Abstract
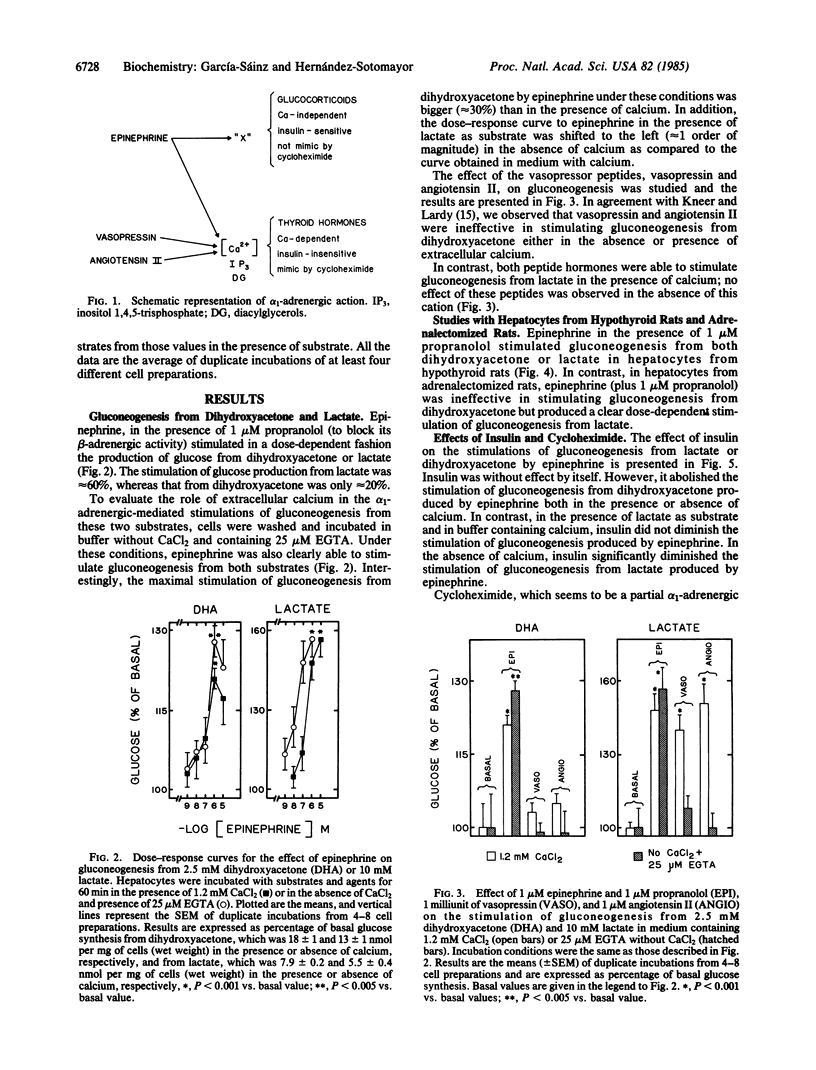
We have previously suggested that the effects of alpha 1-adrenergic agents on hepatocyte metabolism involve two mechanisms: (i) a calcium-independent insulin-sensitive process that is modulated by glucocorticoids and (ii) a calcium-dependent insulin-insensitive process that is modulated by thyroid hormones. We have studied the effect of epinephrine (plus propranolol) on gluconeogenesis from lactate and dihydroxyacetone. It was observed that the adrenergic stimulation of gluconeogenesis from lactate seemed to occur through both mechanisms, whereas when the substrate was dihydroxyacetone the action took place exclusively through the calcium-independent insulin-sensitive process. This effect was absent in hepatocytes from adrenalectomized rats, suggesting that it is modulated by glucocorticoids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggerbeck M., Guellaen G., Hanoune J. Adrenergic receptor of the alpha 1-subtype mediates the activation of the glycogen phosphorylase in normal rat liver. Biochem Pharmacol. 1980 Feb 15;29(4):643–645. doi: 10.1016/0006-2952(80)90389-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Blackmore P. F., Steiner K. E., Exton J. H. Effects of adrenalectomy on hormone action on hepatic glucose metabolism. Reciprocal change in alpha- and beta-adrenergic activation of hepatic glycogen phosphorylase and calcium mobilization in adrenalectomized rats. J Biol Chem. 1979 Apr 10;254(7):2428–2433. [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. alpha-Adrenergic-mediated accumulation of adenosine 3':5' monophosphate in calcium-depleted hepatocytes. J Biol Chem. 1977 Dec 10;252(23):8645–8651. [PubMed] [Google Scholar]
- Chisholm A. B., Allan E. H., Titheradge M. A. Regulation of mitochondrial pyruvate carboxylation in isolated hepatocytes by acute insulin treatment. Biochem J. 1983 Aug 15;214(2):451–458. doi: 10.1042/bj2140451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S., García-Sáinz J. A. Alpha 1-adrenoceptor activation stimulates ureogenesis in rat hepatocytes. Eur J Pharmacol. 1981 Jul 10;72(4):387–390. doi: 10.1016/0014-2999(81)90582-3. [DOI] [PubMed] [Google Scholar]
- Corvera S., García-Sáinz J. A. Hypothyroidism abolishes the glycogenolytic effect of vasopressin, angiotensin II and A23187 but not that of alpha 1-adrenergic amines in rat hepatocytes. FEBS Lett. 1983 Mar 21;153(2):366–368. doi: 10.1016/0014-5793(83)80644-9. [DOI] [PubMed] [Google Scholar]
- Corvera S., García-Sáinz J. A. Vasopressin and angiotensin II stimulate ureogenesis through increased mitochondrial citrulline production. Life Sci. 1982 Nov 29;31(22):2493–2498. doi: 10.1016/0024-3205(82)90755-x. [DOI] [PubMed] [Google Scholar]
- Corvera S., Hernandez-Sotomayor S. M., Garcia-Sainz J. A. Modulation by thyroid status of cyclic AMP-dependent and Ca2+-dependent mechanisms of hormone action in rat liver cells. Possible involvement of two different transduction mechanisms in alpha 1-adrenergic action. Biochim Biophys Acta. 1984 Feb 17;803(1-2):95–105. doi: 10.1016/0167-4889(84)90060-0. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaye J. P., Hughes B. P., Blackmore P. F., Exton J. H. Insulin inhibition of alpha-adrenergic actions in liver. Biochem J. 1981 Mar 15;194(3):949–956. doi: 10.1042/bj1940949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K., Florio V. A., Twible D. A. The role of calcium ion as a mediator of the effects of angiotensin II, catecholamines, and vasopressin on the phosphorylation and activity of enzymes in isolated hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7147–7156. [PubMed] [Google Scholar]
- Hernández-Sotomayor S. M., García-Sáinz J. A. Adrenergic regulation of ureogenesis in hepatocytes from adrenalectomized rats. Possible involvement of two pathways of signal transduction in alpha 1-adrenergic action. FEBS Lett. 1984 Jan 30;166(2):385–388. doi: 10.1016/0014-5793(84)80118-0. [DOI] [PubMed] [Google Scholar]
- Hue L., Felíu J. E., Hers H. G. Control of gluconeogenesis and of enzymes of glycogen metabolism in isolated rat hepatocytes. A parallel study of the effect of phenylephrine and of glucagon. Biochem J. 1978 Dec 15;176(3):791–797. doi: 10.1042/bj1760791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Bahena J., Villalobos-Molina R., García-Sáinz J. A. Cycloheximide: an adrenergic agent. Life Sci. 1982 May 17;30(20):1757–1762. doi: 10.1016/0024-3205(82)90310-1. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kneer N. M., Lardy H. A. Regulation of gluconeogenesis by norepinephrine, vasopressin, and angiotensin II: a comparative study in the absence and presence of extracellular Ca2+1. Arch Biochem Biophys. 1983 Aug;225(1):187–195. doi: 10.1016/0003-9861(83)90022-x. [DOI] [PubMed] [Google Scholar]
- Kneer N. M., Wagner M. J., Lardy H. A. Regulation by calcium of hormonal effects on gluconeogenesis. J Biol Chem. 1979 Dec 10;254(23):12160–12168. [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Age-related changes in the control of hepatic cyclic AMP levels by alpha 1- and beta 2-adrenergic receptors in male rats. J Biol Chem. 1983 Apr 25;258(8):5103–5109. [PubMed] [Google Scholar]
- Morgan N. G., Waynick L. E., Exton J. H. Characterisation of the alpha 1-adrenergic control of hepatic cAMP in male rats. Eur J Pharmacol. 1983 Dec 9;96(1-2):1–10. doi: 10.1016/0014-2999(83)90522-8. [DOI] [PubMed] [Google Scholar]
- Pushpendran C. K., Corvera S., García-Sáinz J. A. Effect of insulin on alpha1-adrenergic actions in hepatocytes from euthyroid and hypothyroid rats. Possible involvement of two pathways in alpha1-adrenergic actions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):451–459. doi: 10.1016/0006-291x(84)91324-x. [DOI] [PubMed] [Google Scholar]
- Strickland W. G., Blackmore P. F., Exton J. H. The role of calcium in alpha-adrenergic inactivation of glycogen synthase in rat hepatocytes and its inhibition by insulin. Diabetes. 1980 Aug;29(8):617–622. doi: 10.2337/diab.29.8.617. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., White A. C., Aspry K., Cutts J., Fain J. N. Stimulation by vasopressin and alpha-catecholamines of phosphatidylinositol formation in isolated rat liver parenchymal cells. J Biol Chem. 1980 Mar 10;255(5):1938–1944. [PubMed] [Google Scholar]
- Whitton P. D., Rodrigues L. M., Hems D. A. Stimulation by vasopressin, angiotensin and oxytocin of gluconeogenesis in hepatocyte suspensions. Biochem J. 1978 Dec 15;176(3):893–898. doi: 10.1042/bj1760893. [DOI] [PMC free article] [PubMed] [Google Scholar]


