Abstract
Chloroquine (CQ), a bitter tasting drug widely used in treatment of malaria, is associated gastrointestinal side effects including nausea or diarrhea. In the present study, we investigated the effect of CQ on electrolyte transport in rat ileum using the Ussing chamber technique. The results showed that CQ evoked an increase in short circuit current (ISC) in rat ileum at lower concentration (≤5×10−4 M ) but induced a decrease at higher concentrations (≥10−3 M). These responses were not affected by tetrodotoxin (TTX). Other bitter compounds, such as denatoniumbenzoate and quinine, exhibited similar effects. CQ-evoked increase in ISC was partly reduced by amiloride(10−4 M), a blocker of epithelial Na+ channels. Furosemide (10−4 M), an inhibitor of Na+-K+ -2Cl− co-transporter, also inhibited the increased ISC response to CQ, whereas another Cl− channel inhibitor, CFTR(inh)-172(10−5M), had no effect. Intriguingly, CQ-evoked increases were almost completely abolished by niflumic acid (10−4M), a relatively specific Ca2+-activated Cl− channel (CaCC) inhibitor. Furthermore, other CaCC inhibitors, such as DIDS and NPPB, also exhibited similar effects. CQ-induced increases in ISC were also abolished by thapsigargin(10−6M), a Ca2+ pump inhibitor and in the absence of either Cl− or Ca2+ from bathing solutions. Further studies demonstrated that T2R and CaCC-TMEM16A were colocalized in small intestinal epithelial cells and the T2R agonist CQ evoked an increase of intracelluar Ca2+ in small intestinal epithelial cells. Taken together, these results demonstrate that CQ induces Cl− secretion in rat ileum through CaCC at low concentrations, suggesting a novel explanation for CQ-associated gastrointestinal side-effects during the treatment of malaria.
Introduction
Chloroquine (CQ) is a drug commonly used for prevention and treatment of malaria. Use of this drug has been expanded for the treatment of other diseases, such as rheumatoid arthritis, systemic lupus erythematous and other related disorders. CQ is usually well tolerated,however, gastrointestinal side effects including nausea or diarrhea have been described [1], [2], [3], [4]. The underlying mechanisms for these side effects are unclear.
CQ is a synthetic bitter-tasting compound. Many bitter-taste receptors,which are believed to function as gatekeepers in the oral cavity to detect and prevent the ingestion of poisonous bitter-tasting compounds, are expressed in mammalian testis [5] and lung [6]. In addition, bitter taste receptors are expressed in the intestinal tract,which is involved in sensing of food components [7], [8], [9], [10], [11], [12], [13]. Kaji et al reported that the bitter compound, 6-PTU, evoked anion secretion in the large intestines of humans and rats [8]. Intestinal transepithelial ion transport is regulated by diverse systems, including the enteric nervous system (ENS) and a variety of gut hormones and cytokines, responding to mechanical and chemical stimuli [14]. In this study, we investigated the effect of CQ on electrolyte transport in rat ileum as assessed with the ussing chamber technique. Our results showed that CQ induces Cl− secretion in rat ileum through CaCC at low concentraions and that these effects might not involve the neural pathway. These findings provide a novel explanation for the gastrointestinal side-effects of CQ-associated with the treatment of malaria.
Materials and Methods
Animals and Tissue Preparation
All experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Shandong University, and the study was approved by the Medical Ethics Committee for Experimental Animals, Shandong University, China (number ECAESDUSM 2012029). Adult male Wistar rats (Animal Center of Shandong University, China), weighing between 200 and 250g, were used for this study. Animals were fasted overnight,but permitted free access to water before experiments. They were anesthetized with ether and decapitated. Tissue preparation was according to that described previously [15]. Segments of ileum were cut along the mesenteric border, and luminal contents were gently removed. Tissues were pinned flat on a Sylgard-lined Petri dish with mucosal surface facing down. To obtain mucosal-submucosal preparations, serosa and muscularis were gently stripped away. During preparation, tissues were bathed in ice-cold Krebs solution(bathing solution) and continuously oxygenated with a gas mixture of 95%O2 and 5%CO2. The Krebs solution contained (in mM): 120.6 NaCl, 5.9 KCl, 2.5 CaCl2,1.2 KH2PO4, 1.2 MgCl2, 15.4 NaHCO3 and 11.5 glucose.
Short-circuit Current Measurement
Short-circuit current (ISC) was measured in vitro in Ussing chambers. The tissue preparations were mounted between the 2 halves of the Ussing chambers (exposed area of 0.50 cm2), equipped with water-jacketed gas lifts. They were bathed on both sides with 5 mL Krebs solution, gassed with 95% O2 and 5% CO2, pH adjusted to 7.4, and maintained at 37°C by circulating the solution through a reservoir during the experiments. The tissue was continuously voltage-clamped to zero potential difference by the application of external current, with compensation for fluid resistance. The baseline value of the electrical parameters was determined as the mean over the 3 min immediately prior to drug administration. The tissues were allowed to equilibrate to these conditions for approximately 30 min to stabilize the ISC prior to the addition of drugs. The transepithelial potential difference for each preparation was measured with Ag/AgCl reference electrodes (P2020S; Physiologic Instruments, San Diego, Calif) connected to a preamplifier that was, in turn, connected to a voltage clamp amplifier (VCC MC4; Physiologic Instruments, San Diego, Calif). The change in the short circuit current (ΔISC) was calculated on the basis of the value before and after the stimulation and was normalized as the current per unit area of epithelium (µA/cm2). To check tissue viability, tissues were stimulated by carbachol(CCh).
The bitter compounds, CQ, denatoniumbenzoate, and quinine were added to the serosal bathing solution and changes in ISC were measured. With the exception of quinine, 5-nitro-2-(3-phenylpropylamino) benzoic acid(NPPB) and 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid(DIDS) (dissolved inDMSO), each drug was dissolved in distilled water and added to the bath to provide the desired molarconcentration. Amiloride(10−4M), CFTR(inh)-172(10−5M), niflumic acid(10−4M), furosemide (10−4M), thapsigargin(10−6M), Cl– free solution, and Ca2+ free solution were used to investigate the ion component of CQ-evoked ISC changes. The Cl– free solution contained (in mM): 117 Na-gluconate, 4.7 K-gluconate, 8 Ca-(gluconate)2,1.2 Mg-(gluconate)2,1.2 NaH2PO4, 25 NaHCO3, and11 glucose. The Ca2+-free EDTA solution contained (in mM): 117 NaCl, 4.7 KCl, 2.5 MgCl2.6H2O,1.2 NaH2PO4, 25 NaHCO3, 3 EDTA and11 glucose. These solutions were bubbled with a gas mixture of 95% O2 and 5% CO2 and buffered at pH7.2–7.4. Tetrodotoxin (TTX-10−6M) was used to test the influence of neural pathway.
Immunohistochemistry
IEC-18 cells were used after 48h in culture. Specifically,the cells were fixed in 4% paraformaldehyde for 10 min and rinsed three times with th PBS,and were blocked by 10% donkey serum for 1h at room temperature. Then, the cells were incubated with mouse anti-TMEM16A(1∶100; Santa Cruz) or anti-TAS2R10(1∶300;Abcam) diluted with 10% donkey serum over night at 4°C. Next, the cells were incubated with. Alexa Fluor 568-conjugated donkey anti-rabbit(1∶5000; Invitrogen) and Alexa Fluor 488-conjugated donkey anti-mouse IgG (1∶5000;Invitrogen) at room temperature for 1h followed by washes with PBS. The DAPI (1∶1,000) was used to stain the nucleus. The coverslips were mounted with75% glycerol.Negative controls were stained without primary antibodies.
Chemical
All drugs were purchased from Sigma-Aldrich Corp.(St.Louis,MO,USA). With the exception of CFTR(inh)-172 and thapsigargin (dissolved in DMSO), each drug was dissolvedin distilled water. The volume of dissolved drugs in H2O or DMSO added to the bathing solutions did not exceed 15 µl (0.3% of bathing solution). The 0.3% DMSO did not affect ISC in rat tissues that was used in our study.
Data Analysis and Statistics
All data are expressed as means ±SE.The n values represent the numbers of animals.
One-way ANOVA or unpaired Student’s t-tests were used to determine whether there were significant differences in basal electrical parameters among the tissue elements. P<0.05 was considered statistically significant.
Results
CQ Evoked an Increase in ISC in Rat Ileum
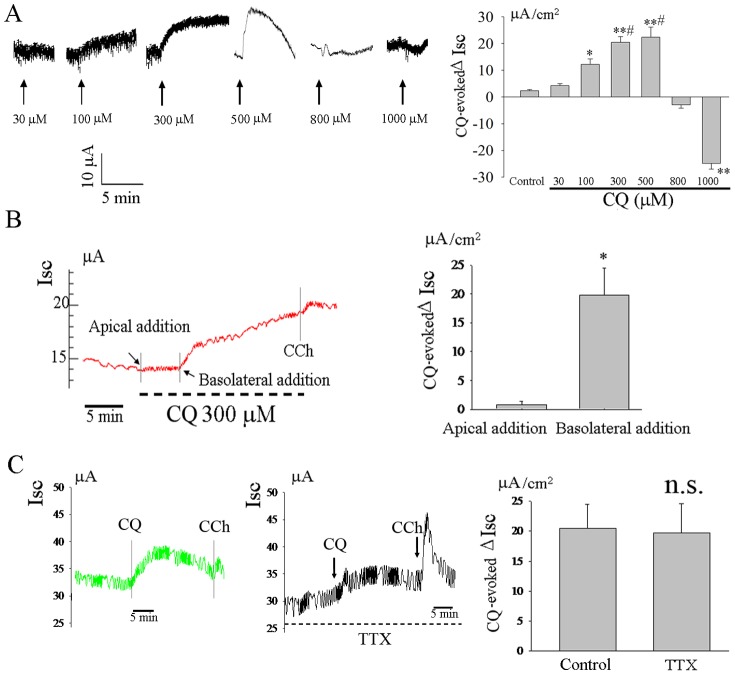
CQ dose-dependently increased basal ISC at low concentrations(≤5×10−4 M), however, it markedly decreased basal ISC at high concentrations (≥10−3 M) (n = 6, Figure 1A). To further investigate the underlying mechanism of this CQ-induced ISC response in rat ileum, CQ (3×10−4 M) was added to either the mucosal or serosal bathing solutions. The CQ-induced ISC response was completely absent when CQ was added to the mucosal bathing solution (n = 6, Figure 1B).
Figure 1. CQ evoked an increase in ISC in rat ileum.
CQ dose-dependently increased basal ISC at low concentrations(≤5×10−4 M), however, it markedly decreased basal ISC at high concentrations (≥10−3 M) (n = 6, A). The serosal addition of CQ (3×10−4M) increased basal ISC, whereas CQ to mucosal bathing solution had no effect on basal ISC (n = 6,B). The CQ-induced increase in ISC was not influenced by TTX(n = 4,C). *P<0.05; **P<0.01 compared with control or apical addition; # P<0.05 compared with 100 µM group; n.s: no significance by the one-way ANOVA or unpaired t-test.
Effect of TTX on the CQ-evoked Increases ISC in Rat Ileum
The ENS plays an important role in the regulation of intestinal epithelial ion transport. To investigate the involvement of the ENS in the serosal CQ-induced ISC response. TTX(10−6M) was added to the serosal bathing solution 15 min before the addition of CQ. TTX did not affect the CQ-induced increase in ISC (n = 4, Figure 1C).
Effects of Amiloride, CFTR(inh)-172, Furosemide, Niflumic Acid, DIDS and NPPB on CQ -evoked Increase in ISC in Rat Ileum
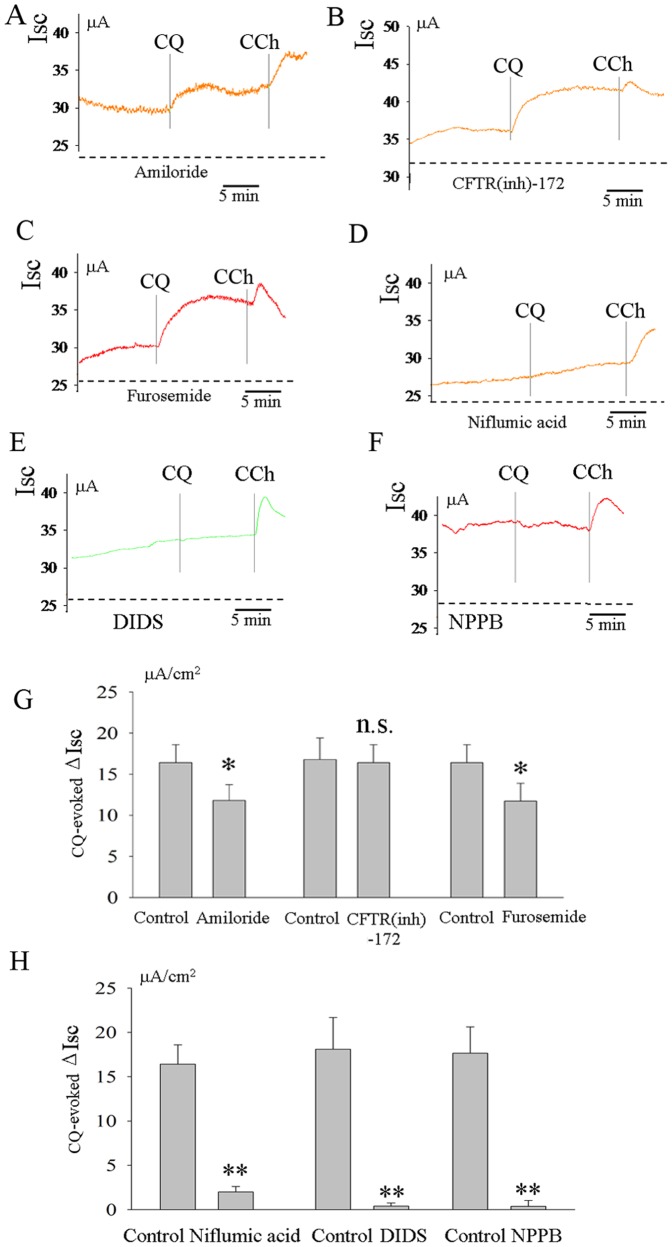
These experiments were designed to investigate the ion components of ISC induced by CQ.Two preparations of ileum from one rat were used for the control and experimental groups. Amiloride (10−4 M), a blocker of epithelial Na+ channels, was added to mucosal bathing solution 15 min before the serosal application of CQ and partly reduced the CQ-evoked ISC from 16.5±2.3 µA/cm2 in the control group to 12.3±2.1 µA/cm2 in the experimental group (P<0.05 by paired t-test, n = 7, Figures 2A and 2G). CFTR(inh)-172, an inhibitor of cystic fibrosis transmembrane conductance regulator (CFTR), was also added to the mucosal bathing solution 15 min before the application of CQ, but did not affect the CQ-evoked increase in ISC (n = 5, Figures 2B and 2G). The serosal addition of the Cl− transporter inhibitor, furosemide (10−4M), partly reduced the CQ-evoked increase (from 16.2±2.8 µA/cm2 to 12.7±1.8 µA/cm2 (P<0.05, n = 4, Figures 2C and 2G). Intriguingly, CQ-evoked increases were almost completely abolished by niflumic acid (10−4M), a relatively specific Ca2+-activated Cl− channel (CaCC) inhibitor (Figures 2D and 2H). To further confirm the specific target of CQ, we tested the effect of another two well-known CaCC inhibitors (NPPB and DIDS) on CQ-evoked response in Isc. The results showed that NPPB and DIDS also completely blocked the CQ-evoked increase in Isc (Figures 2E and 2F).
Figure 2. Effects of amiloride, CFTR(inh)-172, niflumic acid and furosemide on CQ-evoked increase in ISC in rat ileum.
The CQ-evoked increase in ISC was partly inhibited by a blocker of epithelial Na+ channels, amiloride(10−4M)(n = 7, A and G). Cl− channel inhibitor CFTR(inh)-172(10−5M) did not affect this CQ-evoked response (n = 5,B and G). An inhibitor of Na+-K+ -2Cl− cotransporter, furosemide (10−4M) also reduced the CQ-evoked increase in ISC (n = 4, C and G). Niflumic acid (10−4M), DIDS and NPPB almost completely abolished the CQ-evoked increase in Isc(n = 9, D-F and H). *P<0.05; **P<0.01;n.s: no significance by unpaired t-test.
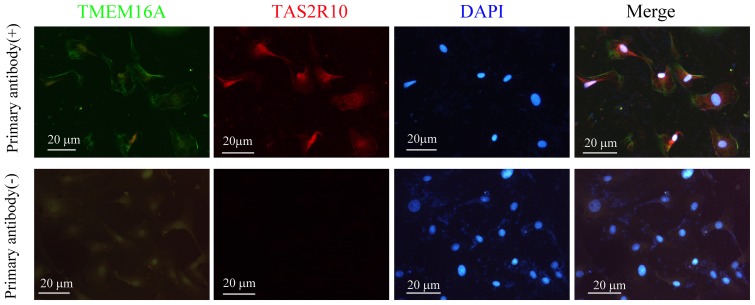
Colocalization of CaCC-TMEM16A and Bitter Receptor T2R in Small Intestinal Epithelial Cells
To identify whether CaCC or T2R was localized in rat small intestinal epithelial cells, we carried out immunohistochemical study in IEC-18 cells. In our present study, we revealed that T2R and TMEM16A were colocalized in small intestinal epithelial cells (Figure 3).
Figure 3. Colocalization of CaCC-TMEM16A and bitter receptor T2R in small intestinal epithelial cells.
Immunohistochemistry was showing TMEM16A and bitter receptor TAS2R10 in rat ileum epithelial cell line IEC-18.
Effects of Cl− and Ca2+-free Solution on CQ-evoked Increase in ISC in Rat Ileum
TAS2R agonists such as saccharin, CQ and denatonium evoke increased intracellular Ca2+ concentrations in human airway smooth muscle [6]. In our present study, we also revealed that CQ triggered an increase of intracellular Ca2+ concentrations, which would be expected to activate the CaCC and then evoke Cl− secretion (Figure S1). To further confirm the ionic basis for the increases in ISC evoked by CQ, Cl–free and Ca2+-free solutions were used. The serosal CQ-induced ISC responses were tested in the absence of Cl− and Ca2+ from the Krebs solutions.The CQ-induced increases in ISC were greatly reduced. (from 16.3±2.2 to 3.1±0.8 µA/cm2, P<0.01, n = 6, Figure 4A) in the absence of Cl−. In the absence of Ca2+, the CQ-induced increases in ISC were totally abolished, and a decrease in basal ISC was seen (n = 5, Figure 4B). Similar results were obtained with thapsigargin(10−6M), a Ca2+ pump inhibitor(n = 6, Figure 4C).
Figure 4. Effects of Cl− and Ca2+-free solution on CQ-evoked increase in ISC in rat ileum.
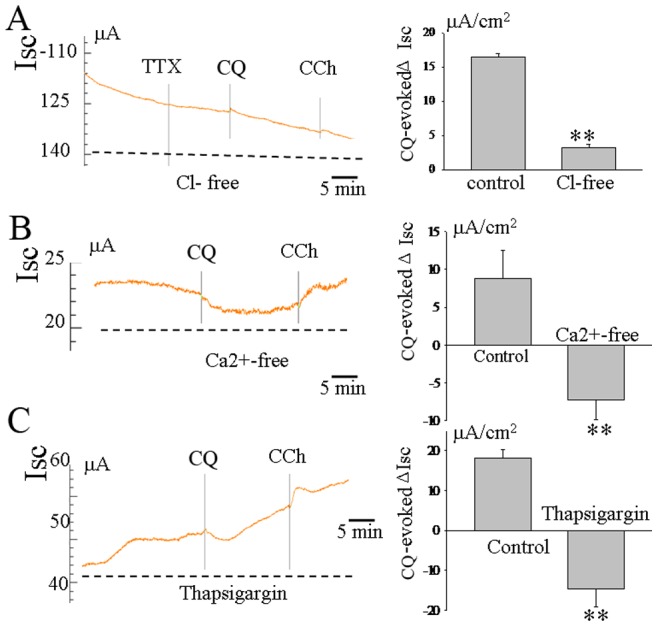
CQ-induced increases in ISC was greatly reduced in the absence of Cl−(n = 6,A). In the absence of Ca2+, CQ-induced increases in ISC were totally abolished, whereas a decrease in basal ISC was seen in this Ca2+-free condition(n = 5,B). A similar effect was obtained in response to pretreatment with thapsigargin(10−6M), a Ca2+ pump inhibitor(n = 6, C). **P<0.01 by unpaired t-test.
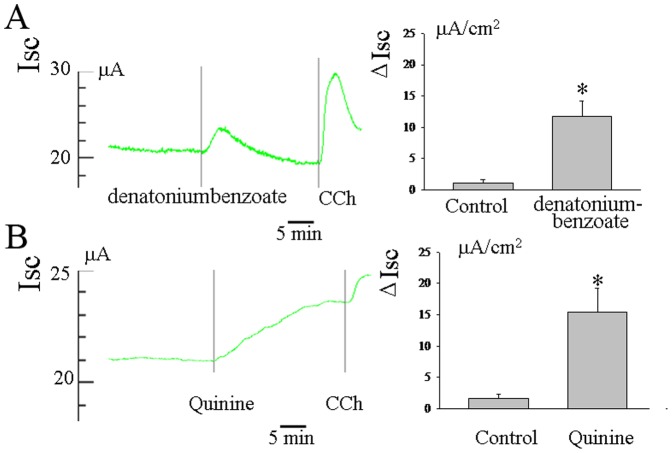
Effects of other Bitter Compounds on Basal ISC in Rat Ileum
To examine whether other bitter compounds also evoke secretory responses, we measured the effect of denatoniumbenzoate (10−4M) and quinine(10−4M) on the basal ISC in rat ileum as assessed with the Ussing chamber technique. The data showed that the serosal addition of both denatoniumbenzoate and quinine also induced an increase in Isc(n = 5, Figure 5).
Figure 5. Effect of other bitter compounds such as denatoniumbenzoate and quinine in basal ISC in rat ileum.
Other compounds, such as denatoniumbenzoate and quinine also induced an increase in Isc(A and B). *P<0.05 by unpaired t-test.
Discussion
In the present study, we demonstrated the action of a bitter taste receptor ligand, CQ, on electrolyte transport in rat ileum. Our results indicate that CQ induces Cl− secretion probably by stimulating CaCC in rat ileum at low concentrations (≤3×10−4 M), and that such effects do not appear to involve a neural pathway.
CQ Evoked Cl- Secretion in Rat Ileum
CQ(10−4M) evoked an increase in basal ISC in rat ileum. Consistent with previous research [16], addition of CQ (10−4M) to the mucosal surface of ileum did not alter basal electrolyte transport, whereas electrolyte transport was affected after addition of CQ to the solution bathing the serosal surface. However, in contrast to our study, these authors reported that addition of CQ (10−4M) to the serosal surface reduced ISC as determined in vitro using a rabbit ileum. Although we also demonstrated that CQ reduced ISC in rat ileum at high concentration (≥10−3 M), this discrepancy might be attributable to the different animal models used in these two studies. The ileum of different species may have different sensitivity to chloroquine due to the different type of bitter taste receptor or the different expression level of CaCC-TMEM16A. Interestingly, our study revealed that the expression level of CaCC-TMEM16A was different among rabbit and rat(data not shown). Next, we investigated the underlying mechanisms of CQ-evoked increase of ISC. Although our results also supported the concept that Na+ absorption was involved in the CQ-induced increase of Isc, the finding that amiloride decreased this response by only 25%, hinted that other(not Na+) ion components were involved in the CQ-evoked increased in Isc. Since it had been reported that 6-PTU, a similar bitter compound,evokes anion secretion [8], we tested the effects of some Cl− inhibitors,such as CFTR(inh)-172, furosemide, niflumic acid, DIDS and NPPB on CQ-evoked ISC responses. CFTR is known as an apical membrane Cl− channel in epithelial cells [17], [18]. Our results revealed that CFTR(inh)-172 did not affect the CQ-evoked increase in ISC . The Na+-K+-2Cl− co-transporter is known as the predominant transporter at basolateral membrane for Cl− uptake into epithelial cells [19]. The intracellular-free chloride concentration is maintained by a Na+-K+-2Cl− co-transporter that actively accumulates Cl−. In the present study, furosemide, a Na+-K+-2Cl− co-transporter inhibitor, reduced the CQ-evoked ISC response by 22%. CaCCs are plasma membrane proteins involved in various important physiological processes. In epithelial cells, CaCC activity mediates the secretion of Cl−. TMEM16A is recently identified as CaCC [20], [21]. In our present study, we demonstrated that CaCC channel inhibitors, such as niflumic acid, DIDS and NPPB, almost completely abolished the CQ-evoked increase of ISC, and CaCC-TMEM16A was widely expressed in small intestinal epithelial cells. Given that the CQ-induced increases in ISC were largerly abolished in Cl−-free conditions, it would seem reasonable to conclude that Cl− secretions through CaCC are involved in the ISC response to CQ.
The Role of Ca2+
Based upon our results, it appears that the CQ-evoked increase in ISC was not dependent on the neural pathway. CQ increases rabbit ileal Ca2+ content [16] and evokes increased intracelluar Ca2+ of rat small epithelial cells, which suggest that this effect may involve intracelluar Ca2+. The bitter taste receptor,T2R, is expressed not only in the taste buds but also in intestinal tissues and we have reported that T2Rs are widely distributed in rat ileum epithelium [13]. In our present study, we revealed that T2R and TMEM16A were colocalized in small intestinal epithelial cells. T2Rs belong to the family of G protein coupled receptors(GPCRs) and activate phospholipase C (PLCβ2), to produce inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) [22]. IP3 binds to IP3 receptors and elicits a release of Ca2+ from the sarcoplasmic reticulum (SR) [23]. Moreover, taste receptor cell responses to the bitter stimulus, denatonium, involve Ca2+ influx via store-operated channels [24]. It has been reported that CQ increases rabbit ileal calcium content [16] and increases intracellular Ca2+ concentrations [6], [9]. The present results showed that the CQ-evoked ISC increase in ratileum was highly dependent on either intracellular or extracellular calcium. Therefore, a possible underlying mechanism of this CQ-evoked secretory response is that intestinal epithelial cell responses to this bitter simulus enables Ca2+ entry via store-operated Ca2+ release-activated Ca2+ channels(CRAC) that drives Cl− secretion by activating CaCC (Figure 6). Other bitter compounds, such as quinine and denatoniumbenzoate had the same effect as CQ. However, it remains unclear which types of T2R are activated by CQ. Interestingly in the absence of extracellar Ca2+ or in the presence of thapsigargin, CQ dereased basal ISC. As mentioned above, high concentration of CQ also induced a decrease in ISC. These results suggest that the Ca2+-activated Cl− channel may not be the only target of CQ. It is known that CQ is a membrane-stabilizer and can be trapped in the cell. Thus the CQ-evoked decrease in ISC under these conditions may represent a non-specific action of this bitter compound.
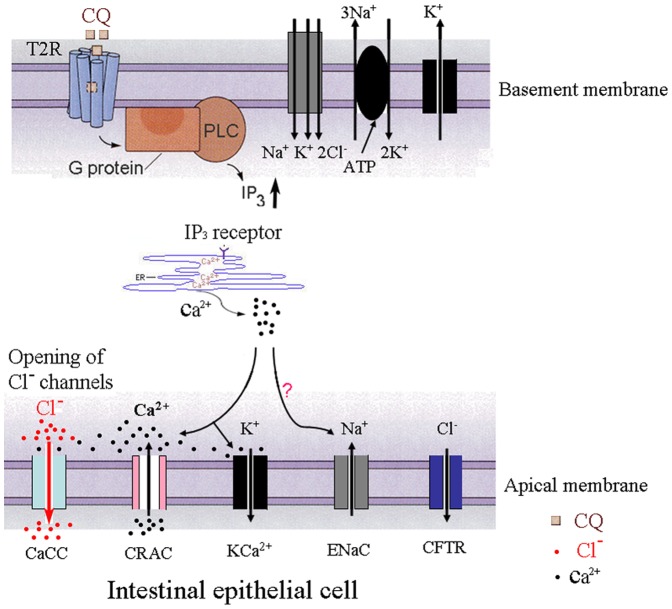
Figure 6. A working model of CQ in intestinal epithelial cells.
CQ binds to T2Rs activating a G-protein to produce phospholipase C (PLC) in the basement membrane of intestinal epithelial cells followed by Ca2+ release from the sarcoplasmic reticulum (SR). Local Ca2+ entry through store-operated Ca2+ release-activated Ca2+ channels(CRAC) drives Cl− secretion by stimulating Ca2+-activated Cl− channels(CaCC) in the apical membrane of intestinal epithelial cells. The intracellular-free chloride concentration is maintained by a Na+-K+-2Cl− co-transporter that actively accumulates Cl−.
In conclusion, the present results indicate that within the rat ileum, Cl− secretion induced by CQ at low concentrations involved CaCC through a T2R chemical-sensing mechanism in rat ileum. Such effects do not appear to be dependent on a neural pathway. Taken together, our results suggest that fluid secretion stimulated by CQ-induced Cl− secretion appears to be responsible for gastrointestinal side-effects of this bitter compound in the treatment of malaria.
Supporting Information
CQ evoked an increase in intracellular Ca2+ in rat ileum epithelial cell line IEC-18 by single cell Ca2+ imaging analysis. *P<0.05; compared with control by paired t-test.
(TIF)
Acknowledgments
We thank Dr. Xiao Yu (University of Shandong) for comments on the manuscript;Dr. Chuanyong Liu (University of Shandong) for comments on some experiments.
Funding Statement
This work was supported by grants to Jingxin Li from the National Natural Science Foundation of China (grant no.31171108). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fogh S, Schapira A, Bygbjerg IC, Jepsen S, Mordhorst CH, et al. (1988) Malaria chemoprophylaxis in travellers to east Africa: a comparative prospective study of chloroquine plus proguanil with chloroquine plus sulfadoxine-pyrimethamine. Br Med J (Clin Res Ed) 296: 820–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steffen R, Fuchs E, Schildknecht J, Naef U, Funk M, et al. (1993) Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting east Africa. Lancet 341: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 3. Barrett PJ, Emmins PD, Clarke PD, Bradley DJ (1996) Comparison of adverse events associated with use of mefloquine and combination of chloroquine and proguanil as antimalarial prophylaxis: postal and telephone survey of travellers. Bmj 313: 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Durrheim DN, Gammon S, Waner S, Braack LE (1999) Antimalarial prophylaxis–use and adverse events in visitors to the Kruger National Park. S Afr Med J 89: 170–175. [PubMed] [Google Scholar]
- 5. Xu J, Cao J, Iguchi N, Riethmacher D, Huang L Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod 19: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hofer D, Puschel B, Drenckhahn D (1996) Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A 93: 6631–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaji I, Karaki S, Fukami Y, Terasaki M, Kuwahara A (2009) Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. Am J Physiol Gastrointest Liver Physiol 296: G971–981. [DOI] [PubMed] [Google Scholar]
- 9. Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, et al. (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A 99: 2392–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rozengurt E (2006) Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291: G171–177. [DOI] [PubMed] [Google Scholar]
- 11. Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, et al. (2006) Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291: G792–802. [DOI] [PubMed] [Google Scholar]
- 12. Jeon TI, Zhu B, Larson JL, Osborne TF (2008) SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest 118: 3693–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jing F, Liu M, Yang N, Liu Y, Li X, et al. Relaxant effect of chloroquine in rat ileum: possible involvement of nitric oxide and BKCa. J Pharm Pharmacol 65: 847–854. [DOI] [PubMed] [Google Scholar]
- 14. Furness JB, Kunze WA, Clerc N (1999) Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol 277: G922–928. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Li XF, Hua G, Xu JD, Zhang XH, et al. Colonic submucosal 5-HT(3) receptor-mediated somatostatin-dependent secretoinhibitory pathway is suppressed in water-immersion restraint stressed rats. Eur J Pharmacol 656: 94–100. [DOI] [PubMed] [Google Scholar]
- 16. Fogel R, Sharp GW, Donowitz M (1982) Chloroquine stimulates absorption and inhibits secretion of ileal water and electrolytes. Am J Physiol 243: G117–126. [DOI] [PubMed] [Google Scholar]
- 17. Thiagarajah JR, Verkman AS (2003) CFTR pharmacology and its role in intestinal fluid secretion. Curr Opin Pharmacol 3: 594–599. [DOI] [PubMed] [Google Scholar]
- 18. Basavappa S, Vulapalli SR, Zhang H, Yule D, Coon S, et al. (2005) Chloride channels in the small intestinal cell line IEC-18. J Cell Physiol 202: 21–31. [DOI] [PubMed] [Google Scholar]
- 19. Isenring P, Forbush B 3rd (1997) Ion and bumetanide binding by the Na-K-Cl cotransporter. Importance of transmembrane domains. J Biol Chem 272: 24556–24562. [DOI] [PubMed] [Google Scholar]
- 20. Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, et al. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594. [DOI] [PubMed] [Google Scholar]
- 22. Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, et al. (2001) Bitter taste transduced by PLC-beta(2)-dependent rise in IP(3) and alpha-gustducin-dependent fall in cyclic nucleotides. Am J Physiol Cell Physiol 280: C742–751. [DOI] [PubMed] [Google Scholar]
- 23. Ogura T, Mackay-Sim A, Kinnamon SC (1997) Bitter taste transduction of denatonium in the mudpuppy Necturus maculosus. J Neurosci 17: 3580–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogura T, Margolskee RF, Kinnamon SC (2002) Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol 87: 3152–3155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CQ evoked an increase in intracellular Ca2+ in rat ileum epithelial cell line IEC-18 by single cell Ca2+ imaging analysis. *P<0.05; compared with control by paired t-test.
(TIF)