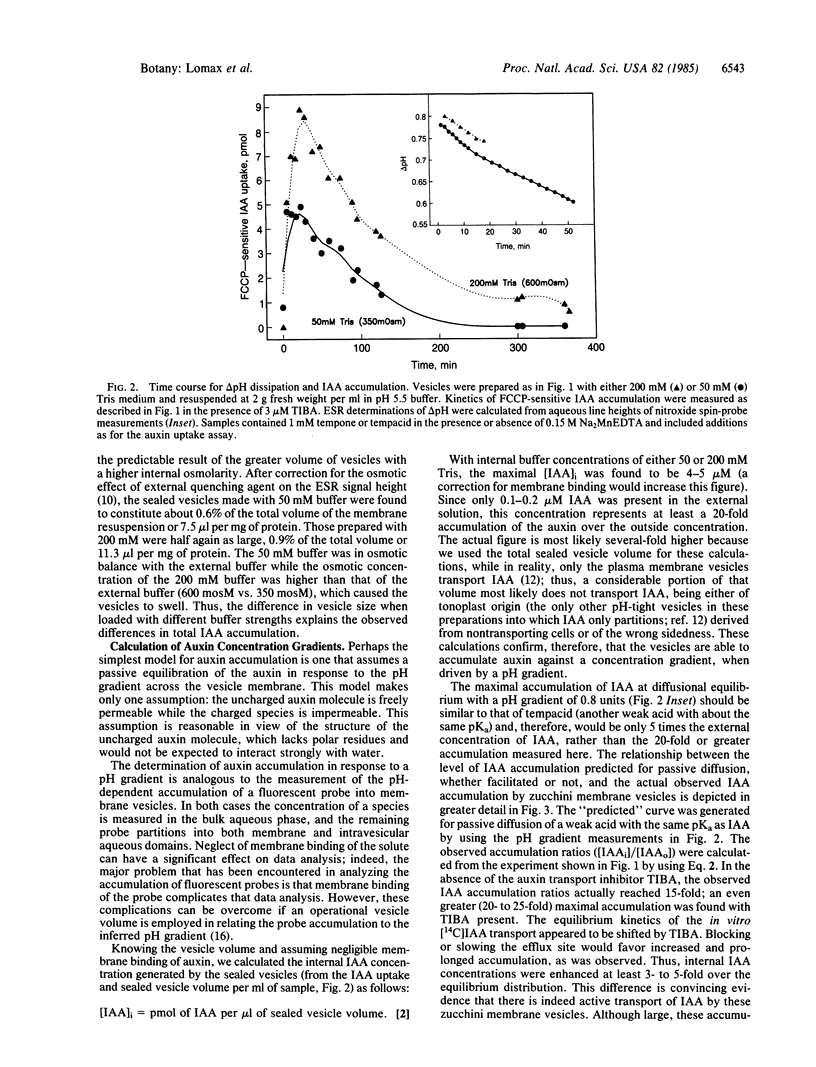
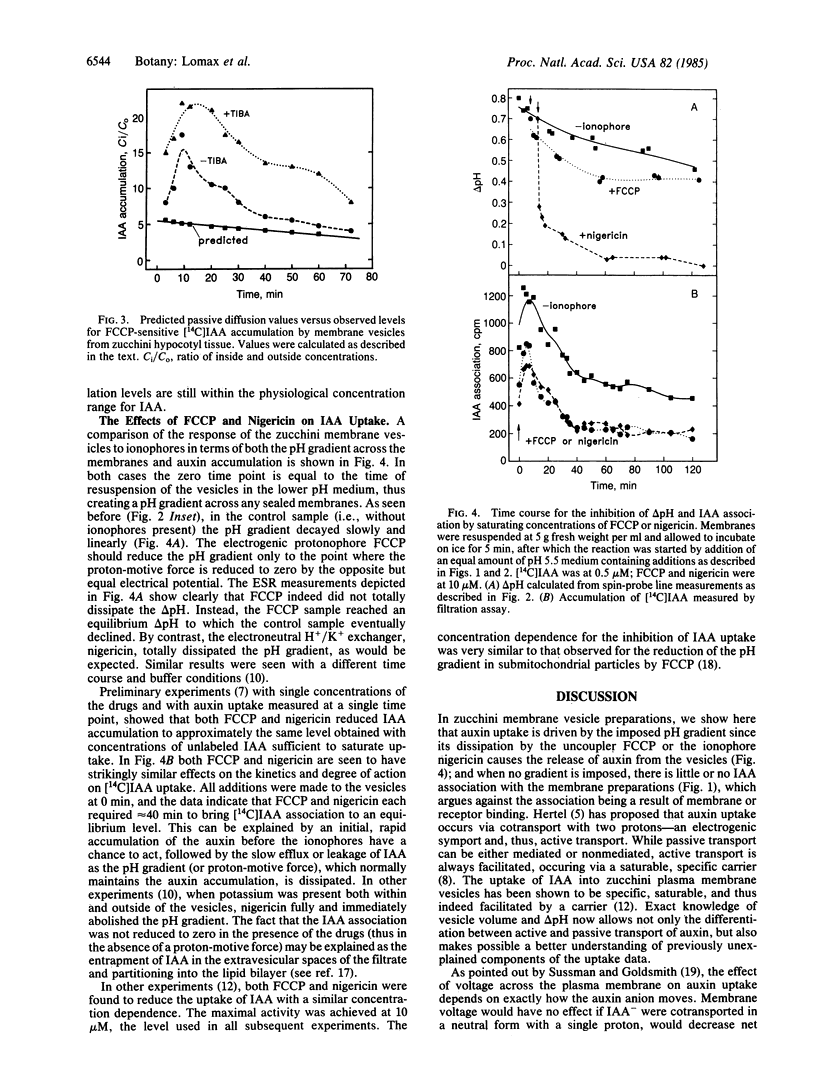
Abstract
Closed and pH-tight membrane vesicles prepared from hypocotyls of 5-day-old dark-grown seedlings of Cucurbita pepo accumulate the plant growth hormone indole-3-acetic acid along an imposed proton gradient (pH low outside, high inside). The use of electron paramagnetic spin probes permitted quantitation both of apparent vesicle volume and magnitude of the pH gradient. Under the experimental conditions used, hormone accumulation was at minimum 20-fold, a value 4 times larger than what one would predict if accumulation reflected only diffusional equilibrium at the measured pH gradient. It is concluded that hormone uptake is an active process, with each protonated molecule of hormone accompanied by an additional proton. Experiments with ionophores confirm that it is the pH gradient itself which drives the uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldsmith M. H., Goldsmith T. H., Martin M. H. Mathematical analysis of the chemosmotic polar diffusion of auxin through plant tissues. Proc Natl Acad Sci U S A. 1981 Feb;78(2):976–980. doi: 10.1073/pnas.78.2.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melandri B. A., Mehlhorn R. J., Packer L. Light-induced proton gradients and internal volumes in chromatophores of Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1984 Nov 15;235(1):97–105. doi: 10.1016/0003-9861(84)90258-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The driving force for proton(s) metabolites cotransport in bacterial cells. FEBS Lett. 1976 Jul 15;66(2):159–163. doi: 10.1016/0014-5793(76)80493-0. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Wade A. G., Ward P. J. First impressions of the use of meptazinol in general practice. J Int Med Res. 1981;9(1):74–78. doi: 10.1177/030006058100900113. [DOI] [PubMed] [Google Scholar]



