Abstract
TIS108 is a triazole-type strigolactone (SL)-biosynthesis inhibitor that reduces the level of 2′-epi-5-deoxystrigol (epi-5DS) in rice. Here we report the effects of TIS108 on Arabidopsis. Treatment of TIS108 increased the number of branches and repressed root hair elongation as was observed in SL-deficient mutants, and co-application of GR24, a synthetic SL analog, recovered the TIS108-induced phenotype to that of wild-type. In addition, MAX3 and MAX4 genes in the SL-biosynthesis pathway were upregulated in TIS108-treated Arabidopsis, probably due to feedback regulation caused by SL deficiency. These results indicate that TIS108 is an effective tool for regulating SL production in Arabidopsis.
Keywords: plant hormone, strigolactone, plant growth regulator, biosynthesis inhibitor, arabidopsis
Strigolactones (SLs) are terpenoid lactones produced in many plant species and have various functions: germination stimulants of root parasitic weeds, root-derived signals that induce hyphal branching in arbuscular mycorrhizal fungi, and a plant hormone that inhibits shoot branching and regulates root morphology.1-4 In the past, several branching mutants have been identified as SL biosynthesis and signaling mutants. To date, it is known that the biosynthesis of SLs is controlled by one b-carotene isomerase (D27), two carotenoid cleavage dioxygenases, CCD7 (MAX3, RMS5 D17/HTD1, DAD3) and CCD8 (MAX4, RMS1, D10, DAD1) and one cytochrome P450 monooxygenase (MAX1).5-7 More recently, Alder et al. reported that three enzymes, D27, CCD7 and CCD8, produce an SL-like chemical named carlactone from b-carotene in vitro and proposed a SL-biosynthesis pathway.7 However, the enzymatic function of MAX1 has not been uncovered.
As described above, at least one step of SL biosynthesis is thought to be mediated by one cytochrome P450 enzyme,8 and therefore P450 inhibitors may inhibit SL biosynthesis in Arabidopsis. Previously, we chose triazole derivatives as these have been shown to act as efficient inhibitors of P450 monooxygenase and reported two SL-biosynthesis inhibitors, 2,2-dimethyl-7-phenoxy-4-(1H-1,2,4-triazol-1-yl)heptan-3-ol (TIS13)9 and 6-phenoxy-1-phenyl-2-(1H-1,2,4-triazol-1-yl) hexan-1-one (TIS108)10 (Fig. 1A). Although TIS13 inhibits the production of epi-5DS, which is a major SL, in rice, TIS13-treated plants show a severe dwarf phenotype which does not recover following the application of SL; this suggests the possibility that TIS13 inhibits the biosynthesis of other plant hormones such as gibberellin (GA) and/or brassinosteroid (BR).9 To develop specific SL-biosynthesis inhibitors, a structure-activity relationship study on TIS13 derivatives was performed by estimating the level of epi-5DS and growth retardation in rice, which led to the identification of a specific SL-biosynthesis inhibitor, TIS10810 (Fig. 1). However, it is unclear whether TIS108 inhibits the production of SLs in Arabidopsis. In this study, to identify the effect of TIS108 on SL function in Arabidopsis, we examined the morphological changes in TIS108-treated Arabidopsis.
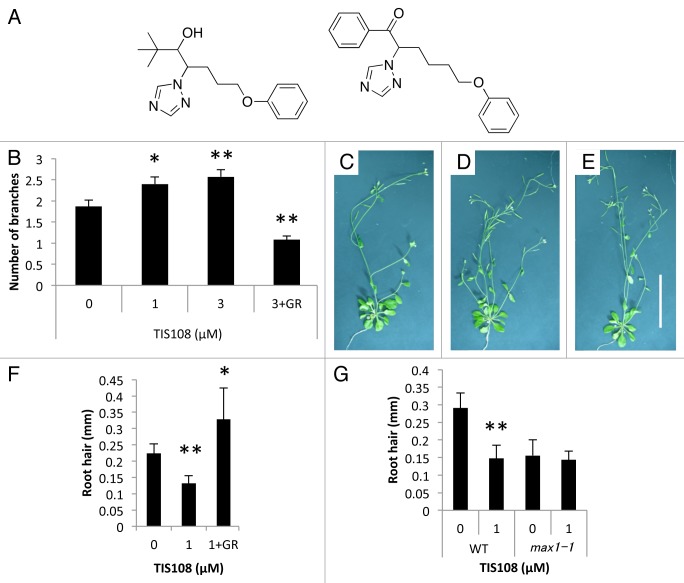
Figure 1. Effects of TIS108 on Arabidopsis. (A) Structure of SL biosynthesis inhibitors, TIS13 (left) and TIS108 (right). (B) The number of branches in TIS108-treated Arabidopsis. The data are mean ± SE of 8 to 14 samples. (C–E) Arabidopsis treated with 0 µM TIS108 (C), 3 µM TIS108 (D) and 3 µM TIS108 and 5 µM GR24 (E). Scale bar indicates 5 cm. (F–G) The length of root hairs in TIS108-treated Arabidopsis. Y-axis as: length of root hairs. The data are mean ± SD of 4 to 7 biological replicates. Note: * and ** means are statistically different from that of the control treatment, determined by t-tests (p < 0.05 and p < 0.01, respectively). GR:, 5 µM GR24.
SLs are known inhibitors of shoot branching in plants.3,4 Therefore, we estimated the effect of TIS108 on shoot branching of Arabidopsis (Fig. 1). In Arabidopsis, SL-biosynthesis mutants (max mutants) exhibit bushier shoots than wild-type plants and this phenotype is recovered by 5 µM SL treatment.3,4 TIS108-treated wild-type plants showed an increased-branching phenotype in a dose-dependent manner within the concentration range of 1–3 µM (Fig. 1B–E). In addition, treatment of 5 µM GR24 inhibited the TIS108-induced bushier shoots. Furthermore, no side effects, such as dwarfism, were observed in plants treated with TIS108 at these concentrations (Fig. 1B–D).
Recently, two reports demonstrated that SLs regulate root hair elongation.11,12 On the basis of these results, we estimated the effect of TIS108 on root hair elongation. In TIS108-treated wild-type plants, root hair elongation was suppressed, whereas root hair elongation was not affected by TIS108 in the SL-deficient mutants (Fig. 1E). In addition, this phenotype was rescued by co-application of 5 µM GR24 (Fig. 1F). These results suggest the possibility that the phenotype of TIS108-treated plants was caused by SL deficiency induced by the inhibition of SL production.
Next, we estimated the effect of TIS108 on Arabidopsis gene expression. Previous analysis revealed that the transcription levels of several genes related to SL biosynthesis, such as MAX3 (At2g44990) and MAX4 (At4g32810), were upregulated in several SL-biosynthesis and SL-insensitive mutants.3,4,13 On the basis of these results, we examined the mRNA levels of two SL-biosynthesis genes (MAX3 and MAX4) and a SL-signaling (MAX2) gene in Arabidopsis roots grown in the presence of TIS108 using real-time quantitative RT-PCR. MAX3 and MAX4 genes were upregulated in TIS108-treated plants, while the expression level of the MAX2 gene, which was not affected by the level of SL, did not change (Fig. 2). These results strongly suggest that TIS108 regulates number shoot and the expression of SL-biosynthesis genes through the inhibition of SL biosynthesis.
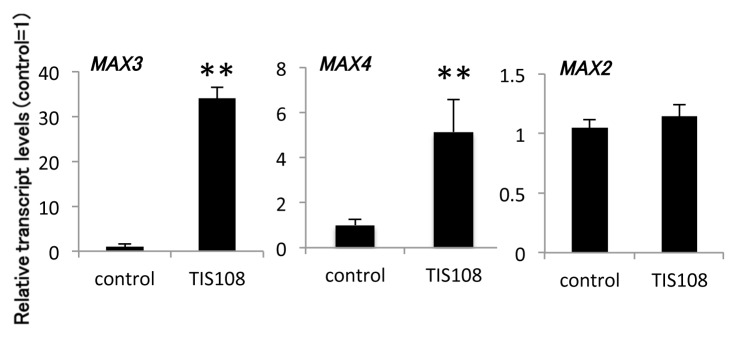
Figure 2. Relative expression of SL-biosynthesis/signaling genes in roots of adult plants. The expression level of each transcript was normalized using UBC (At5g25760) and is displayed relative to the expression level in the control (= 1). The data are mean ± SD of three biological replicates. * and ** Means arestatistically different from that of the wild-type in the control treatment, determined by t-tests (p < 0.05 and p < 0.01, respectively).
TIS108-treated Arabidopsis showed changes in both phenotype and gene expression that were similar to those of SL-deficient Arabidopsis mutants, namely, more branches, suppressed root hair elongation and higher expression of MAX3 and MAX4 mRNA than in control plants. In addition, the TIS108-induced phenotype was nullified by the addition of 5 µM GR24. These results suggest the possibility that TIS108 also inhibits SL biosynthesis in Arabidopsis, although we could not detect any SLs in Arabidopsis because the endogenous level of SLs might be very low (data not shown).
In several cases, triazole derivatives inhibit diverse P450s. For example, uniconazole-P can affect the biosynthesis of GA and BR, as well as the deactivation of abscisic acid.14,15 Therefore, secondary effects of TIS108 may also play a role in plants, but Figure 1 indicates that TIS108 treatment resulted in few morphological changes, except for the bushy phenotype at 3 µM in Arabidopsis. This result shows that TIS108 is a specific SL-biosynthesis inhibitor at this concentration with regard to plant height.
TIS108 inhibits SL biosynthesis, but its target site(s) is still unknown. Given the fact that at least one P450 (MAX1) is involved in SL biosynthesis in Arabidopsis and that various plants have MAX1 homologs, the target site(s) of TIS108 could be MAX1 homolog(s). Recently, it was reported that D27, CCD7 and CCD8 produce carlactone from b-carotene in vitro and the SL-biosynthesis pathway was proposed.7 According to that report, carlactone may be subsequently converted to strigolactone by MAX1 and unknown enzyme(s). Carlactone analysis in plants treated with TIS108 may be of great help for identifying the target site(s). However, there may be other P450 enzymes that play an important role in SL biosynthesis. In the near future, it will be important to identify the target site of TIS108.
In this report, we indicated that the phenotype of TIS108-treated Arabidopsis is similar to that of max mutants. As SL biosynthesis inhibitor is capable of being applied to various plants, TIS108 should be a useful tool for investigating the function of SLs, not only in other plants, but also in tissues, organs and biochemical processes. Moreover, in view of the potency of GA-biosynthesis inhibitors16,17 and BR-biosynthesis inhibitors18 for the selection of new mutants, SL-biosynthesis inhibitors should present a useful approach to find new branching pathways or other novel mutants.
Acknowledgment
This work was supported by a grant from the Program for the Core Research for Evolutional Science and Technology (CREST) Program of Japan Science and Technology Agency (JST) to T.A. and JSPS (Grant-in-Aid for Research Activity Start-up) to S.I.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24193
References
- 1.Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science. 1966;154:1189–90. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–7. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–94. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 4.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 5.Beveridge CA, Kyozuka J. New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol. 2010;13:34–9. doi: 10.1016/j.pbi.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Drummond RS, Martínez-Sánchez NM, Janssen BJ, Templeton KR, Simons JL, Quinn BD, et al. Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE7 is involved in the production of negative and positive branching signals in petunia. Plant Physiol. 2009;151:1867–77. doi: 10.1104/pp.109.146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, et al. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335:1348–51. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 8.Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8:443–9. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Ito S, Kitahata N, Umehara M, Hanada A, Kato A, Ueno K, et al. A new lead chemical for strigolactone biosynthesis inhibitors. Plant Cell Physiol. 2010;51:1143–50. doi: 10.1093/pcp/pcq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito S, Umehara M, Hanada A, Kitahata N, Hayase H, Yamaguchi S, et al. Effects of triazole derivatives on strigolactone levels and growth retardation in rice. PLoS ONE. 2011;6:e21723. doi: 10.1371/journal.pone.0021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruyter-Spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol. 2011;155:721–34. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koltai H. Strigolactones are regulators of root development. New Phytol. 2011;190:545–9. doi: 10.1111/j.1469-8137.2011.03678.x. [DOI] [PubMed] [Google Scholar]
- 13.Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano T, et al. Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotechnol Biochem. 2009;73:2460–5. doi: 10.1271/bbb.90443. [DOI] [PubMed] [Google Scholar]
- 14.Min YK, Asami T, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S. New lead compounds for brassinosteroid biosynthesis inhibitors. Bioorg Med Chem Lett. 1999;9:425–30. doi: 10.1016/S0960-894X(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 15.Saito S, Okamoto M, Shinoda S, Kushiro T, Koshiba T, Kamiya Y, et al. A plant growth retardant, uniconazole, is a potent inhibitor of ABA catabolism in Arabidopsis. Biosci Biotechnol Biochem. 2006;70:1731–9. doi: 10.1271/bbb.60077. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 1993;5:887–96. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nambara E, Keith K, McCourt P, Naito S. Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol. 1994;35:509–13. [PubMed] [Google Scholar]
- 18.Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell. 2002;2:505–13. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]