Abstract
It is unclear how standardized neuropsychological measures of motor function relate to brain volumes of motor regions in autism spectrum disorder (ASD). An all male sample composed of 59 ASD and 30 controls (ages 5–33 years) completed three measures of motor function: strength of grip (SOG), finger tapping test (FTT), and grooved peg-board test (GPT). Likewise, all participants underwent magnetic resonance imaging with region of interest (ROI) volumes obtained to include the following regions: motor cortex (pre-central gyrus), somatosensory cortex (post-central gyrus), thalamus, basal ganglia, cerebellum and caudal middle frontal gyrus. These traditional neuropsychological measures of motor function are assumed to differ in motor complexity with GPT requiring the most followed by FTT and SOG. Performance by ASD participants on the GPT and FTT differed significantly from controls, with the largest effect size differences observed on the more complex GPT task. Differences on the SOG task between the two groups were non-significant. Since more complex motor tasks tap more complex networks, poorer GPT performance by those with ASD may reflect less efficient motor networks. There was no gross pathology observed in classic motor areas of the brain in ASD, as region of interest (ROI) volumes did not differ, but FTT was negatively related to motor cortex volume in ASD. The results suggest a hierarchical motor disruption in ASD, with difficulties evident only in more complex tasks as well as a potential anomalous size-function relation in motor cortex in ASD.
Keywords: autism, network disruption, motor impairment, brain volume differences, motor cortex volume
Although Kanner’s (1943) classic paper outlined the core features of autism that eventually became the triad criteria for the diagnosis of autism—qualitative impairments in social interaction, communication, and repetitive and stereotyped behaviors—he also noted, “… several of the children were somewhat clumsy in gait and gross motor performance…” (p. 248). While most contemporary studies of autism spectrum disorder (ASD) have focused on the core diagnostic features of autism (Geschwind, 2009), clinically described delays in motor development and impairments in motor function are commonplace in ASD where meta-analytic findings have suggested a more central role (Fournier, Hass, Naik, Lodha, & Cauraugh, 2010), described by some as a motor coordination deficit in ASD (Miyahara, 2013). While these reviews have shown motor differences between typically developing (TD) and ASD individuals, most individuals with ASD do not meet criteria for a developmental coordination disorder (Emck et al., 2009). While individuals with ASD may exhibit motor findings, as reviewed by Fournier et al. (2010), Miyahara (2013) and Emck (2009) there is considerable variability in how motor impairments have been assessed in these studies, many of which have been based on simple clinical rating scales and not traditional neuropsychological measures of motor functioning like strength of grip (SOG), the finger tapping test (FTT) or grooved-pegboard test (GPT; see Lezak, Howieson, Bigler & Tranel, 2012).
Studies that have used some of these traditional neuropsychological measures of motor function have found impairments in those with ASD when group compared to TD individuals (see Muller, Schafer, Kuhn, & Przuntek, 2000; Pedersen, Oberg, Larsson, & Lindval, 1997; Sachdev, Hume, Toohey, & Doutney, 1996); however, other studies have reported either mixed findings or an absence of any motor difference (Hardan, Kilpatrick, Keshavan, & Minshew, 2003; Jansiewicz, et al., 2006; Kern et al., 2011; Minshew, Goldstein, & Siegel, 1997; Rumsey & Hamburger, 1988; Szatmari, Tuff, Finlayson, & Bartolucci, 1990; Weimer, Schatz, Lincoln, Ballantyne, & Trauner, 2001; Williams, Goldstein, & Minshew, 2006).
The lack of consistent motor findings in ASD across studies, including the aforementioned studies that did use standardized neuropsychological measures, likely relates to: design and methodological differences across studies; the level of motor complexity being assessed; the clinical heterogeneity of autism samples; utilization of a single motor task versus multiple measures; the use of non-standardized versus standardized measures; differences in sample sizes; differences in age ranges (i.e., childhood, adolescence, adulthood, lifespan); and differences in how autism severity is associated with cognitive factors (see Carcani-Rathwell, Rabe-Hasketh, & Santosh, 2006) since as Goldman and colleagues (2009) observed, lower cognitive ability was associated with greater motor impairment. Furthermore, given the heterogeneity of autism and its expression (Rapin, 1991), a universal motor impairment would not necessarily be expected.
Unfortunately, some of these limitations are inescapable given the nature and constraints of research with this clinical population. Yet, what is clear from the literature is that motor deficits in some fashion likely are associated with and related to ASD (Bhat, Landa, Galloway, 2011; Bonnet & Gao, 1996; Downey & Rapport, 2012; Dziuk et al., 2007; Emck, Bosscher, van Wieringen, Doreleijers, & Beek, 2012; Fournier, Hass, Naik, Lodha, & Cauraugh, 2010; Gowen & Hamilton, 2012; Jansiewicz et al., 2006; Landa et al., 2012; Miyahara, 2013; Nobile et al., 2011; Travers, Powell, Klinger, & Klinger, 2012). If so it is important to characterize motor performance in ASD using standardized neuropsychological measures like SOG, FTT and GPT.
While these standardized motor tasks represent the behavioral output related to motor function, they also reflect integrity of known motor regions of the brain such as primary motor cortex and other cortical and subcortical regions that support motor function (Lezak et al. 2012). What has not been systematically investigated in ASD, or even in TD indiviudals is how anatomical indices of known motor regions relate to motor control. Using contemporary neuroimaging methods to calculate region of interest (ROI) volume of key brain areas involved in motor function provides an anatomical metric about motor system integrity (see Draganski & Bhatia, 2010; Hervé et al., 2005). However, few studies have been systematic in the application of traditional neuropsychological measures of motor functioning in examining motor areas in ASD let alone neuroimaging findings (Mostofsky et al., 2009; Qiu, Adler, Crocetti, Miller, & Mostofsky, 2010). Furthermore because of the distributed anomalies that may be present in neuroimaging findings associated with ASD, it is important when ROI anatomical studies are performed that multiple regions be examined rather than one or just a few (Frith, 2003; Zielinski et al., 2012). Also, a variety of issues relate to morphological differences in the developing ASD brain where altered developmental trajectories may result in different volumes depending on age (Courchesne and Pierce, 2005; Courchesne, Carper, & Akshoomoff, 2003). Disruption of early brain development, even if that ROI eventually normalizes in volume with age, has the potential to significantly influence circuitry and resultant function (Polsek et al., 2011). Regardless of these developmental factors, assessing ROI volumes of key brain areas is an established quantitative method used as a marker of neural integrity (Tofts, 2008; Jara, 2013).
Given the need for characterization of ASD motor performance in comparison TD individuals on standardized neuropsychological tests and whether ROI differences in the volumes of classic motor areas are observed in ASD, the current investigation had three aims: (1) describe motor function based on traditional neuropsychological SOG, FTT and GPT measures in a large sample of individuals with ASD spanning child to adulthood, (2) compare neuroimaging-derived volumes of classic motor ROIs between ASD and typically developing participants, and (3) explore whether same or different relationships between neuroimaging identified motor ROIs were observed in ASD participants and TD controls. ROI volumes were derived from the automated FreeSurfer method, to include primary motor and sensory cortices, premotor regions of the frontal cortex, basal ganglia (caudate, globus pallidus, and putamen combined), thalamus, and cerebellum.
The GPT involves motor dexterity, visual processing, speed, attention, and continuous monitoring of accuracy, whereas FTT predominately measures simple motor coordination and dexterity and SOG essentially measures only upper extremity muscle strength (Lezak et al., 2012; Strauss, Sherman, & Spreen, 2006). Based in part on what and how these measures assess motor function, a hierarchy of motor control may be inferred. GPT performance reflects a more cognitively demanding task that would require a greater level of multiple and integrated motor regions than FTT and FTT requires greater motor complexity than SOG to carry out the task (Haaland & Delaney, 1981). Indeed, evidence for a motor hierarchical model is provided by factor analytic findings suggesting that FTT and pegboard type tasks measure independent dimensions of manual proficiency (Baser & Ruff, 1987; Stanford & Barratt, 1996). Nonetheless, all of these motor tasks exhibit some degree of association (Schear & Sato, 1989; Strauss, Sherman, & Spreen, 2006), suggesting a common neural substrate likely involving the corticospinal system and associated motor pathways (Triggs et al., 2000).
From this hierarchical perspective of motor control, with increased motor system complexity necessary to perform the task, if ASD is associated with deficits in neural connectivity (Geschwind, 2009) then the greatest likelihood for motor differences between ASD and TD groups would be with the GPT and FTT tasks, with the least likelihood for differences in SOG. We hypothesized that participants with ASD would exhibit lower motor performance compared to TD participants, in particular with the FTT and GPT measures, and that correlates of motor functioning and neuroanatomical ROIs would differ between the two groups.
Method
Subjects and Assessment
Ascertainment
Participants diagnosed with autism and control subjects were a subset from the first wave of data collection of a longitudinal investigation of brain development. Participant selection from the larger sample was based on having complete motor data and time point 1 magnetic resonance imaging (MRI) performed in proximity to motor testing. Details of subject ascertainment are outlined in Alexander et al. (2007). All facets of this investigation were undertaken with the understanding and written consent of each subject or legal guardian, with the approval of the University of Utah and Brigham Young University Institutional Review Boards, where testing was performed, and in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association.
Subject groups
All subjects were male, ages 5–33 years. All subjects had a nonverbal standard IQ score greater than 65 on formal psychometric assessment using either the Differential Ability Scales (Elliot, 1990), Wechsler Intelligence Scale for Children–III (Wechsler, 1991), Wechsler Adult Intelligence Scale-III (Wechsler, 1997) or the Wechsler Abbreviated Scales of Intelligence (Wechsler, 1999). Fifty-nine (59) participants formed the ASD group with 30 participants in the TD control group. Since this was a longitudinal project, for subjects with multiple test dates, neuropsychological data were taken on the date closest to the neuroimaging test date and first test administration. Subject demographics are presented in Table 1.
Table 1.
Demographic Information
| ASD (n = 59) |
Typically-developing (n = 30) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t | p | |
| Age in years | 15.61 | 7.48 | 5.00- 33.17 |
15.29 | 6.48 | 5.17- 26.17 |
0.20 | 0.84 |
| Head Circumference (cm) |
55.54 | 2.36 | 50.60- 60.20 |
55.40 | 2.30 | 49.20- 59.30 |
0.27 | 0.79 |
| Handedness Inventory |
61.89 | 52.24 | −100.00 to 100.00 |
69.90 | 42.98 | −80.00- 100.00 |
−0.72 | 0.47 |
| Wechsler FIQ | 100.30 | 17.71 | 58.00- 137.00 |
120.23 | 16.72 | 95.00- 153.00 |
−5.09** | 0.00 |
| Wechsler PIQ | 101.38 | 17.01 | 64.00- 129.00 |
118.03 | 19.02 | 88.00- 155.00 |
−4.15** | 0.00 |
| Wechsler VIQ | 98.54 | 21.34 | 51.00- 138.00 |
117.57 | 14.83 | 94.00- 151.00 |
−4.79** | 0.00 |
Note. = p<.05
p<.01. Edinburgh Handedness inventory on a scale from −100 (left-handed) to 100 (right-handed).
FIQ = Full Scale IQ, PIQ = Performance IQ, VIQ = Verbal IQ
Autism Spectrum Disorder (ASD)
All cases of autism were idiopathic with all meeting ASD criteria as described. Autism was diagnosed based on the findings of the Autism Diagnostic Interview-Revised (ADI-R), a semi-structured, investigator-based interview with good reliability and validity (Lord et al., 2000) as well as directly assessed with the Autism Diagnostic Observation Schedule-Generic (ADOS-G), a semi-structured play and interview session designed to elicit social, communication, and stereotyped repetitive behaviors characteristic of autism (Lord et al., 2000). ADI-R and ADOS-G descriptive information is presented in Table 2. Where multiple administrations of these measures were available, since most of the participants were part of a longitudinal investigation, the administration on the date closest to the motor test administration was used. Diagnostic classification was based on available ADI-R and ADOS-G findings along with Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV; American Psychiatric Association, 1994) criteria, with the following breakdown: lifetime diagnosis autistic disorder of 52 and pervasive developmental disorder, not otherwise specified of 7. History, physical exam, Fragile-X gene testing, and karyotype were performed on all subjects to exclude medical causes of autistic phenotypes.
Table 2.
Characterization of the Autism and Control sample
| ASD | Typically-developing | |||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | |
| ADOS S+C: Module 1 | 1 | 17.00 (0) | 0 | 0 | ||
| ADOS S+C: Module 2 | 5 | 17.20 (4.15) | 12–23 | 1 | 0 (0) | 0 |
| ADOS S+C: Module 3 | 22 | 14.77 (3.62) | 7–21 | 11 | 1.55 (1.44) | 0–4 |
| ADOS S+C: Module 4 | 31 | 12.32 (3.90) | 1–20 | 16 | .75 (1.00) | 0–3 |
| ADI-R Soc | 51 | 20.11 (5.71) | 6–30 | 0 | ||
| ADI-R Com | 51 | 15.65 (4.64) | 7–25 | 0 | ||
| ADI-R RSB | 51 | 7.02 (2.36) | 2–12 | 0 | ||
Note. ADOS S+C = Autism Diagnostic Observation Schedule: Social and Communication Total. The ADOS consists of 4 modules and the individual being evaluated is given just one module, depending on expressive language level and chronological age. Each module has different cut-off scores and as such should not be considered equivalent. Two control participants had incomplete ADOS data, which is not reported above. ADI-R Soc = Autism Diagnostic Interview, Revised: Reciprocal Social Interactions; ADI-R Com = Autism Diagnostic Interview, Revised: Language/Communication; ADI-R RSB = Autism Diagnostic Interview, Revised: Restricted, Repetitive, and Stereotyped Behaviors and Interests. Four participants in the autism sample were missing ADI-R data and no ADI-R data were available for the control group.
Control sample
TDC participants had no developmental, neurological, or clinical history for major psychiatric disorders, and completed an ADOS-G assessment to ensure none met criteria for ASD.
Neuroimaging
Volumetric studies were based on magnetic resonance images acquired on a Siemens Trio 3.0 Tesla scanner at the University of Utah. A 12-channel, receive-only RF head coil was used to obtain 3D T1-weighted image volumes with 1mm isotropic resolution using an MP-RAGE sequence (TI = 900 msec, TR = 2300 msec, TE = 2.91 msec, flip angle = 9 degrees, sagittal, field of view = 25.6 cm, matrix = 256 × 256 × 160). Imaging from three ASD subjects could not be used and were not part of the quantitative MRI analyses.
Volumetric image analysis
All analyses were performed with FreeSurfer, version 5.1 (http://surfer.nmr.mgh.harvard.edu/) and followed the methods detailed by Bigler et al. (2010). The following gray matter ROIs were examined: precentral gyrus, postcentral gyrus, caudal middle frontal area (i.e., supplementary motor area), thalamus, cerebellum, and basal ganglia. Whole brain white and gray matter and intracranial volume were also estimated.
Justification for including these ROIs comes from Moritz, Haughton, Cordes, Quigley, and Meyerand (2000), who demonstrated involvement of the precentral and postcentral gyri, supplementary motor area, cerebellum, thalamus, and putamen during a FTT task using fMRI techniques. SOG has been shown to be associated with activation of the postcentral gyri and supplementary motor area as well as cerebellum (Cramer et al., 2002). All of the above motor regions are also the presumed motor control areas involved in the GPT, although GPT performance has not yet been examined from a neuroimaging perspective. Increased levels of motor skill and precision are required to manipulate the small pegs used with the GPT (Lezak, et al., 2012), suggesting coordination of primary motor, basal ganglia and cerbellar regions along with sensory-perceptual feedback to efficiently perform the GPT task.
IQ
Verbal skills are often diminished in autism (Rapin, 1999) along with considerable variability in verbal and performance IQ scores, often resulting in lower IQ in autism samples (Deutsch & Joseph, 2003). However, in disorders where intellectual compromise may be part of the clinical picture for the dependent variable in question, over controlling for IQ may, in fact, limit important findings (see Dennis, Francis, Cirino, Schachar, Barnes &Fletcher, 2009). Likewise, to be as inclusive of the ASD sample being assessed we utilized a liberal lower IQ limit [performance IQ (PIQ) ≥ 64] as the minimal level of intellectual functioning to participate in this investigation. Only three ASD participants had a PIQ score below 70 and only one with a PIQ of 64. To further examine issues related to IQ, because the ASD and control samples were sufficiently large, as part of a secondary comparison, participants with ASD were matched to controls to be within five IQ points, to control for differences that may be specific to IQ. Since the lowest PIQ score in the control sample was 88, in this secondary analysis matching on IQ, none of the lower functioning participants were included in those comparisons.
Head circumference and handedness
Standard occipitofrontal head circumference and handedness based on the Edinburgh Handedness Inventory (Oldfield, 1971) were obtained on all subjects. Demographic findings are reported in Table 1.
Motor tests
Standard FTT and SOG instruments from the Halstead-Retain Battery (Reitan Laboratories: www.reitanlabs.com; see also [Heaton, Grant, & Matthews, 1991]) were used along with the standard GPT (Matthews & Klove, 1964). All participants were administered the SOG, FTT, and GPT as outlined by Reitan and Wolfson (1986), Heaton et al. (1991), and Matthews and Klove (1964). The manual finger tapping board was used for the majority of subjects but for younger children the electronic FTT version was used. The number of taps performed over 10 second epochs was recorded for each trial, separately for each hand. To ensure consistency of responding, a minimum of five trials was administered with the total score required to be within ±5 points. If this was not achieved, the total from all trials were averaged for each hand and both hands combined.
For the GPT, the participant was required to insert pegs in a prescribed order as quickly as possible using the dominant and nondominant hand separately. The number of pegs dropped was also recorded. The score was the time required to place pegs into all 25 holes, and the timing was not interrupted in the event of a dropped peg. For participants over eight years of age, 25 pegs were administered and for aged 5–8 participants, only 10 pegs were administered. To compare all subjects’ motor performance for this test, the standard metric variables of drop rate (number of pegs dropped/total number of peg holes), and unit completion time (number of pegs placed/total completion time) were calculated. The total GPT score was computed by combining the nondominant hand completion time and the dominant hand completion time. SOG was measured by use of a hand dynamometer. The test requires the person to hold the upper part of the dynamometer in the palm of the hand and squeeze the stirrup with the fingers as tightly as possible. The average strength in kilograms of the two trials was recorded for each hand if they were within a 5-point range. If the two trials were not within ±5 points, a third trial was completed and the average of those 3 three trials was used. The total SOG score was computed by combining the nondominant hand mean and the dominant hand mean.
Statistical analysis
Group means were calculated and compared for autism and control subjects, using analysis of covariance (ANCOVA) with age, head circumference, and PIQ as covariates. FTT mean scores for both hands, GPT unit completion time (single peg) and peg drop rate for both hands, and the SOG means for both hands were compared between ASD and control groups. Motor findings were then examined in relation to key neuroimaging identified motor areas based on the volumetric data using partial correlations. FTT, GPT, and SOG totals were used for the partial correlations to reduce family wise error rates, including linear regressions that explored possible group by region interactions. A testwise false-positive error rate was set at 0.05, thus controlling for potential experimentwise errors. Analyses were run using version 20 of the IBM SPSS Statistics package. Clinical impairment on standardized neuropsychological measures is often conservatively defined as falling two standard deviations (z-score = −2.0) below the mean of a normative sample (Lezak et al., 2012; Strauss, Sherman, and Spreen, 2006). For each measure a simple frequency count was determined for who performed at this level of impairment based on normative data derived from the Lafayette Instrument Company (2002), Strauss et al., 2006, Mathiowetz, Wiemer, and Federman (1986), and Nussbaum and Bigler, (1997).
Results
Sample Characteristics
As previously shown in Table 1, no significant differences were found for group-matching variables (age, head circumference, handedness index) except IQ, as expected.
Motor Test Performance in Autism and Controls
SOG, FTT and GPT results, along with effect size differences between the ASD and TDC groups, are summarized in Table 3. Motor tasks were all positively inter-correlated as shown in Table 4. The distributions for SOG and GPT were not normally distributed; however, after applying log transformations homogeneity of variance assumptions were met. Results of SOG, FTT and GPT performance by group are detailed in Table 4. As shown in Table 1 because of the IQ differences between TD controls (TDC) and those with ASD, an IQ matched sample (matching on PIQ) was created from the original sample (within +/− 5 PIQ points) consisting of 24 participants in each group, resulting in no significant difference on PIQ between groups [t(46) = −.63, p = .54]. Even after matching on IQ, there was no difference in the pattern of motor task findings between the IQ matched group and the original sample using PIQ as a covariate; therefore, the findings reported below were based on all subjects with PIQ as a covariate.
Table 3.
Motor Performance ANCOVAs controlling for Age, Head Circumference, and PIQ
| Hand | Mean ASD (SD) N=56 |
Mean TD (SD) N=30 |
F | p | ρη2 |
|---|---|---|---|---|---|
| SOG | |||||
| DH | 27.23(14.94) | 31.34(15.92) | 1.57 | .21 | .019 |
| NDH | 25.56(14.16) | 29.06 (14.87) | 3.47 | .07 | .040 |
| FTT | |||||
| DH | 41.40(8.46) | 44.94(8.87) | 5.90* | .02 | .070 |
| NDH | 38.95(8.27) | 41.95(9.55) | 9.51** | .003 | .110 |
| GPT-Drop Rate | |||||
| DH | 0.03(0.06) | 0.02(0.04) | 0.99 | .32 | .012 |
| NDH | 0.04(0.06) | 0.01(0.02) | 9.32** | .005 | .231 |
| GPT- UCT | |||||
| DH | 3.34(0.96) | 2.70 (0.49) | 18.70** | <.001 | .182 |
| NDH | 3.86 (1.27) | 3.03 (.73) | 19.93** | <.001 | .192 |
Note. TD = typically-developing; ASD = Autism Spectrum Disorder; FTT = Finger Tapping Test; SOG = Strength of Grip; GPT = Grooved Pegboard Test; DH = dominant hand; NDH = nondominant hand; UCT = Unit Completion Time; ρη2 = partial eta squared. Partial eta squared is the default effect size measure reported in SPSS. If you have only one predictor variable, then partial eta squared is equivalent to eta squared. SOG was measured in kilograms.
p<.05
p<.01. SOG-DH, Unit Completion Time-DH and NDH, and Drop Rate-NDH did not pass the homogeneity of variance assumption. Log transformations were performed for these variables and homogeneity assumptions were met. Means for the SOG and GPT-Drop Rate and GPT-UCT are untransformed.
Table 4.
Correlation Matrix of Motor Performance in ASD and TD
p<.05
p<.01
SOG
Participants with ASD had lower SOG scores, which only approached significance for the nondominant hand (see Table 3). For the ASD group, 7 participants (11.8%) were clinically impaired (i.e., z-score ≥ −2.0) for either hand with 3 (10.0%) participants found in the impaired range for the TDC group.
FTT
ASD individuals were significantly slower (i.e., less taps per 10 second epoch) for both hands on the FTT (see Table 3), although effect size differences were nominal to small (Cohen, 1988). For the ASD group, 5 (8.5%) participants were clinically impaired (i.e., z-score ≥ −2.0) for both hands, 6 (10.1%) for the dominant hand only and 8 (13.6%) for the nondominant hand only. No individuals in the TDC group performed at a level meeting clinically impaired cutoffs.
GPT
On the GPT task ASD individuals were also subtly but significantly slower than controls in the time that was required to retrieve a single peg and correctly place it on the board (i.e., UCT) with either hand and had significantly more drops using the nondominant hand, differences that remained significant even after log transformation of the non-normally distributed GPT (see Table 3). As shown in Table 3 for these significant GPT findings, effect size differences were small but substantially larger than what was observed with FTT. For the ASD group concerning total completion time, 2 (3.3%) participants were clinically impaired (i.e., z-score ≥ −2.0) for both hands, 4 (6.7%) for the dominant hand only and 2 (3.3%) for the nondominant hand only. No individuals in the TDC group below the clinical cutoff.
Age Effects and Motor Performance
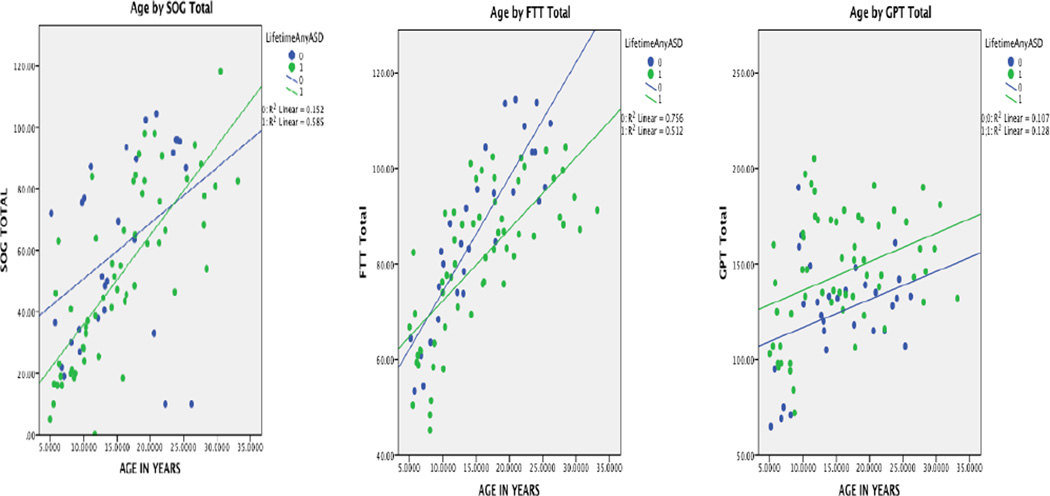
Because of the age range examined in this investigation, group comparisons by age and motor performance were undertaken and plotted as shown in Figure 1, which revealed cross-sectional similarities and differences for ASD and TDC participants. Motor performance is clearly age-dependent regardless of group. The ASD and TDC slopes for cross-sectional age-related changes were similar for FTT and GPT performance (although longitudinal investigations will be more apt at detecting true age-related changes in these motor measures). As shown in Figure 1, overall FTT performance is slower by just a few taps per 10 second epoch. However, for GPT performance, while the regression lines have similar slopes at all age levels ASD subjects are slower. Using an arbitrary cut point based on visual inspection of approximately 145 seconds to completion, 29/59 (49.2%) of the ASD subjects had slower times but only 6/30 (20%) of controls.
Figure 1.
Age by motor task performance plots. Green = ASD; Blue = TDC
ROI Volumes in Autism and Controls
ANCOVA comparing the following motor areas including the precentral gyrus, postcentral gyrus, caudal middle frontal area (i.e. supplementary motor area), thalamus, cerebellum, and basal ganglia volumes revealed no significant volume differences between the two groups (Table 5), after controlling for age, head circumference, and PIQ. There were no differences between the groups in total gray matter volume, total white matter volume, and intracranial volume using the same covariates (data not shown).
Table 5.
ROI ANCOVAs controlling for Age, Head Circumference, and PIQ
| Structure | Mean ASD (SD) N=59 |
Mean TD (SD) N=30 |
F | p | ρη2 |
|---|---|---|---|---|---|
| Thalamus | 16.92 (1.46) | 17.05 (2.17) | 0.09 | .76 | .001 |
| Precentral | 30.84 (4.51) | 31.48 (3.78) | 0.35 | .55 | .004 |
| Postcentral | 21.28 (3.83) | 21.91 (2.93) | 1.06 | .31 | .013 |
| Caudal Middle Frontal | 17.26 (3.44) | 17.98 (3.30) | 0.18 | .67 | .002 |
| Basal Ganglia | 25.46 (2.44) | 25.02 (3.07) | 2.38 | .13 | .030 |
| Cerebellum | 124.97 (13.34) | 127.55 (14.38) | 0.01 | .92 | <.001 |
Note. ROI volumes combine left and right hemisphere volumes for each ROI structure. ROI units are measured in centimeters cubed. Basal Ganglia comprised the caudate, putamen, and pallidum volumes. ρη2 = partial eta squared. Partial eta squared is the default effect size measure reported in SPSS. If you have only one predictor variable, then partial eta squared is equivalent to eta squared. TD = typically-developing; ASD = Autism Spectrum Disorder.
p<.05
p<.01.
Relationship between ROI Volume and Motor Performance
The relationship between motor ROI volumes and motor performance was examined using partial correlations controlling for age and head circumference; Table 6. To reduce the number of statistical comparisons, right and left motor performance scores were combined into a total score for SOG, FTT and GPT yielding a single motor index for each measure. Bilateral ROI volumes were also combined to create a total volume for each motor ROI. In ASD, the FTT score was negatively correlated with the volume of the precentral gyrus (r = −.30, p = .025), indicating that as brain volume increased, motor performance decreased (Table 5). The FTT-precentral gyrus volume relationship was not significantly different in ASD and TDC participants; there was no significant group by precentral gyrus interaction (t = −.54, p = .59). No ROI volumes were significantly correlated with motor performance for TDC participants. Partial correlations between total white matter volume and motor performance were all insignificant regardless of group (Table 7). Similarly, individual ROI volumes for gray matter and white matter did not show significant correlations with overall motor performance.
Table 6.
Partial Correlations between motor ROI volumes and motor performance for ASD – controlling for Age and Head Circumference
| Structure | Finger Tapping | Grooved Pegboard (Completion Time) |
Strength of Grip (kgs) |
|||
|---|---|---|---|---|---|---|
| ASD | TD | ASD | TD | ASD | TD | |
| Precentral gyrus | −.30* | .08 | −.25 | −.07 | −.07 | −.20 |
| Postcentral gyrus | −.19 | .28 | −.08 | −.09 | −.15 | .07 |
| Caudal Middle Frontal | −.18 | .10 | −.19 | .03 | −.06 | −.10 |
| Thalamus | −.06 | −.20 | −.10 | −.19 | −.12 | −.16 |
| Cerebellum | −.18 | −.09 | −.13 | −.08 | .04 | −.12 |
| Basal Ganglia | −.15 | −.14 | .26 | −.11 | .08 | −.30 |
Note. ROI volumes combine left and right hemisphere volumes for each ROI structure. ASD = Autism Spectrum Disorder; TD = typically-developing; ASD = Autism Spectrum Disorder.
p<.05.
Table 7.
Partial Correlations between total white matter volume and motor performance for ASD – controlling for Age and Head Circumference
| Structure | Finger Tapping | Grooved Pegboard (Completion Time) |
Strength of Grip (kgs) |
|||
|---|---|---|---|---|---|---|
| ASD | TD | ASD | TD | ASD | TD | |
| TD | ||||||
| Total White Matter Volume | −.02 | .03 | .03 | −.14 | −.14 | −.29 |
Note. ASD = Autism Spectrum Disorder; TD = typically-developing; ASD = Autism Spectrum Disorder.
p<.05
Discussion
Two major aims of this investigation were to characterize motor function in a large sample of individuals with ASD spanning child to adulthood using traditional neuropsychological SOG, FTT and GPT measures and determine if any gross differences in ROI volumes derived from classic motor areas of the brain were present. As a group, ASD participants exhibited reduced motor performance on standard FTT and GPT neuropsychological tasks compared to age- and sex-matched (all males) TD counterparts, even after controlling for differences in IQ. Although significant, the magnitude of difference was generally subtle, as effect size differences were only small for the comparisons that yielded significant differences. Likewise, only a small number of individuals in the ASD group exhibited what could be described as clinical impairment on all three motor tasks, in contrast to the TDC group. Anatomically, there were no gross volumetric differences in key motor areas between the ASD and control participants. Thus, the observed diminished finger oscillation speed and dexterous motor control on the FTT and GPT tasks in the ASD group cannot be attributed to gross anatomical difference within key motor regions. Motor cortex volume was negatively correlated with FTT performance in ASD but not in controls. Although SOG was lower in the ASD group it did not differ significantly from controls. The findings of reduced FTT and GPT performance in ASD, tasks that require more dexterous motor control than grip strength, fits with the supposition that where motor dysfunction may occur in ASD is at higher levels of motor integration. Furthermore, it was only with the GPT task that the differences were present at all age levels (see Figure 1).
Furthermore, the lack of significance with SOG but with FTT and GPT would be expected if ASD is, in part, related to abnormal networks and connectivity, since both FTT and GPT would require larger integrated networks for motor performance than basic strength. Motor deficits in ASD could also be related to a disturbance in functional organization of the motor cortices (Muller et al., 2001). The one observation of a negative correlation between motor cortex and FTT would be consistent with an overgrowth theory of autism, where overgrowth may be associated with less efficient networks (Amaral, Dawson, & Geschwind, 2011; Courchesne, Webb, & Schumann, 2011), and therefore, larger size associated with poorer function. Mostofsky and colleagues (2007), relying on clinical motor exam findings only, observed that increased white matter volume of motor cortex in autism was associated with abnormal clinical motor findings. In the current study, volume differences of motor cortex were not observed, but nonetheless, a larger motor cortex was associated with reduced FTT speed in ASD participants. It is possible that subtle overgrowth and/or lack of pruning within neural systems that control motor function leads to inefficiency (Miyoshi & Okada, 2004) and to the general negative relationships of motor cortex volume and FTT in ASD. The normal developmental process of synaptic pruning has been postulated to be compromised in autism (Frith, 2003; Schultz, Klin, & Lombroso, 2002), disrupting how activity and experience support the organization of functional networks in typical brain development (Just, Keller, Malave, Kana, & Varma, 2012; Kandel et al., 2000). Just, Keller, Malave, Kana, and Varma (2012) have postulated that the developing autistic brain may not support an appropriate balance between maturation and experience. Errors in pruning may interfere with the emergence of the specialized functions of one or more of a set of neuroanatomical structures (see Pelphrey, Shultz, Hudac, and Vander Wyk (2011). In TD individuals normal pruning could help eliminate faulty connections and optimize coordinated neural functioning, which compromised pruning might fail to accomplish. Compromised pruning processes possibly result in some degree of aberration in typical anatomical size-function relations that adversely influences communication among cortical regions (Just et al., 2012). However, there is much to be discovered about development, pruning, white matter connectivity and cortical maturation in ASD (Cassanova et al., 2006). While the findings of larger motor cortex being associated with poorer motor function in this ASD sample fits with aberrant development and potential faulty pruning and/or overgrowth, the precise meaning of these findings is unknown.
Hadders-Algra (2008) postulated that the relationship between preterm birth injury, white matter damage and motor deficits in children diagnosed with ASD was a problem of connectivity. Ligam et al. (2009) have shown a greater incidence of thalamic pathology in periventricular leukomalacia (PVL) associated with prematurity, and speculate about the relation between thalamic damage, white matter pathology and autism (see also Limperopoulos et al., 2008). Since PVL has the potential to disrupt neuronal migration, the potential thalamic damage and disrupted white matter connectivity being related to ASD is intriguing. Furthermore, Nair, Traiber, Shukla, Shih and Muller (2013) recently reported impaired thalomocortical connectivity in ASD. In the current study, prematurity was excluded as a risk factor for the ASD cohort examined. Thalamic volume in the ASD cohort did not differ from the TDC group and therefore no gross thalamic pathology was found. Thalamic volume did not significantly relate to any motor measurement nor did white matter volume. As such, the current findings are unlikely related to any gross neural insult or abnormality, but these methods do not specifically address issues of connectivity and development. Unfortunately, ROI volumes likely represent poor proxies as indices of brain connectivity and studies that employ more direct measures of functional connectivity should be conducted in relation to motor performance in ASD (see Miyahara, 2013).
While SOG is primarily a basic measure of the integrity of the corticospinal tract (Schulz et al., 2012), functional MRI (fMRI) studies also indicate that SOG performance results in some basal ganglia and cerebellar activation (Cramer, et al., 2002; Keisker, Hepp-Reymond, Blickenstorfer, & Kollias, 2010; van Nuenen, Kuhtz-Buschbeck, Schulz, Bloem, & Siebner, 2012). Nonetheless, this SOG network is far more simple than the activation patterns observed during FTT performance as assessed by functional neuroimaging (De Guio, Jacobson, Molteno, Jacobson, & Meintjes, 2012; Lopez-Larson et al., 2012; Witt, Laird, & Meyerand, 2008), where coordinated finger movement has been shown to depend on widespread motor connections (Jin, Lin, & Hallett, 2012; Roessner et al., 2012).
Because of the motion restrictions imposed by fMRI studies of motor function, the SOG and FTT methods have been readily adapted to the scanner environment, but not the GPT task. Neuroimaging lesion studies as well as low frequency transcranial magnetic stimulation associated with GPT performance have found correlations between GPT proficiency and connectivity between motor, premotor and supplemental motor cortices, corpus callosum and cerebellum (Franc et al., 2011; Kodl et al., 2008; Miall & Christensen, 2004; Otten et al., 2012) where reduced parietal white matter integrity on DTI was associated with worse GPT performance. These latter findings may be best explained by GPT performance not only requires motor skill but guided attention as well (Lezak, et al., 2012). The preceding considerations suggest that the GPT task requires far greater neural complexity than SOG. The significant differences observed with FTT and GPT in autism, and that these tasks depend on both short- and long-range connectivity, is consistent with the postulated functional connectivity problems associated with ASD (Dowell, Mahone, & Mostofsky, 2009; Mostofsky & Ewen, 2011).
ROI Volumes and Motor Function
Previous voxel-based morphometry studies found enlargements in the right postcentral gyrus, right medial frontal gyrus, and the right posterior lobe of cerebellum (Ke et al., 2008) and decreases in the left precentral gyrus (Cauda et al., 2011) and left supplementary motor area (Mengotti et al., 2011) in autism when compared with controls. The Mengotti et al. (2011) and Ke et al. (2008) studies differ from the current study in that they have much smaller sample sizes and a smaller age range. Cauda et al. (2011) was a meta-analysis of 16 studies with a total of 350 ASD subjects (which included high-functioning autism, Asperger’s Syndrome, and unspecified diagnoses), and male and female subjects. None of the above studies examined motor function with the SOG, FTT or GPT tasks systematically as have been done in this investigation.
Size-function relationships may emerge in brain development potentially reflecting optimal size (e.g., volume) of a given structure that may best relate to cognitive function as measured by neuropsychological variables (Koscik & Tranel, 2012). However, ROI volume relationships with neuropsychological functioning are complex, particularly in individuals with neuropsychiatric disorders (Crespo-Facorro, Barbadillo, Pelayo-Teran, & Rodriguez-Sanchez, 2007). Because of the complexities of brain development and individual differences in cognitive and neurobehavioral functioning, brain volumetry may not correlate with neuropsychological variables in a consistent and systematic pattern. Given the general lack of ROI volume relationships, it is likely that such an approach as used in the current investigation may simply be insensitive in detecting motor network abnormalities. Therefore, given prior reports of white matter pathway abnormalities in autism (Lainhart & Lange, 2011; Mak-Fan et al., 2012; Weinstein et al., 2011), it may be that measures more directly assessing white matter connectivity, such as diffusion tensor imaging (DTI) and functional connectivity MRI, may provide improved understanding of motor abnormalities in autism. In fact, those investigations are underway with this cohort.
Limitations and Conclusions
The autism sample was all male and comprised of high functioning individuals who do not represent those with lower functioning abilities, or females with autism. As already stated this investigation did not directly assess connectivity where direct measures of motor performance using functional MRI techniques may yield much more specific findings related to motor differences in ASD compared to controls. Also, this study did not assess presence of clinical motor signs and their relationship to objective neuropsychological measures of motor functioning.
Despite these limitations the current findings do fit with a hierarchical model of impaired functioning in autism where basic motor ability is preserved, but as motor complexity increases, a greater likelihood of impaired motor function is observed in ASD. As Miyahara (2013) posited, it is more meaningful and clinically practical to use standard assessments thereby insuring common metrics used by researchers and clinicians in the study of ASD. Using all three standardized neuropsychological measures (i.e., SOG, FTT, GPT), as in the current study provides a motor profile that hopefully will be useful to clinicians assessing motor function in ASD. As shown in Figure 1, regardless of age there were consistently more individuals with ASD that exhibited slowed performance times on the GPT than observed in control participants. The hierarchical model of motor control used in the current investigation as well as the findings reported herein, provide information and heuristics to further explore motor findings associated with ASD.
Acknowledgments
The project described was supported by Grant Numbers RO1 MH080826 (JEL, EDB, ALA, NL), RO1 MH084795 (JEL, PTF, NL), and KO8 MH092697 (JSA) from the National Institute Of Mental Health; Grant Numbers T32 HD07489 (BGT) and P30 HD003352-45 (Waisman Center Core Grant) from the Eunice Kennedy Shriver NICHD, The Hartwell Foundation (BGT), and the Primary Children’s Foundation Early Career Development Award (BAZ). Support from the Poelman Foundation to Brigham Young University for autism research is gratefully acknowledged. The authors report no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health & Development, or the National Institutes of Health. We thank former members of the Utah Autism CPEA for their assistance during the early stages of this project. We sincerely thank the children, adolescents, and adults with autism and the individuals with typical development, who participated in this study, and their families. The assistance of Tracy J. Abildskov with image analysis and Jo Ann Petrie, Ph.D. with manuscript preparation is gratefully acknowledged.
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Amaral D, Dawson G, Geschwind DH. Autism spectrum disorders. Oxford: Oxford University Press; 2011. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders-DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. DSM-5 Development. [Online]. American Psychiatric Association. Available online at: http://www.dsm5.org.
- Baser CN, Ruff RM. Construct validity of the San Diego Neuropsycholgocial Test Battery. Archives of Clinical Neuropsychology. 1987;2:13–32. [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Wilde EA, McCauley SR, Li X, Merkley TL, Fearing MA, Newsome MR, Scheibel RS, Hunter JV, Chu Z, Levin HS. Diffuse damage in pediatric traumatic brain injury: A comparison of automated versus operator-controlled quanitification methods. NeuroImage. 2010;50:1017–1026. doi: 10.1016/j.neuroimage.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Bonnet KA, Gao XK. Asperger syndrome in neurologic perspective. Journal of Child Neurology. 1996;11:483–489. doi: 10.1177/088307389601100615. [DOI] [PubMed] [Google Scholar]
- Carcani-Rathwell I, Rabe-Hasketh S, Santosh PJ. Repetitive and stereotyped behaviours in pervasive developmental disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:573–581. doi: 10.1111/j.1469-7610.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Schmitz C. Minicolumnar abnormalities in autism. Acta neuropathologica. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geda E, Sacco K, D'Agata F, Duca S, Geminiani G, et al. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:1304–1313. doi: 10.1136/jnnp.2010.239111. [DOI] [PubMed] [Google Scholar]
- Cohen J. 2nded. Hillsdale, NJ: Erlbaum; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23(2–3):153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Webb SJ, Schumann C. From toddlers to adults: the changing landscape of the brain in autism. In: Amaral D, Dawson G, Geschwind GDH, editors. Autism spectrum disorders. Oxford: Oxford University Press; 2011. pp. 611–631. [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, et al. Motor cortex activation is related to force of squeezing. Human Brain Mapping. 2002;16:197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Barbadillo L, Pelayo-Teran JM, Rodriguez-Sanchez JM. Neuropsychological functioning and brain structure in schizophrenia. International Review of Psychiatry. 2007;19:325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- De Guio F, Jacobson SW, Molteno CD, Jacobson JL, Meintjes EM. Functional magnetic resonance imaging study comparing rhythmic finger tapping in children and adults. Pediatric Neurology. 2012;46:94–100. doi: 10.1016/j.pediatrneurol.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch CK, Joseph RM. Brief report: cognitive correlates of enlarged head circumference in children with autism. Journal of Autism and Developmental Disorders. 2003;33:209–215. doi: 10.1023/a:1022903913547. [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23:563–570. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey R, Rapport MJ. Motor activity in children with autism: a review of current literature. Pediatric Physical Therapy. 2012;24:2–20. doi: 10.1097/PEP.0b013e31823db95f. [DOI] [PubMed] [Google Scholar]
- Draganski B, Bhatia KP. Brain structure in movement disorders: A neuroimaging perspective. Current Opinion in Neurology. 2010;23:413–419. doi: 10.1097/WCO.0b013e32833bc59c. [DOI] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Elliot CD. Differential ability scales. San Antonio, TX: The Pyschological Corporation; 1990. [Google Scholar]
- Emck C, Bosscher R, Beek P, Doreleijers T. Gross motor performance and self-perceived motor competence in children with emotional, behavioural, and pervasive developmental disorders: a review. Developmental Medicine & Child Neurology. 2009;51(7):501–517. doi: 10.1111/j.1469-8749.2009.03337.x. [DOI] [PubMed] [Google Scholar]
- Emck C, Bosscher RJ, van Wieringen PC, Doreleijers T, Beek PJ. Psychiatric symptoms in children with gross motor problems. Adapted Physical Activity Quarterly: APAQ. 2012;29:161–178. doi: 10.1123/apaq.29.2.161. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long-standing type 1 diabetes. Diabetes. 2011;60:315–319. doi: 10.2337/db10-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. What do imaging studies tell us about the neural basis of autism. Autism: Neural basis and treatment possibilities. 2003:149–176. [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annual Review of Medicine. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Wang C, Salgado MW, Greene PE, Kim M, Rapin I. Motor stereotypies in children with autism and other developmental disorders. Developmental Medicine and Child Neurology. 2009;51:30–38. doi: 10.1111/j.1469-8749.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- Gowen E, Hamilton A. Motor abilities in autism: A review using a computational context. Journal of Autism and Developmental Disorders. 2012 Jun;22 doi: 10.1007/s10803-012-1574-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Delaney HD. Motor deficits after left or right hemisphere damage due to stroke or tumor. Neuropsychologia. 1981;19:17–27. doi: 10.1016/0028-3932(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M. Reduced variability in motor behaviour: An indicator of impaired cerebral connectivity? Early Human Development. 2008;84:787–789. doi: 10.1016/j.earlhumdev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Kilpatrick M, Keshavan MS, Minshew NJ. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. Journal of Child Neurology. 2003;18:317–324. doi: 10.1177/08830738030180050801. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Hervé PY, Mazoyer B, Crivello F, Perchey G, Tzourio-Mazoyer N. Finger tapping, handedness and grey matter amount in the Rolando's genu area. NeuroImage. 2005;25:1133–1145. doi: 10.1016/j.neuroimage.2004.12.062. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jara H. New Jersey: World Scientific; 2013. Theory of quantitative magnetic resonance imaging. [Google Scholar]
- Jin SH, Lin P, Hallett M. Reorganization of brain functional small-world networks during finger movements. Human Brain Mapping. 2012;33:861–872. doi: 10.1002/hbm.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4th ed. New York: McGraw-Hill; 2000. [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Ke X, Hong S, Tang T, Zou B, Li H, Hang Y, et al. Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport. 2008;19:921–925. doi: 10.1097/WNR.0b013e328300edf3. [DOI] [PubMed] [Google Scholar]
- Keisker B, Hepp-Reymond MC, Blickenstorfer A, Kollias SS. Differential representation of dynamic and static power grip force in the sensorimotor network. The European Journal of Neuroscience. 2010;31:1483–1491. doi: 10.1111/j.1460-9568.2010.07172.x. [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Troutman MR, Davis G, King PG, et al. Autism severity and muscle strength: A correlation analysis. Research in Autism Spectrum Disorders. 2011;5:1011–1015. [Google Scholar]
- Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. Brain evolution and human neuropsychology: the inferential brain hypothesis. Journal of the International europsychological Society: JINS. 2012;18:394–401. doi: 10.1017/S1355617712000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafayette Instrument Company. Grooved pegboard test user instructions. 2002 Retrieved from http://www.si-instruments.com.au/industry/download/lafayette-current-version-grooved-pegboard-test-32025-lafayette-32025-grooved-pegboard-test-manual-pdf.html.
- Lainhart JE, Lange N. Increased neuron number and head size in autism. JAMA: The Journal of the American Medical Association. 2011;306:2031–2032. doi: 10.1001/jama.2011.1633. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Bauman M. Journal of Child Psychology and Psychiatry. 53: 2012. Latent class analysis of early developmental trajectory in baby siblings of children with autism; pp. 986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5th ed. Oxford, New York: Oxford University Press; 2012. [Google Scholar]
- Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, Kinney HC. Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatric research. 2009;65:524–529. doi: 10.1203/PDR.0b013e3181998baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Sullivan NR, Soul JS, Robertson RL, Moore M, du Plessis AJ. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 2008;121(4):758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, Yurgelun-Todd DA. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Research. 2012;202:224–232. doi: 10.1016/j.pscychresns.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Mak-Fan KM, Morris D, Vidal J, Anagnostou E, Roberts W, Taylor MJ. White matter and development in children with an autism spectrum disorder. Autism. 2012 Jun;14 doi: 10.1177/1362361312442596. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Wiemer DM, Federman SM. Grip and pinch strength: norms for 6-to 19-year-olds. The American Journal of Occupational Therapy. 1986;40(10):705–711. doi: 10.5014/ajot.40.10.705. [DOI] [PubMed] [Google Scholar]
- Matthews C, Klove H. Instruction manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- Mengotti P, D'Agostini S, Terlevic R, De Colle C, Biasizzo E, Londero D, et al. Altered white matter integrity and development in children with autism: A combined voxel-based morphometry and diffusion imaging study. Brain Research Bulletin. 2011;84:189–195. doi: 10.1016/j.brainresbull.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Miall RC, Christensen LO. The effect of rTMS over the cerebellum in normal human volunteers on peg-board movement performance. Neuroscience Letters. 2004;371:185–189. doi: 10.1016/j.neulet.2004.08.067. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society: JINS. 1997;3:303–316. [PubMed] [Google Scholar]
- Miyahara M. Meta Review of Systematic and Meta Analytic Reviews on Movement Differences, Effect of Movement Based Interventions, and the Underlying Neural Mechanisms in Autism Spectrum Disorder. Frontiers in Integrative Neuroscience. 7:16. doi: 10.3389/fnint.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi S, Okada M. Storage capacity diverges with synaptic efficiency in an associative memory model with synaptic delay and pruning. IEEE Transactions on Neural Networks. 2004;15:1215–1227. doi: 10.1109/TNN.2004.832711. [DOI] [PubMed] [Google Scholar]
- Moritz CH, Haughton VM, Cordes D, Quigley M, Meyerand ME. Whole-brain functional MR imaging activation from a finger-tapping task examined with independent component analysis. AJNR American Journal of Neuroradiology. 2000;21:1629–1635. [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Ewen JB. Altered connectivity and action model formation in autism is autism. The Neuroscientist. 2011;17:437–448. doi: 10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Schafer S, Kuhn W, Przuntek H. Correlation between tapping and inserting of pegs in Parkinson's disease. The Canadian Journal of Neurological Sciences. 2000;27:311–315. doi: 10.1017/s0317167100001062. [DOI] [PubMed] [Google Scholar]
- Muller RA, Pierce K, Abrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biological Psychiatry. 2001;49:665–76. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Perego P, Piccinini L, Mani E, Rossi A, Bellina M, et al. Further evidence of complex motor dysfunction in drug naive children with autism using automatic motion analysis of gait. Autism. 2011;15:263–283. doi: 10.1177/1362361309356929. [DOI] [PubMed] [Google Scholar]
- Nussbaum NL, Bigler ED. Halstead-Reitan neuropsychological test batteries for children. In: Reynolds CR, Fletcher-Janzen E, editors. Handbook of Clinical Child Neuropsychology. New York and London: Plenum Press; 1997. [Google Scholar]
- Oberman LM, McCleery JP, Hubbard EM, Bernier R, Wiersema JR, Raymaekers R, et al. Developmental changes in mu suppression to observed and executed actions in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012 Feb;4 doi: 10.1093/scan/nsr097. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otten ML, Mikell CB, Youngerman BE, Liston C, Sisti MB, Bruce JN, et al. Motor deficits correlate with resting state motor network connectivity in patients with brain tumours. Brain. 2012;135:1017–1026. doi: 10.1093/brain/aws041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SW, Oberg B, Larsson LE, Lindval B. Gait analysis, isokinetic muscle strength measurement in patients with Parkinson's disease. Scandinavian Journal of Rehabilitation Medicine. 1997;29:67–74. [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsek D, Jagatic T, Cepanec M, Hof PR, Simic G. Recent Developments in Neuropathology of Autism Spectrum Disorders. Transl Neurosci. 2011;2(3):256–264. doi: 10.2478/s13380-011-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH. Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:539–551. doi: 10.1016/j.jaac.2010.02.012. 551 e531–534. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autistic children: Diagnosis and clinical features. Pediatrics. 1991;87:751–760. [PubMed] [Google Scholar]
- Rapin I. Autism in search of a home in the brain. Neurology. 1999;52:902–904. doi: 10.1212/wnl.52.5.902. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. In: Wedding D, Horton AM Jr, Webster JS, editors. The neuropsychology handbook: Behavioral and clinical perspectives. New York, NY US: Springer Publishing Co; 1986. pp. 134–160. [Google Scholar]
- Roessner V, Wittfoth M, August JM, Rothenberger A, Baudewig J, Dechent P. Finger tapping-related activation differences in treatment-naive pediatric Tourette syndrome: a comparison of the preferred and nonpreferred hand. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2012 doi: 10.1111/j.1469-7610.2012.02584.x. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological findings in high-functioning men with infantile autism, residual state. Journal of Clinical and Experimental Neuropsychology. 1988;10:201–221. doi: 10.1080/01688638808408236. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Hume F, Toohey P, Doutney C. Negative symptoms, cognitive dysfunction, tardive akathisia and tardive dyskinesia. Acta Psychiatrica Scandinavica. 1996;93:451–459. doi: 10.1111/j.1600-0447.1996.tb10677.x. [DOI] [PubMed] [Google Scholar]
- Schear JM, Sato SD. Effects of visual acuity and visual motor speed and dexterity on cognitive test performance. Archives of Clinical Neuropsychology. 1989;4:25–32. [PubMed] [Google Scholar]
- Schultz RT, Klin A, Lombroso PJ. Genetics of childhood disorders: XLIII. Autism, part 2: Neural foundations. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1259. doi: 10.1097/00004583-200210000-00018. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke. 2012;43:2248–2251. doi: 10.1161/STROKEAHA.112.662619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MS, Barratt ES. Verbal skills, finger tapping, and cognitive tempo define a second-order factor of temporal information processing. Brain and Cognition. 1996;31:35–45. doi: 10.1006/brcg.1996.0023. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman ES, Spreen O. 3rd. ed. New York, NY US: Oxford University Press; 2006. A compendium of neuropsychological tests: Administration, norms, and commentary. [Google Scholar]
- Szatmari P, Tuff L, Finlayson MA, Bartolucci G. Asperger's syndrome and autism: Neurocognitive aspects. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:130–136. doi: 10.1097/00004583-199001000-00021. [DOI] [PubMed] [Google Scholar]
- Travers BG, Powell PS, Klinger LG, Klinger MR. Motor difficulties in Autism Spectrum Disorder: Linking symptom severity and postural stability. Journal of Autism and Developmental Disorders. 2012 Nov;8 doi: 10.1007/s10803-012-1702-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tofts P. Quantitative MRI of the Brain. N.Y: Wiley; [Google Scholar]
- Triggs WJ, Calvanio R, Levine M, Heaton RK, Heilman KM. Predicting hand preference with performance on motor tasks. Cortex. 2000;36:679–689. doi: 10.1016/s0010-9452(08)70545-8. [DOI] [PubMed] [Google Scholar]
- van Nuenen BF, Kuhtz-Buschbeck J, Schulz C, Bloem BR, Siebner HR. Weight-specific anticipatory coding of grip force in human dorsal premotor cortex. The Journal of Neuroscience. 2012;32:5272–5283. doi: 10.1523/JNEUROSCI.5673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-III (WISC-III) San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-III (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: Evidence for a deficit in proprioception. Journal of Developmental and Behavioral Pediatrics. 2001;22:92–101. doi: 10.1097/00004703-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, et al. Abnormal white matter integrity in young children with autism. Human Brain Mapping. 2011;32:534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychology. 2006;12:279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. NeuroImage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Anderson JS, Froehlich AL, Prigge MB, Nielsen JA, Cooperrider JR…, Lainhart JE. scMRI reveals large-scale brain network abnormalities in autism. PLoS One. 2012;7(11):e49172. doi: 10.1371/journal.pone.0049172. [DOI] [PMC free article] [PubMed] [Google Scholar]