50 CRLB values for each metabolite at each B0. The quantification precision for each metabolite at a given B0 was calculated as the average of these 50 CRLB values.
The SNR of the simulated spectra was chosen in order to obtain accurate estimates of the CRLBs at all magnetic fields. If SNR is too low, many low concentration metabolites cannot be reliably fitted. If SNR is too high, estimates of CRLBs for high concentration metabolites are very small (< 2%) and are not precise enough because of rounding in LCModel. Therefore, two simulations were performed. One simulation was performed with ‘low’ SNR (SNR of NAA peak measured in the frequency domain was 25±1 at 4T) to estimate CRLBs for Cr, PCr, Glu, NAA and Ins as well as the sums tCho = PCho+GPC, tCr = Cr+PCr and Glx = Glu+Gln. A second simulation was performed with ‘high’ SNR (SNR of NAA peak in the frequency domain was 100±4 at 4T) to determine CRLBs for all other metabolites.
T1 effects were neglected because T1 relaxation times show only modest increases with B0 (1). For example, assuming a TR of 5s and an increase in the T1 of tCr-CH3 from 1.39s at 1.5T to 1.75s at 9.4T (1) would result in a negligible 3% loss in SNR at 9.4T compared to 1.5T due to T1 saturation.
RESULTS
SNRfreq becomes nearly constant as the B0 field increases
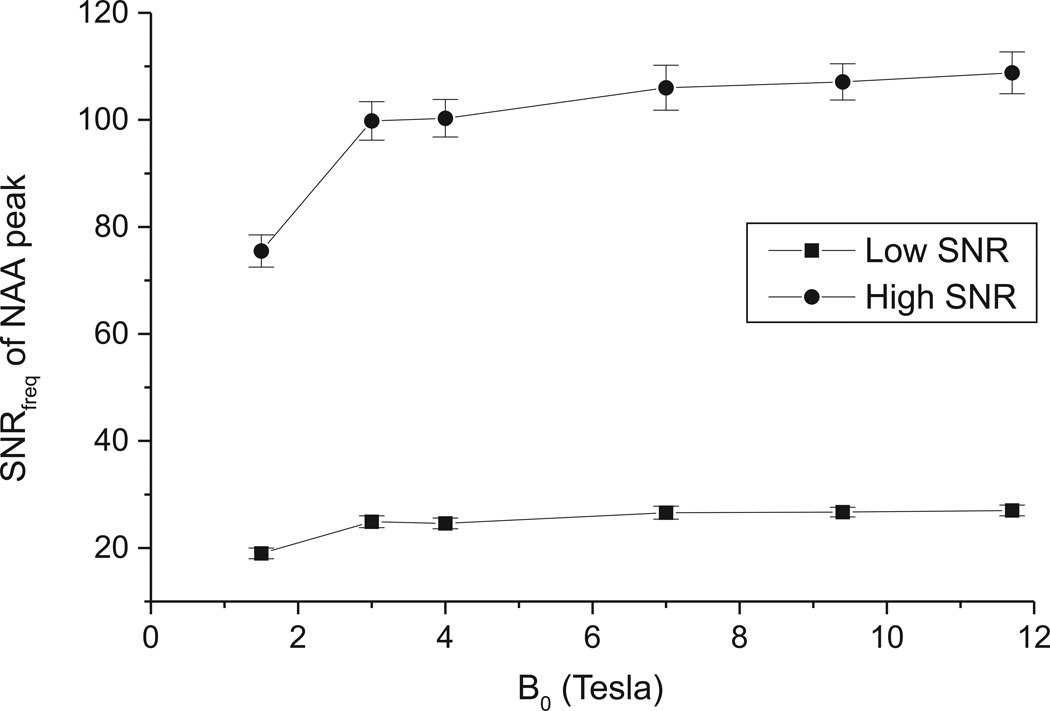
In our simulated 1H NMR spectra, both the linewidth (in Hz) and the SNR in the time domain were assumed to increase linearly with B0. With those two assumptions, SNRfreq of singlets, defined as peak height relative to RMS noise, was found to level off and showed only modest increase above 3–4T (Figure 2). This is explained by the fact that the increase in SNRtime is offset by the increase in linewidth. Quantitatively, SNRfreq is proportional to SNRtime/ΔνHz, where ΔνHz is the spectral linewidth in Hz. With our assumptions: SNRtime=C·B0 and ΔνHz=D·B0 + E where C, D and E are constants. It follows that SNRfreq is proportional to C·B0/(D·B0+E). When B0 increases, then D·B0>>E and SNRfreq becomes nearly constant. For example using experimental values for the tCr linewidth at 3.03 ppm, the linewidth of tCr in the human brain (including the small chemical shift difference between Cr and PCr) can be expressed as ΔνHz = 1.31B0 + 0.27 (1) or in ppm, Δνppm = ΔνHz/γB0 = 0.031 + 0.006/B0. For B0>3T, the second term becomes negligible (< 5%) resulting in a constant linewidth (in ppm) of ~0.031 ppm as well as constant SNRfreq. The above formula is valid for tCr, and the constants would be slightly different for other singlets, but NAA shows a similar behavior, with a plateau in SNRfreq above 3–4T (Figure 2).
Figure 2.
SNRfreq as a function of B0 measured as NAA peak intensity relative to RMS noise using both the low and high SNR conditions. Error bars represent SD.
Quantification precision continues to increase with B0 at very high field
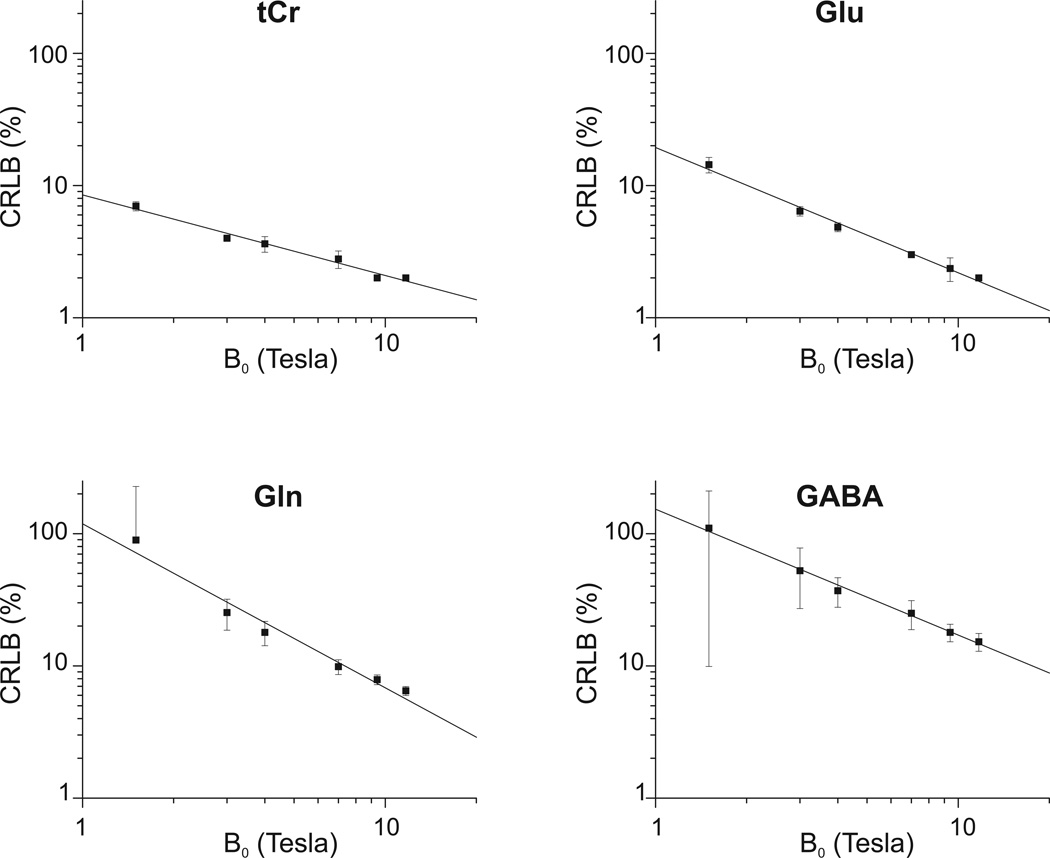
In spite of the fact that SNRfreq become nearly constant at very high field, simulations show that the quantification precision (estimated by CRLBs) continues to increase substantially for all metabolites as B0 increases. Figure 3 shows examples of this improvement for tCr, Glu, Gln and GABA.
Figure 3.
Mean CRLBs for selected metabolites (tCr, Glu, Gln and GABA) obtained from Monte-Carlo simulations. The data are plotted in logarithmic scale. Solid lines represent the best fits. Error bars represent SD of 50 CRLB values obtained from Monte-Carlo simulations. Note that values for tCr and Glu were obtained with the “low SNR” condition and values for Gln and GABA with the “high SNR” condition.
To quantify the improvement of CRLBs as a function of B0, these plots were fitted with a function in the form CRLB ∝ B0α or, in logarithm coordinates, with a linear function log(CRLB) = α·log(B0)+C, with α and C as free parameters. Results show a close linear relationship between log(CRLB) and log(B0) for all metabolites (R2 ranging from 0.96 to 1 for all metabolites, except R2=0.88 for glucose and R2=0.93 for sIns). The fitted slope ranged from α=−0.42 (for glucose) to α=−1.05 (for glutamine) (Table 1). A slope value of −1.0 corresponds to CRLB ∝ 1/B0, whereas −0.5 corresponds to .
Table 1.
Slope (α) and R2 correlation coefficient obtained after fitting the CRLBs as a function of B0 using a power regression function i.e. σ·B0α (equivalent to a linear fit in logarithmic coordinates).
| Spin systems | Metabolites | Slope, α | R2 |
|---|---|---|---|
| Mostly singlet | NAA† | −0.67 | 0.94 |
| Creatine† | −0.62 | 0.96 | |
| Phosphocreatine† | −0.57 | 0.96 | |
| Scyllo-inositol | −0.56 | 0.93 | |
| Total choline† | −0.51 | 1.00 | |
| Total creatine† | −0.61 | 0.98 | |
| J-coupled | Alanine | −0.75 | 0.98 |
| Ascorbate | −0.67 | 0.98 | |
| Aspartate | −0.44 | 0.96 | |
| Glucose | −0.42 | 0.88 | |
| GABA | −0.97 | 1.00 | |
| Glutamate† | −0.95 | 0.99 | |
| Glutamine | −1.05 | 0.99 | |
| Glutathione | −0.87 | 0.97 | |
| Glycerophosphorylcholine | −0.92 | 0.99 | |
| Lactate | −0.72 | 0.98 | |
| Myo-Inositol† | −0.52 | 0.96 | |
| NAAG | −0.70 | 1.00 | |
| Phosphethanolamine | −0.87 | 0.99 | |
| Phosphocholine | −0.80 | 1.00 | |
| Taurine | −0.78 | 1.00 | |
| Glutamate + glutamine† | −0.86 | 0.99 |
The † sign indicates values obtained using the “low SNR” condition. The SD for the slope was < 0.1 for all metabolites.
These findings are supported by theory. Indeed, theoretical calculations of CRLB for a singlet predict that, if SNRtime and ΔνHz both increase linearly with B0 (i.e constant SNRfreq) then the CRLB of the estimated concentration decreases as (17). Our simulation results show that, for metabolites appearing primarily as singlets in the 1H NMR spectrum, the slope α was close to −0.5 (−0.61 for tCr, −0.51 for tCho and −0.56 for sIns), which is in good agreement with theoretical predictions.
For other metabolites that appear as multiplets with complex J-coupled spectral patterns, such theoretical calculations are generally not possible. However, the increase in quantification precision was higher than for singlets in most cases, with the slope α generally comprised between −0.5 and −1. Metabolites that showed the highest increase in quantification precision with B0 were glutamine (α=−1.05), glutamate (α=−0.95) and GABA (α=−0.97). This reflects the simplification of spectral pattern and reduced overlap of these metabolites at high field, whereas they overlap strongly at lower field (Figure 1), Similarly, reduced overlap between the J-coupled resonances of GPC and PCho at high field contributed to higher α values for these two metabolites (α=−0.92 for GPC and α=−0.80 for PCho). However, we found exceptions to this trend: for Glc (α=−0.42), Asp (α=−0.44), or Ins (α=−0.52), the decrease in CRLBs with B0 was more modest. Therefore, the actual improvement in quantification precision with B0 is strongly dependent on the spectral pattern of each metabolite.
The number of metabolites that can be quantified reliably increases with B0
The improvement in quantification precision with B0 resulted in a steady increase in the number of metabolites that could be quantified reliably, using CRLB < 25% as a threshold (Table 2). With the ‘low SNR’ simulation condition, which is typical of in vivo MRS in human brain, five metabolites could be quantified reliably with CRLB < 25% (tCr, tCho, NAA, Glx and Ins) at 1.5T, six metabolites (Cr, PCr, tCho, NAA, Glx and Ins) at 3T and up to sixteen metabolites at 11.7T (Table 2). The remaining three metabolites (Asc, Glc and sIns) had CRLBs around 30% at 11.7T. Although this is only valid for those particular SNR conditions, it illustrates how gains in quantification precision translate into increased neurochemical information content.
Table 2.
List of metabolites that can be quantified reliably with CRLB < 25% at different B0 using the ‘low SNR’ simulation condition. While this result is valid only for this particular SNR condition, it illustrates how gains in quantification precision translate into a higher number of metabolites that can be quantified reliably.
| Magnetic Field (T) |
1.5 | 3 | 4 | 7 | 9.4 | 11.7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of metabolites |
5 | 6 | 8 | 12 | 14 | 16 | ||||||
| Metabolite | CRLB (%) |
Metabolite | CRLB (%) |
Metabolite | CRLB (%) |
Metabolite | CRLB (%) |
Metabolite | CRLB (%) |
Metabolite | CRLB (%) |
|
| NAA | 8 | NAA | 4 | NAA | 3 | NAA | 2 | NAA | 2 | NAA | 2 | |
| tCho | 18 | tCho | 12 | tCho | 11 | tCho | 8 | tCho | 7 | tCho† | 6 | |
| Ins | 10 | Ins | 6 | Ins | 6 | Ins | 4 | Ins | 4 | Ins | 3 | |
| Glx | 13 | Glx | 7 | Glx† | 5 | Glx† | 3 | Glx† | 3 | Glx† | 2 | |
| tCr | 7 | tCr† | 4 | tCr† | 4 | tCr† | 3 | tCr† | 2 | tCr† | 2 | |
| Cr | 13 | Cr | 12 | Cr | 9 | Cr | 7 | Cr | 7 | |||
| PCr | 13 | PCr | 12 | PCr | 10 | PCr | 8 | PCr | 7 | |||
| Glu | 5 | Glu | 3 | Glu | 2 | Glu | 2 | |||||
| Gln | 18 | Gln | 10 | Gln | 8 | Gln | 6 | |||||
| GSH | 24 | GSH | 16 | GSH | 13 | GSH | 12 | |||||
| NAAG | 17 | NAAG | 14 | NAAG | 12 | |||||||
| PE | 19 | PE | 15 | PE | 13 | |||||||
| GPC | 23 | GPC | 21 | GPC | 18 | |||||||
| Tau | 24 | Tau | 17 | Tau | 14 | |||||||
| GABA | 18 | GABA | 15 | |||||||||
| Asp | 22 | Asp | 19 | |||||||||
| Lac | 22 | |||||||||||
| PCho | 23 | |||||||||||
| Ala | 24 | |||||||||||
Note that metabolites representing the sum of two other metabolites were not counted when their individual components could be quantified separately For instance, both Glu and Gln were reliably quantified at 4T such that Glx was not counted. Those metabolites are marked with a † sign.
DISCUSSION
Comparison between human brain and rat brain
In human brain, we show here that both peak SNR in the frequency domain (SNRfreq) and the linewidth expressed in ppm (Δνppm) are expected to become nearly constant above 3T. A similar prediction has been made in the rat brain, where the increase in linewidth (in ppm) was shown to become nearly constant at high field (11,18). According to de Graaf and colleagues (11), the linewidth of tCr in rat brain becomes nearly constant above 9.4T and converges to ~0.018 ppm (compared with ~0.031 ppm in human brain). Interestingly, the B0 threshold above which Δνppm and SNRfreq level off is higher in rat brain (9.4T) than in human brain (3T). This is due to the longer T2 relaxation times of metabolites and smaller microscopic B0 inhomogeneity in rat brain compared to the human brain, resulting in smaller metabolites linewidth in the rat brain.
Relevance of assumptions used in simulations
Assumption on SNR
Simulations in the present study assumed a linear 1:1 increase in SNRtime with B0, based on the expected theoretical gain in intrinsic SNR (13). However, the actual gain in SNRtime with B0 is strongly dependent on experimental factors such as the performance of RF coils used or the spatial distribution of B1 field. Even if experimental conditions (RF chain, RF coil geometry, etc) are kept nearly identical, the distribution of the B1 field inevitably changes with B0, especially at high field. As such, any increase in SNRtime with B0 measured experimentally in human brain is valid only for a specific coil geometry and voxel location, even if all other experimental conditions are perfectly matched.
For example, a previous study reported a ~2-fold increase in SNRfreq at 7T versus 4T (10), in apparent contradiction with the results of the present simulation study, which predicts very little improvement in SNRfreq from 4T to 7T. However, a 2-fold increase in SNRfreq, together with the reported increase in tCr linewidth from 5.9Hz at 4T to 9.8Hz at 7T, would correspond to a 3.3-fold increase in SNRtime for tCr. This experimentally measured factor was much higher than the factor of 1.75 (i.e. 7/4) assumed in our simulations based on theoretical predictions (12,13). One possible explanation for the higher than expected gain in SNRtime measured experimentally is that different RF coils were used at 4T and 7T, with slightly different geometry and design, which could favor the 7T coil. Alternatively, even when using very similar surface coils at 4T and 7T, a larger portion of the available SNR may be concentrated close to the coil as B0 increases. Indeed, when comparing images at 4T and 7T in (10), SNR clearly drops more quickly at 7T than at 4T when going away from the coil.
While the assumption of a linear increase of SNRtime with B0 may not hold true in all particular cases, our simulations give useful insight into the gains in quantification precision that can be expected at high field for every metabolite. Simulations make it possible to have a consistent SNR hypothesis across all B0 values, and therefore identify general trends not easily identified from experimental data due to the difficulty to match experimental conditions at multiple B0.
In addition, since CRLBs are inversely proportional to SNRtime for a given B0 (17), results can easily be extrapolated using a different assumption for SNRtime = f(B0). For example, if SNRtime varied as B01.2 instead of B0, all α coefficients in Table 1 would be simply multiplied by 1.2.
Assumption on very short TE
Our simulations are valid for very short TE sequences for which the loss of signal from T2 relaxation during TE can be neglected. At high field, however, such losses from T2 relaxation may become significant. For similar experimental conditions, the minimum TE tends to increase at high field due to increased power requirements, and T2 relaxation times become shorter. This is particularly true for MRS sequences on clinical scanners, which are generally not optimized to minimize TE.
State-of-the-art research sites, in contrast, recognizing that minimizing TE is critical to harness gains in quantification precision at high-field, have used MRS methodology that keeps relatively short TE even at 7T and 9.4T (1,9,15). However, even in those studies, loss of signal due to T2 relaxation may not be completely negligible at high field.
Our results can be extended to take into account the additional loss of signal fromT2 relaxation by taking advantage of the fact that all CRLB values obtained in our simulations are inversely proportional to SNR at each B0 (17). Therefore, corrected CRLBs can be derived from our initial, uncorrected CRLB values as CRLBcorr=CLRBuncorr*SNRuncorr/SNRcorr with CRLBuncorr proportional to B0α and α coefficients given in Table 1. If SNRuncorr/SNRcorr is expressed empirically as B0β, then CRLBcorr is proportional to B0α+β and coefficients α in Table 1 simply need to be corrected by adding the term β.
Values of TE(min) and even T2 relaxation times depend on the specific sequence used. Therefore, we estimated the signal loss using approximate values of TE(min) and T2 relaxation times at each B0 for the two MRS sequences currently being used in our center: STEAM and semi-LASER (19).
For STEAM, coefficients α in Table 1 need to be corrected by adding a term β=0.06 (average of 0.05 for NAA and 0.07 for tCr) (See Supplemental Data for an example). For semi-LASER, coefficients α in Table 1 need to be corrected by a term β=0.2 (average of 0.18 for NAA and 0.21 for tCr).
Assumption on optimal shimming and spectral quality
The 1H NMR spectra used in the current study were simulated assuming perfect B0 shimming in the human occipital lobe in vivo. Shimming imperfections (i.e. B0 inhomogeneities that cannot be compensated for by 2nd order shim coils) increase linearly with B0, and these imperfections may be significant in areas of the brain other than the occipital lobe and/or in large voxels. Therefore the present study provides the maximum achievable improvement of quantification precision at high field for perfectly shimmed voxels.
It was also assumed that there was no baseline distortion and no lipid contamination due to imperfect outer volume suppression. Achieving such optimal conditions is not always possible experimentally, since spectral quality is strongly dependent on the pulse sequence and B0 shimming algorithm used, the brain region under investigation, and any movement from the subject.
CRLB as estimator of quantification precision
Finally, it should be kept in mind that CRLBs represent only a lower bound of the experimental error. Nonetheless, CRLBs are generally good estimators of the quantification precision of a given metabolite when spectral noise is the main contributor to experimental error, as is the case when SNR for this metabolite is relatively low. At higher SNR, intra-subject experimental error typically becomes dominated by other experimental factors such as movement.
CONCLUSION
In conclusion, the quantification precision of metabolites is expected to continue to improve in the human brain with field strength up to 11.7T, even though SNRfreq does not increase substantially above 3T. The improvement in quantification precision is dependent on the spectral pattern of each metabolite. In general, greater improvement in quantification precision is obtained for J-coupled metabolites than for singlets as B0 increases.
Supplementary Material
Acknowledgement
This work was supported by NIH grants P41RR008079, P41EB015894, P30NS057091, R01NS38672 and the W.M. Keck Foundation. The authors thank Dr. Gülin Öz and Dr. Malgorzata Marjanska for careful reading of the manuscript and helpful suggestions, as well as Dr. Pierre-Francois van de Moortele for insightful discussions.
REFERENCES
- 1.Deelchand DK, Moortele P-FVd, Adriany G, Iltis I, Andersen P, Strupp JP, Thomas Vaughan J, Ugurbil K, Henry P-G. In vivo 1H NMR spectroscopy of the human brain at 9.4 T: Initial results. J Magn Reson. 2010;206(1):74–80. doi: 10.1016/j.jmr.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruetter R, Weisdorf SA, Rajanayagan V, Terpstra M, Merkle H, Truwit CL, Garwood M, Nyberg SL, Ugurbil K. Resolution improvements in in vivo 1H NMR spectra with increased magnetic field strength. J Magn Reson. 1998;135(1):260–264. doi: 10.1006/jmre.1998.1542. [DOI] [PubMed] [Google Scholar]
- 3.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med. 2001;45(5):765–769. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 4.Gonen O, Gruber S, Li BS, Mlynarik V, Moser E. Multivoxel 3D proton spectroscopy in the brain at 1.5 versus 3.0 T: signal-to-noise ratio and resolution comparison. AJNR Am J Neuroradiol. 2001;22(9):1727–1731. [PMC free article] [PubMed] [Google Scholar]
- 5.Inglese M, Spindler M, Babb JS, Sunenshine P, Law M, Gonen O. Field, Coil, and Echo-Time Influence on Sensitivity and Reproducibility of Brain Proton MR Spectroscopy. AJNR Am J Neuroradiol. 2006;27(3):684–688. [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarci K, Reynolds G, Petersen RC, Boeve BF, Knopman DS, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Jack CR. Proton MR Spectroscopy in Mild Cognitive Impairment and Alzheimer Disease: Comparison of 1.5 and 3 T. AJNR Am J Neuroradiol. 2003;24(5):843–849. [PMC free article] [PubMed] [Google Scholar]
- 7.Bartha R, Drost DJ, Menon RS, Williamson PC. Comparison of the quantification precision of human short echo time 1H spectroscopy at 1.5 and 4.0 Tesla. Magn Reson Med. 2000;44(2):185–192. doi: 10.1002/1522-2594(200008)44:2<185::aid-mrm4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Otazo R, Mueller B, Ugurbil K, Wald L, Posse S. Signal-to-noise ratio and spectral linewidth improvements between 1.5 and 7 Tesla in proton echo-planar spectroscopic imaging. Magn Reson Med. 2006;56(6):1200–1210. doi: 10.1002/mrm.21067. [DOI] [PubMed] [Google Scholar]
- 9.Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61(6):1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 10.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62(4):868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Graaf R, Brown P, McIntyre S, Nixon T, Behar K, Rothman D. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn Reson Med. 2006;56(2):386–394. doi: 10.1002/mrm.20946. [DOI] [PubMed] [Google Scholar]
- 12.Redpath TW. Signal-to-noise ratio in MRI. Br J Radiol. 1998;71(847):704–707. doi: 10.1259/bjr.71.847.9771379. [DOI] [PubMed] [Google Scholar]
- 13.Hoult DI. Rotating frame zeugmatography. J Magn Reson. 1979;33:183–197. doi: 10.1002/mrm.1910010306. [DOI] [PubMed] [Google Scholar]
- 14.Henry PG, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn Reson Med. 2006;55(2):250–257. doi: 10.1002/mrm.20764. [DOI] [PubMed] [Google Scholar]
- 15.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 16.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 17.Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. Cramér-Rao bound expressions for parametric estimation of overlapping peaks: influence of prior knowledge. J Magn Reson. 2000;143(2):311–320. doi: 10.1006/jmre.1999.2002. [DOI] [PubMed] [Google Scholar]
- 18.Mlynarik V, Cudalbu C, Xin L, Gruetter R. 1H NMR spectroscopy of rat brain in vivo at 14.1Tesla: improvements in quantification of the neurochemical profile. J Magn Reson. 2008;194(2):163–168. doi: 10.1016/j.jmr.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Oz G, Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magnetic Resonance in Medicine. 2011;65(4):901–910. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.