Abstract
The NAD-synthesizing enzyme NMNAT2 is critical for axon survival in primary culture and its depletion may contribute to axon degeneration in a variety of neurodegenerative disorders. Here we discuss several recent reports from our laboratory that establish a critical role for NMNAT2 in axon growth in vivo in mice and shed light on the delivery and turnover of this survival factor in axons. In the absence of NMNAT2, axons fail to extend more than a short distance beyond the cell body during embryonic development, implying a requirement for NMNAT2 in axon maintenance even during development. Furthermore, we highlight findings regarding the bidirectional trafficking of NMNAT2 in axons on a vesicle population that undergoes fast axonal transport in primary culture neurites and in mouse sciatic nerve axons in vivo. Surprisingly, loss of vesicle association boosts the axon protective capacity of NMNAT2, an effect that is at least partially mediated by a longer protein half-life of cytosolic NMNAT2 variants. Analysis of wild-type and variant NMNAT2 in mouse sciatic nerves and Drosophila olfactory receptor neuron axons supports the existence of a similar mechanism in vivo, highlighting the potential for regulation of NMNAT2 stability and turnover as a mechanism to modulate axon degeneration in vivo.
Keywords: NMNAT2, Wallerian degeneration, axon growth, axon survival, axonal transport, neurodegeneration, palmitoylation, ubiquitin proteasome
Introduction
Axon pathology is a major contributor to nervous system dysfunction in many neurodegenerative diseases. Indeed, axonal dysfunction, and an impairment of axonal transport in particular, are often observed at very early stages of disease before the onset of symptoms and before widespread neurodegeneration. This suggests a potential causative role of axonal impairments for the resulting neurodegeneration.1-3 The spontaneous mutant WldS mouse, in which the degeneration of an axon distal to a site of injury (Wallerian degeneration) is delayed significantly, has helped establish the concept that axon degeneration in many neurodegenerative conditions is mechanistically related to Wallerian degeneration.4 The WldS gene is a chimera that arises from a triplication on mouse chromosome four5 and consists of the coding sequence for the N-terminal 70 amino acids of the ubiquitin conjugation factor Ube4b fused, via an 18 amino acid linker, to the full coding region of Nmnat1 (Nicotinamide mononucleotide adenylyltransferase 1).6 Expression of this chimeric protein in mice,6 rats,7 zebrafish,8,9 Drosophila10,11 or in human dorsal root ganglion neurons in primary cutlture12 is sufficient for the WLDS phenotype of delayed Wallerian degeneration.
Even though initially only detected in the nucleus,13 recent results show that the WLDS protein is also present in axons in vivo.14 Moreover, its exclusion from the nucleus, and the resulting stronger accumulation in the cytoplasm, increases its protective properties, suggesting a non-nuclear site of action.14 Recent work in our laboratory established NMNAT2, which shares its NAD-synthesizing enzymatic activity with the WLDS fusion protein, as a critical survival factor that prevents spontaneous degeneration in primary culture neurites. Its constant supply from the cell body into axons is required for axon survival and its rapid depletion after a block of axonal transport appears to be sufficient to trigger Wallerian degeneration.15 Due to its very short half-life, any disruption in NMNAT2 axonal transport could result in spontaneous axon degeneration. Indeed, the ability of WLDS to protect against axon degeneration in several neurodegenerative disease models suggests that a relative lack of NMNAT2 supply could contribute to axon degeneration in these conditions, though the role of NMNAT2 in disease remains to be tested (see Fig. 1).15 Based on these findings, it is important to understand the axonal trafficking of the endogenous survival factor NMNAT2, its role in axon maintenance in vivo, as well as the factors that determine its turnover and axon protective capacity.
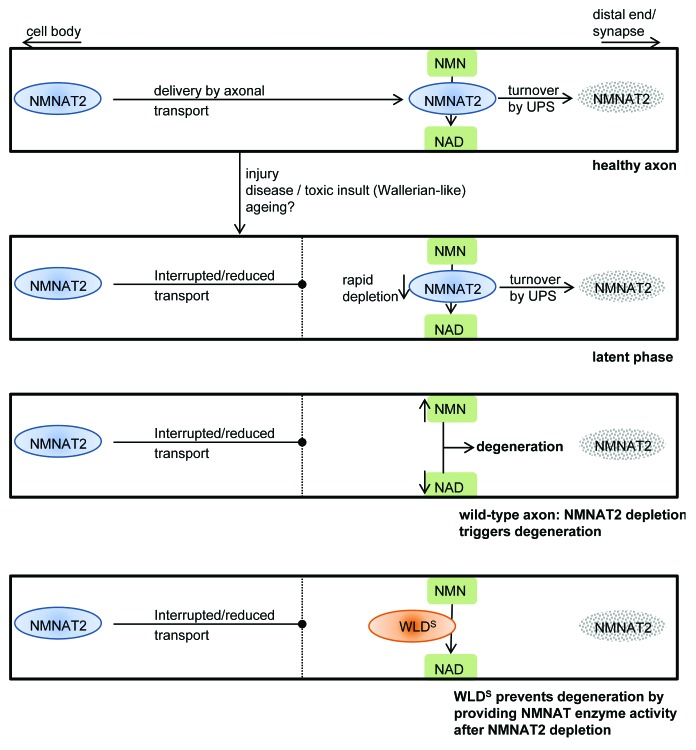
Figure 1. Model of NMNAT2 depletion as a trigger of Wallerian degeneration and axon protection by WLDS. In a healthy axon, NMNAT2 is rapidly turned over by the ubiquitin proteasome system (UPS). In order to carry out its enzymatic function, the conversion of NMN to NAD, it is constantly replenished from the cell body by axonal transport. Axonal transport can be blocked or reduced through injury of the axon, through disease processes that affect the efficiency of transport or during normal aging. Due to its short half-life, NMNAT2 is rapidly depleted distally to a site of injury or block of axonal transport. The resulting lack of the critical NMNAT enzyme activity triggers a downstream signaling cascade culminating in Wallerian degeneration. As WLDS has a much longer half-life than NMNAT2, and as these two proteins share the same enzymatic activity, WLDS, if present in the axon, can substitute the critical enzyme activity after depletion of NMNAT2, resulting in a significantly prolonged latent phase of degeneration.
Role of NMNAT2 for Axon Maintenance in Vivo
In order to confirm the importance of NMNAT2 for axon survival in vivo, we generated conditional Nmnat2 gene trap mice, similar to the NMNAT2-deficient Blad mutant mice recently described.16 Homozygous Nmnat2gtE/gtE mice lacked any detectable NMNAT2 expression. Consistent with a requirement for NMNAT2 for axon survival in vivo, axons in both peripheral and central nervous systems were truncated at short distances beyond the cell body.17 While analysis of the Blad mutant mice led to the suggestion of a degenerative axon defect in the absence of NMNAT2, our data indicate an early developmental defect in axon extension instead. The inability to detect degenerated fragments of distal axons, together with repeated imaging of primary culture neurite outgrowth, suggest that these axons never extended more than a few millimeters beyond the cell body.17 This indicates that NMNAT2 is required during development and axons fail to grow normally in its absence. The short axon stumps that were supported in this condition were most likely maintained by the presence of NMNAT1, whose enzymatic activity is localized within the nucleus. Exchange of NAD and related metabolites between the proximal axon and cell body through simple diffusion could thus support the limited axon extension found in the absence of NMNAT2. In further support of an NMNAT-dependent axon maintenance model, gross morphological defects as well as the truncation of peripheral and central nervous system axons in Nmnat2gtE/gtE mice were rescued by expression WLDS in a dose-dependent manner, with WLDS homozygotes surviving even into adulthood.17 This confirms the ability of WLDS to directly substitute for NMNAT2 in both axon growth and maintenance in vivo.
The above findings imply a novel developmental role for NMNAT2. However, it is conceivable that the mechanism that limits axon outgrowth in the absence of NMNAT2 is closely related to the axon degeneration pathway triggered by depletion of NMNAT2 after axotomy or disruption of axonal transport. If NMNAT2 levels are critical for axon maintenance from the outset, axons extending beyond a threshold distance where NMNAT2 levels become limiting for axon survival may degenerate at their distal extremities.
Given the short half-life and critical role of NMNAT2 in axon maintenance, any reduction in NMNAT2 supply could put axons at risk of degeneration.15 The well-documented reduction in axonal transport during aging18 and disease-associated disruptions to axonal transport2 could thus synergize to deplete NMNAT2 in distal axons sufficiently to induce axon degeneration. In agreement with such a model, loss of NMNAT2 expression from one allele (resulting in a maximum 50% decrease in protein expression) was sufficient to deplete NMNAT2 below its critical threshold and induce spontaneous axon degeneration.17
Interestingly, however, our data also indicate that axons can undergo compensatory changes that allow them to grow and survive in the presence of otherwise sub-threshold levels of NMNAT2. Compound heterozygotes carrying two independent NMNAT2 knockdown alleles express approximately 25% of wild-type levels of NMNAT2, well below the 50% level at which we observed spontaneous degeneration after loss of expression from one allele. Interestingly, however, these compound heterozygous mice are overtly normal, fertile and live until at least 12 months of age.17 This surprising result indicates that downstream elements of the axon degeneration pathway can, to some degree, adapt to lower levels of NMNAT2 and maintain axon integrity in a situation where such low levels of NMNAT2 are present from early development onwards. Identification of the mechanism(s) responsible for these compensatory changes could open up novel avenues to delay axon degeneration when NMNAT2 supply is limited through impairments of axonal transport. In particular, it will be important to determine if these changes can be induced in mature axons to allow axon survival at what would otherwise be sub-threshold NMNAT2 levels.
Axonal Trafficking and Turnover of NMNAT2
Previously, NMNAT2 was shown to associate with the Golgi apparatus in HeLa cells and this association depends on an intact C164/C165 dual-cysteine palmitoylation site in its central ISTID (isoform specific targeting and interaction) domain.19,20 Additionally, NMNAT2 was seen to traffic bidirectionally along primary culture neurites on particles and at speeds compatible with fast axonal transport.15 In one recent report, we confirmed the vesicular nature of this transport organelle by showing co-migration of NMNAT2 with Golgi and synaptic vesicle markers, suggesting a Golgi-derived vesicle population as the mode of entry for NMNAT2 into axons. Additionally, we confirmed that the C164/165 palmitoylation site was required for vesicular axonal transport of NMNAT2.21 Based on the known short half-life of NMNAT2 and its critical role in axon maintenance, we envisaged a model in which a vesicle-bound population of NMNAT2 would be shielded from turnover during transport, as it takes many hours or even days to reach the distal ends of long axons. Interestingly, we found the opposite to be the case. Cytosolic NMNAT2 mutants lacking palmitoylation and vesicle association had a longer protein half-life than the vesicle-bound, wild-type form.21 Similarly, fluorescently tagged NMNAT2-Venus in mouse peripheral nerves in vivo was stabilized significantly after loss of central ISTID regions that mediate vesicle attachment.22 The observed increase in half-life was at least partially mediated by reduced levels of ubiquitination of the cytosolic forms and resulted in an improved level of axon protection by these mutants in primary culture neurites. Importantly, these changes were reverted when cytosolic mutants were re-targeted to vesicle membranes by various means, indicating that association with vesicle membranes promotes NMNAT2 ubiquitination and turnover.21 Given that we found palmitoylation to be critical for NMNAT2 membrane association, the enzymes mediating NMNAT2 palmitoylation and, potentially, depalmitoylation may thus be of interest when trying to modulate NMNAT2 subcellular localization and turnover.
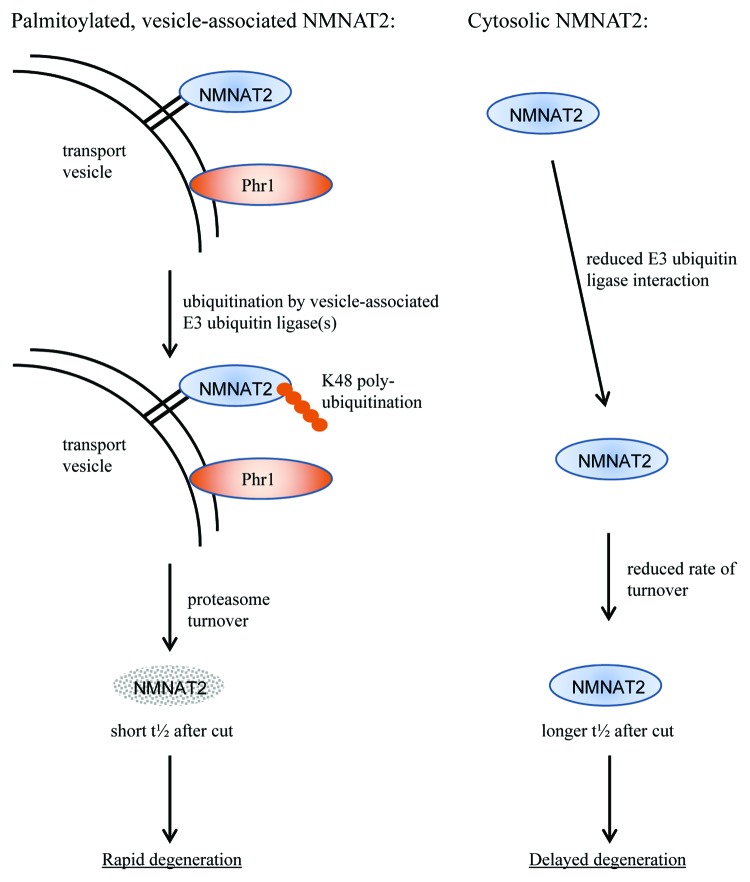
The precise mechanism behind the elevated ubiquitination of vesicle-associated NMNAT2 is as yet unclear. However, one interesting possibility is the localization of a relevant ubiquitin ligase enzyme to the NMNAT2 transport vesicle (see Figure 2). In support of this idea, we found that re-targeting cytosolic NMNAT2 mutants to the mitochondrial outer membrane did not induce NMNAT2 ubiquitination, suggesting a mechanism that is specific for vesicle membranes.21
Figure 2. Model of the mechanism of vesicle association–induced ubiquitination and turnover of NMNAT2. Palmitoylation promotes membrane association of NMNAT2, thus bringing it into close proximity with Phr1 and/or other putative E3 ubiquitin ligases. The close association and confinement to a 2D space strongly promote NMNAT2 K48-linked poly-ubiquitination and rapid turnover by the proteasome. Thus, NMNAT2 half-life is short, resulting in rapid depletion after axotomy or interruption of axonal transport and, ultimately, rapid axon degeneration. In contrast, cytosolic NMNAT2 encounters relevant E3 ubiquitin ligases much less frequently, resulting in lower levels of K48-linked poly-ubiquitination and a slower rate of UPS-mediated turnover. Consequently, the half-life of cytosolic NMNAT2 is prolonged, resulting in a longer latent phase and delayed axon degeneration.
Effects of palmitoylation on the rate of turnover have been reported for several target proteins, including cell surface receptors,23-26 the oncogene TBC1D3,27 the intracellular trafficking protein Sortilin28 and the S. cerevisiae Tlg1 SNARE protein.29 For all these, palmitoylation appears to result in stabilization of the target protein, in contrast to our findings regarding NMNAT2. The effects on protein stability in the above cases appear to be mediated by palmitoylation-dependent regulation of the relative subcellular localization of target proteins with respect to their relevant E3 ubiquitin ligases, effectively limiting the accessibility of target proteins to ubiquitin ligases. It is possible that a converse mechanism operates for NMNAT2, such that instead of shielding it from ubiquitination, palmitoylation could place NMNAT2 in the proximity of its E3 ubiquitin ligase.
Interestingly, a candidate E3 ubiquitin ligase for NMNAT2 has recently been identified. First, Drosophila dNmnat was characterized as a target of the E3 ligase Highwire. In the absence of Highwire both dNmnat and ectopically expressed mammalian NMNAT2 were more stable in axons, and axons were preserved significantly longer after axotomy.30 Another study then reported delayed axon degeneration in conditional knockout mice for Phr1 (MYCBP2), the murine homolog of Highwire. Steady-state levels of NMNAT2 were elevated in these mice and this increase in NMNAT2 levels was necessary for axon protection.31 Together, these findings establish Phr1 as a candidate E3 ubiquitin ligase for NMNAT2. Interestingly, Phr1 was also identified in a proteomics analysis performed on different classes of axonal transport vesicles and found to associate with a specific sub-population of vesicles.32 These data are consistent with a model in which palmitoylation and vesicle association promote NMNAT2 ubiquitination and turnover by bringing it into proximity with Phr1 and, perhaps, other relevant E3 ubiquitin ligases on axonal transport vesicle membranes (Fig. 2).
Axon protection in the absence of candidate E3 ubiquitin ligases for dNmnat/NMNAT2,30,31 the delay of axon degeneration in the presence of inhibitors of the ubiquitin-proteasome system33 (UPS) and our findings regarding the localization-dependent turnover and axon protective capacity of NMNAT221,22 all support NMNAT2 protein stability as a promising target for modulating axon degeneration. The most useful step for intervention in NMNAT2 ubiquitination and turnover remains to be determined. However, targeting the UPS as a whole is unlikely to be advantageous as long-term inhibition and dysfunction of the UPS actually contribute to neurodegenerative disease.34 Similarly, interference with E3 ligases might be difficult, especially given the critical roles of Phr1 during development35-38 and its other identified downstream targets.39-41 Thus, modulation of NMNAT2 subcellular localization, perhaps through interference in its palmitoylation status, could be an interesting alternative.
We found that loss of exon 6-encoded central ISTID sequences boosted NMNAT2 protective capacity both in mouse primary culture neurites and in Drosophila axons in vivo. Interestingly, the degree of protection, at least in primary culture, was stronger than that achieved by expression of WLDS.21,22 This finding illustrates that the survival factor NMNAT2, normally an endogenous, labile protein that is rapidly lost after its supply from the cell body ceases, can be changed into a highly axon protective molecule.
The mechanism by which loss of the central ISTID region mediates this strong increase in protective capacity remains to be elucidated. Interestingly, the central ISTID-deficient protein was no more stable than a simple exon 6 point mutant, at least in HEK 293T cells, suggesting that mechanisms other than protein stability, such as an altered enzymatic activity or protein-protein interactions, may mediate this change.21 On the other hand, protein turnover mechanisms could differ in the axon. In agreement with this, proteasome inhibition only partially arrests NMNAT2 turnover in primary culture neurites but completely stops it in HEK 293T cells.15 Similarly, the continued turnover of NMNAT2 in the absence of Phr131 could point to an alternative, non-UPS route that partially mediates NMNAT2 degradation (or, of course, to the presence of additional E3 ubiquitin ligases targeting NMNAT2, which remain to be identified). Thus, the central ISTID sequence could be involved in such a putative, alternative pathway for NMNAT2 turnover in axons. Given the strong effects of loss of the central ISTID region on its axon protective capacity, it will be very interesting to elucidate the underlying mechanism.
Interestingly, existing data could be interpreted to suggest that NMNAT2 does not need to be present in the axon indefinitely to delay Wallerian degeneration. In our transgenic mice, levels of NMNAT2-Venus protein fall significantly by 72 h after cut, yet axons are very strongly protected up to 14 d after cut.22 As above, the continued turnover of NMNAT2 in the absence of Phr1 is a similar example—NMNAT2 is still lost fairly rapidly after axotomy in neurites deficient of Phr1, yet axons are protected for prolonged periods of time in vivo.31 These findings could suggest that NMNAT enzymatic activity only needs to be present in the axon during the initial phase after axotomy. If this is the case, it might imply that one or more downstream executors of the axon degeneration pathway are also unstable and undergo rapid turnover within the first few hours after cut. As long as a sufficient, above-threshold amount of NMNAT2 “outlasts” them, protection could be achieved. Although not an executor molecule, the recent finding that the rate of turnover of SCG10 influences the progression of Wallerian degeneration42 supports the idea that relative levels and stabilities of both positive and negative regulators of the axon degeneration pathway could be critical in determining the duration of axon survival. Alternatively, protein turnover may slow down sufficiently after cut so that the higher starting levels of NMNAT2 in the above situations mean that sufficient quantities remain in the axon at later times to ensure axon survival.
Open Questions
One of the main questions arising from our model is how neurons are able to supply sufficient quantities of extremely labile NMNAT2 into axons to avoid spontaneous axon degeneration. With a half-life of around 45 min,21 the velocity of fast axonal transport means that a fraction of less than 1% of all NMNAT2 would arrive at the distal end of a moderately long, 4 cm mouse peripheral axon. The scale of the problem becomes massive in a long human peripheral nerve of one meter length, with fast axonal transport taking several days to arrive at the distal end. Importantly, the half-life of human NMNAT2 has not yet been assessed and could differ from that of mouse NMNAT2 in order to support much longer axons and the resulting longer transport times in human peripheral nerves. However, this appears unlikely as there is very high sequence conservation and we found human NMNAT2 to be no more protective than mouse NMNAT2 against neurite degeneration after axotomy when expressed in mouse primary culture neurites.21 Additionally, human dorsal root ganglion neurites degenerate within 5 h after cut in primary culture,12 further arguing against a longer half-life of human NMNAT2. Perhaps more likely, the half-life of NMNAT2 could be different in axons in vivo as compared with primary culture neurites. Indeed, the longer latent phase of Wallerian degeneration in vivo supports this idea and suggests that NMNAT2 could be more stable and require a longer time to be depleted sufficiently after axotomy to trigger degeneration. Currently, in the absence of suitable antibodies, there is no data that we are aware of that would support this claim. Our findings regarding turnover of NMNAT2-Venus in sciatic nerves do not adequately address this issue either, as we have shown that the YFP Venus tag most likely stabilizes the protein.22 If NMNAT2 is indeed more stable in vivo, the relevant mechanisms remain to be elucidated, but the presence of non-neuronal cells is one potential factor that could contribute to this effect.
Another possibility that has not been explored in-depth so far is that NMNAT2 turnover might accelerate in axons after cut. Such a longer half-life in uninjured axons would allow sufficient quantities of NMNAT2 to reach the distal ends of intact axons to ensure axon maintenance. Alternatively, palmitoylation could target NMNAT2 to multiple vesicle populations with distinct effects on its half-life, as other cargoes have been shown to associate with multiple vesicle populations with only partially overlapping sets of associated proteins.32 One could envisage a system in which one class of vesicles delivers NMNAT2 into the axon without reducing its half-life (e.g., a Phr1-deficient vesicle population), while association with another class of vesicles (e.g., a Phr1-containing vesicle population) could promote its removal from the cytosol, ubiquitination and turnover. Elucidating the precise identity of vesicle sub-populations that NMNAT2 associates with and perhaps the identification of alternative E3 ubiquitin ligases or of ubiquitin–proteasome independent turnover pathways for NMNAT2 will help answer the question of how NMNAT2 is turned over and how healthy axons ensure a sufficient supply to prevent spontaneous degeneration.
It is worth noting that local synthesis of NMNAT2 in the axon seems unlikely to contribute significantly to its replenishment. Several large-scale studies identified hundreds of axonal mRNAs, but Nmnat2 mRNA was not found,43-46 suggesting that, at least in primary culture neurites, it is either absent from axons or present at very low, sub-detection abundance. Moreover, cell body but not axonal protein synthesis is required for axon survival,15 suggesting that most NMNAT2 is supplied by the cell body. Additionally, the inability of isolated axons to maintain a sufficient supply of NMNAT2 to ensure axon survival after axotomy does of course in itself argue against a substantial contribution of local synthesis to axonal levels of NMNAT2.
Another, related, “frequently asked question” is why NMNAT2 is so unstable in the first place. One possibility is that a stable cytoplasmic NMNAT enzyme in axons could have—so far uncharacterized—adverse effects. The short half-life would then result from balancing this adverse effect with the risk of axon degeneration. However, the observation that mice expressing stable, axonally targeted NMNATs (such as WLDS, NMNAT2-Venus and NMNAT2Δex6-Venus) are overtly healthy and normal until at least 12 months of age suggests that there is no obvious adverse effect associated with the possession of a stable, cytosolic NMNAT enzyme in axons. Thus, a second, and perhaps more likely, possibility is that rapid turnover of NMNAT2 and the resulting fast Wallerian degeneration are beneficial. Peripheral nerve regeneration in many species of mammal is remarkably efficient and functional recovery is relatively quick, with obvious implications for the animal’s evolutionary fitness. As regeneration of peripheral nerve axons is impaired when degeneration is delayed,47-51 rapid degeneration probably facilitates rapid regeneration and functional recovery in a situation of peripheral nerve injury where a distal stump that has been separated from the proximal axon and cell body is of no further use. Unfortunately, peripheral nerve regeneration in humans is not as efficient as that observed in many other species of mammal.52 Additionally, Wallerian-like degeneration affects axons in the central nervous system, where spontaneous regeneration and functional recovery are rare and spatially limited. Thus, the problem from a human point of view is that this system puts axons at risk of degeneration that are not irreversibly damaged. In situations where axonal transport is sufficiently impaired, distal NMNAT2 levels could fall below critical levels, triggering the degeneration pathway in axons that are still continuous with their cell bodies. Preventing this Wallerian-like degeneration in situations where maintenance of the existing axon is more beneficial than the attempt to regenerate a new one is likely to produce clinical benefits in many neurodegenerative conditions.
One area of clinical importance where this could apply is chemotherapy-induced peripheral neuropathy. Several chemotherapy drugs have been shown to impair axonal transport in vitro and in vivo.53-56 Additionally, the ability of WLDS expression to prevent axon degeneration and peripheral neuropathy induced by paclitaxel in mice, and by vincristine in primary culture, suggests a Wallerian-like mechanism of axon degeneration.57-59 Given our recent findings about the role of NMNAT2 in axon maintenance and the mechanism of axon protection by WLDS by substituting for NMNAT2,15 one mechanism by which these microtubule-targeting drugs cause peripheral neuropathy could be a reduced supply of NMNAT2 into distal axons. Modulation of the axon survival factor NMNAT2 through alterations to its half-life or axonal delivery may be a promising route to address these issues.
Together these recent reports confirm important roles for endogenous NMNAT2 for axon growth and survival in vivo and show that variant NMNAT2 forms that lack central ISTID sequences can preserve injured axons in mammalian primary culture and in vivo, both in mice and Drosophila, with an efficacy matching or even exceeding that of WLDS. These sequences seem to be closely linked to turnover of NMNAT2, which appears to involve vesicle targeting and ubiquitin-proteasome mediated turnover. The E3 ligase Highwire/Phr1 is a strong candidate for mediating this process at least partially. Modulation of NMNAT2 subcellular localization and turnover could thus present a novel avenue to address axon degeneration in situations of an impaired axonal supply of this critical axon survival factor.
Glossary
Abbreviations:
- ISTID
isoform-specific targeting and interaction domain
- NMNAT
nicotinamide mononucleotide adenylyltransferase
- UPS
ubiquitin-proteasome system
- WLDS (WldS)
Wallerian degeneration slow protein (mouse strain/ gene)
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/27049
References
- 1.Adalbert R, Coleman MP. Axon pathology in age-related neurodegenerative disorders. Neuropathol Appl Neurobiol. 2012;39:90–108. doi: 10.1111/j.1365-2990.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 2.Millecamps S, Julien J-P. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–76. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- 3.De Vos KJ, Grierson AJ, Ackerley S, Miller CCJ. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–73. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 4.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–67. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–90. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 7.Adalbert R, Gillingwater TH, Haley JE, Bridge K, Beirowski B, Berek L, Wagner D, Grumme D, Thomson D, Celik A, et al. A rat model of slow Wallerian degeneration (WldS) with improved preservation of neuromuscular synapses. Eur J Neurosci. 2005;21:271–7. doi: 10.1111/j.1460-9568.2004.03833.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin SM, O’Brien GS, Portera-Cailliau C, Sagasti A. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137:3985–94. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y, Yan T, Zheng J, Ge X, Mu Y, Zhang Y, Wu D, Du J-L, Zhai Q. Overexpression of Wld(S) or Nmnat2 in mauthner cells by single-cell electroporation delays axon degeneration in live zebrafish. J Neurosci Res. 2010;88:3319–27. doi: 10.1002/jnr.22498. [DOI] [PubMed] [Google Scholar]
- 10.Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O’Leary DDM, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–95. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–81. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Kitay BM, McCormack R, Wang Y, Tsoulfas P, Zhai RG. Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLD(S)-mediated axon protection independent of axonal mitochondria. Hum Mol Genet. 2013;22:1601–14. doi: 10.1093/hmg/ddt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang C, Bernardes-Silva M, Coleman MP, Perry VH. The cellular distribution of the Wld s chimeric protein and its constituent proteins in the CNS. Neuroscience. 2005;135:1107–18. doi: 10.1016/j.neuroscience.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 14.Beirowski B, Babetto E, Gilley J, Mazzola F, Conforti L, Janeckova L, Magni G, Ribchester RR, Coleman MP. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009;29:653–68. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks AN, Lorenzetti D, Gilley J, Lu B, Andersson K-E, Miligan C, Overbeek PA, Oppenheim R, Bishop CE. Nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2) regulates axon integrity in the mouse embryo. PLoS One. 2012;7:e47869. doi: 10.1371/journal.pone.0047869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilley J, Adalbert R, Yu G, Coleman MP. Rescue of peripheral and CNS axon defects in mice lacking NMNAT2. J Neurosci. 2013;33:13410–24. doi: 10.1523/JNEUROSCI.1534-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman M. Molecular signaling how do axons die? Adv Genet. 2011;73:185–217. doi: 10.1016/B978-0-12-380860-8.00005-7. [DOI] [PubMed] [Google Scholar]
- 19.Mayer PR, Huang N, Dewey CM, Dries DR, Zhang H, Yu G. Expression, localization, and biochemical characterization of nicotinamide mononucleotide adenylyltransferase 2. J Biol Chem. 2010;285:40387–96. doi: 10.1074/jbc.M110.178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau C, Dölle C, Gossmann TI, Agledal L, Niere M, Ziegler M. Isoform-specific targeting and interaction domains in human nicotinamide mononucleotide adenylyltransferases. J Biol Chem. 2010;285:18868–76. doi: 10.1074/jbc.M110.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milde S, Gilley J, Coleman MP. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol. 2013;11:e1001539. doi: 10.1371/journal.pbio.1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milde S, Fox AN, Freeman MR, Coleman MP. Deletions within its subcellular targeting domain enhance the axon protective capacity of Nmnat2 in vivo. Sci Rep. 2013;3:2567. doi: 10.1038/srep02567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Z, Ni Y, Szabo G, Linden J. Palmitoylation of the recombinant human A1 adenosine receptor: enhanced proteolysis of palmitoylation-deficient mutant receptors. Biochem J. 1999;342:387–95. doi: 10.1042/0264-6021:3420387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percherancier Y, Planchenault T, Valenzuela-Fernandez A, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. Palmitoylation-dependent control of degradation, life span, and membrane expression of the CCR5 receptor. J Biol Chem. 2001;276:31936–44. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- 25.Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, Kanellopoulos JM. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 2009;23:795–805. doi: 10.1096/fj.08-114637. [DOI] [PubMed] [Google Scholar]
- 26.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–20. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong C, Lange JJ, Samovski D, Su X, Liu J, Sundaresan S, Stahl PD. Ubiquitination and degradation of the hominoid-specific oncoprotein TBC1D3 is regulated by protein palmitoylation. Biochem Biophys Res Commun. 2013;434:388–93. doi: 10.1016/j.bbrc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumaresq-Doiron K, Jules F, Lefrancois S. Sortilin turnover is mediated by ubiquitination. Biochem Biophys Res Commun. 2013;433:90–5. doi: 10.1016/j.bbrc.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 29.Valdez-Taubas J, Pelham H. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 2005;24:2524–32. doi: 10.1038/sj.emboj.7600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012;10:e1001440. doi: 10.1371/journal.pbio.1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 2013;3:1422–9. doi: 10.1016/j.celrep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LSB, Cavalli V. Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem. 2009;284:34628–39. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–25. doi: 10.1016/S0896-6273(03)00429-X. [DOI] [PubMed] [Google Scholar]
- 34.Dennissen FJ, Kholod N, van Leeuwen FW. The ubiquitin proteasome system in neurodegenerative diseases: culprit, accomplice or victim? Prog Neurobiol. 2012;96:190–207. doi: 10.1016/j.pneurobio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Hagarman JA, O’Brien TP. An essential gene mutagenesis screen across the highly conserved piebald deletion region of mouse chromosome 14. Genesis. 2009;47:392–403. doi: 10.1002/dvg.20510. [DOI] [PubMed] [Google Scholar]
- 36.Burgess RW, Peterson KA, Johnson MJ, Roix JJ, Welsh IC, O’Brien TP. Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice. Mol Cell Biol. 2004;24:1096–105. doi: 10.1128/MCB.24.3.1096-1105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloom AJ, Miller BR, Sanes JR, DiAntonio A. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev. 2007;21:2593–606. doi: 10.1101/gad.1592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vo BQ, Bloom AJ, Culican SM. Phr1 is required for proper retinocollicular targeting of nasal-dorsal retinal ganglion cells. Vis Neurosci. 2011;28:175–81. doi: 10.1017/S0952523810000386. [DOI] [PubMed] [Google Scholar]
- 39.Klinedinst S, Wang X, Xiong X, Haenfler JM, Collins CA. Independent pathways downstream of the Wnd/DLK MAPKKK regulate synaptic structure, axonal transport, and injury signaling. J Neurosci. 2013;33:12764–78. doi: 10.1523/JNEUROSCI.5160-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–20. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Holland S, Coste O, Zhang DD, Pierre SC, Geisslinger G, Scholich K. The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J Biol Chem. 2011;286:3671–80. doi: 10.1074/jbc.M110.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A. SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A. 2012;109:E3696–705. doi: 10.1073/pnas.1216204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gumy LF, Yeo GSH, Tung Y-CL, Zivraj KH, Willis D, Coppola G, Lam BYH, Twiss JL, Holt CE, Fawcett JW. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willis D, Li KW, Zheng J-Q, Chang JH, Smit AB, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–91. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poon MM, Choi S-H, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–9. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zivraj KH, Tung YCL, Piper M, Gumy L, Fawcett JW, Yeo GSH, Holt CE. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J Neurosci. 2010;30:15464–78. doi: 10.1523/JNEUROSCI.1800-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen S, Bisby MA. Long-term consequences of impaired regeneration on facial motoneurons in the C57BL/Ola mouse. J Comp Neurol. 1993;335:576–85. doi: 10.1002/cne.903350409. [DOI] [PubMed] [Google Scholar]
- 48.Chen S, Bisby MA. Impaired motor axon regeneration in the C57BL/Ola mouse. J Comp Neurol. 1993;333:449–54. doi: 10.1002/cne.903330310. [DOI] [PubMed] [Google Scholar]
- 49.Bisby MA, Chen S. Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain Res. 1990;530:117–20. doi: 10.1016/0006-8993(90)90666-Y. [DOI] [PubMed] [Google Scholar]
- 50.Court F, Alvarez J. Nerve regeneration in Wld(s) mice is normalized by actinomycin D. Brain Res. 2000;867:1–8. doi: 10.1016/S0006-8993(00)02140-5. [DOI] [PubMed] [Google Scholar]
- 51.Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. Eur J Neurosci. 1994;6:420–8. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 52.Höke A. Mechanisms of Disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448–54. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 53.LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–9. doi: 10.1016/j.neuro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theiss C, Meller K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell Tissue Res. 2000;299:213–24. doi: 10.1007/s004410050019. [DOI] [PubMed] [Google Scholar]
- 55.Nakata T, Yorifuji H. Morphological evidence of the inhibitory effect of taxol on the fast axonal transport. Neurosci Res. 1999;35:113–22. doi: 10.1016/S0168-0102(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 56.Goshima Y, Usui H, Shiozawa T, Hida T, Kuraoka S, Takeshita S, Yamashita N, Ichikawa Y, Kamiya Y, Gotoh T, et al. Computational analysis of the effects of antineoplastic agents on axonal transport. J Pharmacol Sci. 2010;114:168–79. doi: 10.1254/jphs.09352FP. [DOI] [PubMed] [Google Scholar]
- 57.Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Ann Neurol. 2002;52:442–7. doi: 10.1002/ana.10300. [DOI] [PubMed] [Google Scholar]
- 58.Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, Asress S, Adalbert R, Silva A, Bridge K, Huang XP, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14:116–27. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki Y, Vohra BPS, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009;29:6526–34. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]