Abstract
Background
Researches have revealed that the endothelial nitric oxide synthase (eNOS) gene G894T polymorphism is associated with the risk of Myocardial infarction (MI), but the results remain conflicting.
Objective and Methods
A meta-analysis was conducted to investigate the association between eNOS G894T polymorphism and MI. Published studies from PubMed, Embase, CNKI and CBM databases were retrieved. The pooled odds ratios (ORs) for the association between eNOS G894T polymorphism and MI and their corresponding 95% confidence intervals (CIs) were estimated using the random- or fixed- effect model.
Results
A total of 34 studies including 8229 cases and 12839 controls were identified for the meta-analysis. The eNOS G894T polymorphism was significantly associated with MI under a homozygous genetic model (OR = 1.41, 95% CI = 1.08–1.84; P = 0.012), a recessive genetic model (OR = 1.35, 95% CI = 1.06–1.70; P = 0.014), a dominant genetic model (OR = 1.18, 95% CI = 1.04–1.34; P = 0.009). In the subgroup analysis by ethnicity (non-Asian and Asian), no significant association was observed between eNOS G894T polymorphism and MI risk among non-Asians (P>0.05), but a positive significant association was found among Asians (P<0.05).
Conclusions
The eNOS G894T polymorphism is associated with increased MI risk in Asians. The results indicate that ethnicity plays important roles in the association between eNOS G894T polymorphism and MI.
Introduction
Myocardial infarction (MI) is a complex syndrome determined by multiple predisposing genetic and environmental factors. Previous studies have investigated the association of genetic variants in DNA repair pathways, lipid-related pathways, fibrinolytic system, renin angiotensin aldosterone system and nitric oxide synthase with MI risk [1], [2], [3], [4], [5].
There are several forms of nitric oxide synthase such as neuronal nitric oxide synthase (nNOS), endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS). The vascular nitric oxide (NO), mainly produced by eNOS, is a critical molecule in regulating the vascular system, including the inhibition of the platelet aggregation and adhesion and reduction of vascular smooth muscle cell proliferation [6]. Furthermore, overproduction of NO can inhibit DNA repair and cause DNA damage [7], which plays an important role in the occurrence of MI [3]. NO regulation may result from the functional eNOS genetic polymorphisms. The eNOS gene is mapped on human chromosome 7q35–36 and contains 26 exons and 25 introns. The eNOS G894T polymorphism, a coding region variant, results in a Glu298Asp substitution and decreases the NO levels [8].
To date, studies on the association of eNOS G894T polymorphism with MI clinical phenotype have been extensively explored. However, the results still remain inconclusive and conflicting. Some studies found that the allele T of eNOS G894T polymorphism was the risk factor for MI, but others had the opposite results. Therefore, in the current study, a meta-analysis from 34 individual studies with a total of 21068 subjects including 8229 cases and 12839 controls was performed to get a more precise estimation of the association between eNOS G894T polymorphism and MI.
Materials and Methods
Publication Search and Inclusion Criteria
We searched the electronic databases PubMed, Embase, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biomedical Literature Database (CBM) using the following search terms: (myocardial infarction or myocardial infarct) and (endothelial nitric oxide synthase) and (polymorphism or mutation or variant), without restriction on language. The included articles were published before September 2013. All eligible studies were retrieved, and their references were examined manually for other potentially relevant studies.
The inclusion criteria were as follows: a) case-control design. b) the association of eNOS G894T polymorphism with MI should be evaluated. c) the genotype data was available in the cases and controls. d) the control subjects must be in agreement with the Hardy-Weinberg equilibrium (HWE).
Data Extraction
All data were independently collected from the included studies according to a standardized protocol by two investigators. The discrepancies during data extraction were resolved by consensus. The same data in different studies were used only once. The following information was extracted: first author’s name, publication year, original country, ethnicity, sample size, and number of genotype in cases and controls.
Statistical Analysis
The association between eNOS G894T polymorphism and MI was assessed using crude odds ratio (OR) with 95% confidence interval (CI). The pooled ORs were determined for homozygous model (TT versus GG), heterozygous model (GT versus GG), recessive model (TT versus GT/GG), dominant model (GT/TT versus GG). The Z test was used to assess the pooled OR with the significance set at P<0.05. HWE was assessed using the Chi-square test in control groups. The presence of between-study heterogeneity was evaluated by using the I2 statistic test, which does not inherently depend on the number of studies in the meta-analysis and is preferable to the test of heterogeneity [9]. The value of I2 ranged from 0–100%. If obvious heterogeneity was observed among the studies (I2>50%), the random-effects model (the DerSimonian and Laird method) was used to calculate the pooled OR and 95% CI [10]. Otherwise, the fixed-effects model (the Mantel-Haenszel method) was adopted for the meta-analysis [11]. Subgroup analyses according to the ethnicity and the total sample size were also performed to evaluate the association. When stratified by total sample size, we defined the large group if the sample size was more than 1000 and the small group if the sample size was less than 400, otherwise was the medium group. Meta-regression was performed to explore the sources of between-study heterogeneity. The study ethnicity, total sample size, control sample size, MI sample size, ratio of MI sample size to control sample size, publication year were regarded as the potential confounding factors. Sensitivity analyses were conducted to evaluate the effect of individual study on pooled results and assess the stability of results. The potential publication bias was detected with Begg’s funnel plot [12], and the funnel plot asymmetry was assessed by Egger’s linear regression test [13]. All statistical analyses were performed using the STATA 12.0 software (StataCorp, College Station, TX, USA).
Results
Characteristics of Eligible Studies
Our meta-analysis was performed according to guidelines of the “Preferred Reporting Items for Systematic reviews and Meta-Analyses” (PRISMA) statement (Supplement S1) [14]. A total of 417 relevant papers were yielded by the literature search, among which 34 studies met the inclusion criteria, including 8229 MI cases and 12839 controls. As is showed in the flow diagram (Supplement S2), 372 papers were excluded owing to the obvious irrelevance. We reviewed the full texts of the remaining 45 articles. Among them, 2 were reviews, 4 were duplicated publications, 3 had no controls, 4 had insufficient data for calculation of OR and 95% CI and 1 was deviated from the HWE. At last, a total of 34 studies for the association between eNOS G894T polymorphism and MI risk were obtained in the final meta-analysis. Data collected from the included studies were summarized in the Table 1. Those included studies in Japan, Australia, France, Northern Ireland, United Kingdom, Germany, Turkey, Greece, Korea, Brazil, Sweden, Poland, Hungary, Mexico, India, Egypt, Netherlands and China.
Table 1. Characteristics of eligible studies included in the meta-analysis.
| First author | Year | Country | Ethnicity | Sample size (Case/Control) | MI | Control | MAF | HWE of control | ||||
| GG | GT | TT | GG | GT | TT | |||||||
| Shimasaki et al [15] | 1998 | Japan | Asian | 285/607 | 225 | 59 | 1 | 526 | 80 | 1 | 0.068 | 0.254 |
| Hibi et al [16] | 1998 | Japan | Asian | 226/357 | 189 | 32 | 5 | 295 | 62 | 0 | 0.087 | 0.072 |
| Cai et al [17] | 1998 | Australia | Caucasian | 95/478 | 54 | 35 | 6 | 244 | 197 | 37 | 0.283 | 0.751 |
| Poirier et al [18] | 1999 | France | Caucasian | 368/421 | 163 | 156 | 49 | 148 | 219 | 54 | 0.388 | 0.051 |
| Poirier et al [18] | 1999 | Northern Ireland | Caucasian | 163/155 | 55 | 76 | 32 | 58 | 72 | 25 | 0.394 | 0.738 |
| Hingorani et al [19] | 1999 | United Kingdom | Caucasian | 249/183 | 97 | 107 | 45 | 86 | 81 | 16 | 0.309 | 0.617 |
| Cai et al [20] | 1999 | Australia | Caucasian | 306/457 | 134 | 137 | 35 | 220 | 182 | 55 | 0.319 | 0.072 |
| Song et al [21] | 2000 | China | Asian | 114/104 | 89 | 18 | 7 | 90 | 13 | 1 | 0.072 | 0.501 |
| Wang et al [22] | 2001 | Taiwan | Asian | 114/218 | 97 | 17 | 0 | 177 | 38 | 3 | 0.101 | 0.560 |
| Gardemann et al [23] | 2002 | Germany | Caucasian | 1277/533 | 565 | 561 | 151 | 256 | 227 | 50 | 0.307 | 0.975 |
| Wei et al [24] | 2002 | China | Asian | 51/108 | 40 | 9 | 2 | 98 | 10 | 0 | 0.046 | 0.614 |
| Aras et al [25] | 2002 | Turkey | Caucasian | 76/117 | 43 | 28 | 5 | 60 | 48 | 9 | 0.282 | 0.888 |
| Qi et al [26] | 2003 | China | Asian | 107/81 | 82 | 16 | 9 | 68 | 13 | 0 | 0.080 | 0.432 |
| Schmoelzer et al [27] | 2003 | Austria | Caucasian | 126/248 | 60 | 54 | 12 | 121 | 102 | 25 | 0.306 | 0.609 |
| Agema et al [28] | 2004 | Netherlands | Caucasian | 356/574 | 174 | 157 | 25 | 216 | 270 | 88 | 0.389 | 0.811 |
| Zhan et al [29] | 2005 | China | Asian | 37/172 | 25 | 12 | 0 | 141 | 31 | 0 | 0.090 | 0.194 |
| Antoniades et al [30] | 2005 | Greece | Caucasian | 228/519 | 97 | 99 | 32 | 255 | 217 | 47 | 0.300 | 0.932 |
| Yu et al [31] | 2006 | China | Asian | 120/264 | 98 | 22 | 0 | 237 | 26 | 1 | 0.053 | 0.752 |
| Chao et al [32] | 2006 | China | Asian | 41/150 | 25 | 11 | 5 | 119 | 29 | 2 | 0.110 | 0.877 |
| Jo et al [33] | 2006 | Korea | Asian | 129/803 | 104 | 23 | 2 | 667 | 131 | 5 | 0.088 | 0.600 |
| Sampaio et al [34] | 2007 | Brazil | Mixed | 115/104 | 56 | 46 | 13 | 52 | 45 | 7 | 0.284 | 0.509 |
| Andrikopoulos et al [35] | 2008 | Greece | Caucasian | 1602/727 | 722 | 701 | 179 | 352 | 297 | 78 | 0.312 | 0.199 |
| Odeberg et al [36] | 2008 | Sweden | Caucasian | 318/85 | 179 | 121 | 18 | 43 | 32 | 10 | 0.306 | 0.296 |
| Vasilakou et al [37] | 2008 | Greece | Caucasian | 49/161 | 30 | 16 | 3 | 76 | 74 | 11 | 0.298 | 0.212 |
| Gluba et al [38] | 2009 | Poland | Caucasian | 278/134 | 140 | 118 | 20 | 62 | 61 | 11 | 0.311 | 0.454 |
| Szabó et al [39] | 2009 | Hungary | Caucasian | 118/384 | 39 | 58 | 21 | 200 | 161 | 23 | 0.270 | 0.204 |
| Isordia-Salas et al [40] | 2010 | Mexico | Mixed | 180/180 | 104 | 62 | 14 | 134 | 42 | 4 | 0.139 | 0.742 |
| Angeline et al [41] | 2010 | India | Asian | 100/100 | 56 | 30 | 14 | 67 | 31 | 2 | 0.175 | 0.462 |
| Dafni et al [42] | 2010 | Greece | Caucasian | 204/218 | 83 | 94 | 27 | 108 | 95 | 15 | 0.287 | 0.334 |
| Katakami et al [43] | 2010 | Japan | Asian | 226/3593 | 182 | 43 | 1 | 3045 | 533 | 15 | 0.078 | 0.103 |
| Gad et al [44] | 2012 | Egypt | Caucasian | 104/101 | 52 | 47 | 5 | 59 | 34 | 8 | 0.248 | 0.333 |
| Zigra et al [45] | 2013 | Greece | Caucasian | 107/103 | 50 | 46 | 11 | 50 | 42 | 11 | 0.312 | 0.626 |
| Narne et al [46] | 2013 | India | Asian | 73/121 | 42 | 29 | 2 | 84 | 35 | 2 | 0.162 | 0.442 |
| Arun et al [47] | 2013 | India | Asian | 287/279 | 213 | 62 | 12 | 190 | 82 | 7 | 0.172 | 0.597 |
MI: Myocardial infarction; MAF: minor allele frequency; HWE: Hardy-Weinberg equilibrium.
Results of Meta-analysis
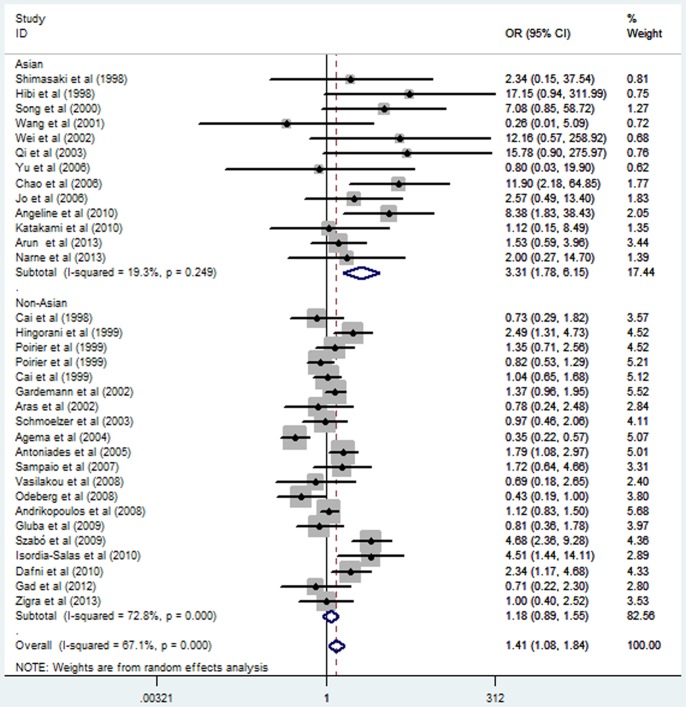
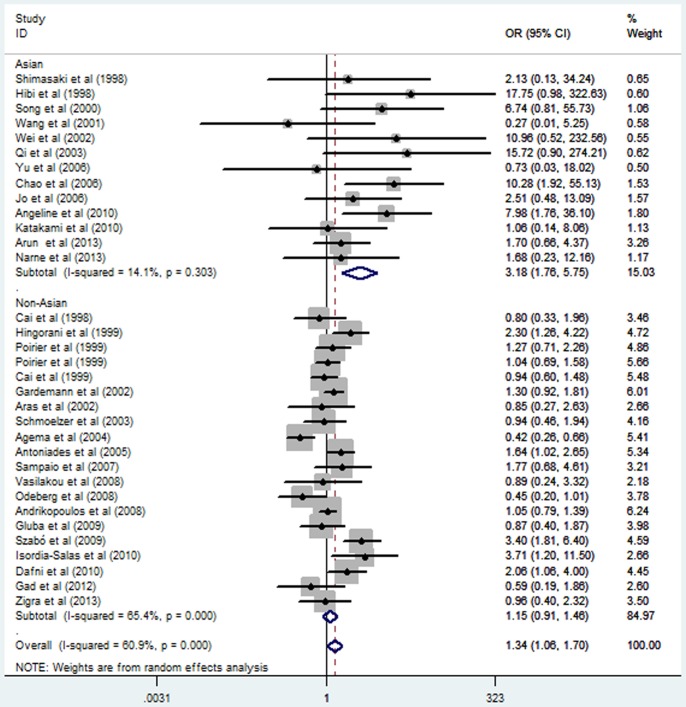
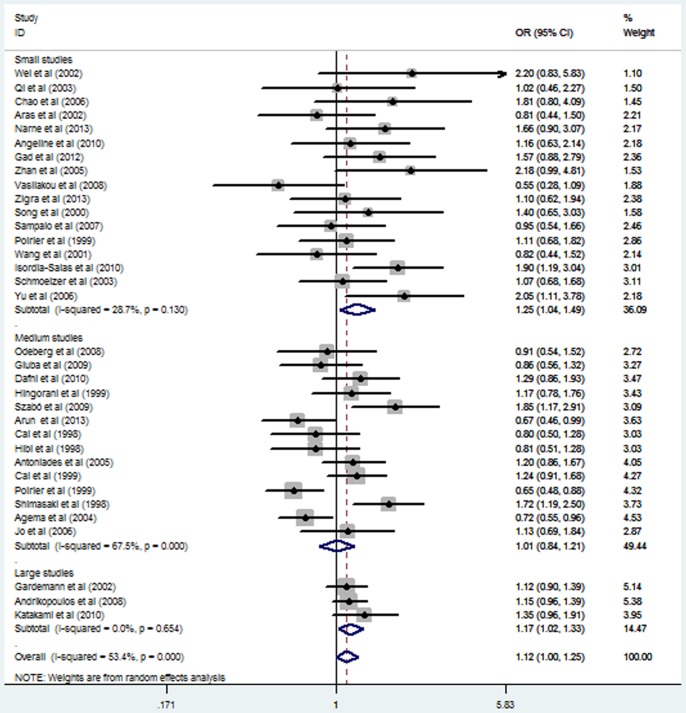
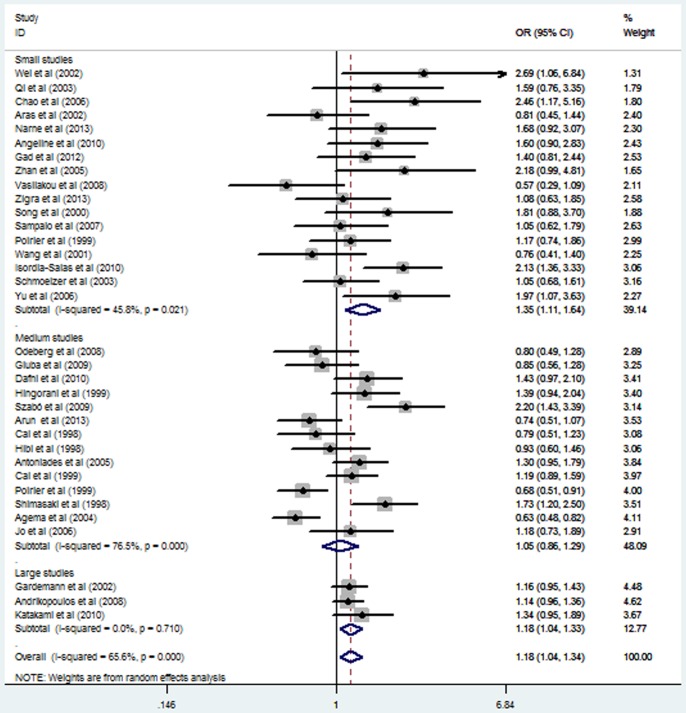
A significant association between eNOS G894T polymorphism and MI was found under a homozygous genetic model (OR = 1.41, 95% CI = 1.08–1.84; P = 0.012), a heterozygous genetic model (OR = 1.12, 95% CI = 1.00–1.25; P = 0.054), a recessive genetic model (OR = 1.35, 95% CI = 1.06–1.70; P = 0.014), a dominant genetic model (OR = 1.18, 95% CI = 1.04–1.34; P = 0.009) (Table 2).
Table 2. Pooled ORs and 95% CIs of the association between eNOS G894T polymorphism and MI.
| TT vs. GG | GT vs. GG | TT vs. GT/GG | TT/GT vs. GG | |||||||||
| OR (95% CI) | I2 (%) | P-value | OR(95% CI) | I2 (%) | P-value | OR(95% CI) | I2 (%) | P-value | OR(95% CI) | I2 (%) | P-value | |
| Overall | 1.41(1.08–1.84) | 67.1 | 0.012 | 1.12(1.00–1.25) | 53.4 | 0.054 | 1.34(1.06–1.70) | 60.9 | 0.014 | 1.18(1.04–1.34) | 65.6 | 0.009 |
| Ethnicity | ||||||||||||
| Asian | 3.44(2.15–5.49) | 19.3 | 0.000 | 1.26(1.02–1.57) | 50.1 | 0.032 | 3.41(2.14–5.43) | 14.1 | 0.000 | 1.40(1.13–1.74) | 52.8 | 0.002 |
| Non-Asian | 1.18(0.89–1.55) | 72.8 | 0.250 | 1.05(0.93–1.20) | 54.4 | 0.430 | 1.15(0.91–1.46) | 65.4 | 0.236 | 1.08(0.93–1.25) | 69.1 | 0.322 |
| Sample size | ||||||||||||
| Small | 1.67(1.26–2.21) | 48.0 | 0.000 | 1.24(1.07–1.43) | 28.7 | 0.005 | 1.58(1.21–2.07) | 45.1 | 0.001 | 1.32(1.15–1.52) | 45.8 | 0.000 |
| Medium | 1.30(0.83–2.03) | 80.1 | 0.256 | 1.01(0.84–1.21) | 67.5 | 0.902 | 1.27(0.87–1.86) | 75.0 | 0.221 | 1.05(0.86–1.29) | 76.5 | 0.626 |
| Large | 1.22(0.97–1.52) | 0.0 | 0.087 | 1.16(1.02–1.33) | 0.0 | 0.023 | 1.14(0.92–1.42) | 0.0 | 0.219 | 1.18(1.04–1.33) | 0.0 | 0.011 |
OR: odds ratio; 95% CI: 95% confidence interval. P-value was for pooled ORs. When I2<50%, it was for fixed effect model, otherwise it was for random effect model. Small study: studies with less than 400 participants; Medium study: studies with more than 400 and less than 1000 participants; Large study: studies with more than 1000 participants.
Subgroup analysis stratified by ethnicity also suggested a significant association between eNOS G894T polymorphism and MI in the Asian subgroup under a homozygous genetic model (OR = 3.44, 95% CI = 2.15–5.49; P = 0.000), a heterozygous genetic model (OR = 1.26, 95% CI = 1.02–1.57; P = 0.032), a recessive genetic model (OR = 3.41, 95% CI = 2.14–5.43; P = 0.000), and a dominant genetic model (OR = 1.40, 95% CI = 1.13–1.74; P = 0.002). In contrast, no significant association was observed in the non-Asian subgroup under any of the genetic models (P>0.05) (Table 2; Figures 1 and 2;Supplements S3 and S4). Stratified analyses by the total sample size also suggested that eNOS G894T polymorphism increased the MI risk both in large sample size studies and small sample size studies (Table 2; Figures 3 and 4).
Figure 1. Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a homozygous genetic model (TT vs. GG) stratified by ethnicity.
Figure 2. Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a recessive genetic model (TT vs. GG/GT) stratified by ethnicity.
Figure 3. Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a heterozygous genetic model (GT vs. GG) stratified by the total sample size.
Figure 4. Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a dominant genetic model (TT/GT vs. GG) stratified by the total sample size.
Sources of Heterogeneity
Under homozygous and recessive genetic models, meta-regression revealed that ethnicity was the sources of between-study heterogeneity (P = 0.007, P = 0.004 respectively), which was consistent with subgroup analyses results in homozygous and recessive genetic models (Table 2; Figures 1 and 2). Moreover, under the dominant genetic model, meta-regression showed that ethnicity might be the sources of between-study heterogeneity (P = 0.058), which was also consistent with subgroup analyses results in the dominant genetic model (Table 2). In addition, subgroup analyses revealed that the heterogeneity was significantly reduced in the small sample size group and large sample size group in all genetic models, suggesting that the total sample size was the source of heterogeneity (Table 2; Figures 3 and 4).
Sensitivity Analysis
A single study was excluded each time to evaluate the effect of individual study on the combined ORs and 95% CIs. The omission of any single study did not make significant difference in the pooled effects of homozygous, heterozygous, recessive and dominant genetic models, suggesting a high stability of our meta-analysis results (data not shown).
Publication Bias
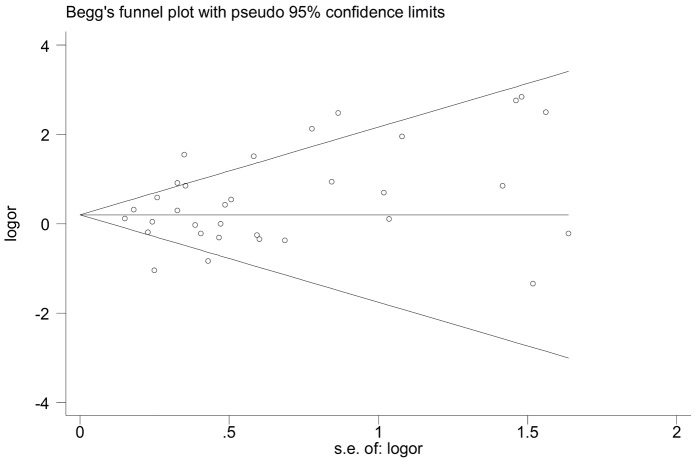
Publication bias of the selected articles was assessed by the Begg’s funnel plot and Egger’s test. The shape of the funnel plot did not show obvious publication bias (Figure 5). Similarly, no evidence of publication bias was observed by Egger’s test (P = 0.075 for homozygous genetic model; P = 0.299 for heterozygous genetic model; P = 0.118 for dominant genetic model; P = 0.055 for recessive genetic model).
Figure 5. Funnel plot for studies of the association of myocardial infarction and eNOS G894T polymorphism under a homozygous genetic model (TT vs. GG).
Discussion
In the current meta-analysis with 8229 cases and 12839 controls, we found that there were significant associations between eNOS G894T polymorphism and MI: OR = 1.41 for the homozygous genetic model, OR = 1.12 for the heterozygous genetic model, OR = 1.35 for the recessive genetic model, and OR = 1.18 for the dominant genetic model. Further stratified analysis revealed that the eNOS G894T polymorphism was significantly associated with MI in the Asian subgroup (P<0.05), but not in the non-Asian subgroup (P>0.05). The results indicated that ethnicity played important roles in the association of eNOS G894T polymorphism with MI risk.
A number of association studies have investigated the association between the eNOS G894T polymorphism and the risk of coronary artery disease (CAD), MI, coronary spasms and hypertension [5], [48], [49]. Mechanism study has also suggested the mutation was functional in the production of NO [8]. Human study showed that blood pressure decrease in the eNOS 894TT carriers was greater than the other genotypes carriers after the exercise training [50]. Therefore, subjects carrying the eNOS 894TT genotype may have low NO in vivo and are more susceptible to endothelial dysfunction, which might increase the risk of MI. The present meta-analysis results of homozygous and recessive genetic models can account for the above hypothesis. Nevertheless, the number of TT genotype is relatively small in Asia populations and the 95%CI line of the pooled OR for Asia populations is longer than that for non-Asia population studies in Figure 1 and 2. So the results of homozygous and recessive genetic models in Asia populations need to be further confirmed in future.
Conflicting results have been reported in investigating the association of the eNOS G894T polymorphism with MI. To our knowledge, our meta-analysis represents the first one focusing on the association between eNOS G894T polymorphism and the risk of MI. In 2004, Casas et al [51]. performed a meta-analysis to evaluate the association between eNOS G894T polymorphism and ischemic heart disease (IHD) including MI and CAD. They found that individuals homozygous for the eNOS 894T allele were at moderately increased risk of IHD. In 2012, the meta-analysis results of Zhang indicated that eNOS G894T polymorphism was associated with CAD risk among Asia population [5]. However, the above two meta-analysis did not evaluate the association between eNOS G894T polymorphism and MI. Our meta-analysis provided a precise result regarding the association of eNOS G894T polymorphism with MI risk.
Between-study heterogeneity is common and should be explored in the meta-analysis. In the current study, significant heterogeneity was found in the association of eNOS G894T polymorphism with MI risk. Therefore, meta-regression and subgroup analyses were performed to explore the sources of between-study heterogeneity. The results indicated that ethnicity was the source of heterogeneity in the homozygous and recessive genetic models and total sample size was the source of heterogeneity in all genetic models. Sensitivity analysis revealed that the omission of any single study did not have significant impact on the overall meta-analysis estimate. Furthermore, in the meta-analysis, funnel plot did not reflect considerable asymmetry and Egger’s test also indicated no obvious publication bias. All these made the meta-analysis results reliable to some extent.
There are some limitations in this meta-analysis. First, our meta-analysis was based primarily on the unadjusted ORs with 95% CIs and the potential confounding factors were not available. Second, gene-gene and gene-environment interactions may play important roles in the function of eNOS G894T polymorphism, but the effect was not addressed in our meta-analysis.
In conclusion, this meta-analysis demonstrated that the eNOS G894T polymorphism was associated with increased risk of MI. Further stratification by ethnicity indicated the association between the polymorphism and MI was restricted in the Asians. However, large-scale studies well designed for the gene-gene and gene-environment interactions information are needed to be conducted to elucidate the associations in future.
Supporting Information
PRISMA 2009 Checklist.
(DOC)
PRISMA 2009 Flow Diagram.
(DOC)
Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a heterozygous genetic model (GT vs. GG) stratified by ethnicity.
(TIF)
Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a dominant genetic model (TT/GT vs. GG) stratified by ethnicity.
(TIF)
Funding Statement
The work was supported by the National Scientific Foundation of China (No. 81273595, 81202594, 81001445), the “863” Project (No. 2012AA02A518), the Scientific Foundation of Hunan (No. 11K073, 10JJ4020). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Verschuren JJ, Trompet S, Deelen J, Stott DJ, Sattar N, et al. (2013) Non-homologous end-joining pathway associated with occurrence of myocardial infarction: gene set analysis of genome-wide association study data. PLoS One 8: e56262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Song C, Pedersen NL, Reynolds CA, Sabater-Lleal M, Kanoni S, et al. (2013) Genetic variants from lipid-related pathways and risk for incident myocardial infarction. PLoS One 8: e60454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gong LL, Peng JH, Han FF, Zhu J, Fang LH, et al. (2012) Association of tissue plasminogen activator and plasminogen activator inhibitor polymorphism with myocardial infarction: a meta-analysis. Thromb Res 130: e43–e51. [DOI] [PubMed] [Google Scholar]
- 4. Franco E, Palumbo L, Crobu F, Anselmino M, Frea S, et al. (2007) Renin-angiotensin-aldosterone system polymorphisms: a role or a hole in occurrence and long-term prognosis of acute myocardial infarction at young age. BMC Med Genet 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang K, Bai P, Shi S, Zhou B, Wang Y, et al. (2012) The G894T polymorphism on endothelial nitric oxide synthase gene is associated with increased coronary heart disease among Asia population: evidence from a Meta analysis. Thromb Res 130: 192–197. [DOI] [PubMed] [Google Scholar]
- 6. Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 7. Chien YH, Bau DT, Jan KY (2004) Nitric oxide inhibits DNA-adduct excision in nucleotide excision repair. Free Radic Biol Med 36: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 8. Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, et al. (2002) The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J Hypertens 20: 2023–2027. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 11. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 12. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 13. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimasaki Y, Yasue H, Yoshimura M, Nakayama M, Kugiyama K, et al. (1998) Association of the missense Glu298Asp variant of the endothelial nitric oxide synthase gene with myocardial infarction. J Am Coll Cardiol 31: 1506–1510. [DOI] [PubMed] [Google Scholar]
- 16. Hibi K, Ishigami T, Tamura K, Mizushima S, Nyui N, et al. (1998) Endothelial nitric oxide synthase gene polymorphism and acute myocardial infarction. Hypertension 32: 521–526. [DOI] [PubMed] [Google Scholar]
- 17. Cai H, Wang X, Colagiuri S, Wilcken DE (1998) A common Glu298–>Asp (894G–>T) mutation at exon 7 of the endothelial nitric oxide synthase gene and vascular complications in type 2 diabetes. Diabetes Care 21: 2195–2196. [DOI] [PubMed] [Google Scholar]
- 18. Poirier O, Mao C, Mallet C, Nicaud V, Herrmann SM, et al. (1999) Polymorphisms of the endothelial nitric oxide synthase gene - no consistent association with myocardial infarction in the ECTIM study. Eur J Clin Invest 29: 284–290. [DOI] [PubMed] [Google Scholar]
- 19. Hingorani AD, Liang CF, Fatibene J, Lyon A, Monteith S, et al. (1999) A common variant of the endothelial nitric oxide synthase (Glu298–>Asp) is a major risk factor for coronary artery disease in the UK. Circulation 100: 1515–1520. [DOI] [PubMed] [Google Scholar]
- 20. Cai H, Wilcken DE, Wang XL (1999) The Glu-298–>Asp (894G–>T) mutation at exon 7 of the endothelial nitric oxide synthase gene and coronary artery disease. J Mol Med (Berl) 77: 511–514. [DOI] [PubMed] [Google Scholar]
- 21.Song J (2000) Association study of Alu repeat insertion/deletion polymorphism in tissue plasminogen activator gene and the Glu-Asp298 mutation at exon7 of the endothelial nitric oxide synthase gene with myocardial infarction. Chinese Academy Of Medical Science press 51p.
- 22. Wang CL, Hsu LA, Ko YS, Ko YL, Lee YH (2001) Lack of association between the Glu298Asp variant of the endothelial nitric oxide synthase gene and the risk of coronary artery disease among Taiwanese. J Formos Med Assoc 100: 736–740. [PubMed] [Google Scholar]
- 23. Gardemann A, Lohre J, Cayci S, Katz N, Tillmanns H, et al. (2002) The T allele of the missense Glu(298)Asp endothelial nitric oxide synthase gene polymorphism is associated with coronary heart disease in younger individuals with high atherosclerotic risk profile. Atherosclerosis 160: 167–175. [DOI] [PubMed] [Google Scholar]
- 24. Wei D, Shan J, Chen Z, Shi Y (2002) The G894T mutation of the endothelial nitric oxide synthase gene is associated with coronary atherosclerotic heart disease in Chinese. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 19: 471–474. [PubMed] [Google Scholar]
- 25. Aras O, Hanson NQ, Bakanay SM, Tsai MY, Gulec S (2002) Endothelial nitric oxide gene polymorphism (Glu298Asp) is not associated with coronary artery disease in Turkish population. Thromb Haemost 87: 347–349. [PubMed] [Google Scholar]
- 26. Qi J, Ma Y, Li M, Yuan D, Zeng D (2003) Endothelial nitric oxide synthase gene polymorphism and acute myocardial infarction. Chinese Journal of Practical Internal Medicine 23: 470–473. [Google Scholar]
- 27. Schmoelzer I, Renner W, Paulweber B, Malaimare L, Iglseder B, et al. (2003) Lack of association of the Glu298Asp polymorphism of endothelial nitric oxide synthase with manifest coronary artery disease, carotid atherosclerosis and forearm vascular reactivity in two Austrian populations. Eur J Clin Invest 33: 191–198. [DOI] [PubMed] [Google Scholar]
- 28. Agema WR, de Maat MP, Zwinderman AH, Kastelein JJ, Rabelink TJ, et al. (2004) An integrated evaluation of endothelial constitutive nitric oxide synthase polymorphisms and coronary artery disease in men. Clin Sci (Lond) 107: 255–261. [DOI] [PubMed] [Google Scholar]
- 29. Zhan Y, Di Q, Cheng Y, Ding X (2005) Correlation between Glu298Asp polymorphism of vascular endothelial nitric oxide synthase gene and myocardial infarction in the elderly Chinese. Chinese Journal of Clinical Rehabilitation 9: 177–179. [Google Scholar]
- 30. Antoniades C, Tousoulis D, Vasiliadou C, Pitsavos C, Chrysochoou C, et al. (2005) Genetic polymorphism on endothelial nitric oxide synthase affects endothelial activation and inflammatory response during the acute phase of myocardial infarction. J Am Coll Cardiol 46: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 31.Yu H (2006) The relations of gene polymorphisms of eNOS and FVll with Coronary Heart Disease in Henan Han population. Zhengzhou University press 21p.
- 32.Chao YX (2006) Association of the endothelial nitric oxide synthase gene polymorphism with essential hypertension and its cardiocerebrovascular complications. Shandong University press 35p.
- 33. Jo I, Moon J, Yoon S, Kim HT, Kim E, et al. (2006) Interaction between -786TC polymorphism in the endothelial nitric oxide synthase gene and smoking for myocardial infarction in Korean population. Clin Chim Acta 365: 86–92. [DOI] [PubMed] [Google Scholar]
- 34. Sampaio MF, Hirata MH, Hirata RD, Santos FC, Picciotti R, et al. (2007) AMI is associated with polymorphisms in the NOS3 and FGB but not in PAI-1 genes in young adults. Clin Chim Acta 377: 154–162. [DOI] [PubMed] [Google Scholar]
- 35. Andrikopoulos GK, Grammatopoulos DK, Tzeis SE, Zervou SI, Richter DJ, et al. (2008) Association of the 894G>T polymorphism in the endothelial nitric oxide synthase gene with risk of acute myocardial infarction. BMC Med Genet 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Odeberg J, Larsson CA, Rastam L, Lindblad U (2008) The Asp298 allele of endothelial nitric oxide synthase is a risk factor for myocardial infarction among patients with type 2 diabetes mellitus. BMC Cardiovasc Disord 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vasilakou M, Votteas V, Kasparian C, Pantazopoulos N, Dedoussis G, et al. (2008) Lack of association between endothelial nitric oxide synthase gene polymorphisms and risk of premature coronary artery disease in the Greek population. Acta Cardiol 63: 609–614. [DOI] [PubMed] [Google Scholar]
- 38. Gluba A, Banach M, Rysz J, Piotrowski G, Fendler W, et al. (2009) Is polymorphism within eNOS gene associated with the late onset of myocardial infarction? A pilot study. Angiology 60: 588–595. [DOI] [PubMed] [Google Scholar]
- 39. Szabo GV, Kunstar A, Acsady G (2009) Methylentetrahydrofolate reductase and nitric oxide synthase polymorphism in patients with atherosclerosis and diabetes. Pathol Oncol Res 15: 631–637. [DOI] [PubMed] [Google Scholar]
- 40. Isordia-Salas I, Leanos-Miranda A, Borrayo-Sanchez G (2010) The Glu298ASP polymorphism of the endothelial nitric oxide synthase gene is associated with premature ST elevation myocardial infarction in Mexican population. Clin Chim Acta 411: 553–557. [DOI] [PubMed] [Google Scholar]
- 41. Angeline T, Isabel W, Tsongalis GJ (2010) Endothelial nitric oxide gene polymorphisms, nitric oxide production and coronary artery disease risk in a South Indian population. Exp Mol Pathol 89: 205–208. [DOI] [PubMed] [Google Scholar]
- 42. Dafni C, Drakoulis N, Landt O, Panidis D, Reczko M, et al. (2010) Association of the eNOS E298D polymorphism and the risk of myocardial infarction in the Greek population. BMC Med Genet 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katakami N, Kaneto H, Matsuoka TA, Takahara M, Imamura K, et al. (2010) Accumulation of gene polymorphisms related to oxidative stress is associated with myocardial infarction in Japanese type 2 diabetic patients. Atherosclerosis 212: 534–538. [DOI] [PubMed] [Google Scholar]
- 44. Gad MZ, Abdel RM, Hashad IM, Abdel-Maksoud SM, Farag NM, et al. (2012) Endothelial nitric oxide synthase (G894T) gene polymorphism in a random sample of the Egyptian population: comparison with myocardial infarction patients. Genet Test Mol Biomarkers 16: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zigra AM, Rallidis LS, Anastasiou G, Merkouri E, Gialeraki A (2013) eNOS gene variants and the risk of premature myocardial infarction. Dis Markers 34: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Narne P, Ponnaluri KC, Singh S, Siraj M, Ishaq M (2013) Association of the genetic variants of endothelial nitric oxide synthase gene with angiographically defined coronary artery disease and myocardial infarction in South Indian patients with type 2 diabetes mellitus. J Diabetes Complications 27: 255–261. [DOI] [PubMed] [Google Scholar]
- 47. Arun KA, Umamaheswaran G, Padmapriya R, Balachandar J, Adithan C (2013) Endothelial nitric oxide synthase gene polymorphisms and the risk of acute myocardial infarction in a South Indian population. Mol Biol Rep 40: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 48. Yoshimura M, Yasue H, Nakayama M, Shimasaki Y, Sumida H, et al. (1998) A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet 103: 65–69. [DOI] [PubMed] [Google Scholar]
- 49. Niu W, Qi Y (2011) An updated meta-analysis of endothelial nitric oxide synthase gene: three well-characterized polymorphisms with hypertension. PLoS One 6: e24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rankinen T, Rice T, Perusse L, Chagnon YC, Gagnon J, et al. (2000) NOS3 Glu298Asp genotype and blood pressure response to endurance training: the HERITAGE family study. Hypertension 36: 885–889. [DOI] [PubMed] [Google Scholar]
- 51. Casas JP, Bautista LE, Humphries SE, Hingorani AD (2004) Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation 109: 1359–1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.
(DOC)
PRISMA 2009 Flow Diagram.
(DOC)
Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a heterozygous genetic model (GT vs. GG) stratified by ethnicity.
(TIF)
Forest plot of myocardial infarction associated with eNOS G894T polymorphism under a dominant genetic model (TT/GT vs. GG) stratified by ethnicity.
(TIF)