Abstract
Background
Recent advances in Bell’s palsy (BP) were reviewed to assess the current trends in its management and prognosis.
Material/Methods
We retrieved the literature on BP using the Cochrane Database of Systematic Reviews, PubMed, and Google Scholar. Key words and phrases used during the search included ‘Bell’s palsy’, ‘Bell’s phenomenon’, ‘facial palsy’, and ‘idiopathic facial paralysis’. Emphasis was placed on articles and randomized controlled trails (RCTs) published within the last 5 years.
Results
BP is currently considered the leading disorder affecting the facial nerve. The literature is replete with theories of its etiology, but the reactivation of herpes simplex virus isoform 1 (HSV-1) and/or herpes zoster virus (HZV) from the geniculate ganglia is now the most strongly suspected cause. Despite the advancements in neuroimaging techniques, the diagnosis of BP remains one of exclusion. In addition, most patients with BP recover spontaneously within 3 weeks.
Conclusions
Corticosteroids are currently the drug of choice when medical therapy is needed. Antivirals, in contrast, are not superior to placebo according to most reliable studies. At the time of publication, there is no consensus as to the benefit of acupuncture or surgical decompression of the facial nerve. Long-term therapeutic agents and adjuvant medications for BP are necessary due to recurrence and intractable cases. In the future, large RCTs will be required to determine whether BP is associated with an increased risk of stroke.
Keywords: Bell Palsy, Herpes Simplex Virus Protein Vmw65, Hydroxycorticosteroids
Background
Bell’s palsy is an acute, ipsilateral, facial nerve (CN VII) paralysis of unknown etiology that results in weakness of the platysma and muscles of facial expression [1]. Nicolaus Friedrich, an 18th century professor of medicine at Wurzburg, may have been the first to publish a case report of a facial nerve paralysis of unknown origin [2]. He gave an account of 3 middle-aged adults who had a similar onset of acute or subacute unilateral facial paralysis, which gradually improved over a period of weeks to months [2]. His clinical findings, De paralysis Musculorum Faciei Rheumatica, were first published in 1798 in the German medical literature.
Two years later, this paper was reviewed in English and published in the Annals of Medicine in Edinburgh. Around that time, a young Charles Bell (Figure 1) was studying medicine at the institution, and may have read Friedrich’s paper [3]. Bell later studied the function of the facial nerve in animals [2]. While practicing surgery in London, he encountered many cases of unilateral facial nerve paralysis and published his first report in 1821 [4]. Interestingly, his most famous and widely quoted account of facial paralysis was published in 1828, in which he told the story of a man who had been tossed by a bull. The ensuing puncture wound led to a lasting paralysis of his facial nerve [2].
Figure 1.

Sir Charles Bell (Used with permission from Van Gijn, 2011).
Although Bell’s initial publication on facial paralysis came after Friedreich’s account by 23 years, Grzybowski argues that Bell deserves the credit for differentiating peripheral from central facial nerve paralysis [4]. Today, the term Bell’s palsy is synonymous with idiopathic peripheral facial paralysis.
Recent advances in neuroimaging techniques such as MRI have equipped modern physicians with a considerable advantage over their predecessors in respect to visualizing the facial nerve. However, to our knowledge, there is no consensus on the etiological explanation or the preferred long-term treatment option for Bell’s palsy. In this review, we aim to provide the most comprehensive understanding of Bell’s palsy to date, with emphasis on clinical implications and the preferred management strategies based on reports from the recent literature.
Epidemiology
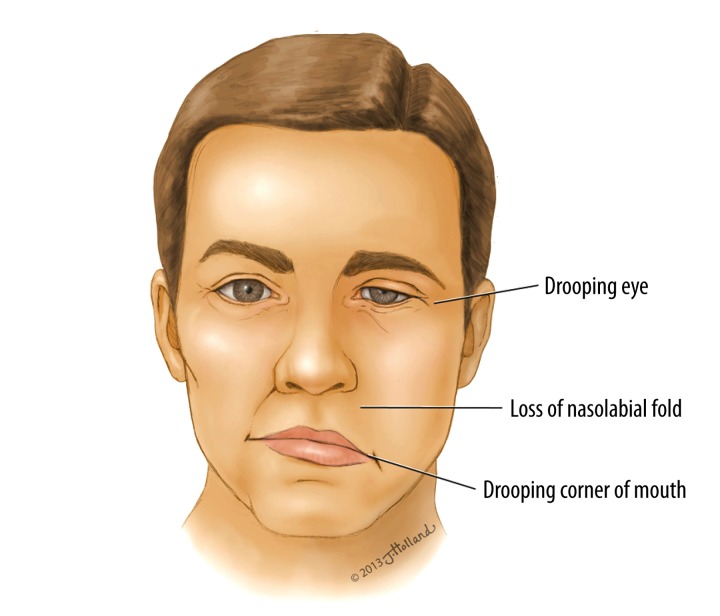
Bell’s palsy (Figure 2) is the most common disorder affecting the facial nerve [5] and is responsible for about 80% of all facial mononeuropathies [6]. Ramsey Hunt syndrome, which is a complication of the varicella-zoster virus infection, is the second leading cause of hemifacial paralysis [7]. Epidemiological studies report that Bell’s palsy affects 11–40 persons per 100,000 each year, with peak incidence usually between the ages of 15 and 50 years [8–10]. In the United States alone, more than 60 000 cases are diagnosed annually [11], with similar incidence rates reported among males and females [12]. Pregnant women, often during the third trimester and early postpartum periods, have also been shown to have higher incidence and risk of Bell’s palsy – up to 3 times greater compared to the general population [5,13]. Other susceptible groups include diabetics [1,14–16], the elderly [17–19], and patients with hypothyroidism [6].
Figure 2.
Drawing representing a man with Bell’s palsy showing right facial hemiparalysis.
Anatomy
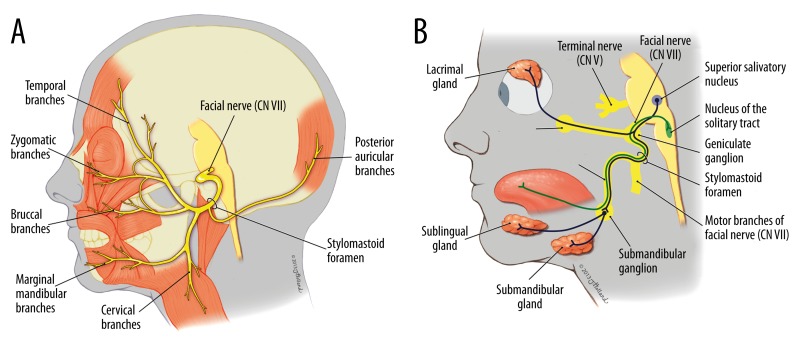
Knowledge of the course and function of the facial nerve (CN VII) is crucial to understanding the pathophysiology of Bell’s palsy. The nerve provides efferent motor innervation to the muscles of the face, the stapedius, and posterior belly of the digastric muscles (Figure 3A) [7]. In addition, sensory and parasympathetic fibers travel with the facial nerve. These parasympathetic fibers supply the lacrimal gland via the greater superficial petrosal nerve, and the submandibular glands via chorda tympani (Figure 3B). It is therefore not surprising that all of these fibers are susceptible to paralysis if the facial nerve is affected.
Figure 3.
(A) Schematic diagram of the motor innervation of the facial nerve. (B) Schematic diagram of the parasympathetic innervation of the facial nerve.
CN VII enters the temporal bone at the internal acoustic meatus, continues via the fallopian canal, and exits through the stylomastoid foramen. The narrowest portion of the fallopian canal is at the lateral end of the internal auditory canal [7]. The most probable mechanism of Bell’s palsy is an inflammatory process of the facial nerve leading to its compression along this narrow segment of the fallopian canal [20,21]. This inflammation initially causes a temporary loss of sensory or motor function, but can lead to permanent nerve degeneration later [20].
Mechanism and Etiology
The mechanism of Bell’s palsy has been the subject of fierce debate for decades, with the underlying cause of neuropathy remaining elusive despite several proposed theories. One theory describes BP as an acute demyelinating disease, which may have a similar pathogenic mechanism as Guillain-Barré syndrome [22,23]. It has been suggested that they both represent an inflammatory demyelinating neuritis in which BP can be considered a mononeuritic variant of Guillain-Barré [23,24].
Based on recent reports, the suspected etiology could be due to the reactivation of latent herpes viral infections in the geniculate ganglia, and their subsequent migration to the facial nerve [9,25]. Herpes simplex virus 1 (HSV-1) and herpes zoster virus (HZV) may be the causative agents, with HZV believed to be the more aggressive virus since it spreads across the nerve by means of satellite cells [9]. These reports are in agreement with the earlier findings of Murakami et al., who successfully isolated HSV-1 DNA from the endoneural fluid of the facial nerve by polymerase chain reaction (PCR) during the acute phase of Bell’s palsy [26]. As previously mentioned, the literature supports the idea of herpes simplex virus-mediated inflammation leading to nerve compression and clinical features such as facial paralysis [25,27,28].
Recently, the inactivated intranasal influenza (flu) vaccine has also been linked with Bell’s palsy [29]. Mutsch et al. performed a matched case-control study with case-series analysis, during which they looked at whether the 773 Bell’s palsy patients had also received the flu vaccine [29]. After adjusting for other variables, they reported that a strong temporal and specific association did exist; the risk of developing Bell’s palsy in patients who received the vaccine was nearly 19 times that of control groups without flu vaccine. Mutsch’s study found peak incidence of Bell’s palsy at 31 to 60 days after vaccination. From that data, it has been suggested that the activation of Bell’s palsy is not due to a direct toxic effect from the vaccine, but rather an autoimmune disorder or reactivation of HSV [30]. It is worth noting that the vaccine is no longer in clinical use. There was also no association of the palsy with parenteral flu vaccines.
Other known documented infectious causes of Bell’s palsy include: adenovirus, Coxsackie virus, cytomegalovirus, Epstein-Barr virus, influenza, mumps, and rubella [31]. Rickettsia is a rare infectious cause [32]. Suggested non-infectious causes of Bell’s palsy include autoimmune processes such as Hashimoto’s encephalopathy [33,34], ischemia from atherosclerosis leading to facial nerve edema [6,18,19], and familial origin, with about 4% to 8% of Bell’s palsy patients reported to have an associated family history [5].
Clinical Presentation
Bell’s palsy typically presents with a sudden and rapid onset of unilateral facial weakness, often within a few hours [7]. In fact, the symptoms can be so startling that most of the affected individuals either think they have had a stroke or a serious brain lesion [9]. It is important to note that up to 60% of these patients report a preceding viral illness [35].
Initially, partial palsy is reported by most patients, with maximum facial weakness often seen within 2 days [7,9]. Patients may also complain of ipsilateral earache as well as numbness of the face, tongue, and ear [25]. Moreover, cases of hyperacusis (possibly from stapedial muscle dysfunction), tinnitus, taste disturbances (most likely from injury to nervus intermedius proximal to geniculate ganglion), and decreased lacrimation have also been reported [5,6,9].
The motor deficit is almost always unilateral in Bell’s palsy [6], with both the upper and lower parts of the face affected. This helps to distinguish the disorder from a central supranuclear lesion, in which paresis occurs only in the lower facial muscles [6].
An often overlooked psychological component may also exist in patients presenting with acute Bell’s palsy, in which the acute onset of the condition can drastically affect social activity [36].
Diagnosis and Investigations
Because Bell’s palsy involves the peripheral facial nerve, there is often impaired ipsilateral movement of the affected side of the face, drooping of the eyebrow and corner of the mouth, as well as the loss of the ipsilateral nasolabial fold (Figure 2). Bell’s phenomenon – the upward movement of the eye on attempted closure of the lid due to weakness of the orbicularis oculi – is a pathognomonic sign [6,37].
Apart from diffuse facial nerve paralysis, Bell’s palsy is typically characterized by an acute onset, with symptoms presenting within several days and eventual resolution by 4 months. A progressive and prolonged course with frequent relapses and no recovery suggests neoplastic process [38].
Bell’s palsy must be differentiated from other facial paralysis, as there is a 13–20% rate of misdiagnosis [7]. Conditions that may mimic Bell’s palsy include CNS neoplasms, stroke, HIV infection, multiple sclerosis, Guillain-Barré syndrome, Ramsay-Hunt syndrome, Melkersson-Rosenthal syndrome, Lyme disease, otitis media, cholesteatoma, sarcoidosis, trauma to the facial nerve, autoimmune diseases such as Sjogren’s syndrome, and metabolic disorders such as diabetes mellitus [5,7,20,39]. Bell’s palsy is typically diagnosed by exclusion, and a thorough history and physical exam is needed to rule out other treatable or intracranial lesions [5]. Ear function should routinely be tested using tuning forks and standard pneumatic otoscopes. Clinical evidence of herpes zoster infection may help aid in the diagnosis of Bell’s palsy. However, vesicular lesions may be absent in the presence of pre-herpetic neuralgia in a clinical condition termed Zoster sin herpete [9].
The modified House-Brackmann scale (1985) is a clinical tool used to document the degree of facial paralysis and to predict probability of recovery. It assesses gross facial features and symmetry, both at rest and during motion. The grading is from 1 to 6, with the latter being total paralysis [7,40]. Patients who present with some observable facial movement and incomplete paralysis are expected to have uniformly good recovery. Patients with a House-Brackmann score of 6 may have prolonged or incomplete recovery [41]. The Sunnybrook facial grading system, Yanagihara grading system, and Sydney grading system represent regional alternatives to the House-Brackmann scale and have similar reliability, although reported ease of use varies [42–46]. The House-Brackmann scale is currently the most widely used and accepted.
Laboratory and imaging studies are not routinely needed in the diagnosis of Bell’s palsy and are only recommended in patients with recurrence, or if there has been no improvement after more than 3 weeks of therapy [47].
It is still recommended that patients with Bell’s palsy be referred to a neurologist or otolaryngologist as soon as possible to rule out more serious neurological conditions [35]. Serological tests to rule out Lyme disease are essential in endemic areas [5]. It is important to note that while Bell’s palsy is rare in children younger than 10 years old, as many as 50% of the reported cases of facial palsy in this group are due to Lyme disease [5].
Electromyography (EMG) and motor nerve conduction studies of the facial nerve can yield useful information on the viability of the affected nerve, thus aiding in the decision making process regarding treatment and/or surgical management of disease [48]. These electrodiagnostic studies yield information on the amount of evoked action potentials in affected muscles. Using this data, clinicians can estimate the amount of axonal loss. Patients exhibiting greater than 90% axonal degeneration should be considered for surgical decompression, while axonal degeneration of less than 90% has a favorable prognosis [49]. These studies are clinically useful within 2 weeks of complete facial paralysis [1]. After 3 months of onset of symptoms, needle electromyography may be employed to confirm any subclinical signs of re-innervation, thus acting as a prognostic indicator for the possibility of recovery [48].
Up to 5% of all lower motor neuron facial paralysis may be due to benign and malignant neoplasms [50]. Furthermore, a recent longitudinal study in Taiwan found a statistically significant increased risk of cancer in BP patients at 5-year follow-up [51]. If there is clinical suspicion, imaging studies such as CT with contrast or gadolinium-enhanced MRI are useful in ruling out neoplasms [5]. It is suggested that any case of BP without resolution within 4 months or first presenting 4 months after symptom onset undergo contrast-enhanced imaging of the parotid gland, temporal bone, and brain. Repeat imaging is indicated if symptoms persist at 7 months without a readily identifiable cause. Biopsy of affected tissue adjacent to the facial nerve may then be considered if imaging is negative at 7 months [52].
Management of Bell’s Palsy
Because the exact cause is still unknown, Bell’s palsy has no prevention or cure [39]. Thus, attempts at management over the years have been geared toward reducing inflammation to the facial nerve and/or preventing corneal complications stemming from paresis of the facial muscles [7].
Protecting the cornea from excessive dryness and abrasions should be addressed by the clinician through proper patient education. The cornea of patients with BP is especially at risk of drying because of improper lid closure and decreased tear production. Prescription of hourly lubricating eye drops and eye ointment during sleep are recommended [9]. In addition, the clinician should be prepared to provide psychological support in the early stages of management [9].
Both the preferred and optimum medical therapies for Bell’s therapy are still deliberated, with several randomized controlled trials (RCTs) showing mixed results [25,35]. The past 2 decades have consequently seen an increased number of trials, not only with anti-herpetic medications and corticosteroids, but also alternative techniques such as acupuncture and physical therapy. The quest for these alternative therapies probably stemmed from early data that failed to show any significant effect of drugs in treating Bell’s palsy. A Cochrane review of 4 RCTs conducted in 2004, for instance, showed that corticosteroids were no better than placebo [53], and another review of 3 trials in the same year also concluded that antivirals had no effect in the resolution of Bell’s palsy [54].
Recently, however, evidence has been mounting in support of corticosteroid as the treatment of choice [55–63]. Two RCTs by Sullivan et al. on 496 patients, and 1 on 839 patients by Engstrom et al., showed that early treatment with prednisone significantly improved the recovery of facial nerve function at 3- and 9-month intervals [58,62]. These studies also confirmed that the use of antivirals was inconsequential, whether given alone or with prednisone. Moreover, they noted that measuring each patient’s baseline severity of the palsy could provide information on which patients are more likely to benefit from corticosteroid treatment.
A meta-analysis by de Almeida et al. on 10 trials, assessing the efficacy of prednisolone versus placebo in the management of Bell’s palsy, also reported an overall significant benefit to treatment using prednisolone, with the number needed to treat (NNT) at 11 [57]. These findings were corroborated by a recent Cochrane review of 8 RCTs with over 1500 patients (2010), which validated the benefit of treating Bell’s palsy patients with corticosteroids. Of the 754 patients who were treated with corticosteroids, the authors noted that only 23% had incomplete recovery of facial motor function at 6 months, compared to 33% of patients who were given the placebo. In addition, they noted that the patients who had received corticosteroids had a significant reduction in motor synkinesis during follow-up compared to their counterparts [64].
Antiviral agents against HSV have also been extensively used to treat Bell’s palsy, and their effectiveness has been increasingly disputed based on recent studies. A 2009 Cochrane systematic review of 7 high-quality trials with 1987 participants concluded that anti-herpetic antivirals provide no significant benefit compared with placebo in generating complete recovery from Bell’s palsy [65]. Although antivirals alone do not provide a clear benefit, the efficacy of corticosteroids plus antivirals is less clear. Several studies have found no significant benefit from using combined antiviral and glucocorticoid treatment [66]. Recently, a network meta-analysis of 6 studies with a total of 1805 patients from 1996 to 2008 showed that a combination of corticosteroids with antivirals had a marginal benefit over corticosteroids alone [61]. However, since the findings did not reach statistical significance, the authors concluded that prednisone remains the single best evidence-based treatment for Bell’s palsy [61]. A 2013 study in Korea found a statistically significant improvement in patients with severe facial palsy when given famciclovir plus steroids within 1 week of presentation vs. steroids alone [67]. These results are promising, but it is unlikely that additional studies will significantly change current clinical practice due to the modest benefit of adding antivirals [68]. It has been proposed that research efforts should instead focus on the dosage and timing of steroid treatment, and treatment outcomes in specified age groups [68].
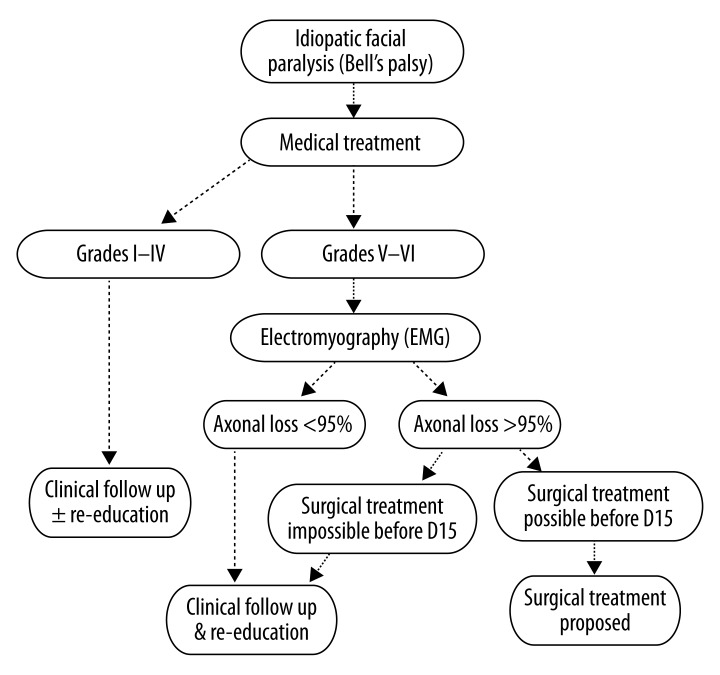
The literature shows no consensus for the benefit of, or indication for, surgery in the treatment of Bell’s palsy [46,69,70–73]. Risks associated with surgical decompression include seizures, unilateral hearing loss, CSF leak, and facial nerve injury [9,71,73]. Surgical decompression of the facial nerve therefore remains highly controversial and should only be considered in refractory cases, given the low-quality and inconclusive nature of the reports on its use. The proposed algorithm for managing severe Bell’s palsy is shown in Figure 4.
Figure 4.
Proposed algorithm for the management of severe Bell’s palsy. FP, facial palsy; EMG, electromyography; D15, fifteen days after onset of palsy (Redrawn and modified with permission from Bodenez et al., 2010).
Despite the advocacy of its use by some authors, acupuncture has not been proven to have any effect on either the recovery process or long-term morbidity from Bell’s palsy [74]. A recent 2013 RCT by Xu, however, does show promise in the utilization of acupuncture alongside glucocorticoid treatment. Patients undergoing intense acupuncture stimulation and glucocorticoid treatment had better 6 month outcomes according to the House-Brackmann scale vs. patients receiving standard acupuncture plus glucocorticoids [75]; the findings are promising, but additional high quality trials will be required before its efficacy can be validated.
Electrical nerve stimulation is a proposed method of accelerating recovery in BP patients through invoked muscle stimulation. Further studies will be required to assess clinical applicability [76–79].
Prognosis
Most patients with Bell’s palsy regain normal function with or without medical therapy, often within 3 weeks [6,80]. In some cases, full recovery takes up to 9 months [5], but up to 30% are left with potentially disfiguring facial weakness, involuntary movements, and/or persistent lacrimation, requiring further interventions [25,39,71]. A delay in the diagnosis and administration of medications could play a role in residual weakness of the face and mouth, but other factors such as severity of the inflammation and compression of the facial nerve are equally significant. Age and the degree of facial paralysis are other reported prognostic factors, with younger patients and those with partial facial palsy gaining almost full restoration [5]. Patients who exhibit signs of recovery within 21 days of symptom onset have a favorable prognosis [81.
Electrodiagnostic testing is helpful in identifying severe cases of Bell’s palsy [6]; however, it must be done in a timely manner because decompression of the facial nerve later than 14 days after onset of symptoms does not alter the prognosis of Bell’s palsy [82]. It must also be remembered that almost all patients with Bell’s palsy do regain some function within 3 months of onset; hence, all affected individuals should be re-evaluated at that time to rule out tumor as the cause of the facial paresis.
The recurrence rate of Bell’s palsy is about 12% of cases, with 36% re-experiencing paralysis on the same side [5,39]. Multiple recurrences are relatively rare, occurring in about 3% of cases. BP recurrence does not seem to be correlated with prognosis [83].
Complications of Bell’s Palsy
Long-term complications can develop from Bell’s palsy. For instance, when nerve fibers have been damaged, they may aberrantly regenerate by connecting with the lacrimal ducts instead of the salivary glands. This can lead to lacrimation while eating, or “crocodile tears” [6]. Similarly, regenerating motor neurons can innervate inappropriate muscles, which leads to abnormal movements or facial synkinesis [6]. Botulinum toxin injection and facial reanimation through cosmetic surgery are among the proposed methods of treating such long-term sequelae [9,84–86]. Long-term patient satisfaction and quality of life may be monitored using the Facial Clinimetric Evaluation Scale (FaCE) in conjunction to the House-Brackmann scale in these instances [87].
Most recently, Chiu et al. reported that Bell’s palsy can increase the risk of non-hemorrhagic stroke [88]. In a population-based cohort study of 7506 Bell’s palsy patients and 22 158 non-BP patients followed for 2 years, they found that Bell’s palsy patients were at an increased risk (4%) of stroke compared to the general population (1.6%) after adjusting for co-morbidities [88]. Their finding can partly be explained by the fact that previous studies have shown an increased risk of stroke in patients with HSV-1 and HZV [89]. These pathogens have been suggested to cause inflammation as well as atherosclerosis and vasculopathy in the cerebral vasculature [90,91].
Conclusions
Based on our review, the cause of Bell’s palsy remains elusive, but herpetic reactivation is now the most strongly suspected cause. Its clinical presentation is well-documented in the literature. However, the diagnosis is still one of exclusion. While prior systematic reviews showed no difference between medical therapy and placebo in treating Bell’s palsy, results of recent RCTs strongly recommend corticosteroids as the treatment of choice. Antiviral therapy is of no significant help according to most reliable studies. Surgical treatment of Bell’s palsy is still controversial, and should only be used for refractory cases.
Acknowledgements
The authors wish to thank Jessica Holland, MS, Medical Illustrator in the Department of Anatomical Sciences, St Georges University, Grenada, West Indies, for the creation of her illustrations used in this publication.
Footnotes
Source of support: Self financing
References
- 1.Gilden DH. Clinical practice. Bell’s Palsy. N Engl J Med. 2004;351(13):1323–31. doi: 10.1056/NEJMcp041120. [DOI] [PubMed] [Google Scholar]
- 2.Bird TD, Nicolaus A. Friedreich’s description of peripheral facial nerve paralysis in 1798. J Neurol Neurosurg Psychiatry. 1979;42(1):56–58. doi: 10.1136/jnnp.42.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman LM. The Founders of Neurology. Springfield (IL): Charles C. Thomas; 1970. [Google Scholar]
- 4.Grzybowski A, Kaufman MH. Sir Charles Bell (1774–1842): contribution to neuro-ophalmology. Acta Opthalmologica Scandinavica. 2007;85:897–901. doi: 10.1111/j.1600-0420.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 5.Wolfson AB. Narwood-Nuss’ Clinical Practice of Emergency Medicine. 5th ed. Philadelphia (PA): Lippincott Williams & Williams; 2009. [Google Scholar]
- 6.Goroll AH, Mulley AG. Primary care medicine: office evaluation and management of the adult patient. 6th ed. Philadelphia (PA): Lippincott Williams & Williams; 2009. [Google Scholar]
- 7.Runge MS, Greganti MA. Netter’s Internal Medicine. 2nd ed. Philadelphia (PA): Elsevier; 2009. [Google Scholar]
- 8.De Diego-Sastre JI, Prim-Espada MP, Fernandez-Garcia F. The epidemiology of Bell’s palsy. Rev Neurol. 2005;41:287–90. [PubMed] [Google Scholar]
- 9.Holland NJ, Weiner GM. Recent developments in Bell’s palsy. [Review] BMJ. 2004;329(7465):553–57. doi: 10.1136/bmj.329.7465.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prescott CA. Idiopathic facial nerve palsy (the effect of treatment with steroids) J Laryngol Otol. 1988;102:403–7. doi: 10.1017/s0022215100105201. [DOI] [PubMed] [Google Scholar]
- 11.Morris AM, Deeks SL, Hill MD, et al. Annualized incidence and spectrum of illness from an outbreak investigation of Bell’s palsy. Neuroepidemiology. 2002;21(5):255–61. doi: 10.1159/000065645. [DOI] [PubMed] [Google Scholar]
- 12.Bosco D, Plastino M, Bosco F, et al. Bell’s palsy: a manifestation of prediabetes? Acta Neurol Scand. 2011;123(1):68–72. doi: 10.1111/j.1600-0404.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 13.Hilsinger RL, Adour KK, Doty HE. Idiopathic facial paralysis, pregnancy, and the menstrual cycle. Ann Otol Rhinol Laryngol. 1975;84(4 Pt 1):433–42. doi: 10.1177/000348947508400402. [DOI] [PubMed] [Google Scholar]
- 14.Adour KK, Byl FM, Hilsinger RL, Jr, et al. The true nature of Bell’s palsy: analysis of 1,000 consecutive patients. Laryngoscope. 1978;88(5):787–801. doi: 10.1002/lary.1978.88.5.787. [DOI] [PubMed] [Google Scholar]
- 15.Mountain RE, Murray JA, Quaba A, Maynard C. The Edinburg facial palsy clinic: a review of three years’ activity. J R Coll Surg Edinb. 1994;39(5):275. [PubMed] [Google Scholar]
- 16.Riga M, Kefalidis G, Danielides V. The role of diabetes mellitus in the clinical presentation and prognosis of bell palsy. JABFM. 2012;25(6):819–26. doi: 10.3122/jabfm.2012.06.120084. [DOI] [PubMed] [Google Scholar]
- 17.Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265(7):743–52. doi: 10.1007/s00405-008-0646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merwarth HR. The occurence of peripheral facial paralysis in hypertension vascular disease. Ann Intern Med. 1942;17:298–30. [Google Scholar]
- 19.Raff MC, Asbury AK. Ischemic mononeuropathy and mononeuropathy multiplex in diabetes mellitus. N Engl J Med. 1968;279:17–22. doi: 10.1056/NEJM196807042790104. [DOI] [PubMed] [Google Scholar]
- 20.Jackson CG, von Doersten PG. The facial nerve. Current trends in diagnosis, treatment, and rehabilitation. Med Clin North Am. 1999;83(1):179–95. doi: 10.1016/s0025-7125(05)70096-1. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Li Y, Yuan X, Lin Z. Bell’s palsy may have relations to bacterial infection. Med Hypotheses. 2009;72(2):169–70. doi: 10.1016/j.mehy.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Aviel A, Ostfeld E, Burstein R, et al. Peripheral blood T and B lymphocyte subpopulations in Bell’s palsy. Ann Otol Rhinol Laryngol. 1983;92:187–91. doi: 10.1177/000348948309200218. [DOI] [PubMed] [Google Scholar]
- 23.Greco A, Gallo A, Fusconi M, et al. Bell’s palsy and autoimmunity. Autoimmun Rev. 2012;12:323–28. doi: 10.1016/j.autrev.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Chaco J. Subclinical pheripheral nerve involvement in unilateral Bell’s palsy. Am J Phys Med. 1973;52:195–97. [PubMed] [Google Scholar]
- 25.Schirm J, Mulkens PS. Bell’s palsy and herpes simplex virus. APMIS. 1997;105:815–23. doi: 10.1111/j.1699-0463.1997.tb05089.x. [DOI] [PubMed] [Google Scholar]
- 26.Murakami S, Mizobuchi M, Nakashiro Y, et al. Bell’s palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124(1):27–33. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Baringer JR. Herpes simplex virus and Bell palsy. Ann Intern Med. 1996;124(1 Pt 1):63. doi: 10.7326/0003-4819-124-1_part_1-199601010-00010. [DOI] [PubMed] [Google Scholar]
- 28.Peitersen E. Bell’s palsy: The spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;(549):4–30. [PubMed] [Google Scholar]
- 29.Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 30.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N Engl J Med. 2004;350(9):860–61. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 31.Morgan M, Nathwani D. Facial palsy and infection: the unfolding story. Clin Infect Dis. 1992;14(1):263. doi: 10.1093/clinids/14.1.263. [DOI] [PubMed] [Google Scholar]
- 32.Bitsori M, Galanakis E, Papadakis CE, Sbyrakis S. Facial nerve palsy associated with Rickettsia conorii infection. Arch Dis Child. 2001;85(1):54. doi: 10.1136/adc.85.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaitkin BM, May M, Podvinec M, et al. Idiopathic (Bell’s) palsy, herpes zoster cephalicus, and other facial nerve disorders of viral origin. In: May M, Schaitkin BM, editors. The facial nerve: May’s. 2nd ed. New York: Thieme Medical; 2000. pp. 319–38. [Google Scholar]
- 34.Lei H, Mei L, Long XH, et al. A case of Hashimoto’s encephalopathy misdiagnosed as viral encephalitis. Am J Case Rep. 2013;14:366–69. doi: 10.12659/AJCR.889312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benecke JE. Facial paralysis. Otolaryngol Clin North Am. 2002;35:357–65. doi: 10.1016/s0030-6665(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 36.Weir AM, Pentland B, Murray J, et al. Bell’s Palsy: The effecrt on self image, mood state and social activity. Clin Rehabil. 1993;7:88. [Google Scholar]
- 37.Gondivkar S, Parikh V, Parikh R. Herpes zoster oticus: A rare clinical entity. Contemp Clin Dent. 2010;2(1):127–29. doi: 10.4103/0976-237X.68588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boahene DO, Olsen KD, Driscoll C, et al. Facial nerve paralysis secondary to occult malignant neoplasms. Otolaryngol Head Neck Surg. 2004;130(4):459. doi: 10.1016/j.otohns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Shannon S, Meadow S, Horowitz SH. Are drug therapies effective in treating Bell’s palsy? J Fam Pract. 2003;52:156–59. [PubMed] [Google Scholar]
- 40.House J, Brackmann D. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–47. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 41.Danner CJ. Facial Nerve Paralysis. Otolaryngol Clin N Am. 2008;4:619–32. doi: 10.1016/j.otc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Berg T, Jonsson L, Engstrom M. Agreement between the Sunnybrook, House-Brackmann, and Yanagihara facial nerve grading systems in Bell’s palsy. Otol Neurotol. 2004;25(6):1020–26. doi: 10.1097/00129492-200411000-00027. [DOI] [PubMed] [Google Scholar]
- 43.Coulson SE, Croxson, Adams RD, O’Dwyer NJ. Reliability of the “Sydney,” “Sunnybrook,” and “House Brackmann” facial grading systems to assess voluntary movement and synkinesis after facial nerve paralysis. Otolaryngol Head Neck Srug. 2005;132(4):543–49. doi: 10.1016/j.otohns.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Marsk E, Bylund N, Jonsson L, et al. Prediction of nonrecovery in Bell’s palsy using Sunnybrook grading. Laryngoscope. 2012;122(4):901–90. doi: 10.1002/lary.23210. [DOI] [PubMed] [Google Scholar]
- 45.Yanagihara N, Gyo K, Yumoto E, Tamaki M. Transmastoid decompression of the facial nerve in Bell’s palsy. Arch Otolaryngol. 1979;105(9):530–34. doi: 10.1001/archotol.1979.00790210028006. [DOI] [PubMed] [Google Scholar]
- 46.Yanagihara N, Hato N, Murakami S, Honda N. Transmastoid decompression as a treatment of Bell palsy. Otol Head Neck Surg. 2001;24(3):282–86. doi: 10.1067/mhn.2001.112309. [DOI] [PubMed] [Google Scholar]
- 47.Bodenez C, Bernat I, Willer JC, et al. Facial nerve decompression for idiopathic Bell’s palsy: report of 13 cases and literature review. J Laryngol Otol. 2010;124(3):272–78. doi: 10.1017/S0022215109991265. [DOI] [PubMed] [Google Scholar]
- 48.Valls-Sole J. Electrodiognostic studies of the facial nerve in a peripheral facial palsy and femifacial spasm. Muscle Nerve. 2007;36(1):14. doi: 10.1002/mus.20770. [DOI] [PubMed] [Google Scholar]
- 49.Shularick NM, Mowry SE, Soken H, Hansen MR. Is electroneurography beneficial in the management of bell’s palsy? Laryngoscope. 2013;123:1066–67. doi: 10.1002/lary.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selesnick SH, Patwardhan A. Acute facial paralysis: evaluation and early management. Am J Otolaryngol. 1994;15:387–408. doi: 10.1016/0196-0709(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 51.Sheu JJ, Keller JJ, Lin H. Increased risk of cancer after Bell’s palsy: a 5-year follow up study. J Neurooncol. 2012;110(2):215–20. doi: 10.1007/s11060-012-0954-9. [DOI] [PubMed] [Google Scholar]
- 52.Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell’s palsy: finding occult malignancy in acute-onset facial paralysis. Am J Otolaryngol. 2010;31(5):339–42. doi: 10.1016/j.amjoto.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) Cochrane Database System Rev. 2004;4:CD001942. doi: 10.1002/14651858.CD001942.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Allen D, Dunn L. Aciclovir or valaciclovir for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2004;3:CD001869. doi: 10.1002/14651858.CD001869.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Adour KK, Ruboyianes JM, Von Doersten PG, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized controlled trial. Ann Otol Rhinol Laryngol. 1996;105(5):371–78. doi: 10.1177/000348949610500508. [DOI] [PubMed] [Google Scholar]
- 56.Browning GG. Bell’s palsy: a review of three systematic reviews of steroid and anti-viral therapy. Clin Otolaryngol. 2010;35(1):56–58. doi: 10.1111/j.1749-4486.2010.02084.x. [DOI] [PubMed] [Google Scholar]
- 57.De Almeida JR, Al Khabori M, Guyatt GH, et al. Combined corticosteroid and antiviral treatment for Bell’s palsy: a systematic review and meta-analysis. JAMA. 2009;302(9):985–93. doi: 10.1001/jama.2009.1243. [DOI] [PubMed] [Google Scholar]
- 58.Engstrom M, Berg T, Stjernquist-Desatnik A, et al. Prednisolone and valaciclovir in Bell’s palsy: a randomized, double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2008;7(11):993–1000. doi: 10.1016/S1474-4422(08)70221-7. [DOI] [PubMed] [Google Scholar]
- 59.Hato N, Yamada H, Kohno H, et al. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol & Neurotol. 2007;28(3):408–13. doi: 10.1097/01.mao.0000265190.29969.12. [DOI] [PubMed] [Google Scholar]
- 60.Kawaguchi K, Inamura H, Abe Y, et al. Reactivation of herpes simplex virus type 1 and varicella-zoster virus and therapeutic effects of combination therapy with prednisolone and valacyclovir in patients with Bell’s palsy. Laryngoscope. 2007;117(1):147. doi: 10.1097/01.mlg.0000248737.65607.9e. [DOI] [PubMed] [Google Scholar]
- 61.Numthavaj P, Thakkinstian A, Dejthevaporn C, Attia J. Corticosteroid and antiviral therapy for Bell’s palsy: a network meta-analysis. BMC Neurol. 2011;11:1. doi: 10.1186/1471-2377-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan FM, Swan IR, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med. 2007;357(16):1598–607. doi: 10.1056/NEJMoa072006. [DOI] [PubMed] [Google Scholar]
- 63.Yeo SG, Lee YC, Park DC, Cha CI. Acyclovir plus steroid alone in the treatment of Bell’s palsy. Am J Otolaryngol. 2008;29(3):163. doi: 10.1016/j.amjoto.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Salinas RA, Alvarez G, Daly F, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) Cochrane Database System Rev. 2010;3:CD001942. doi: 10.1002/14651858.CD001942.pub4. [DOI] [PubMed] [Google Scholar]
- 65.Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis) Cochrane Database System Rev. 2009;4:CD001869. doi: 10.1002/14651858.CD001869.pub4. [DOI] [PubMed] [Google Scholar]
- 66.Quant EC, Jeste SS, Muni RH, et al. The benefits of steroids versus steroids plus antiviruals for treatment of Bell’s palsy: a meta-analysis. BMJ. 2009;339:b3354. doi: 10.1136/bmj.b3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HY, Bryun JY, Park MS, Yeo SG. Steroid-antiviral treatment improves the recovery rate in patients with severe bell’s palsy. Am J Med. 2013;126(4):336–41. doi: 10.1016/j.amjmed.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 68.Gronseth GS, Paduga R. Evidence-based guideline update: Steroids and antivirals for Bell palsy. Neurology. 2012;79:2209–13. doi: 10.1212/WNL.0b013e318275978c. [DOI] [PubMed] [Google Scholar]
- 69.Adour KK. Decompression for Bell’s palsy: why I don’t do it. Eur Arch Otorhinolaryngol. 2002;259(1):40–47. doi: 10.1007/pl00007528. [DOI] [PubMed] [Google Scholar]
- 70.Friedman RA. The surgical management of Bell’s palsy: a review. Am J Otol. 2000;21(1):139–44. doi: 10.1016/s0196-0709(00)80090-3. [DOI] [PubMed] [Google Scholar]
- 71.Grogan P, Gronseth G. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review) Neurology. 2001;56:830–36. doi: 10.1212/wnl.56.7.830. [DOI] [PubMed] [Google Scholar]
- 72.May M, Klein SR, Taylor FH. Idiopathic (Bell’s) facial palsy: natural history defies steroid or surgical treatment. Laryngoscope. 1985;95(4):406–9. doi: 10.1288/00005537-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 73.McAllister K, Walker D, Donnan PT, Swan I. Surgical interventions for the early management of Bell’s palsy. Cochrane Database Syst Rev. 2011;2:CD007468. doi: 10.1002/14651858.CD007468.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Chen N, Zhou M, He L, et al. Acupuncture for Bell’s palsy. Cochrane Database Syst Rev. 2010;8:CD002914. doi: 10.1002/14651858.CD002914.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu S, Huang B, Zhang C, et al. Effectiveness of strengthened stimulation during acupuncture for the treatment of Bell palsy: a randomised controlled trial. CMAJ. 2013;185(6):473. doi: 10.1503/cmaj.121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gittins J, Martin K, Sheldrick J, et al. Electrical stimulation as a therapeutic option to improve eyelid function in chronic facial nerve disorders. Invest Ophthalmol Vis Sci. 1993;40(3):547. [PubMed] [Google Scholar]
- 77.Targan RS, Alon G, Kay SL. Effect of long-ter electrical stimulation on motor recovery and improvement of clinical residuals in patients with unresolved facial nerve palsy. Otolaryngol Head Neck Surg. 2000;122(2):246. doi: 10.1016/S0194-5998(00)70248-8. [DOI] [PubMed] [Google Scholar]
- 78.Schrode LW. Treatent of facial muscles affected by Bell’s palsy with high-voltage electrical muscle stimulation. J Manipulative Physiol Ther. 1993;16(5):347. [PubMed] [Google Scholar]
- 79.Toffola ED, Tinelli C, Lozza A, et al. Choosing the best rehabilitation treatment for Bell’s palsy. Eur J Rehabil Med. 2012;48:635–43. [PubMed] [Google Scholar]
- 80.Peitersen E. The natural history of Bell’s palsy. Am J Otol. 1982;4(2):107–11. [PubMed] [Google Scholar]
- 81.Jabor MA, Gianoli G. Management of Bell’s palsy. J La State Med Soc. 1996;148(7):279. [PubMed] [Google Scholar]
- 82.Gantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell’s palsy. Laryngoscope. 1999;109(8):1177–88. doi: 10.1097/00005537-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 83.Pitts DB, Adour KK, Hilsinger RL. Recurrent Bell’s palsy: analysis of 140 patients. Laryngoscope. 1988;98(5):535. doi: 10.1288/00005537-198805000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Douglas RS, Gausas RE. A systematic comprehensive approach to management of irreversible facial paralysis. Facial Plast Surg. 2003;19(1):107. doi: 10.1055/s-2003-39135. [DOI] [PubMed] [Google Scholar]
- 85.Ito H, Ito H, Nakano S, Kusaka H. Low-dose subcutaneous injection of botulinum toxin type A for facial synkinesis and hyperlacrimation. Acta Neurol Scand. 2007;115(4):271. doi: 10.1111/j.1600-0404.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 86.Nava-Castaneda A, Tovilla-Canales JL, Boullosa V, et al. Duration of botulinum toxin effect in the treatment of crocodile tears. Opthal Plast Reconstr Surg. 2006;22(6):453. doi: 10.1097/01.iop.0000244515.07925.99. [DOI] [PubMed] [Google Scholar]
- 87.Ng JH, Ngo RW. The use of the facial clinimetric evaluation scale as a patient based grading system in bell’s palsy. Laryngoscope. 2013;123:1256–60. doi: 10.1002/lary.23790. [DOI] [PubMed] [Google Scholar]
- 88.Chiu YN, Yen MF, Chen LS, Pan SL. Increased risk of stroke after Bell’s palsy: a population-based longitudinal follow-up study. J Neurol Neurosurg Psychiatry. 2012;83(3):341–43. doi: 10.1136/jnnp.2011.240390. [DOI] [PubMed] [Google Scholar]
- 89.Elkind MS, Ramakrishnan P, Moon YP, et al. Infectious burden and risk of stroke: the northern Manhattan study. Arch Neurol. 2010;67(1):33–38. doi: 10.1001/archneurol.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voorend M, van der Ven AJ, Kubat B, et al. Limited role for C. pneumoniae, CMV and HSV-1 in cerebral large and small vessel atherosclerosis. Open Neurol J. 2008;2:39–44. doi: 10.2174/1874205X00802010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40(11):3443–48. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 92.Van Gijn J. Charles Bell (1774–1842) J Neurol. 2011;258(6):1189–90. doi: 10.1007/s00415-011-5912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]