Abstract
Background
Gestational diabetes mellitus (GDM) occurs in 3–5% of all pregnancies. GDM increases both maternal and fetal risks, causes fetal macrosomia, and hence increases the rates of caesarean sections and delivery complications such as shoulder dystocia. An early predictive marker and consequent early treatment could be beneficial, so amniotic fluid insulin and C-peptide have been examined in several studies. Increased amniotic fluid insulin in early amniocentesis between the 14th and 20th gestational week predicted a later GDM. A potential direct association with fetal macrosomia remains to be determined.
Material/Methods
This retrospective study investigated amniotic fluid insulin/C-peptide from amniocenteses between 14 and 20 weeks of gestation in correlation with fetal birth weight, type of delivery, and complications. To focus on effects of fetal hyperinsulinism apart from therapeutic confounders, we included patients who did not participate in GDM screening. Insulin and C-peptide were measured in 144 samples of frozen amniotic fluid. Birth weight, type of delivery, complications, and birth injuries were noted.
Results
Birth weights ranged from 760 g to 4410 g with a mean weight of 3424 g at an average of 40 weeks gestation. The mean amniotic fluid insulin was 4.36 μU/ml and the mean C-peptide concentration was 0.076 ng/ml. There was no correlation between amniotic fluid insulin or C peptide and birth weight, type of delivery, complications, and birth injuries.
Conclusions
Amniotic fluid insulin and C-peptide are unsuitable as predictive marker for fetal macrosomia, type of delivery, complications, or birth injuries.
Keywords: amniocentesis, gestational diabetes, macrosomia, amniotic fluid, insulin, C-peptide
Background
Diabetes mellitus (DM) is a chronic hyperglycemia caused by impaired insulin secretion. Diabetes mellitus type I (DM I) has absolute insulin deficiency in contrast to diabetes mellitus type II (DM II) which has relative insulin deficiency. Gestational diabetes mellitus (GDM) is defined as impaired glucose tolerance with onset during pregnancy. GDM shows pathophysiologic mechanisms similar to DM II [1]. Causal conditions are genetic predisposition, obesity, and lifestyle. During the second part of pregnancy, an increasing insulin resistance occurs due to changes in adipokinin, leptin, and TNF-alpha release [2]. The prevalence varies from 0.6% to 20% over the last 20 years. In Germany, the prevalence in 2010 was about 3.7% with a distinct increase since 2002 [3].
There are various GDM complications for both mother and child: increasing numbers of maternal urinary tract infections and vaginal Candida infections were observed [4]. There is an increase in risk for preterm birth [5,6] and pregnancy-induced hypertension (PIH) [6]. Fetal macrosomias, caesarean section, shoulder dystocia, birth injuries, and severe postpartum hemorrhage (HAPO [7,8]) occur more frequently in GDM patients. Various studies have shown that 35–60% of patients with GDM develop DM II or impaired glucose tolerance during the following 10 years [9–13].
Chronic maternal hyperglycemia causes increased fetal insulin secretion by pancreatic β-cell hypertrophia. The fetus thus develops macrosomia or – as a result of acute and chronic oxygen deficiency – higher risks for growth restriction or even intrauterine fetal death [14]. Macrosomia also increases shoulder dystocia rates [15], birth injuries, and increased caesarean section rates. Lower surfactant-production leads to respiratory complications [16,17].
Maternal hyperglycemia leads to β-cell proliferation of the fetus and hence to hyperinsulinism. C-peptide is a byproduct of insulin synthesis. Several studies showed a correlation between increased amniotic fluid insulin or C-peptide and GDM. Falluca et al. found increased levels of amniotic fluid C-peptide in patients who developed GDM [18], whereas Carpenter and Star provided evidence for increased amniotic fluid insulin in patients who later develop GDM [19,20].
In contrast, in a prospective study our group could not demonstrate any correlation between amniotic fluid insulin, C-peptide, and the development of GDM [21].
All things considered, the role of increased amniotic fluid insulin and C-peptide and the development of a GDM remain to be elucidated. Therefore, in this study we investigated the correlation between amniotic fluid insulin, C-peptide, and fetal birth weight, as well as type of delivery and birth complications. We included only women who underwent an amniocentesis between 14 and 22 weeks of gestation. We included women who did not participate in GDM diagnostics to eliminate confounding effects of GDM therapy. The data from these further unselected patients were collected before the general German GDM screening started in 2012.
Material and Methods
We retrospectively analyzed singleton pregnancy and delivery data of 144 women who underwent an amniocentesis at the Würzburg University Hospital, Department of Obstetrics and Gynecology. The indication for amniocentesis in 77.1% was maternal age >35 years. We only included women with normal fetal chromosomal findings and normal amniotic fluid alpha fetoprotein (AFP) who did not undergo testing for GDM and hence did not receive any therapy. Exclusion criteria were fetal growth retardation or infections.
The amniocentesis was performed between the 14th and 20th week gestation. We extracted 15 ml of amniotic fluid for chromosome analysis and AFP testing. Insulin was measured using an Iod-125 radioimmunoassay kit (Schering, Berlin, Germany) using the protocol for serum samples. Radioactively labeled Insulin was used in a defined concentration, which competes with the sample of insulin to be measured. The specific primary anti-insulin antibody binds less of the radioactively marked insulin the more sample insulin is bound. To clear the sample of free antigen, the antigen-antibody complexes were precipitated by a secondary antibody against the primary antibody. After the binding, reaction according to the manufacturer’s instructions, the radioactivity was assessed by a scintillation counter.
The radioactive tracer Insulin-I-125 and an anti-insulin mouse immunoglobulin were diluted with distilled water. For the generation of a standard curve, a serial dilution of a known Insulin sample was performed. We incubated 100 μl of sample with 900 μl of diluted tracer. After 18 hours of incubation at room temperature, the insulin-Ig complexes were precipitated. The supernatant was discarded and radioactivity was measured with a gamma counter. Using the standard curve, we calculated the insulin concentrations. The lower detection limit was 4.2 μU/ml
The use of specific antibodies makes this assay precise; it can distinguish small differences in concentration due to the sensitive detection of radioactivity.
C-peptide was measured using an immunoradiometric assay (IRMA-C-PEP Kit). This kit uses 2 monoclonal antibodies specific for different epitopes on the C-peptide antigen. The capture antibody was coated on a well, whereas the signaling antibody with I-125 is soluble. Both antibodies bind simultaneously to the sample insulin. Again, a serial dilution was used to obtain the standard curve. After incubation, we measured the radioactivity with a gamma counter and calculated the C-peptide concentrations according to the standard curve. The lowest concentration to be detected was 0.036 ng/ml.
Data concerning the pregnancies and births were noted and analyzed using SPSS 18.0.2 for Windows and Microsoft Excel 2010. A p value ≤0.05 was considered statistically significant.
Results
The mean newborn birth weight was 3420 g, whereas the subgroup of mature newborns showed a mean of 3530 g. The maternal age ranged from 19 to 44 years with a mean age of 35.6 years. The total rate of vaginal deliveries was 81.9% (of which 71.5% were spontaneous and 10.4% were operative), and the cesarean section rate was 18.1%. The overall rate of premature birth was 7.6%.
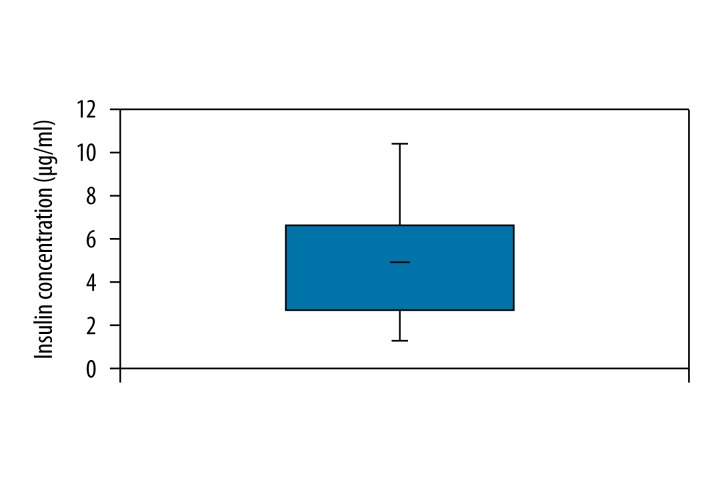
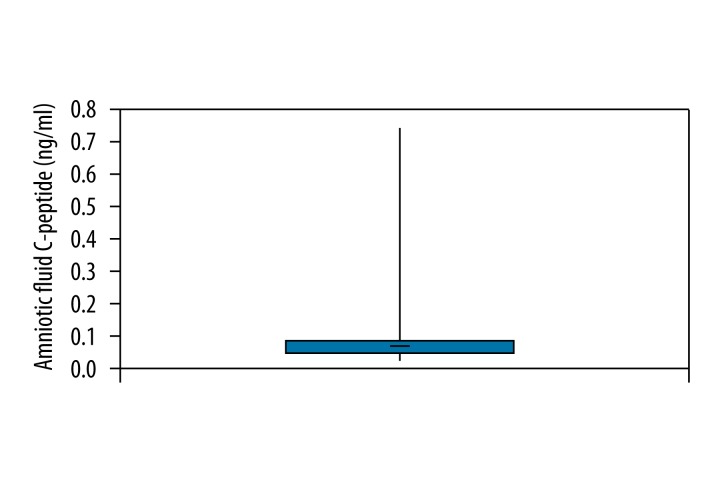
Amniotic fluid insulin was measured in 140 samples. The mean concentration was 4.364 μU/ml, ranging from 0.9 μU/ml to 9.9 μU/ml (Figure 1). Amniotic fluid C-peptide was measured in 57 samples. Concentrations ranged from 0.022 ng/ml to 0.721 ng/ml and the mean was 0.076 ng/ml (Figure 2).
Figure 1.
Overall amniotic fluid insulin.
Figure 2.
Overall amniotic fluid C-peptide.
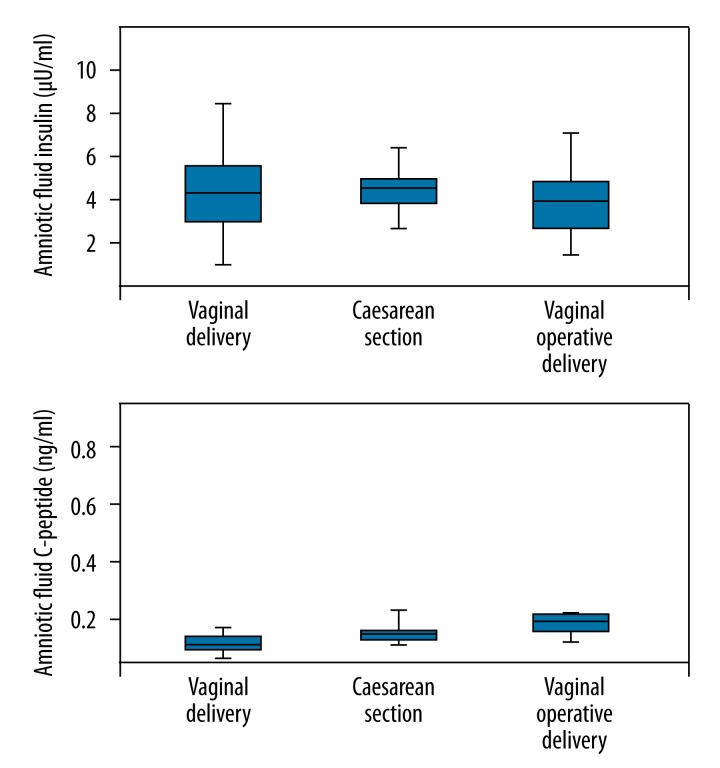
There was no significant correlation between the amniotic fluid insulin or C-peptide values and different delivery modes (Figure 3).
Figure 3.
Amniotic fluid insulin vs. type of delivery.
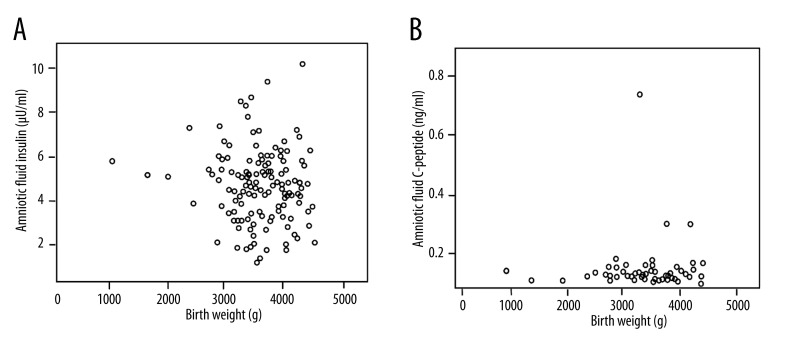
Regarding the newborn birth weight, there was no correlation with amniotic fluid insulin or C-peptide (Figure 4).
Figure 4.
(A) Amniotic fluid insulin vs.birth weight. (B) Amniotic fluid C-peptide vs.birth weight.
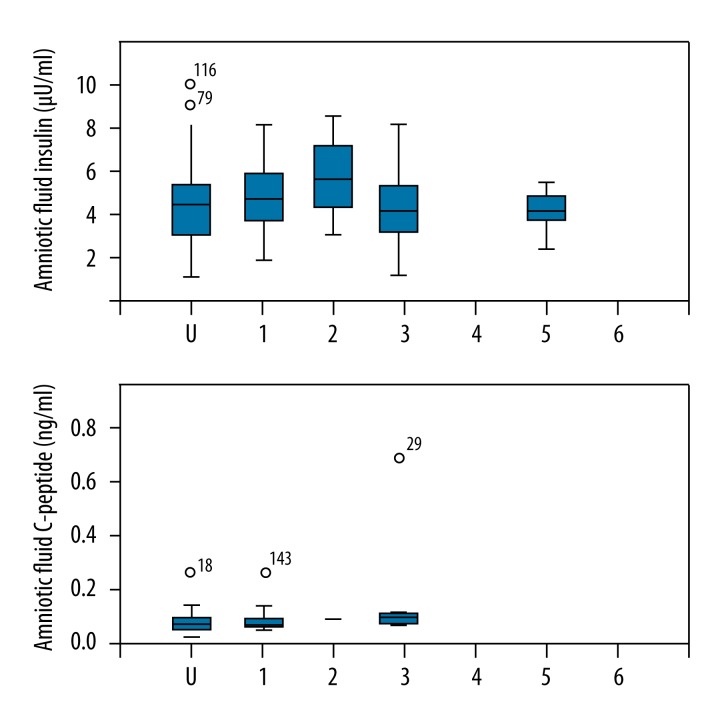
The concentrations of amniotic fluid insulin and C-peptide were compared with the collected birth injuries data (e.g. perineal tears grade I to III, effects on the clitoris, labia, or vaginal tears) (Figure 5).
Figure 5.
Amniotic fluid insulin and C-peptide vs. birth injuries. U – undertermined, 1 – no injury, 2 – perineal tear grade I–II, 3 – perineal tear grade III, 4 – episiotomy, 5 – affection of clitoris or labia, 6 – vaginal tear.
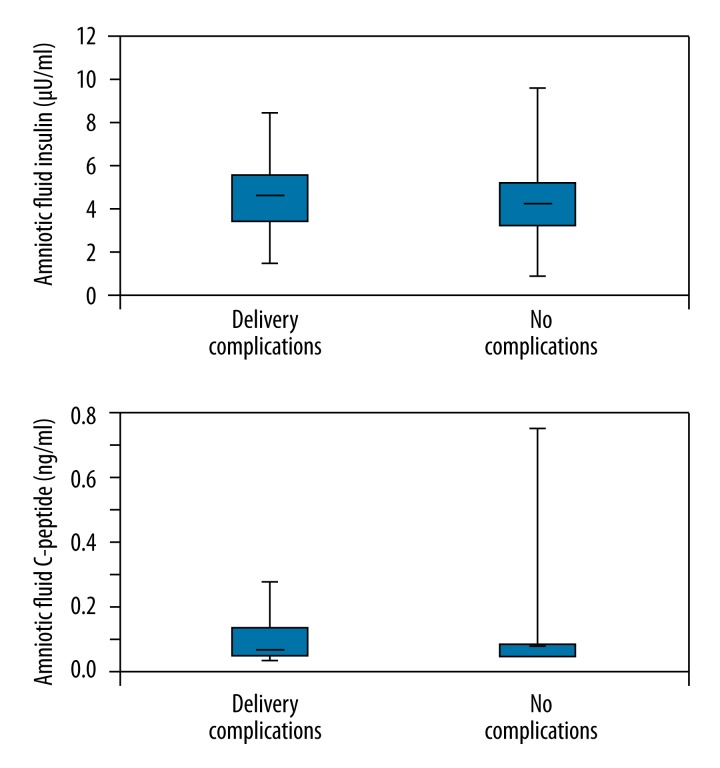
Only 10 patients had delivery complications. These were fever in 3 cases, major postpartum bleeding in 3 cases, 1 case with intraoperative complications, and 3 cases without complications. Between the group with complications and the control group there was no significant difference in amniotic fluid insulin or C-peptide (Figure 6).
Figure 6.
Amniotic fluid insulin and C-peptide vs. delivery complications.
Discussion
According to the Pedersen hypothesis, maternal hyperglycemia caused by a GDM leads to fetal hyperglycemia and hence to fetal hyperinsulinism. Reiher et al. analyzed age-dependent insulin secretion and β-cell number of the fetal pancreas in diabetic and non-diabetic women and found significant fetal β-cell hypertrophia in diabetic women [22]. The amniotic fluid insulin is of fetal origin [23,24].
During pregnancy, glucosuria, polyhydramnios, and fetal macrosomia are possible indicators. The German Society of Obstetrics and Gynecology (DGGG) suggests screening for GDM, which was established in December 2011.
The HAPO 2008 study showed a distinct correlation between increased maternal blood glucose and fetal outcome, especially macrosomia and shoulder dystocia, which led to adaptation of thresholds for the oGT in venous blood plasma [3].
Because a predictive or early detection marker for the development of a GDM would be beneficial to indicate increased risk as soon as the early second trimester, several authors examined the correlation between amniotic fluid glucose, insulin, and C-peptide. Glucose is transported through the placenta, whereas amniotic fluid insulin is believed to be produced by the fetus [24]. As pregnancy progresses, insulin levels increase [25].
In a population of 39 patients with GDM and 194 control-patients, Star et al. showed a significant correlation between GDM and increased amniotic fluid insulin in amniocenteses at 14 to 20 weeks gestation. Interestingly, the glucose levels did not differ between cases and controls.
Falluca et al. found increased levels of amniotic fluid C-peptide in patients who developed GDM [18]. For the study, we included 77 control patients and 9 patients with GDM who underwent amniocentesis between 15 and 22 weeks gestation.
Carpenter et al. provided evidence for increased amniotic fluid insulin in patients who developed GDM later on [19,20]. They included 296 patients who underwent an amniocentesis between 14 and 20 weeks gestation.
In contrast to this, our group could not find any correlation between amniotic fluid insulin or C-peptide and the development of a GDM in a prospective study including 90 patients [21].
Multiple investigators (HAPO2009) have found an association between GDM and fetal macrosomia, as well as increased rates of caesarean sections, birth injuries, and delivery complications [8,14,15].
Until recently, the German obstetrical guidelines did not include a general screening for GDM. Therefore, we investigated whether there is an association between amniotic fluid insulin or C-peptide as markers for fetal glucose metabolism and the development of GDM-complications such as fetal macrosomia, delivery complications, and birth injuries. To eliminate treatment for diabetes as a confounding factor, we only included patients who did not participate in GDM testing.
Conclusions
We retrospectively analyzed frozen amniotic fluid samples of 144 patients and could not find correlations between amniotic fluid insulin or C-peptide with fetal macrosomia, delivery mode, and birth complications or injuries. Measuring the amniotic fluid insulin and C-peptide in the early amniocentesis seems to be unsuitable for prediction of GDM complications. The role of these proteins for the prediction of a GDM developing later during pregnancy remains to be determined.
Footnotes
Source of support: Departmental sources
References
- 1.Kjos SL, Buchanan TA. Current concepts: Gestational diabetes mellitus. N Eng J Med. 1999;341(23):1749–56. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 2.Worda C, et al. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191(6):2120–24. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 3.Kleinwechter H, Schäfer-Graf U, Bührer C, et al. Gestationsdiabetes mellitus (GDM) Evidenzbasierte Leitlinie zu Diagnostik, Therapie u. Nachsorge der Deutschen Diabetes-Gesellschaft (DDG) und der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) 2011 [in German] [Google Scholar]
- 4.Bhat M, et al. Determinants of gestational diabetes mellitus: A case control study in a district tertiary care hospital in south India. Int J Diabetes Dev Ctries. 2010;30(2):91–96. doi: 10.4103/0973-3930.62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju H, et al. Borderline gestational diabetes mellitus and pregnancy outcomes. BMC Pregnancy Childbirth. 2008;8:31. doi: 10.1186/1471-2393-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadl HE, et al. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. 2010;27(4):436–41. doi: 10.1111/j.1464-5491.2010.02978.x. [DOI] [PubMed] [Google Scholar]
- 7.Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58(2):453–59. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shand AW, et al. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998–2002. Diabet Med. 2008;25(6):708–15. doi: 10.1111/j.1464-5491.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 9.Lobner K, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. 2006;55(3):792–97. doi: 10.2337/diabetes.55.03.06.db05-0746. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer-Graf UM, et al. How do we reduce the number of cases of missed postpartum diabetes in women with recent gestational diabetes mellitus? Diabetes Care. 2009;32(11):1960–64. doi: 10.2337/dc09-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albareda M, et al. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care. 2003;26(4):1199–205. doi: 10.2337/diacare.26.4.1199. [DOI] [PubMed] [Google Scholar]
- 12.Hunger-Dathe W, et al. Prevalence of impaired glucose tolerance 6 years after gestational diabetes. Exp Clin Endocrinol Diabetes. 2006;114(1):11–17. doi: 10.1055/s-2005-873015. [DOI] [PubMed] [Google Scholar]
- 13.Lauenborg J, et al. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care. 2004;27(5):1194–99. doi: 10.2337/diacare.27.5.1194. [DOI] [PubMed] [Google Scholar]
- 14.Rackham O, Paize F, Weindling AM. Cause of death in infants of women with pregestational diabetes mellitus and the relationship with glycemic control. Postgrad Med. 2009;121(4):26–32. doi: 10.3810/pgm.2009.07.2026. [DOI] [PubMed] [Google Scholar]
- 15.Langer O, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170(4):1036–46. doi: 10.1016/s0002-9378(94)70097-4. discussion 1046–47. [DOI] [PubMed] [Google Scholar]
- 16.Sugahara K, et al. Differential expressions of surfactant protein SP-A, SP-B, and SP-C mRNAs in rats with streptozotocin-induced diabetes demonstrated by in situ hybridization. Am J Respir Cell Mol Biol. 1994;11(4):397–404. doi: 10.1165/ajrcmb.11.4.7917308. [DOI] [PubMed] [Google Scholar]
- 17.Gewolb IH. Effect of high glucose on fetal lung maturation at different times in gestation. Exp Lung Res. 1996;22(2):201–11. doi: 10.3109/01902149609050847. [DOI] [PubMed] [Google Scholar]
- 18.Fallucca F, et al. Amniotic fluid insulin and C peptide levels in diabetic and nondiabetic women during early pregnancy. J Clin Endocrinol Metab. 1996;81(1):137–39. doi: 10.1210/jcem.81.1.8550740. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter MW, et al. Fetal hyperinsulinism at 14–20 weeks and subsequent gestational diabetes. Obstet Gynecol. 1996;87(1):89–93. doi: 10.1016/0029-7844(95)00361-4. [DOI] [PubMed] [Google Scholar]
- 20.Star J, et al. The relationship between second-trimester amniotic fluid insulin and glucose levels and subsequent gestational diabetes. Prenat Diagn. 1997;17(2):149–54. [PubMed] [Google Scholar]
- 21.Zollner U, Ahmadi M, Dietl J. Early diagnosis of gestational diabetes by amniotic fluid insulin levels? Z Geburtshilfe Neonatol. 2011;215(3):98–104. doi: 10.1055/s-0031-1271744. [DOI] [PubMed] [Google Scholar]
- 22.Reiher H, et al. Age-dependent insulin secretion of the endocrine pancreas in vitro from fetuses of diabetic and nondiabetic patients. Diabetes Care. 1983;6(5):446–51. doi: 10.2337/diacare.6.5.446. [DOI] [PubMed] [Google Scholar]
- 23.Greco AV, et al. Fetal origin of amniotic fluid insulin in the human mother. Clin Endocrinol (Oxf) 1980;12(1):67–70. doi: 10.1111/j.1365-2265.1980.tb03134.x. [DOI] [PubMed] [Google Scholar]
- 24.Casper DJ, Benjamin F. Immunoreactive insulin in amniotic fluid. Obstet Gynecol. 1970;35(3):389–93. [PubMed] [Google Scholar]
- 25.Weiss PA, et al. Insulin levels in amniotic fluid of normal and abnormal pregnancies. Obstet Gynecol. 1984;63(3):371–75. [PubMed] [Google Scholar]